Abstract
BACKGROUND
Maternal diabetes can induce a number of developmental abnormalities in laboratory animals and humans, including facial deformities and defects in neural tube closure. The incidence of birth defects in newborns of diabetic women is approximately 3–5 times higher than among non-diabetics. In mice, non-specific activation of the maternal immune system can reduce fetal abnormalities caused by diverse etiologies, including diabetes induced neural tube defects. This study was conducted to determine whether non-specific maternal immune stimulation could reduce diabetes-induced craniofacial defects as well.
METHODS
Maternal immune function was stimulated before streptozocin (STZ) treatment by maternal footpad injection with Freund’s complete adjuvant (FCA), maternal intraperitoneal (i.p.) injection with granulocyte-macrophage colony-stimulating factor (GM-CSF), or maternal i.p. injection with interferon-γ (IFNγ). Streptozocin (200 mg/kg i.p.) was used to induce hyperglycemia (26–35 mmol blood glucose) in female ICR mice before breeding. Fetuses from 12–18 litters per treatment group, were collected at Day 17 of gestation.
RESULTS
Craniofacial defects were observed in fetuses from all hyperglycemic groups. The incidence of defects was significantly decreased in fetuses from dams immune stimulated with IFNγ or GM-CSF. The most common defects were reduced maxillary and mandibular lengths. Both were prevented by maternal stimulation with GM-CSF.
CONCLUSION
Maternal immune stimulation reduced the incidence of diabetic craniofacial embryopathy. The mechanisms for these protective effects are unknown but may involve maternal or fetal production of cytokines or growth factors that protect the fetus from the dysregulatory effects of hyperglycemia.
INTRODUCTION
Diabetes mellitus can induce early fetal mortality and a number of developmental abnormalities in both laboratory animals and humans. The incidence of birth defects in newborns of diabetic women (Type 1 and Type 2) is approximately 3–5 times higher than among non-diabetics (Ewart-Toland et al., 2000). Defects include abnormalities in neural tube closure; and alterations in facial, cardiac, renal, optic and auricular development. The exact mechanism of teratogenesis has not been definitively identified and is an area of debate in diabetes research. Hyperglycemia, inositol deficiency, and perturbation of arachidonic acid metabolism by excessive reactive oxygen species have all been implicated independently or together as possible teratogenic mechanisms as reviewed by Fine et al. (1999) and Eriksson et al. (2003).
In humans and laboratory animals, the majority of developmental defects caused by hyperglycemia during pregnancy are of neural crest origin (Cederberg et al., 2003). Neural crest tissue is derived from the dorsolateral area of the developing neural tube; from there it proliferates and migrates throughout the body forming a variety of adult structures. Cranial neural crest, found around the developing brain, forms many of the craniofacial structures including parts of the brain and meninges, the cranium, orbit and eye, facial and nasal bones, nasal cartilages, jaw bones, thyroid, and great vessels of the heart (Kanzler et al., 2000; Carstens, 2004). Cranial neural crest tissue proliferates and migrates during the 11–16 somite stage (Carstens, 2004) and thus teratogenic insult at this time could result in craniofacial defects.
Non-specific stimulation of the maternal immune system in mice during the peri-conception period seems to have a broad spectrum efficacy for reducing teratogen induced birth defects from a variety of chemical agents, hyperthermia, x-rays, and diabetes mellitus (Nomura et al., 1990; Holladay et al., 2000; Punareewattana et al., 2003; Punareewattana and Holladay, 2004). Maternal immune stimulation reduced or blocked digit and limb defects (Prater et al., 2004), tail malformations, cleft palate (Sharova et al., 2002), and neural tube defects (Torchinsky et al., 1997; Punareewattana et al., 2003; Punareewattana and Holladay, 2004). Diverse means of maternal immune stimulation have proved effective in reducing defects including intraperitoneal (i.p.) injection of inert particles, intrauterine injection of xenogenic lymphocytes or intrauterine or i.p. injection of immunostimulatory cytokines (reviewed by Hrubec et al., 2005). The operating mechanisms by which such immune stimulation reduces fetal dysmorphogenesis are unknown; however, the collective literature suggests the possibility that immunoregulatory cytokines of maternal origin may be effector molecules that normalize dysregulated apoptosis or timing of fetal cell proliferation (Sharova et al., 2000; Punareewattana and Holladay, 2004).
It was demonstrated in our laboratory recently that maternal immune stimulation with the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) or interferon-γ (IFNγ) reduced the incidence of diabetes induced neural tube defects (both exencephaly and rachischisis) and also reduced the ocular defects (Punareewattana and Holladay, 2004). We noticed that these fetal mice exhibited numerous craniofacial defects in addition to the neural tube defects, and that these defects seemed to be diminished in the groups that received maternal immune stimulation. We hypothesized that maternal immune stimulation would reduce the incidence of craniofacial defects in these fetal mice. The immune stimulants used in this present study are all general immune stimulants that activate macrophages or dendritic cells to secrete inflammatory cytokines, which then activate and differentiate other cells of the immune system. Additionally, IFNγ inhibits T helper type-2 cell proliferation, and GM-CSF stimulates growth and differentiation of granulocytes and monocytes.
MATERIALS AND METHODS
Outbred ICR mice (6–8 weeks old) were purchased (Harlan Sprague–Dawley, Indianapolis, IN) and housed individually (males) or at five per cage (females) for a 2-week acclimation period. Mice were given food (NIH 31 open formula) and distilled water ad lib, and were maintained at 22°C, 40–60% relative humidity with a 14:10 light:dark cycle.
Female mice were divided into five groups. Groups 1–3 received one of three immune stimulants followed by streptozocin (STZ) to induce diabetes. Group 4 received STZ (diabetes) only and Group 5 received a non-immunostimulated non-diabetic control. Female mice in the immune stimulated groups received one of the following: Freund’s complete adjuvant (FCA) (Sigma, St. Louis, MO) 20–30 μl, by footpad injection; GM-CSF (Sigma) 8000 U, i.p. injection; or IFN-γ (Gibco BRL) 1000 U, i.p. injection. All immune stimulants were injected twice, first at 1 week and again at 1 day before STZ administration.
Diabetes was chemically induced in the female mice 7 days before mating by i.p. injection with 200 mg/kg STZ (Sigma). Blood glucose (BG) concentration was determined from tail vein blood twice at approximately 3-day intervals before mating (Sigma Glucose Kit), to ensure BG was ≥26 mmol/L. Mice with BG ≥26 mmol/ L were previously found to produce fetuses with increased birth defects (Punareewattana and Holladay, 2004) and were used in this study. Immune stimulation did not affect BG values. Control mice were non-diabetic pregnant mice that did not receive immune stimulations.
For breeding, females were housed overnight with non-diabetic males, and checked for vaginal plugs the next morning, which was designated gestation day (GD) 0. Fetuses were collected on GD 17 and were fixed in 100% ethanol. Individual fetuses from 12–18 litters per treatment group were examined for defects. The incidence of each defect was determined for every litter. Shortened maxillary and mandibular lengths (except gross micrognathia as exhibited in Fig. 2C) were not included in the incidence data as these were often subjective and were better determined by measurement. Five fetuses from hyperglycemic dams demonstrating characteristic lesions, and one control fetus, were photographed and then stained and cleared as follows. Fetal heads were stained with 0.15% Alcian blue in a 4:1 solution of 95% ethanol to glacial acetic acid for 48 hr. Heads were cleared in 1% KOH for 3–4 days until tissue was transparent. They were then counterstained with 0.01% alizarin red in 0.02% KOH for 12 hr. Heads were further cleared in 0.5% KOH until transparent, approximately 1 week, and then transferred through a graded KOH:glycerin series over the next week until in 100% glycerin.
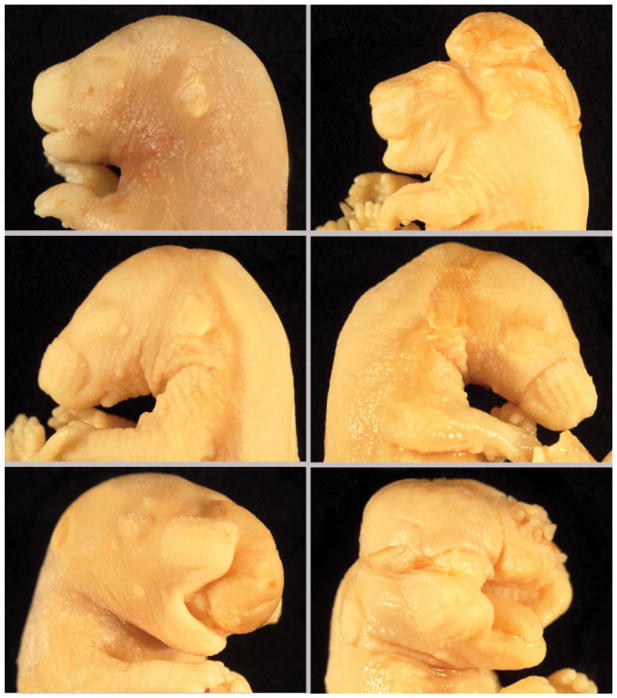
Fig. 2.
Fetal heads at GD 17 demonstrating some of the characteristic craniofacial lesions associated with maternal hyperglycemia. A: Control. B: Exencephaly and brachycephalia. C: Micrognathia. D: Agnathia. E: Cheilognathouranoschisis (cleft lip, palate, and upper jaw) and protrusion of prosencephalon. F: Cheilognathouranoschisis and exencephaly.
Five litters were then selected at random from each treatment group for fetal measurement. Fetuses were placed in lateral recumbency and were measured with an ocular micrometer using a Nikon SMZ stereomicroscope to determine mandibular, maxillary, and crown to rump lengths. Mandibular and maxillary measurements were determined as described by Siman et al. (2000). Measurements were taken from the rostral most point on the muzzle or chin to the base of the skull. Measurements were blind as to treatment group.
Statistical Analysis
One way analysis of variance (ANOVA) (Statistix 8, Analytical Software, Tallahassee, FL) with the mother as the treatment unit was used to determine differences between the treatment groups. When a significant difference was observed (p≤0.05), a Tukey’s means comparison was used to determine differences between treatment groups.
RESULTS
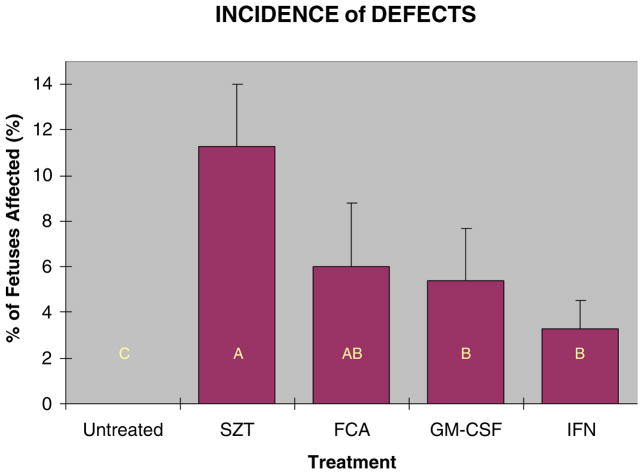
Hyperglycemia of ≥26 mmol/L induced a number of visible craniofacial defects in the fetuses from STZ induced diabetic dams (Table 1). Observed defects included micrognathia, agnathia, brachycephalia, cheilognathouranoschisis (cleft lip, palate, and upper jaw), cheiloschisis (cleft lip), fused lips, microcephaly, and missing rostral/facial region. Neural tube defects and eye defects (open eyes and anophthalmia) were observed but are not included in these data as they have been reported previously for these mice (Punareewattana and Holladay, 2004). Maternal immune stimulation with GM-CSF or IFNγ in conjunction with STZ treatment reduced significantly the number of defects from approximately 11.3% to 5.4% and 3.3% respectively (Fig. 1). These values represent the mean percentages of each litter that had grossly visible micrognathia, agnathia, cheilognathour-anoschisis, cheiloschisis, fused lips, microcephaly, and missing rostral/facial region. The incidence of shortened maxillae and mandible was not included in these counts as it was documented more accurately by measurement. Some of the characteristic lesions are shown in Figures 2 and 3. The cleared and stained heads showed perturbation of a variety of the facial skeletal elements including alterations to the maxillae, premaxillae, and nasal bones, thickened and reduced or absent mandibles, and changes to the frontal, parietal, and other bones affected by severe facial clefts or exencephaly.
Table 1.
Observed Incidence of Defects in Pups From STZ-Induced Diabetic Dams and Diabetic Dams Immune Stimulated Prebreeding With GM-CSF, IFN-γ, or FCA
| Parameter | STZ only n (%) | STZ + GM-CSF n (%) | STZ + IFN- γ n (%) | STZ + FCA n (%) |
|---|---|---|---|---|
| No. of litters | 18 | 13 | 18 | 12 |
| Total no. of fetuses | 200 | 160 | 196 | 119 |
| Defects | ||||
| Microcephaly | 1 (0.5) | 1 (0.63) | 0 (0.0) | 0 (0.0) |
| Cheiloschisis | 1 (0.5) | 1 (0.63) | 1 (0.51) | 0 (0.0) |
| Fused lips | 2 (1.0) | 0 (0.00) | 0 (0.0) | 0 (0.0) |
| Cheilognathouranoschisisa | 4 (2.0) | 3 (1.88) | 1 (0.51) | 1 (0.84) |
| With micrognathiab | 12 (3.0) | 2 (1.25) | 4 (2.04) | 5 (4.2) |
| With agnatha | 2 (1.0) | 1 (0.63) | 0 (0.0) | 0 (0.0) |
| Missing face | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.68) |
| Fetuses with 0 defects | 179 (89.5) | 152 (94.4) | 190 (96.9) | 111 (93.3) |
| Fetuses with 1 defect | 20 (10.0) | 8 (5.6) | 6 (3.1) | 8 (6.7) |
| Fetuses with 2 defectsc | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Litters with no abnormal fetuses | 6 (33.3) | 8 (61.5) | 13 (72.2) | 8 (66.6) |
| Litters with 1 abnormal fetus | 7 (38.9) | 2 (15.4) | 5 (27.7) | 1 (8.3) |
| Litters with 2 abnormal fetuses | 2 (11.1) | 3 (23.1) | 0 (0.0) | 2 (16.7) |
| Litters with 3 abnormal fetuses | 2 (11.1) | 0 (0.0) | 0 (0.0) | 1 (8.3) |
| Litters with 4 abnormal fetuses | 1 (5.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Fetuses with cheilognathouranoschisis were not included in the cheiloschisis count.
These were only fetuses with obvious micrognathia similar to Figure 2C.
Fetuses with cheilognathouranoschisis were counted as only having one defect.
Fig. 1.
Incidence of craniofacial defects (±SE) seen in GD 17 mouse embryos caused by STZ induced maternal hyperglycemia, and reduction in these defects with maternal immune stimulation. Dams were immune stimulated with either FCA (20–30 μl, by footpad injection), GM-CSF (8000 U, i.p. injection), or IFN-γ (1000 U, i.p. injection). Bars with similar letters are not significantly different (p≤0.05).
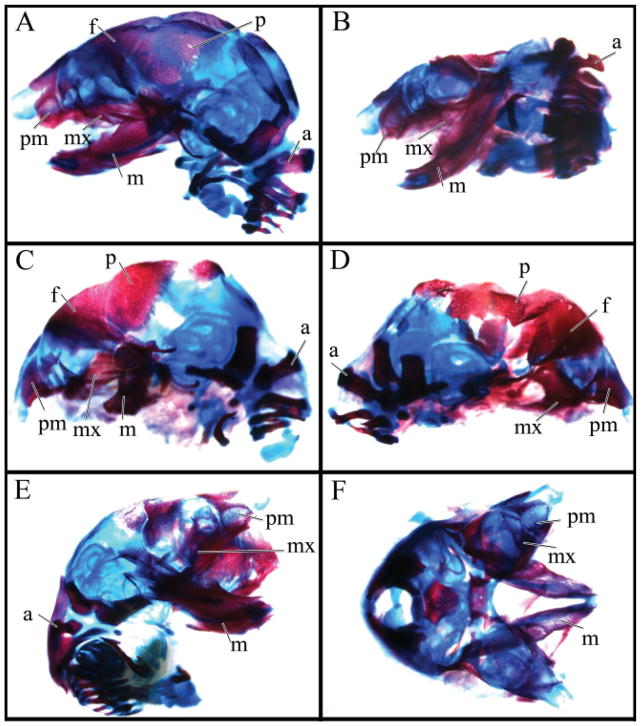
Fig. 3.
Fetal heads from Figure 2 have been cleared and stained with Alcian blue for cartilage and alizarin red for bone. All heads are in the same position and orientation as in Figure 2, except plate F that is a dorsal instead of lateral view to better show the facial clefting. The micrognathic fetus in plate C has a vestigial mandible whereas the agnathic embryo D is lacking a mandible. The following structures are labeled: f, frontal; p, parietal; m, mandible; mx, maxilla; pm, premaxilla; a, atlas.
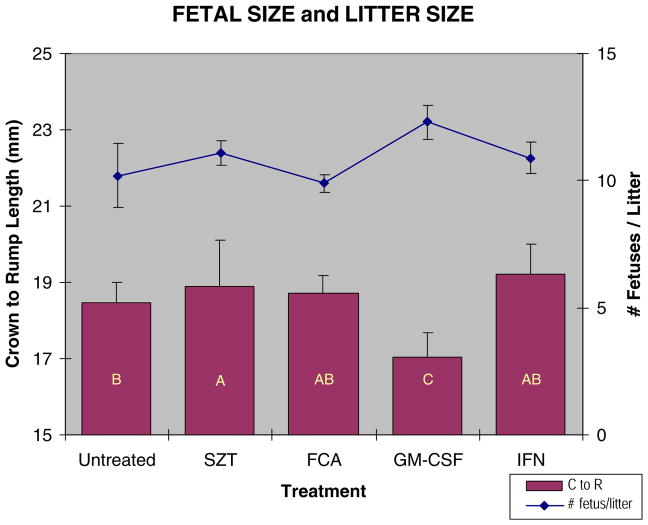
Fetal size varied significantly between the different treatment groups (Fig. 4). Fetuses from diabetic dams tended to be slightly larger than non-diabetic controls; however, fetuses from dams receiving both STZ and GM-CSF were smaller than other fetuses. Litter size also varied between the treatment groups with GM-CSF treated dams having larger litters that may be in part responsible for the smaller fetal size (Fig. 4). Over all treatment groups, however, there was no correlation between fetal size and litter size with a correlation coefficient of r = −0.125.
Fig. 4.
Fetal and litter size for STZ diabetic and immune stimulated diabetic dams. Crown to rump length was measured on GD 17 embryos. Dams were immune stimulated with either FCA (20–30 μl,), GM-CSF (8000 U), or IFN-γ (1000 U). Bars with similar letters are not significantly different (p≤0.05).
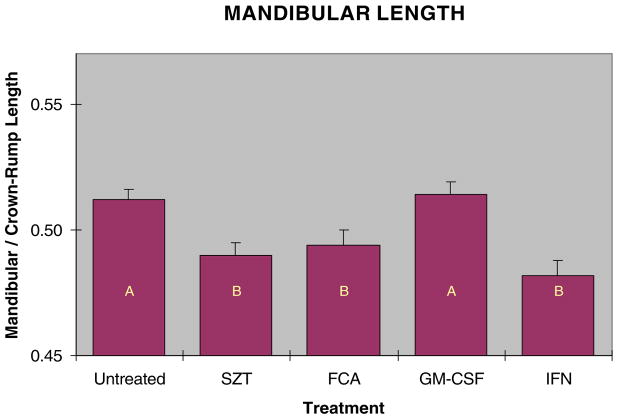
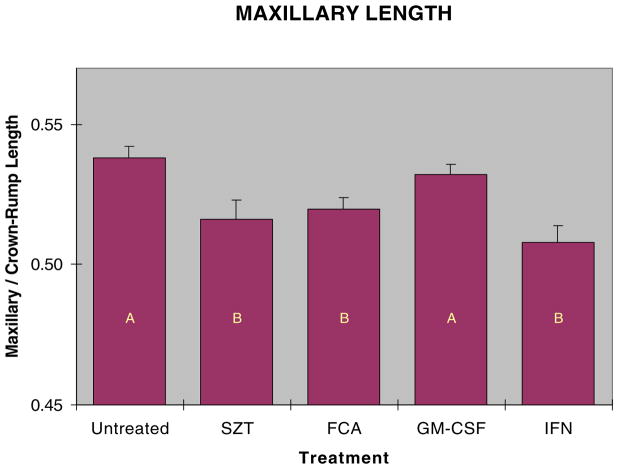
Both mandibular and maxillary lengths were reduced in fetuses from hyperglycemic dams. Because fetuses varied in overall size, direct measurements of the face could not be used to compare fetuses from the different groups, as this would not distinguish shortened facial regions from smaller individuals. Additionally, as both mandibular and maxillary regions were affected, the ratio of these could not be used to accurately describe changes in facial shape, as this ratio would not change if both facial regions were shortened proportionally. We therefore used the ratio of the mandibular or maxillary length to the crown to rump length as the best way to assess relative facial length. Exposure to hyperglycemia in utero decreased fetal mandibular length compared to non-diabetic controls (Fig. 5). Maternal immune stimulation with GM-CSF significantly increased fetal mandibular length compared to STZ treatment alone. Similarly, exposure to hyperglycemia reduced fetal maxillary length (Fig. 6), and stimulation prebreeding with GM-CSF prevented shortening of facial lengths in fetuses of these diabetic mice. Neither the IFNγ nor FCA stimulation significantly prevented craniofacial shortening.
Fig. 5.
Mandibular length measured as a ratio of mandibular to crown to rump length in GD 17 embryos from STZ diabetic and immune stimulated diabetic dams. Dams were immune stimulated with either FCA (20–30 μl,), GM-CSF (8000 U), or IFN-γ (1000 U). Bars with similar letters are not significantly different (p≤0.05).
Fig. 6.
Maxillary length measured as a ratio of maxillary to crown to rump length in GD 17 embryos from STZ diabetic and immune stimulated diabetic dams. Dams were immune stimulated with either FCA (20–30 μl,), GM-CSF (8000 U), or IFN-γ (1000 U). Bars with similar letters are not significantly different (p≤0.05).
DISCUSSION
Normal craniofacial development is a highly complex process that begins early in embryonic development and continues to adulthood. An understanding of the required sequential events during early embryogenesis for normal craniofacial establishment has just occurred in the past 7 or 8 years (Carstens, 2004). Craniofacial dysmorphogenesis is even more complex but has served as a tool for understanding normal developmental processes through the use of genetic knockouts or mutations as recently reviewed by Farlie et al. (2004).
Teratogenic agents known to cause neural tube or craniofacial defects such as retinoic acid and valproic acid have also been used to study craniofacial development. Exposure to retinoic or valproic acid on GD 8–10 induces neural tube and craniofacial defects similar to those observed offspring of the diabetic mice (Taylor et al., 1995; Grant et al., 1997). Neural tube defects are often associated with neural crest lesions, as the neural crest tissue forms and migrates from the developing neural tube at the time of neural tube formation and closure. However, treating these defects as arising from one, albeit complex etiology, is far too simplistic. More than 30 genes are responsible for neural tube defects alone in mice (Juriloff and Harris, 2000), and the craniofacial lesions may appear independently from the neural tube defects (Inoue et al., 2004). Additionally, there seem to be many mechanisms for neural tube defect formation; for example, decreased levels of Pax3 causes neural tube defects in the diabetic pregnancy (Phelan et al., 1997) but Pax3 does not seem to be involved in valproic acid-induced neural tube defects (Wlodarczyk et al., 1996; Williams et al., 1997).
Many craniofacial defects appear concurrently with defects in multiple organ systems as is seen in the human disorders DiGeorge syndrome, velocardiofacial syndrome, and oculo-auriculo-vertebral spectrum or Goldenhar’s syndrome. These syndromes cause defects in facial, palatal, vertebral, cardiac, otic, thymic, and brain development; and arise from defects in neural crest formation and migration (Goldmuntz and Emanuel, 1997; Wang et al., 2002). DiGeorge and velocardiofacial syndromes arise from a specific microdeletion on chromosome 22. There are spontaneous and knockout mouse models that mimic these disorders, although, they are not exact models as their disrupted genes do not map to the same microdeletion on chromosome 22. These mouse models do, however, shed light on the mechanisms involved in craniofacial neural crest lesions. Mice lacking the ETA receptor for endothelin-1 (Et-1) or the Alk2 receptor for bone morphogenic protein (Bmp) display craniofacial defects similar to those reported for the diabetic mice (Clouthier et al., 1998; Dudas et al., 2004).
Normal craniofacial development seems to be quite complex involving many families of patterning genes and growth factors such as Wnt, Bmp, Et-1, FGF, Shh, Sox, Pax, and Zic. Wnts are expressed commonly in the developing vertebrate neural tube and nervous system. Wnt1 and Wnt3 double mutants displayed poorly developed cranial nerve ganglia and a lack of facial bones derived from cranial neural crest tissues (Brault et al., 2001). Bmp signaling is needed for cranial neural crest migration and proliferation into the second and posterior branchial arches affecting the hyoid, stapes, and basioccipital bone formation, and formation of the cranial nerve ganglia. Bmp signaling is not required for migration of neural crest into the first branchial arch (Kanzler et al., 2000). Bmp is needed for induction of polarity of first branchial arch structures, however, and is involved in the formation of other craniofacial lesions such as cleft palate and shortening of frontal and nasal bones (Farlie et al., 2004). Determining the effects of teratogens on craniofacial gene expression is proceeding and it seems that the teratogens have windows of exposure times where they are more likely to disrupt specific genes resulting in differing morphologic defects.
Diabetes affects patterning gene expression in the developing mouse embryo. An example of this is the effect of hyperglycemia on Pax-3, which is involved in caudal neural tube closure and development of the mid and hindbrain (Phelan et al., 1997). Diabetes reduces expression of Pax-3 and increases the number of apoptotic cells in the developing neural tube. In the forebrain, diabetes affects patterning genes and causes developmental perturbations as well. Sonic hedgehog (Shh) and Bmp exert ventralizing and dorsalizing patterning influences respectively. Diabetes reduces Bmp expression and upregulates Shh expression in mice resulting in thicker ventral regions of the forebrain and a syntelencephaly-like presentation (Liao et al., 2004).
Diabetes has also been shown to affect neural crest-derived tissues. Studying the development of cranial nerve ganglia is an elegant way to examine the effect of a teratogen on cranial neural crest, as the neurons of ganglia V, IX and X originate from the neural crest whereas neurons of ganglia VII and VIII originate mainly from epidermal placodes. Cederberg et al. (2003) found that cranial neural crest cells at GD 10 from diabetic rats were delayed in their migration and formation of the cranial nerve ganglia. The delay in ganglionic development represented a specific delay in addition to the more general delay in overall development seen from the experimental diabetic pregnancy. Additionally, more neural crest derived cells died at high glucose concentration than in normal glucose concentration, although apoptosis was not increased (Cederberg et al., 2003). Furthermore, hyperglycemia has been shown to affect normal proliferation and migration of neural crest tissues, reducing the total number of fetal neural crest cells, and reducing their mean migratory distance and their migratory area (Suzuki et al., 1996).
The variety of craniofacial defects observed in the fetuses of diabetic dams in our study was quite large and at the same time the total number of individual specific defects was relatively low. These low incidence rates prevented breaking the data down by defect for statistical analysis, with the exception of shortened mandible and maxillae, which were by far the most common lesions encountered. The micrognathia we observed in the diabetic mice has also been associated with maternal diabetes in rats (Siman et al., 2000). Hyperglycemia could be inhibiting the proliferation and migration of rhombencephalic neural crest causing a reduction in tissue mass that will form the mandible as suggested by previous reports (Suzuki et al., 1996; Cederberg et al., 2003). In some cases however, the fetuses from the diabetic mice in our study were lacking mandibles entirely, indicating further perturbation or severity of the lesion.
Multiple biochemical and signal transduction pathways are modified by diabetes-related hyperglycemia. Cellular levels of myoinositol and arachidonic acid are reduced, leading to diminished prostaglandin E2 (PGE2) levels, whereas reactive oxygen species are generated at higher rates (Reece, 1999). Preventive strategies have been examined in laboratory animals based on knowledge of these pathways, in attempts to reduce diabetic embryopathy associated with high glucose levels. For example, birth defect incidence has been reduced in diabetic mice by dietary supplementation with myoinositol (Baker et al., 1990), arachidonic acid (Goldman et al., 1985; Pinter et al., 1986), lipoic acid (Wiznitzer et al., 1999), or antioxidants including vitamin E (Sivan et al., 1996) and vitamin C (Siman and Eriksson, 1997).
Torchinsky et al. (1997) stimulated uterine immune cells in pregnant mice in an attempt to reduce fetal resorptions associated with diabetes, and made the unexpected observation of significantly reduced malformed fetuses. These authors later reported beneficial effects of maternal immune stimulation on uterine cytokines tumor necrosis factor-alpha (TNFα; Fein et al., 2001) and transforming growth factor-beta (TGFβ; Fein et al., 2002) that show distorted expression in diabetic animals. In this regard, TGFβ crosses the mouse placenta (Gato et al., 2002) and has widely demonstrated importance during fetal development, including in craniofacial formation (Tudela et al., 2002). In what may be a related observation, Sharova et al. (2003) later found a positive correlation between maternal splenic leukocyte TGFβ expression and reduced cleft palate in urethane-treated pregnant mice after immune stimulation.
In conclusion, maternal immune stimulation with IFNγ and GM-CSF reduced the incidence of diabetes induced craniofacial defects; and GM-CSF prevented hyperglycemia induced shortening of craniofacial length. The mechanisms underlying this reduction in diabetes induced craniofacial defects remains unclear but may involve maternal or fetal production of cytokines or growth factors that protect the fetus from the dysregulatory effects of the hyperglycemia.
Acknowledgments
Contract Grant sponsor: NIH; Grant number: NIH K01RR16241-01A1.
References
- Baker L, Piddington R, Goldman A, Egler J, Moehring J. Myoinositol and prostaglandins reverse the glucose inhibition of neural tube fusion in cultured mouse embryos. Diabetologia. 1990;33:593–596. doi: 10.1007/BF00400202. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Carstens MH. Neural tube programming and craniofacial cleft formation. I. The neuromeric organization of the head and neck. Eur J Paediatr Neurol. 2004;8:181–210. doi: 10.1016/j.ejpn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Cederberg J, Picard JJ, Eriksson UJ. Maternal diabetes in the rat impairs the formation of neural-crest derived cranial nerve ganglia in the offspring. Diabetologia. 2003:1245–1251. doi: 10.1007/s00125-003-1100-1. [DOI] [PubMed] [Google Scholar]
- Chan BW, Chan KS, Koide T, Yeung SM, Leung MB, Copp AJ, Loeken MR, Shiroishi T, Shum AS. Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes. 2002;51:2811–2816. doi: 10.2337/diabetes.51.9.2811. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers—animal and human studies. Rev Endocr Metab Disord. 2003;4:79–93. doi: 10.1023/a:1021879504372. [DOI] [PubMed] [Google Scholar]
- Ewart-Toland A, Yankowitz J, Winder A, Imagire R, Cox VA, Aylsworth AS, Golabi M. Oculoauriculovertebral abnormalities in children of diabetic mothers. Am J Med Genet. 2000;90:303–309. [PubMed] [Google Scholar]
- Farlie PG, McKeown SJ, Newgreen DF. The neural crest: basic biology and clinical relationships in the craniofacial and enteric nervous systems. Birth Defects Res C Embryo Today. 2004;72:173–189. doi: 10.1002/bdrc.20013. [DOI] [PubMed] [Google Scholar]
- Fein A, Kostina E, Savion S, Orenstein H, Shepshelovich J, Ornoy A, Torchinsky A, Toder V. Expression of tumor necrosis factor-alpha in the pregnant uterus of diabetic mice: effect of maternal immunopotentiation. Am J Reprod Immunol. 2001;46:161–168. doi: 10.1111/j.8755-8920.2001.460207.x. [DOI] [PubMed] [Google Scholar]
- Fein A, Magid N, Savion S, Orenstein H, Shepshelovich J, Ornoy A, Torchinsky A, Toder V. Diabetes teratogenicity in mice is accompanied with distorted expression of TGF-beta2 in the uterus. Teratog Carcinog Mutagen. 2002;22:59–71. doi: 10.1002/tcm.1039. [DOI] [PubMed] [Google Scholar]
- Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- Gato A, Martinez ML, Tudela C, Alonso I, Moro JA, Formoso MA, Ferguson MW, Martinez-Alvarez C. TGF-beta(3)-induced chondroitin sulphate proteoglycan mediates palatal shelf adhesion. Dev Biol. 2002;250:393–405. [PubMed] [Google Scholar]
- Goldman AS, Baker L, Piddington R, Marx B, Herold R, Egler J. Hyperglycemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proc Natl Acad Sci USA. 1985;82:8227–8231. doi: 10.1073/pnas.82.23.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmuntz E, Emanuel BS. Genetic disorders of cardiac morphogenesis. The DiGeorge and velocardiofacial syndromes. Circ Res. 1997;80:437–443. doi: 10.1161/01.res.80.4.437. [DOI] [PubMed] [Google Scholar]
- Grant JH, Maggio-Price L, Reutebuch J, Cunningham ML. Retinoic acid exposure of the mouse on embryonic day 9 selectively spares derivatives of the frontonasal neural crest. J Craniofac Genet Dev Biol. 1997;17:1–8. [PubMed] [Google Scholar]
- Holladay SD, Sharova LV, Smit BJ, Gogal RM, Jr, Ward DL, Blaylock BL. Non-specific stimulation of the maternal immune system: I. Effects on teratogen-induced fetal malformations. Teratology. 2000;62:413–419. doi: 10.1002/1096-9926(200012)62:6<413::AID-TERA8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hrubec TC, Punareewattana K, Prater MR, Holladay SD. Teratogen induced birth defects in mice: Role of maternal immune stimulation. Cur Topics Toxicol. 2005 in press. [Google Scholar]
- Inoue T, Hatayama M, Tohmonda T, Itohara S, Aruga J, Mikoshiba K. Mouse Zic5 deficiency results in neural tube defects and hypoplasia of cephalic neural crest derivatives. Dev Biol. 2004;270:146–162. doi: 10.1016/j.ydbio.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. Mouse models for neural tube closure defects. Hum Mol Genet. 2000;9:993–1000. doi: 10.1093/hmg/9.6.993. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Liao DM, Ng YK, Tay SS, Ling EA, Dheen ST. Altered gene expression with abnormal patterning of the telencephalon in embryos of diabetic Albino Swiss mice. Diabetologia. 2004;47:523–531. doi: 10.1007/s00125-004-1351-5. [DOI] [PubMed] [Google Scholar]
- Nomura T, Hata S, Kusafuka T. Suppression of developmental anomalies by maternal macrophages in mice. J Exp Med. 1990;172:1325–1330. doi: 10.1084/jem.172.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan SA, Ito M, Loeken MR. Neural tube defects in embryos of diabetic mice: role of the Pax-3 gene and apoptosis. Diabetes. 1997;46:1189–1197. doi: 10.2337/diab.46.7.1189. [DOI] [PubMed] [Google Scholar]
- Pinter E, Reece EA, Leranth CZ, Garcia-Segura M, Hobbins JC, Mahoney MJ, Naftolin F. Arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy. Am J Obstet Gynecol. 1986;155:691–702. doi: 10.1016/s0002-9378(86)80001-1. [DOI] [PubMed] [Google Scholar]
- Prater MR, Strahl ED, Zimmerman KL, Ward DL, Holladay SD. Reduced birth defects caused by maternal immune stimulation in methylnitrosourea-exposed mice: association with placental improvement. Birth Defects Res A. 2004;70:862–869. doi: 10.1002/bdra.20082. [DOI] [PubMed] [Google Scholar]
- Punareewattana K, Holladay SD. Immunostimulation by complete Freund’s adjuvant, granulocyte macrophage colony-stimulating factor, or interferon-γ reduces severity of diabetic embryopathy in ICR mice. Birth Defects Research A. 2004;70:20–27. doi: 10.1002/bdra.10137. [DOI] [PubMed] [Google Scholar]
- Punareewattana K, Sharova LV, Li W, Ward DL, Holladay SD. Reduced birth defects caused by maternal immune stimulation may involve increased expression of growth promoting genes and cytokine GM-CSF in the spleen of diabetic ICR mice. Int Immunopharmacol. 2003;3:1639–1655. doi: 10.1016/S1567-5769(03)00200-5. [DOI] [PubMed] [Google Scholar]
- Reece EA. Maternal fuels, diabetic embryopathy: pathomechanisms and prevention. Semin Reprod Endocrinol. 1999;17:183–194. doi: 10.1055/s-2007-1016225. [DOI] [PubMed] [Google Scholar]
- Sharova LV, Gogal RM, Jr, Sharova AA, Crisman MV, Holladay SD. Immune stimulation in urethane-exposed pregnant mice increases expression of level of spleen leukocyte genes for TGFβ3, GM-CSF and other cytokines that may play a role in reduced chemical-induced birth defects. Int Immunopharmacol. 2002;2:1477–1489. doi: 10.1016/s1567-5769(02)00094-2. [DOI] [PubMed] [Google Scholar]
- Sharova LV, Sharov AA, Sura P, Gogal RM, Jr, Holladay SD. Maternal immune stimulation reduces both placental damage and down-regulated placental growth factor and cell cycle gene expression caused by urethane: are these events related to reduced birth teratogenesis? Int Immunopharmacol. 2003;3:945–955. doi: 10.1016/S1567-5769(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Sharova LV, Sura P, Smith BJ, Gogal RM, Jr, Sharov AA, Ward DL, Holladay SD. Non-specific stimulation of the maternal immune system. II. Effects on fetal gene expression. Teratology. 2000;62:420–428. doi: 10.1002/1096-9926(200012)62:6<420::AID-TERA9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Siman CM, Eriksson UJ. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–1424. doi: 10.1007/s001250050844. [DOI] [PubMed] [Google Scholar]
- Siman CM, Gittenberger-De Groot AC, Wisse B, Eriksson UJ. Malformations in offspring of diabetic rats: morphometric analysis of neural crest-derived organs and effects of maternal vitamin E treatment. Teratology. 2000;61:355–367. doi: 10.1002/(SICI)1096-9926(200005)61:5<355::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sivan E, Reece EA, Wu YK, Homko CJ, Polansky M, Borenstein M. Dietary vitamin E prophylaxis and diabetic embryopathy: morphologic and biochemical analysis. Am J Obstet Gynecol. 1996;175:793–799. doi: 10.1016/s0002-9378(96)80001-9. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Svensson K, Eriksson UJ. High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia. 1996;39:401–411. doi: 10.1007/BF00400671. [DOI] [PubMed] [Google Scholar]
- Taylor LE, Bennett GD, Finnell RH. Altered gene expression in murine branchial arches following in utero exposure to retinoic acid. J Craniofac Genet Dev Biol. 1995;15:13–25. [PubMed] [Google Scholar]
- Torchinsky A, Toder V, Savion S, Shepshelovich J, Orenstein H, Fein A. Immunostimulation increases the resistance of mouse embryos to the teratogenic effect of diabetes mellitus. Diabetologia. 1997;40:635–640. doi: 10.1007/s001250050727. [DOI] [PubMed] [Google Scholar]
- Tudela C, Formoso MA, Martinez T, Perez R, Aparicio M, Maestro C, Del Rio A, Martinez E, Ferguson M, Martinez-Alvarez C. TGF-beta3 is required for the adhesion and intercalation of medial edge epithelial cells during palate fusion. Int J Dev Biol. 2002;46:333–336. [PubMed] [Google Scholar]
- Wang R, Martinez-Frias ML, Graham JM., Jr Infants of diabetic mothers are at increased risk for the oculo-auriculo-vertebral sequence: A case-based and case-control approach. J Pediatr. 2002;141:611–617. doi: 10.1067/mpd.2002.128891. [DOI] [PubMed] [Google Scholar]
- Williams JA, Mann FM, Brown NA. Gene expression domains as markers in developmental toxicity studies using mammalian embryo culture. Int J Dev Biol. 1997;41:359–364. [PubMed] [Google Scholar]
- Wiznitzer A, Ayalon N, Hershkovitz R, Khamaisi M, Reece EA, Trischler H, Bashan N. Lipoic acid prevention of neural tube defects in offspring of rats with streptozocin-induced diabetes. Am J Obstet Gynecol. 1999;180:188–193. doi: 10.1016/s0002-9378(99)70173-0. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk BC, Craig JC, Bennett GD, Calvin JA, Finnell RH. Valproic acid-induced changes in gene expression during neurulation in a mouse model. Teratology. 1996;54:284–297. doi: 10.1002/(SICI)1096-9926(199612)54:6<284::AID-TERA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]