Abstract
Cell-cell and extracellular cell matrix (ECM) interactions provide cells with information essential for controlling morphogenesis, cell-fate specification, and cell death. In animals, one of the major groups of enzymes that degrade the ECM is the matrix metalloproteinases (MMPs). Here, we report the characterization of the cucumber (Cucumis sativus L. cv Marketmore) Cs1-MMP gene encoding such an enzyme likely to play a role in plant ECM degradation. Cs1-MMP has all the hallmark motif characteristics of animal MMPs and is a pre-pro-enzyme having a signal peptide, propeptide, and zinc-binding catalytic domains. Cs1-MMP also displays functional similarities with animal MMPs. For example, it has a collagenase-like activity that can cleave synthetic peptides and type-I collagen, a major component of animal ECM. Cs1-MMP activity is completely inhibited by a hydroxamate-based inhibitor that binds at the active site of MMPs in a stereospecific manner. The Cs1-MMP gene is expressed de novo at the end stage of developmental senescence, prior to the appearance of DNA laddering in cucumber cotyledons leaf discs and male flowers. As the steady-state level of Cs1-MMP mRNA peaks late in senescence and the pro-enzyme must undergo maturation and activation, the protease is probably not involved in nutrient remobilization during senescence but may have another function. The physiological substrates for Cs1-MMP remain to be determined, but the enzyme represents a good candidate for plant ECM degradation and may be involved in programmed cell death (PCD). Our results suggest that PCD occurs only at the culmination of the senescence program or that the processes are distinct with PCD being triggered at the end of senescence.
To develop normally, multicellular organisms need to produce new cells, but of equal importance they also need to be able to eliminate cells in a controlled and systematic fashion. To achieve this, cells have evolved a complex self-destructive program that due to their biochemical and genetic regulation has been termed programmed cell death (PCD). PCD has now been recognized as an indispensable facet of development, defense responses, and tissue sculpting in both animal and plant kingdoms. In animals PCD is often manifest as a stereotypical set of morphological and biochemical changes known as apoptosis. Cells undergoing PCD in plants often exhibit several characteristic death-associated features. Morphologically, the protoplast often condenses, leaving a visible gap between the cell wall and cell membrane. Similarly the nucleus condenses and the nuclear DNA is cleaved by PCD-activated nucleases, frequently into fragments that are multiples of 180 bp (McCabe et al., 1997; Danon and Gallois, 1998). Senescence is also a highly controlled sequence of biochemical and physiological degenerative events whereby nutrients are recycled from senescing cells to other parts of the plant such as meristems, young leaves, developing flowers, or storage tissues (Thomas and Stoddart, 1980; Smart, 1994; Noodén et al., 1997). Because senescence is developmentally programmed and ultimately results in cell death, it has been proposed that it qualifies as a bone fide occurrence of PCD (Gan and Amasino, 1997; Noodén et al., 1997).
Cotyledons of cucumber (Cucumis sativus L. cv Marketmore) provide an excellent experimental system in which to investigate the developmental changes taking place from an early phase of heterotrophic growth through phototrophic growth (Becker et al., 1978) to senescence (Graham et al., 1992; Kim et al., 1997). The development of cucumber cotyledons from germination through to senescence has been characterised at the cellular, molecular, and biochemical levels (Becker et al., 1978; Graham et al., 1992; Kim et al., 1997). Cucumber cotyledons have been invaluable in the study of enzyme regulation and gene expression, and in identifying novel cDNAs of developmentally regulated genes (Becker et al., 1978; Graham et al., 1992; Kim et al., 1997). To define cotyledon development as a model system we have identified seven stages based on chlorophyll content through which the cotyledons pass, including the terminal stage where death of the cells and organ occurs. Using these seven stages we have used cucumber cotyledon development to ask if senescence is an example of PCD and if molecular or biochemical indicators of activated PCD occur during senescence. The markers of PCD we have observed are only apparent at the terminal stage of cotyledon development, becoming apparent as a discreet developmental window at either the very end of or following the senescence program.
Having identified discrete senescent and PCD stages we have used the cotyledon model system to identify genes that are involved in late senescence and early PCD, and may therefore be involved in triggering or regulating PCD in plants. In this paper we describe the first of those genes, a matrix metalloproteinase (MMP), which is expressed late in senescence just before the PCD stage initiates. MMPs have been implicated in extracellular matrix (ECM) degradation during normal or pathological processes. In animals, the controlled remodeling and breakdown of the cell's ECM are important in biological processes such as PCD (Masuda et al., 1998) but also in growth, morphogenesis, cell-fate specification, cell migration, tissue repair, and pathological processes (Werb, 1997). Normal events such as embryogenesis, reproduction, remodeling of bone, and wound healing require controlled synthesis and removal of the various structural proteins, glycoproteins, and proteoglycans that make up the ECM. Degradation of the ECM is associated with several pathological conditions such as arthritis, cancer, and fibrotic diseases, which take place in a destructive manner (Woessner, 1998).
To date, there has been a single report of the existence of a MMP in higher plants. Graham et al. (1991) have purified the enzyme responsible for an EDTA-sensitive azocollase A activity present in crude soybean leaf extracts. They have shown that this enzyme is an MMP, which they have termed SEMP1 (McGeehan et al., 1992), and have reconstructed the SEMP1 cDNA using a PCR approach and RACE (Pak et al., 1997). The function of the soybean enzyme, SEMP1, remains to be clarified.
We report the isolation and characterization of a full-length cDNA encoding a second higher plant MMP in cucumber, which we have termed Cs1-MMP for cucumber MMP 1. We have analyzed the temporal pattern of Cs1-MMP gene expression during cucumber development and have also investigated the enzyme activity. Being able to recognize distinct developmental stages through which cucumber cotyledons pass, has allowed us to distinguish between senescence and PCD. The developmental progression is also useful in identifying genes that are expressed late in senescence or early in PCD. The effectiveness of this has been demonstrated by the isolation of a MMP, which is expressed at the developmental boundary of senescence and PCD in cucumber.
RESULTS
Development of Cucumber Cotyledons
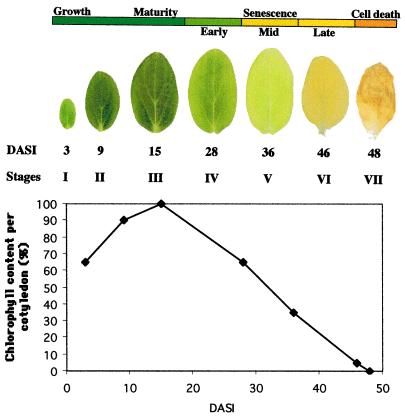
Cucumber cotyledons provide a good model system in which to investigate metabolic and developmental processes, since the changes that occur over time within this tissue do so in the absence of cell division and are relatively uniform throughout the entire organ (Becker et al., 1978). To facilitate these studies and to address questions we had on senescence and PCD, we formalized the developmental phases that the cotyledon passes through from germination through early growth, maturity, and early to late senescence. Additionally, unlike earlier studies using cucumber cotyledons, we included the final stage of cotyledon development where the organ turns brown and collapses. Senescence is often defined by the chlorophyll content of a cell, and one of the advantages of using cucumber cotyledons is that although an organ often contains cells in various stages of development with for example green, yellow, and brown sectors, one can also find cotyledons where the cells are of a uniform color throughout the organ. By carefully searching for and selecting these synchronized cotyledons we were able to characterize the different phases of development on the basis of chlorophyll content and organ color. As can be seen from Figure 1, by d 3 after imbibition the cotyledons are green and just starting their growth phase (stage I). Then they undergo a period of expansion until they are mature around d 15 (stages II and III). By d 28, the cotyledon retains 65% of mature cotyledon chlorophyll content (stage IV); this is the early senescent stage. By d 36, the cotyledon retains 35% of mature cotyledon chlorophyll content (stage V); this is the mid-senescence stage. The cotyledon subsequently becomes yellow in late senescence (stage VI). Following the yellow stage, the cotyledon turns brown and begins to desiccate, causing the organ to shrivel and become crispy (stage VII). This brown stage is accompanied by death of the organ cells.
Figure 1.
The developmental stages of cucumber cotyledons. Stages through which the cotyledons pass from imbibition, through early expansion growth to maturity, early to late senescence, and finally cell death are defined as days after seed imbibition (DASI) on the basis of chlorophyll content and organ color. On d 3, cotyledons are green and just starting their expansion growth phase (stage I), then they undergo a period of expansion until they are mature around d 15 (stage II and III). By d 28, the cotyledon retains 65% of mature cotyledon chlorophyll content (stage IV); this is termed the early senescent stage. By d 36, the cotyledon retains 35% of mature cotyledon chlorophyll content (stage V); this is termed the mid-senescence stage. The cotyledon subsequently becomes yellow in late senescence (stage VI). Following the yellow stage the cotyledon enters a brief stage where it turns brown and becomes crispy (stage VII).
Temporal Activation of PCD in Cucumber Organs and Tissues
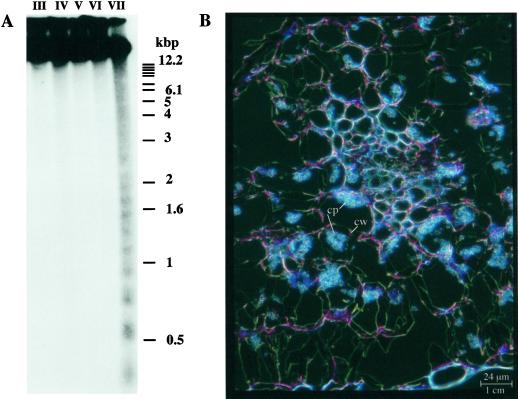
We wanted to determine whether dying cucumber cotyledon cells exhibited any of the characteristic features associated with PCD in higher plants, namely internucleosomal cleavage of DNA and condensation of the protoplast away from the cell wall. Southern-blot analysis showed that internucleosomal cleavage of DNA does occur during the developmental progression of the cotyledons (Fig. 2A). It also demonstrated that the PCD-associated cleavage is restricted exclusively to the final brown stage of cotyledon development. Sectioning and microscopic analysis of these brown cotyledons indicated that the protoplasts of the dead cells had condensed away from the cell wall in a characteristic PCD morphology (Fig. 2B). To demonstrate that PCD was a regular occurrence during or following senescence and is not solely a feature of the cotyledon senescence, we analyzed DNA patterns in a developmental sequence during leaf and cucumber male flower development. In both sequences cell death was accompanied by DNA laddering (data not shown).
Figure 2.
A, Southern-blot analysis showing internucleosomal cleavage of DNA following senescence of cucumber cotyledons. Genomic DNA was extracted from cotyledons (stage III–VII). Five hundred nanograms of genomic DNA was separated by electrophoresis on a 1.5% (w/v) agarose gel. After transfer to a nylon filter, the blot was hybridized to a total cucumber genomic DNA probe prepared by labeling Sau3AI digested DNA fragments. B, Microscopic analysis showing the characteristic PCD morphology. This section through a brown cotyledon (stage VII) was viewed with a Leica microscope and photographed under dark-field. cw, Cell wall; cp, condensed protoplast.
Isolation and Characterization of Genes Associated with the Transition from Senescence to PCD
We were interested in identifying genes that are expressed in late senescence and/or early PCD. To do this, we used a differential screening approach. Construction of two cDNA libraries from RNA of senescent cotyledons (>90% yellow) and RNA of green cotyledons (3 d after seed imbibition) has been previously described (Kim and Smith, 1994; Kim et al., 1997). Differential screening of these two cDNA libraries identified two senescence-related cDNA clones. The characterization of the first clone identified and encoding a putative SPF1-type DNA-binding protein has been reported (Kim et al., 1997). The second cDNA identified showed no significant homology in the databases. Preliminary northern-blot analysis using this cDNA (421 bp) as a probe has revealed a 1.1-kb transcript suggesting that a full-length cDNA would be about three times larger.
Repeated screening of the cDNA library from RNA of senescent fully yellow cotyledons did not allow us to isolate a full-length cDNA. Therefore, we constructed a cucumber genomic library to isolate the corresponding gene. Southern-blot analysis revealed that there was only one copy of this gene in the cucumber genome (data not shown). We isolated 11 genomic λ clones completing three rounds of screening using the partial cDNA as a probe. Restriction mapping of these λ clones allowed us to identify a 6-kb SalI fragment that hybridized to the partial cDNA clone. This fragment was subcloned into a plasmid vector and the DNA sequence was determined for a region of 3 kb.
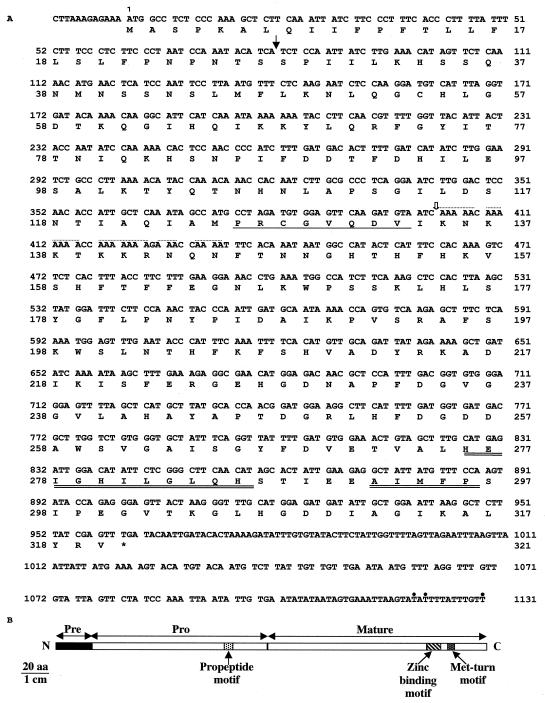
The gene is intronless, has an open reading frame of 960 bp, and encodes a 320-amino acid polypeptide with a calculated molecular mass of 35 kD (Fig. 3). The partial cDNA is located at the 5′ end of the gene and we believe that poly(A) stretches intervening within positions 403 through 434 are responsible for oligo(dT) priming during reverse transcription, leading preferentially to the synthesis of a partial cDNA (421 bp). RACE-PCR (Frohman et al., 1988) was used to map the end of the cDNA, and three alternative polyadenylation sites were identified at the 3′ end of the Cs1-MMP gene.
Figure 3.
Sequence analysis of the Cs1-MMP cDNA. A, Nucleotide sequence of the Cs1-MMP cDNA. The deduced amino acid sequence is shown below the coding sequence and the asterisk indicates the termination codon. The white arrow denotes the 5′ end of the partial cDNA. The dotted line indicates poly(A) stretches intervening within positions 403 through 434 and responsible for oligo(dT) priming during reverse transcription leading to the synthesis of the partial cDNA. The black arrow denotes the putative signal peptide cleavage site. The single-underlined sequence represents the propeptide motif characteristic of the propeptide domain. Double-underlined sequences indicate the zinc-binding consensus sequence and the Met-turn-like motif characteristics of the catalytic domain. Cs1-MMP polyadenylation sites at the 3′ end of the sequence, identified by RACE-PCR, are indicated with a dot. The EMBL data library accession number is AJ133371. B, Schematic representation of the predicted Cs1-MMP protein showing the pre-pro-enzyme, which displays all the hallmark motifs of MMPs, propeptide, zinc binding, and Met-turn motifs.
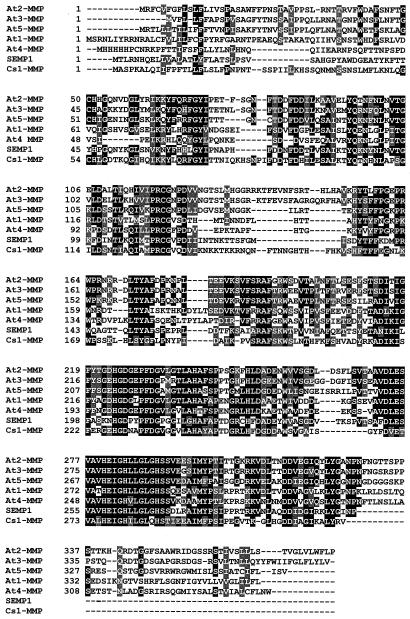
Database searches indicated that the deduced polypeptide shares significant similarity with the metzincin superfamily of zinc metalloproteinases and in particular with the MMP family (Hooper, 1994; Stöcker et al., 1995). It shares 31.6% to 38.6% identity with members of the MMP family recently identified in soybean (Pak et al., 1997) and Arabidopsis (five members available from the genomic sequencing project) (Fig. 4). Since the function is currently unknown for Arabidopsis enzymes, and to be consistent with the naming of animal MMPs, we have named them At-MMPs for Arabidopsis MMPs and numbered them in the order of their discovery in the database.
Figure 4.
Amino acid alignment for the seven higher plant MMPs available to date. The soybean SEMP1 sequence is from Pak et al. (1997). The five Arabidopsis MMP sequences are termed At-MMPs for Arabidopsis MMPs and are numbered in the order of their discovery in the database. Their accession numbers are the following: At1-MMP (Z97341), At2-MMP (AC002062, F20P5.11), At3-MMP (AC002396, F3I6.6), At4-MMP (AF062640 directly submitted to the database by Liu et al. [1998]), and At5-MMP (AC005966, T2K10.2). At1-MMP (chromosome 4), At2-MMP, At3-MMP, and At5-MMP (chromosome 1) come from the genomic sequencing project, whereas At4-MMP is a cDNA sequence. SEMP1 is a secreted protein and Cs1-MMP is also potentially targeted for secretion. At1-MMP, At2-MMP, At3-MMP, and At5-MMP are potential MT-type MMPs based on predictions of localization sites, whereas At4-MMP would have an uncleavable signal peptide and would be retained in the endoplasmic reticulum with a certainty of 55.5% only. At4-MMP may represent a new class of MMPs but its leader sequence, in particular the poly-His following the Met, together with the low homology up to residue 58 looks atypical. However beyond the residue 58, the alignment shows a good degree of homology. This would suggest that there is perhaps a mistake causing a frameshift in the sequence and that At4-MMP would belong to one of the two classes (secreted and MT) already defined.
The deduced Cs1-MMP polypeptide is a pre-pro-enzyme that has all the hallmark motif characteristics of MMPs (Figs. 3 and 4). First, Cs1-MMP has a putative N-terminal signal peptide with a central hydrophobic core and predicted cleavage site between amino acids Ser-27 and Ser-28. We believe that this leader sequence targets the protein for secretion, and this would predict an extracellular localization. Second, it has a propeptide domain that contains a PRCGVPDV-like motif whose Cys is found in all MMPs characterized to date and is normally coordinated to the catalytic zinc ion, maintaining the enzyme in its zymogen form. The dissociation of this bond and its replacement with water in the inactive pro-enzyme “switches” the role of the zinc from a noncatalytic to a catalytic one (Springman et al., 1990; Van Wart and Birkedal-Hansen, 1990). This Cys switch mechanism is followed by a cleavage of the propeptide domain thus generating the active enzyme. Finally, its catalytic domain has a zinc-binding consensus sequence HEXXHXXGXXH, which is followed by a conserved Ser and whose three histidines could be involved in binding the catalytically essential zinc ion. Its catalytic domain also has a Met-turn-like motif ABMYP. The x-ray crystal structure of MMPs from the crayfish, snake venom, and human fibrobast collagenase revealed that such a Met-turn acts as a fourth zinc ligand (Bode et al., 1993; Lovejoy et al., 1994a, 1994b).
Expression of the Cs1-MMP Gene 0
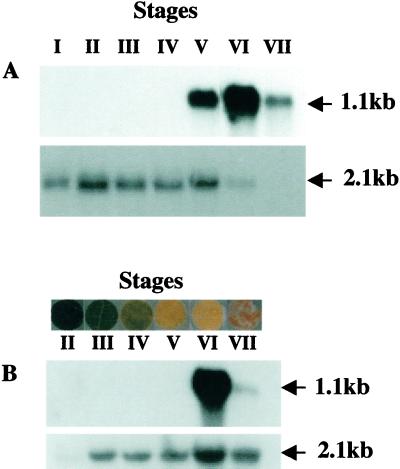
RNA-blot analysis was used to investigate expression of the Cs1-MMP gene during cucumber cotyledon development in a number of staged cotyledon samples harvested after seed imbibition. We describe here the results obtained using the partial cDNA as a probe (Fig. 5A). All these results were confirmed using the full-length cDNA as a probe (data not shown). A maize ubiquitin cDNA probe (Christensen et al., 1992) was hybridized to the same RNA samples as a control. A 1.1-kb transcript was detected late in senescence and was clearly abundant in fully yellow cotyledons (stage VI) and less abundant in fully brown cotyledons (stage VII). The Cs1-MMP gene represents the first gene characterized to date that is expressed de novo at the end stage of developmental senescence and maximal expression occurs 2 d before the appearance of DNA laddering in cucumber cotyledon development.
Figure 5.
Northern-blot analysis of expression of the Cs1-MMP gene. RNA isolated from different developmental stages of cucumber cotyledon (A) and leaf discs (B) were hybridized with the partial Cs1-MMP cDNA (top panel) and the maize Ubi cDNA as a control (bottom panel). Arrows indicate sizes of the transcripts. A, Cotyledons were harvested at the indicated stages as previously described in Figure 1. B, Leaves in which all the cells were at a similar stage of senescence were rare so discs were excised from regions of the leaf where cells were of a uniform color. Stage II refers to RNA isolated from discs excised from fully expanded green leaves from young plants. Stage III refers to RNA isolated from discs excised from mature green leaves isolated from plants that had started flowering. Stages IV and V refer to RNA isolated from discs excised from regions of senescing leaves showing 65% and 35% of green leaf chlorophyll levels, respectively. Stage VI refer to RNA isolated from leaf discs excised from yellow regions. Stage VII refer to RNA isolated leaf discs excised from dead (brown) regions.
We determined whether the Cs1-MMP gene was cotyledon specific or expressed at the boundary of senescence and cell death in whichever organ was undergoing these processes. RNA-blot analysis was used to look at the expression of this gene during cucumber leaf development (Fig. 5B). Cs1-MMP mRNA was only detected in fully yellow leaf discs late in senescence (stage VI) and was still detected in dead leaf discs (stage VII). We also studied the expression of this gene during cucumber male flower development. We have analyzed separately the sterile organs (perianth) and reproductive organs (anthers) of the male flower. The 1.1-kb Cs1-MMP transcript was detected in the perianth of senescing and abscissing male flowers but was not detected in anthers (data not shown).
Activity of the Cs1-MMP Protein
The significance of the action of any enzyme is generally related to the substrates that it acts on and the physiological consequences of these actions. Accordingly animal MMPs have been recognized as a class of enzymes that plays a critical role in ECM turnover and remodeling based on their ability to hydrolyze the major protein components of the ECM (Imper and Van Wart, 1998). The high homology between Cs1-MMP and animal MMP sequences suggested that this enzyme might also play a role in plant ECM degradation. To delineate the functional similarities or differences between plant and animal MMPs, we overexpressed the mature Cs1-MMP protein in Escherichia coli, purified and refolded the His-tagged recombinant enzyme, and tested Cs1-MMP activity using known animal substrates and inhibitors.
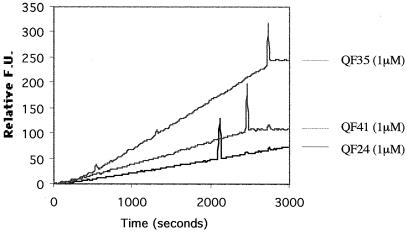
Interstitial collagenase (MMP-1) has the unique capacity to cleave at a specific Gly-Leu/Ile peptide bond in each α-chain of interstitial collagen (Imper and Van Wart, 1998). In order to investigate Cs1-MMP activity, we used a fluorometric assay and tested whether it was able to hydrolyze available quenched fluorescent (QF) peptide substrates. In these substrates, a short sequence of amino acids contains the scissile bond Gly-Leu/Ile, which separates a naturally fluorescent derivative of 7-methoxy-coumarin from a dinitrophenyl group that acts as an internal quencher (Knight et al., 1992). Screening available QF substrates designed on amino acid substitutions surrounding the scissile bond, we have identified that Cs1-MMP efficiently hydrolyzes three of these QF substrates: QF-24, QF-41, and QF-35 (Fig. 6). QF-24, which is an excellent substrate for all MMPs (Knight et al., 1992), is degraded at the lower rate compared with QF-41 and QF-35. QF-41, which is a substrate for fibroblast collagenase and gelatinase B (Knaüper et al., 1996), is hydrolyzed more slowly than QF-35, which is a stomelysin substrate (Murphy et al., 1994). Thus QF-35 was the most efficiently hydrolyzed and the best substrate further to study Cs1-MMP activity.
Figure 6.
Analysis of activity of the Cs1-MMP protein using a fluorometric assay. Relative fluorescence units (F.U.), which are arbitrary units, are given in accordance with time in seconds. Hydrolysis of three QF substrates (QF-24, QF-41, and QF-35) and effect of protease inhibitors on Cs1-MMP activity. Injection spike indicates when each protease inhibitor was added: EDTA-free cocktail of protease inhibitors for the QF-24 assay, hydroxamate-based inhibitor, batimastat (BB94), for QF-41, and QF-35 assays.
To investigate whether the Cs1-MMP activity detected was specific, we first added a cocktail of protease inhibitors to the QF-24 assay. This cocktail was EDTA-free and thus able to inactivate any protease activity except MMP activity. As shown in Figure 6, the rate of fluorescence, corresponding to the hydrolysis of the QF-24 substrate by Cs1-MMP, was unaffected after the injection spike marking the addition of the EDTA-free cocktail of protease inhibitors.
Second, we tested the effect of an hydroxamate-based inhibitor (batimastat [BB94]), which binds reversibly at the active site of MMPs in a stereospecific manner (Beckett et al., 1996). This inhibitor incorporates a zinc binding group (hydroxamic acid) chelating the active site zinc ion and corresponds to a peptide analog of the sequence on the lefthand side of the collagenase cleavage site Gly-Leu/Ile. As shown in Figure 6, the degradation of the QF-41 or the QF-35 substrate by Cs1-MMP was completely inhibited by the addition of 3 μm BB94.
Finally, MMP activity in mammals is regulated by natural endogenous inhibitors. These tissue inhibitors of metalloproteinases (TIMPs) form tight 1:1 stoichiometric complexes with MMPs. Therefore, we investigated whether Cs1-MMP activity was affected by known human TIMPs, TIMP-1, -2, -3, and -4. We found that TIMP-2, -3, and -4 had no effect, whereas TIMP-1 slightly reduced Cs1-MMP activity (data not shown).
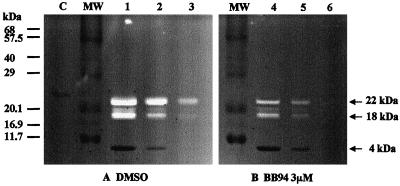
As little as 1 pg of gelatinase can be detected using an SDS-PAGE gel electrophoresis incorporating type-I collagen, as the gelatin substrate, into the gel (Murphy and Crabbe, 1995). We used this gelatin zymography technique to test Cs1-MMP activity and run a range of dilutions (undiluted, 1:5, and 1:50 dilutions) of the refolded enzyme to make sure that the gelatin lysis was in the linear range. The location of the gelatinolytic activity is detectable as a clear band in the blue background of uniform staining. Figure 7A shows that two clear bands of 18 and 22 kD corresponding to gelatinolytic active Cs1-MMP and one small Coomassie Blue-stained band of 4 kD were detected. We believe that Cs1-MMP is self-processing cleaving the N-terminal His-TAG, thus the two clear bands of 18 and 22 kD correspond respectively to processed and unprocessed Cs1-MMP and the 4-kD blue-stained band corresponds to the cleaved N terminus. The gelatin zymography technique is not strictly quantitative but some comparison within the range of dilutions shows a linear decrease from the most concentrated to the most diluted sample. Figure 7B shows the inhibitory effect of BB94 on Cs1-MMP gelatinolytic activity within the same range of dilutions. The results clearly demonstrate that the degradation of type-I collagen is also completely inhibited in the presence of 3 m BB94 for the most diluted enzyme sample. These results clearly indicated that Cs1-MMP has a collagenase-like activity and can degrade a major component of the ECM in animals.
Figure 7.
Gelatin zymography analysis of Cs1-MMP activity. An aliquot (10 L) of the undiluted refolded enzyme (lanes 1 and 4) and of a range of dilution (1:5, lanes 2 and 5; 1:50, lanes 3 and 6) was fractionated by electrophoresis in a 15% (w/v) SDS-acrylamide gel containing type-I collagen as a gelatin substrate. An aliquot of the overexpressed but not refolded protein was also loaded as a control (C). The gel was cut in half and incubated overnight in the assay buffer containing either dimethyl sulfoxide as a control (A) or 3 m BB94 (B).
DISCUSSION
Timing of Developmental PCD during Cucumber Development
DNA laddering is a hallmark of animal apoptosis and has also been described during death of plant cells. It has been shown to occur after abiotic treatment and fungal infection (Ryerson and Heath, 1996) and during carpel senescence (Orzáez and Granell, 1997). We have shown that the PCD hallmark DNA laddering occurs following senescence in cucumber cotyledons and leaves. The laddering occurs only in the final stage (VII) of cotyledon development and is not associated with early, mid-, or late senescence. We also found that internucleosomal DNA cleavage occurs in the later stages of male flower development and seems to be synchronized with the sequential death of the separate whorls of the flower.
We also looked for morphological signs of PCD. Cells undergoing PCD often have their protoplast collapsed away from the cell wall leaving a significant gap between cell wall and cell membrane. McCabe et al. (1997) showed that in cells that encounter a lethal abiotic stress the protoplast collapses away from the cell wall. This morphological collapse is a feature of cells that have died following the hypersensitive response (Levine et al., 1996) and is also a feature of developmental cell deaths, for example, during xylogenesis (Schindler et al., 1995). Cellular collapse has also been reported in senescent cells. Matile and Winkenbach (1971) observed in the corolla of morning glory (Ipomoea purpurea) that the final stages of senescent cell death were preceded by shrinkage of the whole protoplast. The cells of stage VII cotyledons, where DNA processing was taking place, were also seen to display this plant PCD morphology.
DNA laddering and a retracted protoplast are both hallmark features of PCD. Using both of these markers we were able to show temporally where cell death occurred in the developmental sequence of cucumber cotyledons. Our results suggest that senescence and PCD should not be regarded as synonymous terms and that PCD phase occurs only at the culmination of the senescence program or alternatively after the senescence program has been completed in cotyledons. It has been shown that cell death can be uncoupled from defense gene activation during the hypersensitive response (Mittler et al., 1996; del Pozo and Lam, 1998). This poses the question: Do senescence and PCD overlap or can senescence and PCD also be distinguished and uncoupled from each other? Searching for genes that may regulate or initiate PCD and that would therefore be expressed at the boundary of senescence and PCD should provide clues to answer this question. Cs1-MMP encoding an MMP, is the first such gene we have identified that is expressed at the boundary between the two phases.
Characterization of Cs1-MMP, a Gene Expressed at the Boundary of Senescence, and PCD
Mammalian MMPs are divided into four main classes based on their substrates specificities: interstitial collagenases degrading collagens of type I, II, and III; gelatinases degrading type-IV collagen and gelatins; stromelysins and matrilysins degrading not only type-IV collagen but also laminin, fibronectin, and proteoglycans; and membrane-type MMPs (MT-MMPs) (Baramova and Foidart, 1995). Cs1-MMP and SEMP1 share the matrilysin minimal enzyme structure that consists of a signal peptide, a propeptide, and a catalytic domain, whereas other mammalian MMPs have additional fibronectin like repeats within their catalytic domain or hemopexin like repeats as a C-terminal extension.
Unlike mammalian MMP genes, which consist of at least 10 exons and nine introns and are members of a multigene family, Cs1-MMP and SEMP1 genes both lack introns and are present as a single-copy gene in the genome. The soybean MMP (SEMP1) is present in intercellular fluids suggesting it is an extracellular protein (Pak et al., 1997). We believe that the N-terminal putative signal peptide present in the Cs1-MMP protein targets the cucumber MMP for secretion also predicting an extracellular localization. Conversely, four Arabidopsis MMPs identified from the genome sequencing project have a C-terminal extension. Prediction of protein localization sites reveals (certainty of 91.9%) that these Arabidopsis MMPs are putative C-terminal membrane-anchored proteases (Maidment et al., 1999). This suggests that none of these Arabidopsis MMP genes correspond to a Cs1-MMP homolog and, as with mammalian MMP genes, plant MMP genes belong to a multigene family. It also suggests that there are MT-MMPs in plants, and their location at the surface of cells implies that they could play a significant role in the modulation of cell-matrix interactions. In common with mammalian MT-MMPs (Butler et al., 1997; d'Ortho et al., 1997), plant MT-MMPs could be involved in processing the propeptide of secreted MMPs resulting in a metalloproteinase-activation cascade.
Suggested Roles for Plant MMPs
Pak et al. (1997) have shown that SEMP1 gene expression is synchronized with mature stages of leaf development. The SEMP1 mRNA begins to accumulate approximately 10 d after leaf emergence and remains at a constant steady-state level until leaves become senescent. The temporal pattern of protein accumulation parallels that of the mRNA. They have suggested that the soybean MMP may play an important role in tissue remodeling, which occurs during leaf expansion or may alternatively serve a defensive role in plant leaves.
In this paper we have shown by northern-blot analysis that the Cs1-MMP gene is expressed at the boundary of senescence and cell death in cucumber cotyledon development. The Cs1-MMP gene is not expressed in mature green leaf and thus does not seem to correspond to a SEMP1 homolog. Another striking difference between Cs1-MMP and SEMP1 concerns the ability to degrade collagen. We have shown that Cs1-MMP is able to degrade type-I collagen using gelatin zymography, whereas McGeehan et al. (1992) stated that SEMP1 did not cleave collagen even when present at high concentration.
MMP activity requires high levels of regulation in mammals. The propeptide domain has to be processed by the so called “Cys switch” mechanism to generate the activated protease. The MMPs are consequently secreted in a latent form and activated in situ by physiological mechanisms that remain to be clarified (Springman et al., 1990; Van Wart and Birkedal-Hansen, 1990). Additionally, MMP activity is also regulated by endogenous inhibitors. These TIMPs form tight 1:1 stoichiometric complexes with MMPs. Subsequently, the balance between MMPs and TIMP activity is critical for the catabolism of the ECM (Vallon et al., 1997). The mammalian TIMP-1 has an inhibitory effect on both Cs1-MMP and SEMP1. This suggests that a cognate TIMP-like inhibitor might also exist in plants. As the steady-state level of Cs1-MMP mRNA peaks late in senescence and MMP enzyme needs to undergo maturation and activation (processing of the propeptide and possible TIMP-like inactivation), the protease is probably expressed too late to be involved in nutrient remobilization during senescence but may have another function.
One possibility is that Cs1-MMP may be involved in some aspects of PCD that occur soon after it is expressed. Involvement of MMPs in PCD in animals is not without precedent. For example, Masuda et al. (1998) have recently purified and characterized two apoptosis-inducing proteins from hemorrhagic snake venom and shown that both specifically kill vascular endothelial cells in culture. The death of the cells exhibits the characteristic molecular and morphological apoptotic changes (DNA fragmentation and collapsed morphology). Analysis of the partial amino acid sequences of these proteins revealed similarities to members of the metalloprotease/disintegrin family. Additionally, Vu et al. (1998) have shown that gelatinase B (MMP-9) is a key regulator of growth-plate angiogenesis and apoptosis of terminal hypertrophic chondrocytes and suggested that this enzyme might be required to generate signals and release angiogenic molecules sequestered in the ECM. In plants, tracheary element differentiation requires strict coordination of secondary cell wall synthesis and PCD to produce a functional cell corpse. Groover and Jones (1999) have recently proposed a model in which the concomitant secretion of a Ser protease and secondary cell wall precursors might be involved in secondary cell wall synthesis and cell death. Alternatively, the hydrolysis of the primary cell wall during tracheary element differentiation could release a signal molecule triggering cell death, as PCD occurs in response to wall-derived elicitor molecules during the hypersensitive response.
To date the physiological substrates for higher plant MMPs are unknown. Substrates have only been reported in the lower plant species, Chlamydomonas reinhardtii, for a gamete lytic enzyme that displays all the hallmark features of animal MMPs and mediates cell wall release and degradation as a necessary prelude to cell fusion (Inam and Snell, 1988; Kinoshita et al., 1992). The physiological substrate for Cs1-MMP remains to be determined, but the enzyme does display proteolytic activity against an animal ECM component and may act in the plant ECM in an analogous fashion to release signaling molecules. Alternatively, Cs1-MMP could be functionally similar to the enzymes that eliminate cell remnants in animal apoptosis, thereby contributing to the final cleanup of dead plant organs by processing corpses and digesting away cell residues.
MATERIALS AND METHODS
Plant Material
Cucumber (Cucumis sativus L. cv Marketmore) seeds were obtained from W. McNair (Portobello, Edinburgh, UK) and growth conditions were as previously defined (Kim and Smith, 1994). Representative cotyledons were selected on the basis of time from imbibition and uniform color throughout the entire organ. Leaves in which all the cells were at a similar stage of senescence (based on color) were rare so discs were excised from regions of the leaf where cells were of a uniform color. Chlorophyll content was determined as described previously (Arnon, 1949). Cucumber material was photographed with a Nikon 801S camera (Nikon, Tokyo).
Sectioning
Cotyledons slices (2 mm2) were placed in fixative (3% [v/v] paraformaldehyde, 50% [v/v] ethanol, and 5% [v/v] acetic acid) overnight prior to dehydration through ethanol series. Cotyledon material was prepared in polyethylene glycol 1500 as detailed in Marrison and Leech (1992), and 5- to 15-μm sections were cut using a stainless steel blade on a rotary Gallenkamp (Buffalo, NY) microtome. Sections were viewed with a microscope (Leica Microsystems, Wetzlar, Germany) and photographed under dark-field.
Library Construction and Screening
The construction and differential screening of the two cDNA libraries from RNA of senescent cotyledons (>90% yellow) and RNA of green cotyledons (3 d after seed imbibition) have been described previously (Kim and Smith, 1994; Kim et al., 1997). Genomic DNA was extracted according to Draper et al. (1988) and a genomic library of size-fractionated Sau3AI restriction fragments was constructed (Sambrook et al., 1989) in λ-DashII (Stratagene, La Jolla, CA). One million plaques were screened using the Cs1-MMP partial cDNA as a probe. Hybridizations were carried out under standard conditions and filters were washed at high stringency (Sambrook et al., 1989). Positive phages were purified by three rounds of plaque purification. Eleven genomic λ clones were obtained. A 6-kb SalI fragment carrying the Cs1-MMP gene was subcloned into pBluescript KS− (Stratagene) and 3 kb of DNA was sequenced (MWG-Biotech, Milton Keynes, UK). Cs1-MMP mRNA polyadenylation sites were identified by RACE-PCR (Frohman et al., 1988).
Southern- and Northern-Blot Analyses
For Southern blots, genomic DNA was extracted from stage-specific cotyledons according to Draper et al. (1988). DNA was separated on a 1.5% (w/v) agarose gel, transfered to nylon filters for hybridizations, and hybridized to a radioactive probe prepared with Sau3AI digested total genomic DNA. Total RNA was extracted using the protocol described previously (Delorme et al., 1997). RNA was separated on 1.5% (w/v) agarose formaldehyde gels and transferred to nylon filters for hybridizations. DNA probes were prepared using a random primed DNA labeling method (Draper et al., 1988). Hybridizations were carried out under standard conditions and filters were washed at high stringency (Sambrook et al., 1989).
Overexpression in Escherichia coli and Refolding of Cs1-MMP
PCR amplification of the mature enzyme-encoding domain of Cs1-MMP was carried out with the following primers, 5′-ACGGATCCACCTTCTTTGAAGGAAACCTGA-3′ and 5′-TAGTCGACAACTCGATAAAGAGCCTTAATTCC-A-3′and using the PfuTurbo DNA polymerase (Stratagene) according to the instructions of the supplier. The PCR amplified fragment was cloned into the BamHI/SalI sites of pQE30 (Qiagen Inc., Chatsworth, CA) to create a fusion protein containing a 6-His sequence followed by residues 161 to 319 of Cs1-MMP. Expression of the fusion protein in E. coli M15pREP4 strain was carried out as described by the supplier (Qia-expressionist kit, Qiagen Inc.). Purification of the 6-His tagged fusion protein was carried out using either the TALON metal affinity resin (CLONTECH Laboratories, Palo Alto, CA) or a nickel-nitrilo-tri-acetic acid resin (Qia-expressionist kit, Qiagen Inc.) as described by the suppliers and eluted in the presence of imidazole. Refolding was carried out either before or after purification of the 6-His tagged protein using the following buffer: 20 mm Tris (tris[hydroxymethyl]aminomethane)/H2SO4, pH 7.5, 10% (v/v) glycerol, 100 mm Na2SO4, 0.5 μm ZnSO4, 0.02% (w/v) NaN3, and 0.5 mL/L Brij35.
Hydrolysis of a QF Peptide Substrate
The assay was carried out at 37°C using equal volume of concentrated, refolded enzyme within one experiment, in 2.5 mL of buffer (50 mm Tris/HCl, pH 7.5, 10 mm CaCl2, 150 mm NaCl, and 0.05% [v/v] Brij 35) containing 1 μm final substrate, by continuous monitoring using a fluorometer (excitation wavelength of 328 nm, emission wavelength of 393 nm, Luminescence Spectrometer LS50B, Perkin Elmer, Foster City, CA). The instrument was set to zero with substrate buffer (as described in Maidment et al., 1999) and calibrated with 7-methoxycoumarin-Pro-Leu.
Gelatin Zymography
Gelatin (1 mg/mL final concentration) was copolymerized into 15% (w/v) polyacrylamide-SDS gels. Refolded samples were dissolved in unreduced Laemmli buffer (62.5 mm Tris-HCl, pH 6.8, 2% [w/v] SDS, 10% [w/v] glycerol, 5% [v/v] mercaptoethanol, and 0.001% [w/v] bromophenol blue) and loaded on the gel without boiling (Laemmli, 1970). After electrophoresis, the gel was washed in Triton X-100 before incubation overnight at 37°C in an assay buffer (100 mm Tris-HCl, pH 7.5, 30 mm CaCl2, and 0.02% [w/v] NaN3). Subsequently, the gel was stained with Coomassie Blue to detect bands of gelatin digestion.
ACKNOWLEDGMENTS
We thank Gillian Murphy, Vera Knaüper, and Augustin Amour for help with refolding the Cs1-MMP enzyme and testing its activity; we thank British Biotech Pharmaceuticals (Oxford, UK) for allowing us to use the MMP inhibitor BB94 under Gillian Murphy's supervision. We are grateful to Georges Freyssinet and David Cole for constructive discussion and to John Baker for assistance with photography.
Footnotes
This work was supported by Rhône Poulenc (Paris). P.F.M. was funded by a Biotechnology and Biological Science Research Council research grant (to C.J.L.).
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baramova E, Foidart J-M. Matrix metalloproteinase family. Cell Biol Int. 1995;9:239–242. [PubMed] [Google Scholar]
- Becker WM, Leaver CJ, Weir EM, Riezman H. Regulation of glyoxysomal enzymes during germination of cucumber: I. Developmental changes in cotyledonary protein, RNA, and enzyme activities during germination. Plant Physiol. 1978;62:542–549. doi: 10.1104/pp.62.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett RP, Davidson AH, Drummond AH, Huxley P, Whittaker M. Recent advances in matrix metalloproteinase inhibitor research. Drug Dev Today. 1996;1:16–26. [PubMed] [Google Scholar]
- Bode W, Gomis-Rüth F-X, Stöcker W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the ‘metzincins.’. FEBS Lett. 1993;1–2:134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- Butler GS, Will H, Atkinson S, Murphy G. Membrane-type-2 matrix metalloproteinase can initiate the processing of progelatinase A and is regulated by the tissue inhibitors of metalloproteinases. Eur J Biochem. 1997;244:653–657. doi: 10.1111/j.1432-1033.1997.t01-1-00653.x. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript spicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Danon A, Gallois P. UV-C radiation induces apoptotic-like changes in Arabidopsis thaliana. FEBS Lett. 1998;437:131–136. doi: 10.1016/s0014-5793(98)01208-3. [DOI] [PubMed] [Google Scholar]
- Delorme V, Keen CL, Rai KN, Leaver CJ. Cytoplasmic-nuclear male sterility in pearl millet: comparative RFLP and transcript analyses of isonuclear male-sterile lines. Theor Appl Genet. 1997;95:961–968. [Google Scholar]
- del Pozo O, Lam E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol. 1998;8:1129–1132. doi: 10.1016/s0960-9822(98)70469-5. [DOI] [PubMed] [Google Scholar]
- d'Ortho M-P, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad spectrum proteolytic capacities comparable to many matrix metalloprote inases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- Draper J, Scott R, Armitage P, Walden R. Plant Genetic Transformation and Gene Expression: A Laboratory Manual. Oxford: Blackwell Scientific Publications; 1988. pp. 199–261. [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Making sense of senescence. Plant Physiol. 1997;113:313–319. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham IA, Leaver CJ, Smith SM. Induction of malate synthase gene expression in senescent and detached organs of cucumber. Plant Cell. 1992;4:349–357. doi: 10.1105/tpc.4.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Xiong J, Gillikin JW. Purification and developmental analysis of a metalloproteinase from the leaves of Glycine max. Plant Physiol. 1991;97:786–792. doi: 10.1104/pp.97.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Jones AM. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 1999;119:375–384. doi: 10.1104/pp.119.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM. Families of zinc metalloproteinases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- Imper V, Van Wart HE. Substrate specificity and mechanisms of substrate recognition of the matrix metalloproteinases. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. San Diego: Academic Press; 1998. pp. 219–242. [Google Scholar]
- Inam SH, Snell WJ. The Chlamydomonascell wall degrading enzyme, lysin, acts on two substrates within the framework of the wall. J Cell Biol. 1988;106:2211–2221. doi: 10.1083/jcb.106.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-J, Smith SM. Molecular cloning of cucumber phosphoenolpyruvate carboxykinase and developmental regulation of gene expression. Plant Mol Biol. 1994;26:423–434. doi: 10.1007/BF00039551. [DOI] [PubMed] [Google Scholar]
- Kim D-J, Smith SM, Leaver CJ. A cDNA encoding a putative SPF1-type DNA-binding protein from cucumber. Gene. 1997;185:265–269. doi: 10.1016/s0378-1119(96)00665-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fukusawa H, Shimada T, Saito T, Matsuda Y. Primary structure and expression of a gametic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteinases. Proc Natl Acad Sci USA. 1992;89:4693–4697. doi: 10.1073/pnas.89.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaüper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Knight CG, Willenbrock F, Murphy G. A novel coumarin-labeled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Liu CY, Xu H, Graham JS. Cloning and characterization of an Arabidopsis cDNA (accession no. AF062640) homologous to the matrix metalloproteinases (PGR 98-130) Plant Physiol. 1998;117:1127. [Google Scholar]
- Lovejoy B, Cleasby A, Hassell AM, Longley K, Luther MA, Weigl D, McGeehan G, McElroy AB, Drewry D, Lambert MH, Jordan SR. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994a;263:375–377. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- Lovejoy B, Hassell AM, Luther MA, Weigl D, Jordan SR. Crystal structure of recombinant 19-kDa human fibroblast collagenase complexed to itself. Biochemistry. 1994b;33:8207–8217. doi: 10.1021/bi00193a006. [DOI] [PubMed] [Google Scholar]
- Maidment JM, Moore D, Murphy GP, Murphy A, Clark IM. Matrix metalloproteinase homologues from Arabidopsis thaliana. J Biol Chem. 1999;274:34706–34710. doi: 10.1074/jbc.274.49.34706. [DOI] [PubMed] [Google Scholar]
- Marrison JL, Leech RM. Co-immunolocalization of topoisomerase II and chloroplast DNA in developing, dividing and mature wheat chloroplasts. Plant J. 1992;2:783–790. [Google Scholar]
- Masuda S, Hayashi H, Araki S. Two vascular apoptosis-inducing proteins from snake venom are members of the metalloproteinase/disintegrin family. Eur J Biochem. 1998;253:36–41. doi: 10.1046/j.1432-1327.1998.2530036.x. [DOI] [PubMed] [Google Scholar]
- Matile P, Winkenbach F. Function of lysosomes and lysosomal enzymes in the senescing corolla of the morning glory (Ipomoea purpurea) J Exp Bot. 1971;22:759–771. [Google Scholar]
- McCabe PF, Levine A, Meijer P-J, Tapon NA, Pennell RI. A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 1997;12:267–280. [Google Scholar]
- McGeehan G, Burkhart W, Anderegg R, Becherer JD, Gillikin JW, Graham J. Sequencing and characterization of the soybean leaf metalloproteinase. Plant Physiol. 1992;99:1179–1183. doi: 10.1104/pp.99.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Shulaev V, Seskar M, Lam E. Inhibition of programmed cell death in tobacco plants during a pathogen-induced hypersensitive response at low oxygen pressure. Plant Cell. 1996;8:1991–2001. doi: 10.1105/tpc.8.11.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Crabbe T. Gelatinases A and B. Methods Enzymol. 1995;248:470–484. doi: 10.1016/0076-6879(95)48030-7. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nguyen Q, Cockett MI, Atkinson SJ, Allan JA, Knight CG, Willenbrock F, Docherty AJP. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J Biol Chem. 1994;269:6632–6636. [PubMed] [Google Scholar]
- Noodén LD, Guiamet JJ, John I. Senescence mechanisms. Physiol Plant. 1997;101:746–753. [Google Scholar]
- Orzáez D, Granell A. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J. 1997;11:137–144. [Google Scholar]
- Pak J, Liu CY, Huangpu J, Graham JS. Construction and characterization of the soybean leaf metalloproteinase cDNA. FEBS Lett. 1997;404:283–288. doi: 10.1016/s0014-5793(97)00141-5. [DOI] [PubMed] [Google Scholar]
- Ryerson DE, Heath MC. Cleavage of nuclear DNA into oligonucleosomal fragments during cell death induced by fungal infection or by abiotic treatments. Plant Cell. 1996;8:393–402. doi: 10.1105/tpc.8.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schindler T, Bergfeld R, Schopfer P. Arabinogalactan proteins in maize coleoptiles: developmental relationship to cell death during xylem differentiation but not to extension growth. Plant J. 1995;7:25–36. doi: 10.1046/j.1365-313x.1995.07010025.x. [DOI] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytol. 1994;12:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a cysteine switch mechanism for activation. Proc Natl Acad Sci USA. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöcker W, Grams F, Baumann U, Reinemer P, Gomis-Rüth F-X, McKay DB, Bode W. The metzincins-topological and sequential relations between the astacins, adamalysins, serralysins and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Stoddart JL. Leaf senescence. Annu Rev Plant Physiol. 1980;31:83–111. [Google Scholar]
- Vallon R, Müller R, Moosmayer D, Gerlach E, Angel P. The catalytic domain of activated collagenase I (MMP-1) is absolutely required for interaction with its specific inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1) Eur J Biochem. 1997;244:81–88. doi: 10.1111/j.1432-1033.1997.00081.x. [DOI] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;8:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The matrix metalloproteinase family. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. San Diego: Academic Press; 1998. pp. 1–14. [Google Scholar]