Abstract
This study examines the results of neuropsychological testing of 26 active welders and 17 similar controls and their relationship to welders' shortened MRI T1 relaxation time, indicative of increased brain manganese (Mn) accumulation. Welders were exposed to Mn for an average duration of 12.25 years to average levels of Mn in air of 0.11 ± 0.05 mg/m3. Welders scored significantly worse than controls on Fruit Naming and the Parallel Lines test of graphomotor tremor. Welders had shorter MRI T1 relaxation times than controls in the globus pallidus, substantia nigra, caudate nucleus, and the anterior prefrontal lobe. 63% of the variation in MRI T1 relaxation times was accounted for by exposure group. In welders, lower relaxation times in the caudate nucleus and substantia nigra were associated with lower neuropsychological test performance on tests of verbal fluency (Fruit Naming), verbal learning, memory, and perseveration (WHO-UCLA AVLT). Results indicate that verbal function may be one of the first cognitive domains affected by brain Mn deposition in welders as reflected by MRI T1 relaxation times.
I. Introduction
Descriptions of the relationships between manganese (Mn) exposure and neuropsychological dysfunction are reported both in the occupational and environmental literature on Mn (Bowler et al., 2006, Hudnell, 1999, Levi and Nassetta, 2003, Pal et al., 1999, Santamaria et al., 2007, Zoni et al., 2007). Welders are one of the most studied occupational groups for the health effects of Mn (Bowler et al., 2007), due to their relatively well characterized inhalation exposures to very small (sub-micron) Mn-containing particulate and due to welding populations being common and accessible. Mn exposure in welders has been shown to affect both psychomotor function as well as cognition, particularly executive function, sustaining concentration, cognitive flexibility, verbal learning, and working memory (Bowler, Roels, 2007, Bowler et al., 2015). Dose-effect relationships were reported in confined space welders who repaired the San Francisco Bay Bridge and were exposed without respiratory protection and poor ventilation to Mn in air in the range of 0.11-0.46 mg/m3 with 50% of the welders being exposed to Mn levels greater than 0.20 mg/m3 (Bowler et al. 2007). Moreover, there are numerous reports of mood and visuospatial tracking and visuospatial memory problems among welders (Bowler and Lezak, 2015).
Non-human primate studies of the neurotoxic effects of chronic exposure to Mn have supported the findings in humans and showed impairment in fine motor skills, attention and concentration, working memory, and visuospatial abilities, with the parietal and frontal lobes being target sites of Mn accumulation in addition to the basal ganglia (Burton and Guilarte, 2009, Guilarte et al., 2006a, Guilarte, 2013, Guilarte et al., 2008, Schneider et al., 2006, Schneider et al., 2013, 2015).
Few studies to date have examined whether and how neuropsychological impairments are associated with Mn deposition in several specific regions of interest (ROI) in the human brain (Schneider et al., 2006; 2013; 2015). T1-weighted magnetic resonance imaging (MRI) has been suggested as a non-invasive tool to reflect increased brain Mn levels due to Mn exposure both in humans (Dydak and Criswell, 2015, Eriksson et al., 1992, Nelson et al., 1993, Newland et al., 1989) and non-human primates (Burton and Guilarte, 2009, Dorman et al., 2006, Guilarte, 2013, Schneider, Williams, 2015). The MRI signal intensity increases due to the Mn ion's paramagnetic properties, which creates a shortening of the T1 relaxation time (Dydak and Criswell, 2015). Mn exposure has been found to lower the T1 relaxation time in the brain, primarily in the basal ganglia; with visible MRI signal changes in the globus pallidus (Criswell et al., 2012, Kim et al., 1999).
A lower tissue T1 relaxation rate causes a higher signal intensity (“hyperintensity”) in T1-weighted (T1w) MR images. Most studies in the past have used signal intensities in T1w images as an indicator of brain Mn accumulation since measuring T1 relaxation time requires specific MRI sequences. Earlier MRI studies computed a “pallidal index,” (Chang et al., 2010b, Dietz et al., 2001, Jiang et al., 2007, Kim et al., 2007, Shin et al., 2007), which represents the ratio of the T1-weighted signal intensity in the globus pallidus as compared to the signal intensity in frontal white matter. While the globus pallidus shows the most obvious hyperintensities in T1w MRI images, welders show changes in brain metabolism and T1w hyperintensities in many more brain regions due to Mn exposure, as shown recently by Long et al. (2014a). Recent studies have used direct measures of the T1 relaxation time, as, contrary to the pallidal index, it is not confounded by Mn deposition in frontal white matter and is independent of imaging and image processing parameters. (Choi et al., 2007, Criswell, Perlmutter, 2012, Lee et al., 2015).
Previous research has suggested that the frontal lobes integrate connected brain regions and modulate the final motor response (Stuss & Benson, 1984; Stuss & Levine, 2002). The frontal lobes have been associated with complex acts and continuous coordinated movements, such as those required by the Grooved Pegboard test (Lezak et al., 2012). The left frontal lobe, specifically, has been noted to be instrumental in the control of skilled motor movements through frontal-subcortical pathways (Haaland & Yeo, 1989).
MRI findings of lower T1 relaxation times in the globus pallidus, a substructure of the basal ganglia, have frequently been associated with occupational Mn exposure in welders (Criswell et al., 2015, Criswell, Perlmutter, 2012, Dydak et al., 2011, Long, Jiang, 2014a, Yim et al., 1988). It has been shown that presumed Mn deposition in the basal ganglia causes dysfunction associated with the substructures of the globus pallidus, putamen, caudate nucleus, and substantia nigra, which largely affect motor function (Chang et al., 2010a). The substantia nigra, a structure of the extrapyramidal motor system, has been primarily associated with the modulation of movement and has also been associated with Mn toxicity (Dorman et al. 2006, Verina et al. 2011, Guilarte et al. 2013).
Relating to cognitive function, Josephs et al. (2005) found T1 hyperintensities in the basal ganglia to be associated with verbal dysfunction in a study of welders. Neuropsychological testing indicated this dysfunction was related to subcortical or frontal cortex pathways. In addition, Long et al. (2014a) found increased Mn deposition in the frontal cortex of participants exposed to excess levels of Mn. These findings are supported in a review by Guilarte (2013). The frontal cortex has also been associated with impaired motor skills, organization, and disrupted speech production related to verbal skills (Lezak et al., 2012; Stuss & Benson, 1990; Stuss & Levine, 2002).
In a recent study of welders using fMRI, neural activation in the bilateral superior frontal cortex, right-inferior parietal cortex, and bilateral insula cortex was greater in welders than in controls (Seo et al., 2016). In addition, associations were found between deficits in executive function and brain activation in the insula cortex, which is part of a larger network comprising the lateral prefrontal and parietal cortex.
[18F]-Fluoro-L-Dopa Positron Emission Tomography imaging on Mn exposed welders demonstrated significant reduction of [18F]-FDOPA uptake in the caudate nucleus (Criswell et al, 2011). Caudate and frontal cortex are both known to be involved in executive functions, underlined by fMRI findings of decreasing striatal and frontal lobe activities in Parkinson's disease patients with impaired cognitive functions measured by The Tower of London planning task (Lewis et al, 2003). Divac et al. (1967) showed resemblance of the deficits after prefrontal ablation in the animals with lesions at the caudate nucleus. This observation led to the suggestion that executive functions are not governed by prefrontal areas in isolation, but also depend on interaction with frontostriatal and corticostriatal circuits. A meta-analysis of 41 functional MRI (fMRI) studies on executive function in schizophrenic patients further confirms the involvement of these circuits (Minzenberg et al. 2009). Using paradigms such as delayed match-to-sample, go/no-go, sequence recall, and the Wisconsin Card Sorting, several of these studies show that altered brain activities in fronto- and corticostriatal circuits may be considered as reflective of changes in executive dysfunction.
Few studies have examined the association between occupational Mn exposure, brain Mn deposition as measured by neuroimaging, and neurological and neuropsychological performance. In Mn-exposed smelter workers and welders, psychomotor dysfunction, resulting in lower scores on tests of psychomotor speed (Finger Tapping test [FTT]), grip strength (Dynamometer), and tactile function (Grooved Pegboard) has been associated with altered T1-weighted MRI in the basal ganglia in several research reports (Kim, Kim, 1999, Long et al., 2014b).
The present study examines cognitive function in active welders in relation to brain region-specific changes in T1 relaxation times. The objectives of this study are to (1) compare the neuropsychological cognitive and psychomotor test performance of active welders and unexposed control participants from the same factory, and (2) evaluate the relationships between the welders' neuropsychological test performance and MRI T1 relaxation time by brain region. It is hypothesized that (1) overall, welders will have lower scores on neuropsychological tests as well as shorter MRI T1 relaxation times than controls. Based on previous research described above, it is also hypothesized that (2) shorter T1 relaxation times in the regions of the basal ganglia will primarily be associated with lower motor test scores. However, due to the shared neural pathways between these regions and the frontal lobes, associations with cognitive dysfunction, particularly verbal and executive function, may be diffuse and more prominent in this active group of welders, who must maintain skilled motor movements as an occupational skill.
II. Method
1.1. Participants and Recruitment
Thirty welders and 21 unexposed demographically similar controls were recruited as participants from a semi-trailer manufacturer. MRI data was not available for four welders and four controls due to various reasons (claustrophobia, etc). Therefore, all analyses used a sample size of 26 welders and 17 controls.
Recruitment of welders and controls was carried out using similar methods. Several informational events onsite the semi-trailer manufacturing plant were held by the Health and Safety department staff and the research study team. Workers were informed about the study and information recruitment flyers were distributed. Study team members determined the eligibility of interested workers and scheduled appointments to obtain consent and provide additional information about the study. Inclusion criteria for welders consisted of being 18 years of age or older, male, and having worked at the plant for at least three years as full-time welder. Inclusion criteria for non-welders consisted of being 18 years of age or older, male, non-management workers at the same plant, and having no history of welding. Controls had shift work (e.g. in assembly lines), but had no history of exposure to welding fumes nor other significant neurotoxic exposures, such as to solvents or other metals. Both the controls and welders were given the same MRI examination and neuropsychological test battery. The unexposed control group was recruited and tested during the same time frame as the welders.
Exclusion criteria were having a neurological or major medical disorder, reporting known claustrophobia and contra-indication for MRI examination, e.g., metal implants; or being unable to lie still for 1½ hours for any reason, such as having a severe cold with coughing.
The study protocol was approved by the Institutional Review Board (IRB) at Purdue University and confidentiality was assured by assigning study identification numbers and anonymizing all imaging data.
2.2. Work Description
Subjects participating in the study worked in two plant locations, and on several work lines and departments within each plant. The welders from plant 1 primarily worked in a confined space environment welding the inside of tanker trailers, whereas welders from plant 2 worked primarily in open space environments. All welders primarily welded on mild steel using gas metal arc welding – metal inert gas (MIG), which transfers molten filler metal from the end of the filler wire via globules of molten metal (Harris, 2002). This type of welding is also known as machine welding in which the transfer of metal takes place by contact with the filler wire (usually coated in Mn) using an automatic feeder and the base metal. The welders worked at the current facility for an average of 9.49 years (SD = 7.17). The controls worked for an average of 2.43 years (SD = 1.89) in the same factory in positions that varied but included forklift and assembly operations.
2.3. Mn Air Concentration Measurements
To obtain measures of individual exposure to Mn in this study, Mn air concentration was measured utilizing personal respirable air sampling. Each personal respirable air sample was collected over the duration of a full work shift (8 hours) inside the welding helmet for welders and on the shoulder in the personal breathing zone (PBZ) for control subjects. SKC 25 mm aluminum cyclones with an aerodynamic diameter of 4.0 μm cut point, in line with SKC Airchek 52 personal sampling pumps drawing 2.5 L/min of air were utilized. The aluminum cyclone was fitted with a cassette holding a 25mm mixed cellulous ester filter (MCEF) with a pore size of 0.8 μm weight-matched to 50 μg. The cyclone and filters were placed inside the welder's welding helmet. For the control subjects, the cyclone and filters were placed on the shoulder in the personal breathing zone (PBZ). Seventy-seven personal air samples (with an average of six samples per working site) were collected over the duration of the study. The samples were analyzed for Mn using Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
2.4. Exposure Modeling
Since air sampling at a single time point does not take into account factors such as changes of departments (specific working sites) within the factory or respirator use, a recently developed exposure model (Laohaudomchok et al, 2011) was adapted for our study and used to estimate each participant's cumulative exposure index (CEI) over the past three months before the MRI scan as a more accurate estimate of the individual's respirable airborne Mn exposure (Ward et al., 2015). A time window of three months was chosen as Mn deposition in the brain is estimated to have a relatively short biological half-life of only several months (Nelson et al. 1993, Ono et al. 1995), and we hypothesized that MRI T1 would therefore best reflect changes in exposure over this recent time period.
The CEI, given in mg-years/m3, is calculated as the summation of the personal air samples from different departments (working sites within the factory) the subject has worked at over the past three months:
| [Eq. 1] |
Since not every welder was sampled at every working site, a “representative” Mn air concentration was assigned to each working site, calculated as the average over all personal air samples collected at that working site. This average value was then used instead of the single personal air sample for our exposure model:
| [Eq. 2] |
In addition, information on respirator use and arc times (time spent actually welding) are obtained from a detailed work history questionnaire (see below) informing respective weighting factors developed by (Hobson et al., 2011).
Overall, this exposure model, based on personal air samples, is assumed to give a better estimate of Mn exposure the individual may have had over the past three months before the MRI scans and neuropsychological testing, compared to exposure estimates solely based on reported hours of welding and questionnaires only.
2.5. Questionnaire Description
Three short scheduled interviews with each participant were conducted by the study manager (Part 1), the neurologist on the study team (Part 2), and the study team member who calculated individual Mn exposure (EJW) (Part 3), and lasted in total approximately 30 minutes. The interviews used a questionnaire asking about Personal Information (Part 1), Medical History (Part 2), and details provided by the participant on their work history (Part 3).
In Part 1 of the questionnaire, nine questions collected information on demographics and individual lifestyle factors including age, sex, weight, height, race, education, smoking habits, alcohol consumption, and hand dominance. Part 2 consisted of 30 questions collecting information on the participant's medical and psychiatric/neurological history (e.g., complaints of memory, fatigue, headache, depression, sleep, muscle cramps, dizziness, tremor, irritability, libido problems).
Part 3, the work history questionnaire collected information on the number of years which the subjects have welded at the current company, separated by departments, and at past employers. The questionnaire asked very detailed information that can affect exposure including respirator use, arc times (time spent actually welding), base metals, welding types, ventilation, and the amount of space for welding. This information along with the air monitoring data are used in the model to calculate each individual's exposure for the past three months as well as for various other exposure time windows (not presented here). Additionally, we asked about solvent exposures and work history in other industries (i.e. steel manufacturing, battery manufacturing, agriculture etc.), where individuals could be exposed to Mn.
2.6. Neuropsychological Test Battery and Procedure
The neuropsychological tests used were selected for their sensitivity and brevity by a clinical and research neuropsychologist (RB) experienced in assessing effects of Mn exposure as described by (Bowler and Lezak, 2015).
The domain of cognitive flexibility and executive function were tested with the Copy trial of the Rey-Osterrieth Complex Figure Test (Rey-O; Meyers and Meyers, 1995) and the Trails B test (Lezak et al., 2012). In the Rey-O Copy trial, examinees are asked to reproduce a complex stimulus figure, which requires reasoning and planning, both components of executive functioning. Low Copy scores, largely due to repetitions or errors, have been found in patients with lesions localized within the frontal lobes or in those who may have significantly below average educational achievement (Lezak, Howieson, 2012). The Immediate (3-minute delay) and Delayed (30-minute delay) Recall trials (from memory) of the Rey-O were used to assess visuospatial memory. An imaging study in Alzheimer's disease found the Rey-O test scores to appear reflective of posterior temporal-parietal cortex functioning (Melrose et al., 2013).
Part B of the Trail Making Test (Lezak, Howieson, 2012) requires examinees to connect and alternate between consecutively numbered and lettered circles, which, in addition to complex visual scanning, requires cognitive flexibility.
Visuomotor tracking speed was assessed with the Trail Making Test A (Lezak, Howieson, 2012) and the Digit Symbol Coding subtest of the WAIS-III (Wechsler, 1997). The Trails A test requires speeded visuomotor scanning, tracking and connecting a sequence of numbered circles in ascending order.
The Digit Symbol Coding test involves speed of processing visual information, short-term visual memory, and visual scanning. The examinee is required to copy symbols that are paired with numbers in a key. The raw score is the number of squares filled with the correct symbols in 120 seconds and is converted to an age-adjusted scaled score (Wechsler, 1997).
Attention and concentration, working memory, and learning were assessed with the Digit Span subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III; Lezak, Howieson, 2012, Wechsler, 1997). The Digit Span test requires examinees to repeat increasing sequences of numbers in the same (forward) and reverse (backward) order. The easier forward task is a measure of sustained attention, and the more effortful backward task includes a working memory component of storing the numbers and letters and alternating them in sequence (Lezak, Howieson, 2012). An age-adjusted scaled score is computed by summing the number of successfully completed backward and forward sequences.
The WHO-UCLA Auditory Verbal Learning Test (AVLT; Maj et al., 1993) is a verbal list learning and retention task, using 15 common words read to the participant in the same order in five acquisition (learning) trials. A new interference list (List B) is presented in Trial 6, followed by a post-interference recall trial (Trial 7) of the original list. The words included are from universally familiar categories (e.g., body parts) and represent a low cultural bias (Lezak, Howieson, 2012). A difference score between Trial 5 and Trial 1 was created to show the number of words learned across the five learning trials. Any intrusions or repetitions are considered pathognomonic.
Verbal category fluency was assessed with the Fruit Naming test (Bowler and Lezak, 2015). Examinees are asked to name as many fruits as they can within one minute.
Motor function was measured with the bilaterally-administered Finger Tapping Test (FTT) and Grooved Pegboard tests. The FTT is a widely-used measure of psychomotor speed requiring examinees to tap a lever as fast as possible within five 10-second trials alternating between hands. The average number of taps for each hand is converted to a mean total score.
Fine motor dexterity was assessed with the Grooved Pegboard test (Klove, 1963), requiring speeded rotation and insertion of pegs in a 5 × 5 matrix of holes. The number of seconds required to complete the task for each hand is converted to a mean total score.
Graphomotor tremor was assessed with the Parallel Lines test (Bowler and Lezak, 2015). During this task, participants are asked to draw as many vertical straight lines within a provided 2” × 1” rectangle as possible. Drawings are evaluated for qualitative graphomotor tremor.
In addition, a brief test of effort (Rey-15 Item Memory Test) was included (Bowler and Lezak, 2015). A cut-off score of 8 was used for less than optimal effort.
The order of test administration was identical for both groups and all participants. Training and supervision of examiners were provided by the licensed clinical neuropsychologist author. All tests were double-scored according to their manuals and discrepancies between scores were resolved by the neuropsychologist.
2.7. MRI Description
An 8-channel head-coil on a 3 T GE Signa HDxt MRI scanner was used for MRI examination (GE Medical Systems, Waukesha, WI). 3D high-resolution T1-weighted images were acquired for resolving brain anatomy. A fast spoiled gradient echo sequence (FSPGR) was used in sagittal orientation with repetition time = 6.28 ms, echo time = 2.67 ms, inversion time = 400ms, and flip angle = 12°. The matrix size was set to 240 × 240 yielding a resolution of 0.9×0.9×1 mm3.
T1 relaxation mapping
T1 relaxation time mapping using the dual flip angle technique was performed for the assessment of Mn accumulation. Two 3D spoiled gradient echo sequences (SPGR) with flip angles 3° and 17°, respectively, were acquired. Other imaging parameters included: TR/TE = 6.8/1.8 ms, bandwidth = 244 Hz/pixel, matrix dimension = 256 × 192, and resolution = 1×1×2 mm3. The total scan time for each SPGR sequence was 1 minutes and 12 seconds. An inversion recovery sequence (IR-SPGR, TI: 250ms) with the same parameters as the dual flip angle sequences was used to correct the inhomogeneity of the RF field (Deoni, 2007). T1 maps were generated by an in-house program in Matlab (The MathWorks, Natick, MA) using the approach described in (Sabati and Maudsley, 2013). T1 values were calculated as a function of repetition time (TR), flip angle (α) and a factor that is proportional to the equilibrium longitudinal magnetization (ρ) using equation 3:
| [Eq. 3] |
Regions of interest (ROIs)
Measurements of the average T1 values in the globus pallidus, substantia nigra, caudate nucleus, and anterior prefrontal lobe were made using circular ROI of 3 mm2 diameter on T1 maps, using the ROI tool in Matlab and Osirix (Pixmeo, Switzerland). All regions were identified bilaterally by neuroanatomical criteria. The positions of the ROIs are displayed in Figure 1. To reduce variability, two independent ROI measurements were performed per subject by two different individuals and were averaged for use in the data analysis. These bilateral measurements were then averaged to create one T1 value per brain ROI.
Figure 1.
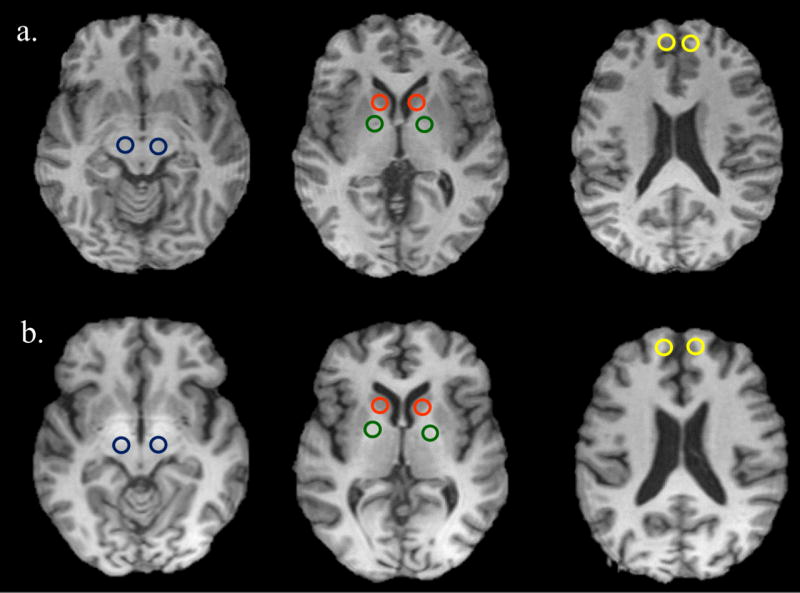
Regions of Interest (ROIs) displayed on T1-weighted images of a control (a) and a welder (b). Color of the ROI: blue for substantia nigra, red for caudate, green for globus pallidus, and yellow for anterior prefrontal lobe. Slight T1 hyperintensities can be discerned in this welder.
2.7. Statistical analyses
All analyses were conducted using SPSS Statistics Version 24. A level of p < 0.05 was used as a threshold for statistical significance for all analyses. Raw neuropsychological test scores were used for all statistical analyses. Independent samples t-tests were used to examine potential differences between welders and controls regarding demographic and exposure characteristics. Chi-square tests were used to examine differences between welders and controls for categorical variables, including ethnicity and the Parallel Lines test. Analysis of covariance (ANCOVA) was used to examine potential neuropsychological test score differences between welders and controls, adjusting for age and education. Although age and education did not differ between the comparison groups, age and education are associated with the neuropsychological tests used and therefore should be included as covariates (Lezak et al., 2012). Adjustments for multiple comparisons were made within each neuropsychological test domain using the Benjamini-Hochberg False Discovery Rate using a critical value of alpha = .05 to provide a more conservative null hypothesis test (Benjamini & Hochberg, 1995; Benjamini, Krieger, & Yekutieli, 2006). The tables show both the unadjusted and adjusted p-values, which reflect the q* value after adjusting for multiple comparisons. Estimates of effects size are conventionally reported using partial eta squared (η2p) to estimate the proportion of variance in test scores attributable to the two participant groups (Cohen, 1988).
Multivariate analysis of variance (MANOVA) was used to examine T1 relaxation time differences between welders and controls. MANOVA is used to determine whether there are differences between independent groups on multiple continuous dependent variables that are correlated, as is the case with MRI T1 relaxation times by brain region.
Partial correlation was used to examine the relationship between MRI T1 relaxation times and neuropsychological test scores, as well as for the relationship between MRI T1 relaxation times and part-3-months exposure (CEI) for the welder group, controlling for age and education. Adjustments for multiple comparisons were made within each neuropsychological domain, taking into account all neuropsychological test scores listed in Table 2, for each of the separate brain ROIs using the Benjamini-Hochberg False Discovery Rate. Unadjusted and adjusted p-values, which reflect the q* value after adjusting for multiple comparisons, are shown. A Pearson correlation was used to investigate the relationship between MRI T1 relaxation times and past-3-months exposure
Statistical considerations
Scores on the Rey-15 Item Test indicate that all but two welders provided valid effort in their responses on the testing. These two welders were excluded from all analyses, resulting in a welder sample size of 30 participants. Four additional welders were excluded from MRI analyses due to absent MRI data - two participants were claustrophobic and two were interrupted during testing by a tornado alarm.
Initial findings indicated that neuropsychological test scores on Rey-Osterrieth Copy, Trails A, and Trails B were not normally distributed. These three tests were log transformed to meet basic assumptions of statistical testing. Although raw scores are reported for the interpretational ease of the reader, log-adjusted data for these three tests were used in all statistical analyses.
3. Results
Comparisons of the demographic, lifestyle, and exposure characteristics between the welders (n = 26) and controls (n =17) are presented in Table 1. Age, education, ethnicity, alcohol consumption, and smoking habits did not significantly differ between welders and controls. The welders' median level ± inter-quartile range (IQR) of respirable Mn was 0.11 ± 0.05 mg/m3, with a mean of 12.25 years of welding experience including former employers (Range: 3-36 years). Welders working in confined space had personal exposures to air Mn levels ranging from 0.5-0.8 mg/m3, whereas Mn exposures on average were considerably lower for welders working in more open work environments, ranging from 0.07-0.15 mg/m3. The median ± IQR for the past-3-months CEI was 0.03 ± 0.03 mg-years/m3 for welders and 0.001 ± 0.001 mg-years/m3 for controls. While we had a large range of exposures due to the two subgroups of welders (open space, confined space), a stratification by subgroups for the following analyses was not reasonable due to the already small sample size.
Table 1. Demographic characteristics and comparison between welders and controls.
| Welders (n = 26) | Controls (n = 17) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Demographic characteristics | Mean | SD | Mean | SD | t | p |
| Age (years) | 40.38 | 11.0 | 36.41 | 10.6 | -1.17 | .247 |
| Education (years) | 12.73 | 1.3 | 12.65 | 1.9 | -0.17 | .865 |
| Number of Cigarettes/Day | 5.27 | 7.9 | 5.65 | 8.7 | 0.15 | .884 |
| Number of Alcoholic Drinks/Week | 3.75 | 5.9 | 2.72 | 2.6 | -0.66 | .515 |
| Years of Welding | 12.25 | 8.2 | 0.00 | 0.0 | - | - |
|
| ||||||
| Ethnicity | n | % | n | % | η2 | p |
|
| ||||||
| Ethnicity | 1.38 | .711 | ||||
| Caucasian | 20 | 76.9 | 14 | 82.4 | ||
| African American | 4 | 15.4 | 3 | 17.6 | ||
| Hispanic | 1 | 3.8 | 0 | 0.0 | ||
| Other | 1 | 3.8 | 0 | 0.0 | ||
|
| ||||||
| Exposure | Median | IQR | Median | IQR | T | p |
|
| ||||||
| Exposure | ||||||
| Air levels (mg/m3) | 0.108 | 0.048 | 0.003 | 0.002 | 20.52 | <0.0001 |
| CEI Past 3 months (mg/m3 × yr.) | 0.031 | 0.028 | 0.001 | 0.001 | 4.96 | <0.0001 |
3.1. Neuropsychological test performance
Table 2 shows the comparison between welders' and controls' test scores on all neuropsychological tests using ANCOVA. The number of intrusions from Trials 6 and 7 of the WHO-UCLA AVLT were included in the correlation analyses because previous research shows that intrusions on Trials 6 and 7 (i.e., interference and post-interference tasks, respectively) may indicate significant verbal retrieval problems in brain-injured persons (Bigler et al., 1989; Lezak et al., 2012). After adjustments for multiple comparisons, welders scored significantly lower than controls on the Fruit Naming test (F = 10.05, padj = .003) and the Parallel Lines test (χ2 = 16.72, padj = .015, Cramer's V = .624). Welders scored lower than controls on Digit Span total (F = 4.57, p = .039, padj = .156) but after adjusting for multiple comparisons, this difference was no longer significant.
Table 2. Neuropsychological test scores and comparison between welders and controls using ANCOVA.
| Domains of function and tests administered | Welders (n = 26) | Controls (n = 17) | F | η2partial | p | padja | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Cognitive Flexibility and Executive Functioning | ||||||||
| Rey-Osterrieth Copy b | 28.50 | 6.7 | 31.47 | 3.6 | 2.14 | .05 | .151 | .302 |
| Trails B b | 70.31 | 34.0 | 60.18 | 18.7 | 0.84 | .02 | .364 | .364 |
| Working Memory/Learning | ||||||||
| WAIS-III Digit Span | 14.77 | 4.0 | 17.47 | 3.7 | 4.57 | .11 | .039 | .156 |
| WHO-UCLA AVLT Correct Trial 1-5 Difference | 4.58 | 1.9 | 5.41 | 1.8 | 1.48 | .04 | .232 | .309 |
| WHO-UCLA AVLT Trial 6 Intrusions | 0.31 | 1.2 | 0.35 | 0.6 | 0.00 | .00 | .989 | .989 |
| WHO-UCLA AVLT Trial 7 Intrusions | 0.04 | 0.2 | 0.18 | 0.4 | 2.08 | .05 | .157 | .309 |
| Visuospatial Memory | ||||||||
| Rey-Osterrieth Immediate Recall | 15.33 | 7.1 | 18.38 | 6.8 | 1.33 | .03 | .256 | .339 |
| Rey-Osterrieth Delayed Recall | 15.38 | 6.1 | 17.81 | 6.8 | 0.94 | .02 | .339 | .339 |
| Visuomotor Tracking Speed | ||||||||
| Trails A b | 25.50 | 7.8 | 24.82 | 8.5 | 0.54 | .00 | .817 | .972 |
| WAIS-III Digit Symbol Coding | 14.36 | 6.1 | 15.41 | 5.4 | 0.00 | .00 | .972 | .972 |
| Verbal Fluency | ||||||||
| Fruit Naming | 12.15 | 3.1 | 15.00 | 3.3 | 10.05 | .21 | .003 | .003 |
| Effort | ||||||||
| Rey-15 Item Test | 13.19 | 2.7 | 13.59 | 2.4 | 0.11 | .00 | .748 | .748 |
| Motor Function | ||||||||
| Finger Tapping dominant hand | 54.99 | 7.3 | 57.54 | 5.7 | 0.33 | .01 | .569 | .784 |
| Finger Tapping nondominant hand | 49.64 | 7.4 | 52.13 | 6.3 | 0.24 | .01 | .629 | .784 |
| Grooved Pegboard dominant hand | 70.40 | 10.3 | 70.24 | 9.6 | 0.24 | .01 | .628 | .784 |
| Grooved Pegboard nondominant hand | 78.53 | 15.8 | 77.76 | 12.7 | 0.08 | .00 | .784 | .784 |
| Parallel Lines | n | % | n | % | χ2=16.72 | - | .005 | .015 |
| Within normal limits | 0 | 0.0 | 5 | 29.4 | ||||
| Borderline-Mild | 4 | 15.4 | 4 | 23.5 | ||||
| Mild | 5 | 19.2 | 6 | 35.3 | ||||
| Mild-Moderate | 8 | 30.8 | 1 | 5.9 | ||||
| Moderate | 7 | 26.9 | 0 | 0.0 | ||||
| Moderate-Severe | 2 | 7.7 | 1 | 5.9 | ||||
Note. Adjusted for age and years of education
Benjamini-Hochberg corrected false discovery rate probabilities shown, adjusted within each neuropsychological domain; p < .05 in bold
Results using log-transformed data to meet assumption of normality
3.2. Brain MRI T1 relaxation time
Table 3 shows the comparison of T1 relaxation times by region of interest (ROI) and results of multivariate analysis of variance (MANOVA) between welders and controls. The MANOVA was significant, Wilk's λ = .61, F(5, 37) = 4.67, p = .002, multivariate η2p = .63, with 63% of the variation in MRI T1 relaxation times accounted for by differences between welders and controls. Specifically, these results show that welders have shorter T1 relaxation times in the globus pallidus, substantia nigra, caudate nucleus, and the anterior prefrontal lobe in welders. The anterior prefrontal lobe demonstrated the largest difference (F = 23.59, p < .001, η2p = .350) between welders and controls of all the studied brain areas.
Table 3. T1 relaxation times by region of interest (ROI) and results of multivariate analysis of variance (MANOVA) between welders and controls.
| Region of Interest (ROI) | Welders (n = 26) | Controls (n = 17) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | η2p | p | |
| Globus Pallidus | 1042.71 | 128.4 | 1169.12 | 113.9 | 10.86 | .209 | .002 |
| Substantia Nigra | 1169.48 | 112.9 | 1254.79 | 138.3 | 4.91 | .107 | .032 |
| Caudate Nucleus | 1326.14 | 140.8 | 1452.64 | 163.0 | 7.32 | .131 | .010 |
| Anterior Prefrontal Lobe | 879.19 | 145.4 | 1099.32 | 145.2 | 23.59 | .350 | <.001 |
Note: Wilk's λ = .61, F(5, 37) = 4.67, p = .002, multivariate η2p = .63
3.3. Correlation between neuropsychological performance and brain MRI T1
Partial correlation was used to analyze the relationship between all neuropsychological test scores listed in Table 2 and MRI T1 relaxation times for each ROI in welders., adjusting for years of age and education (Table 4). Only tests showing a significant correlation (p < 0.05) before adjustment for multiple comparisons are shown in Table 4. Adjustments for multiple comparisons were made within each neuropsychological domain (see Table 2) separately for each of the four brain ROIs. A positive association between lower neuropsychological test performance and lower T1 relaxation time was hypothesized. Negative associations were expected for the number of intrusions (errors) on the WHO-UCLA AVLT as this indicates more intrusions are associated with lower T1 relaxation times.
Table 4. Correlation between neuropsychological test scores and MRI T1 relaxation times for welders.
| Welders (n = 26) | |||
|---|---|---|---|
| pr | p | padja | |
| T1 Globus Pallidus | |||
| WHO-UCLA AVLT Trial 6 Intrusions | -.425 | .039 | .078 |
| WHO-UCLA AVLT Trial 7 Intrusions | -.452 | .026 | .078 |
| T1 Substantia Nigra | |||
| WHO-UCLA AVLT Trial 6 Intrusions | -.465 | .022 | .044 |
| WHO-UCLA AVLT Trial 7 Intrusions | -.529 | .008 | .032 |
| T1 Caudate Nucleus | |||
| Fruit Naming | .446 | .029 | .029 |
| WHO-UCLA AVLT Trial 6 Intrusions | -.462 | .023 | .046 |
| WHO-UCLA AVLT Trial 7 Intrusions | -.550 | .005 | .020 |
| T1 Anterior Prefrontal Lobe | |||
| WHO-UCLA AVLT Trial 7 Intrusions | -.441 | .031 | .120 |
adjusted for age and education
Benjamini-Hochberg-corrected false discovery rate probabilities shown, adjusted within each neuropsychological domain and each region of interest
For welders, there was a positive correlation between Fruit Naming scores and only the caudate nucleus T1 relaxation times. The most frequent finding in the welder group were significant associations between the number of intrusions on the WHO-UCLA AVLT, a measure of verbal learning and memory, and shorter T1 relaxation times in all four ROIs. However, after making adjustments for multiple comparisons, only T1 relaxation times in the substantia nigra and the caudate nucleus were associated with the number of intrusions on Trials 6 (interference) and 7 (post-interference) of the WHO-UCLA AVLT.
3.4. Correlation between neuropsychological performance and Mn exposure
Partial correlation, adjusted for age and education status, was also used to test the relationship between neuropsychological test scores, as listed in Table 2, and the past-3-months CEI. No significant correlations were found for any neuropsychological test scores.
3.5. Correlation between MRI T1 and Mn exposure
Equally, no significant correlation as tested by a simple Pearson correlation, were found between MRI T1 values for any of the four ROIs and past-3-months CEI.
4. Discussion
The use of MRI originated in the late 1970s and quickly became a primary research tool for assessing brain structure and function. Structural neuroimaging using MRI along with traditional functional neuropsychological testing has already changed the application and validity of neuropsychological research as described by Lezak et al. (2012) and Bowler and Lezak (2015).
This is the first study to investigate subtle changes in T1 relaxation times in several brain regions and the relationships these changes have with neuropsychological function in the respective brain region. This study enriches the paucity of knowledge concerning the knowledge of adverse neuropsychological and neurological effects of active welders occupationally exposed to Mn.
In the current study, welders scored significantly worse than controls on tests of verbal fluency (Fruit Naming) and graphomotor tremor (Parallel Lines). In addition, welders had shorter T1 relaxation times compared to the controls in the globus pallidus, substantia nigra, caudate nucleus, and the anterior prefrontal lobe, which support the claim of increased Mn deposition in these brain regions. These findings replicate early studies in animal models showing that excess exposure to Mn leads to particularly high concentrations of Mn in the basal ganglia, specifically in the globus pallidus, caudate/putamen, and substantia nigra (Dorman et al., 2006; Guilarte et al., 2006b). Our results also agree with the report of shortened T1 in more highly exposed welders in the caudate nucleus, putamen, globus pallidus and frontal white matter described by Lee et al. (2015).
4.1. Motor function and brain MRI
In the current study, motor test performance was not associated with MRI T1 relaxation times in any of the examined brain regions. In this study of active welders, precise and speeded skillful movements are an occupational requirement, and therefore motor dysfunction is not likely to be present. Welders in the current study scored similarly to the controls on tests of psychomotor speed on the bilateral FTT. However, when examining graphomotor tremor, welders showed significantly more graphomotor tremors than controls. The neurological effect of having tremor in actively working welders exposed to Mn, who require extraordinary steadiness and stability, is of some concern and is frequently associated with Mn exposure.
Although studies of the effects of Mn in welders in the neurotoxicology literature primarily used motor testing, cognitive impairment may precede motor deficits in active welders, who may have had above average baseline psychomotor speed abilities preceding their Mn exposure. However, this may also indicate a healthy worker effect (Eisen and Robins, 2002), which would mask psychomotor deficits in the absence of baseline testing.
4.2. Cognitive function and brain MRI
Of importance in this study are the findings that the active welders showed associations between verbal intrusions and perseverations and shorter T1 relaxation times in all four brain ROIs studied here. After making adjustments for multiple comparisons, verbal intrusions were only associated with the substantia nigra and caudate nucleus. These findings should be considered preliminary due to the small sample size. Findings of significant associations between verbal intrusions and all brain ROIs before adjustments for multiple comparisons may be replicated using larger samples.
These associations were predicted a priori in light of the multiple brain regions involved in some of the complex, multi-step functions chosen to be tested (e.g., the various connections and pathways for tests of verbal learning requires good function in the frontal lobes, caudate, globus pallidus, nigrostriatal pathway and direct and indirect pathways from the cortex to thalamus through the basal ganglia). The intrusions suggest subtle perseveration and set-shifting problems, which are indicative of executive dysfunction in the Mn exposed welders. This highlights the need for including tests of verbal learning and executive function in neuropsychological studies of occupational Mn exposure to measure verbal learning and memory deficits, intrusions, and perseverations. Intrusions and perseverative errors made by highly skilled workers, such as welders, are pathognomonic for Mn neurotoxicity.
The substantia nigra has been shown to be associated with motor regulation or dysfunction, especially among Parkinson's disease patients, who suffer symptoms similar to those overexposed to Mn (Hudnell, 1999; Pal, 1999). However, the substantia nigra has also been associated with verbal learning, consistent with our results of higher brain Mn levels (with lower relaxation time) in the left substantia nigra. Josephs et al. (2005) reported similar results of verbal dysfunction associated with T1 hyperintensities in the basal ganglia as a result of Mn neurotoxicity.
Results involving the anterior prefrontal lobe from the current study are also consistent with changes found by Guilarte (2013) and Long et al. (2014a) in the frontal cortex of participants exposed to excess levels of Mn. Damage to the frontal lobe has been associated with impaired motor skills and organization as well as disrupted speech production related to verbal abilities (Lezak et al., 2012; Stuss & Benson, 1990; Stuss & Levine, 2002).
Manganese is known to diffuse along established neuronal pathways in the brain (Silva et al, 2008). However, while this study makes the assumption that the shortened T1 is due to increased levels of Mn, little is known about the time course of elevated Mn levels across different brain regions. It is assumed that impaired cognitive and motor function represent a first sign of toxicity in brain regions with observed changes in T1 relaxation times. Yet, the duration of elevated Mn levels in each brain region, a factor not known in this cross-sectional study, is another factor that likely plays a role in functional impairment. This duration factor could explain the finding of significant correlations between T1 and cognitive deficits in the absence of significant group differences (which are representative of one single time point).
Overall, these findings suggest a subtle cognitive decline in active welders' verbal learning, fluency, and memory, which is manifested in several brain regions and subsequent greater levels of intrusions of previously learned stimuli. This dysfunction in the basal ganglia and anterior prefrontal lobe also suggests it may be an early indicator of cognitive impairment, before motor dysfunction becomes apparent.
4.3. Cognitive function and Mn exposure
In this study only the relationship between neuropsychological test scores and a measure of exposure over the past three months prior to the MRI scans were tested. It is not surprising that no significant correlations were found with this short-term measure of exposure, since it is assumed that cognitive effects due to Mn neurotoxicity would rather relate to more long-term, cumulative or higher level exposure metrics. Verbal fluency as indicated by the fruit naming test, seems to be most sensitive even to this short-term exposure metric, indicated by its trending correlation. The relationships to longer-term exposure metrics will be studied in future studies in this group of welders, which also include a longitudinal follow-up. Air Mn levels are a single-time measure of exposure, furthermore not taking into account the use of respirators, and have therefore not been tested for correlations either with neuropsychological test scores nor with MRI T1 levels.
4.4. MRI T1 levels and Mn exposure
In contrast, a significant correlation between MRI T1 levels and past-3-months exposure was hypothesized. In fact, the past-3-months CEI was specifically chosen to match the approximate biological half-life of Mn in the human brain. Yet, no correlation was found. This could be due to the rather small sample size. In addition, the rate of deposition as well as clearance of Mn is likely not linear but of more complex nature (Lee et al. 2015) and is anticipated to be different in different brain regions. Finally, the change in T1 levels was rather small in this cohort of rather low level exposure – only in three welders a change was discerned by eye, without quantitative measures. Thus, at these exposure levels, T1 levels might also be reflecting some confounding influence from iron, which would be masked at higher Mn exposure levels, since Mn is the much stronger T1 contrast agent (Fitsanakis et al. 2006). The relationship of brain Mn deposition with different time windows of exposure needs further studies in the future.
4.5. Limitations
The welders in our study are obviously exposed to multiple metals besides manganese, which could have caused some of the effects observed in this study. Furthermore, other factors, such as shift work or possibly abnormal postures or repetitive movements – though not observed by us – could potentially influence our test results. Therefore, a limitation of this cross-sectional study is the current lack of follow-up data to determine the causal relationship between MRI Mn deposition in the brain and neuropsychological function of the Mn exposed welders over time. This limitation is currently being addressed by a follow-up study, using the same methodology as the current study. Another limitation was the fact that examiners of the neuropsychological test battery were not blinded to the welding status of the subjects for roughly half of the subjects presented here. However, test administration followed a very strict and well-trained protocol. The strict selection of Mn exposed welders and unexposed matched controls from the same facility resulted in a relatively small sample size of 26 welders and 17 controls, which limits statistical power. Results from the current study should therefore serve as preliminary data. Future studies should seek larger samples to replicate the current findings.
4.6. Strengths
This study is one of the few studies investigating subtle changes of MRI T1 relaxation time in several brain regions in Mn exposed subjects. Moreover, the study results show how the altered T1 relaxation time relates to sensitive clinical measures of neuropsychological performance. A methodological strength of this study is the use of matched controls from the same facility and comprehensively measured exposure, including on-site and personal air sampling for a full workday, along with neuroimaging to determine Mn brain deposition in specific ROIs. Clinical neuropsychological testing with selection of brain function domains used in clinical neuropsychology is an additional strength of this study. Additionally, standardized normative scores used in this study also reflect the general practices in clinical neuropsychology and allows a sensitive differentiation in each of the levels of neuropsychological function assessed.
4.7. Directions for Future Research
Studies of Mn will benefit from including additional tests of cognitive flexibility, executive function, working memory, and verbal learning. It would be useful to add additional tests of executive function, including the set-shifting Stroop Interference Test (Lezak, Howieson, 2012). For more highly educated participants, the Wisconsin Card Sorting Test (WCST; Bowler and Lezak, 2015) should be added, which requires planning, abstraction, and having a strategy for efficient organizing principles. The WCST is a test of the ability to shift cognitive sets in response to changing environmental contingencies. Performance on the WCST has been associated with the frontal lobes (Lezak et al., 2012; Stuss et al., 2000).
4.8. Occupational health implications
Given the results of this study of imaging changes, indicative of subtle brain Mn deposition and associations with neuropsychological performance, it is recommended to lower the average respirable Mn air concentration to the currently suggested Threshold Limit Value (TLV) of 0.02 mg/m3 as advised by the American Conference of Governmental Industrial Hygienists (ACGIH, 2013). Although Mn air levels of 0.1 mg/m3, as encountered in this study, are still standard in many welding environments, the threshold for showing subtle effects on cognitive tests is unknown in these relatively low-exposure situations. Frequent air-Mn exposure measurements of the work environments, close proximity to welding processes, together with a very brief functional clinical neuropsychological screening would likely identify early onset of neuropsychological and physical symptoms and inform the management of potential over-exposure of Mn in the workplace. In most field studies, structural MRI cannot be as easily performed on workers because expensive MRI examinations may not be readily available to Mn exposed workers. However, both frequent exposure assessment and occasional brief neuropsychological screening could readily identify a need for a more complete clinical evaluation in adversely affected welders. This would suggest that needed improvements in the workplace environment could be made to improve potential health hazards. Air-Mn exposure reductions could be validated by both personal air sampling and a follow-up screening of the worker's neuropsychological function.
4.9. Conclusions
In summary, the current study shows the presence of shortened T1 relaxation times in welders in specific brain regions of the globus pallidus, substantia nigra, caudate nucleus, and the anterior prefrontal lobe. When measuring neuropsychological performance, Mn exposed welders performed worse than controls on tests of verbal function and graphomotor tremor. Welders' verbal fluency, learning, and perseveration were associated with lower T1 relaxation times in the brain. The findings of lower T1 values, indicative of Mn deposition, together with lower neuropsychological performance suggest the need for careful monitoring of the health implications of Mn over-exposure in order to protect the health and optimal function of welders and other Mn-exposed workers.
Table 5. Correlation between neuropsychological test scores and manganese exposure upon past 3 months for welders.
| pr | p | |
|---|---|---|
| Cognitive Flexibility and Executive Functioning | ||
| Rey-Osterrieth Copy b | -.127 | .518 |
| Trails B b | .067 | .735 |
| Working Memory/Learning | ||
| WAIS-III Digit Span | .010 | .961 |
| WHO-UCLA AVLT Correct Trial 1-5 Difference | .228 | .243 |
| WHO-UCLA AVLT Trial 6 Intrusions | -.030 | .878 |
| WHO-UCLA AVLT Trial 7 Intrusions | .022 | .913 |
| Visuospatial Memory | ||
| Rey-Osterrieth Immediate Recall | .046 | .815 |
| Rey-Osterrieth Delayed Recall | .035 | .861 |
| Visuomotor Tracking Speed | ||
| Trails A b | .195 | .319 |
| WAIS-III Digit Symbol Coding | -.295 | .128 |
| Verbal Fluency | ||
| Fruit Naming | .367 | .055 |
| Motor Function | ||
| Finger Tapping Dominant | -.165 | .401 |
| Finger Tapping Non-Dominant | -.131 | .507 |
| Grooved Pegboard Dominant | .118 | .550 |
| Grooved Pegboard Non-Dominant | .036 | .855 |
| Parallel Lines | -.011 | .957 |
Note. Adjusted for age and years of education
Benjamini-Hochberg corrected false discovery rate probabilities shown, adjusted within each neuropsychological domain
Results using log-transformed data to meet assumption of normality
Table 6. Correlation between manganese exposure (past 3 months) and T1 relaxation times by region of interest (ROI) for welders.
| Region of Interest (ROI) | r | p |
|---|---|---|
| Globus Pallidus | -.102 | .619 |
| Substantia Nigra | -.253 | .212 |
| Caudate Nucleus | .012 | .953 |
| Anterior Prefrontal Lobe | -.079 | .700 |
Highlights.
Manganese exposed welders were studied using MRI and neuropsychological testing
In welders, a shortened MRI T1 relaxation time was found in multiple brain regions
Welders scored worse on tests of verbal fluency and graphomotor tremor
Lower neuropsychological test performance was associated with shorter T1 times
Verbal dysfunction may be an early effect of brain Mn deposition in welders
Acknowledgments
This work is supported by NIH/NIEHS R01 ES020529 (UD) and NIOSH T03 OH008615 (EW). We would like to thank Dr. Donna Mergler and Dr. Yangho Kim for valuable discussions and input on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ACGIH. TLVs and BEIs: Based on the documentation of the Threshold Limit Values for chemical substances and physical agents and Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2013. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;1:491–507. [Google Scholar]
- Bowler RM, Gysens E, Diamond S, Nakagawa M, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–26. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Lezak MD. Neuropsychological evaluation and exposure to neurotoxicants. In: Lotti M, Bleecker ML, editors. Occupational Neurology. Amsterdam: Elsevier; 2015. [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64:167–77. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR. Manganese neurotoxicity: Lessons learned from longitudinal studies in non-human primates. Environ Health Perspect. 2009;117:325–32. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Lee JJ, Seo JH, Song HJ, Kim JH, Bae SJ, et al. Altered working memory process in the manganese-exposed brain. Neuroimage. 2010a;53:1279–85. doi: 10.1016/j.neuroimage.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Chang Y, Woo ST, Kim Y, Lee JJ, Song HJ, Lee HJ, et al. Pallidal index measured with three-dimensional T1-weighted gradient echo sequence is a good predictor of manganese exposure in welders. J Magn Reson Imaging. 2010b;31:1020–6. doi: 10.1002/jmri.22104. [DOI] [PubMed] [Google Scholar]
- Choi DS, Kim EA, Cheong HK, Khang HS, Ryoo JW, Cho JM, et al. Evaluation of MR signal Index for the assessment of occupational manganese exposure of welders by measurement of local proton T1 relaxation time. Neurotoxicology. 2007;28:284–9. doi: 10.1016/j.neuro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, New Jersey: Lawrence Erbaum Associates, Inc.; 1988. [Google Scholar]
- Criswell SR, Nelson G, Gonzalez-Cuyar LF, Huang J, Shimony JS, Checkoway H, et al. Ex vivo magnetic resonance imaging in South African manganese mine workers. Neurotoxicology. 2015;49:8–14. doi: 10.1016/j.neuro.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell SR, Perlmutter JS, Huang JL, Golchin N, Flores HP, Hobson A, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occupational Environmental Medicine. 2012;69:437–43. doi: 10.1136/oemed-2011-100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SCL. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI) J Magn Reson Imaging. 2007;26:1106–11. doi: 10.1002/jmri.21130. [DOI] [PubMed] [Google Scholar]
- Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. Journal of Comparative and Physiological Psychology. 1967;63(2):184–190. doi: 10.1037/h0024348. https://doi.org/10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Wong BA, Dye JA, Robertson ID. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol Sci. 2006;92:219–27. doi: 10.1093/toxsci/kfj209. [DOI] [PubMed] [Google Scholar]
- Dydak U, Criswell SR. Chapter 19 Imaging Modalities for Manganese Toxicity. In: Costa LG, Aschner M, editors. Manganese in Health and Disease: The Royal Society of Chemistry. 2015. pp. 477–512. [Google Scholar]
- Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119:219–24. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen EA, Robins JM. Healthy worker effect. In: El-Shaarawi AH, Piegorsch WW, editors. Encyclopedia of Environmetrics. West Sussex: John Wiley & Sons; 2002. [Google Scholar]
- Eriksson H, Tedroff J, Thuomas K, Aquilonius SM, Hartviq P, Fasth KJ, et al. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66:403–7. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology. 2006;27(5):798–806. doi: 10.1016/j.neuro.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, et al. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006a;202:381–90. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte T, McGlothan J, Degaonkar M, Chen MK, Barker PB, Syversen T, et al. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: A 1H-MRS and MRI Study. Toxicol Sci. 2006b;94:351–8. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu V, Becker KG, Syversen T, et al. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–59. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. Manganese neurotoxicity: New perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Frontiers in Aging Neuroscience. 2013;5:1–10. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Yeo RA. Neuropsychological and neuroanatomic aspects of complex motor control. In: Bigler ED, Yeo RA, Turkheimer E, editors. Neuropsychological function and brain imaging. New York, NY: Springer; 1989. [Google Scholar]
- Harris M. Welding health and safety: a field guide for OEHS professionals. Fairfax, Virginia: American Industrial Hygiene Association; 2002. [Google Scholar]
- Heaton RK, Miller WS, Taylor MJ, Grant I. Psychological Assessment Resources. 2004. Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and caucasian adults. [Google Scholar]
- Hudnell KH. Effects from environmental Mn exposures: a review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20:379–97. [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: 2013. [Google Scholar]
- Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. Neurotoxicology. 2007;28:126–35. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, et al. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–9. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kim EA, Cheong HK, Choi DS, Sakong J, Ryoo JW, Park I, et al. Effect of occupational manganese exposure on the central nervous system of welders: 1H magnetic resonance spectroscopy and MRI findings. Neurotoxicology. 2007;28:276–83. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kim Y, Jeong KS, Song HJ, Lee JJ, Seo JH, Kim GC, et al. Altered white matter microstructural integrity revealed by voxel-wise analysis of diffusion tensor imaging in welders with manganese exposure. Neurotoxicology. 2011;32:100–9. doi: 10.1016/j.neuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim KS, Yang JS, Park IJ, Kim E, Jin Y, et al. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20:901–7. [PubMed] [Google Scholar]
- Klove H. Clinical Neuropsychology. In: Forster FM, editor. The Medical Clinics of North America. New York, NY: Saunders; 1963. [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, et al. Toenail, blood and urine as biomarkers of manganese exposure. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2011;53(5):506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Flynn MR, Du G, Lewis MM, Fry R, Herring AH, et al. T1 relaxation rate (R1) indicates nonlinear Mn accumulation in brain tissue of welders with low-level exposure. Toxicol Sci. 2015 doi: 10.1093/toxsci/kfv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi BS, Nassetta WJ. Neurologic effects of manganese in humans: A review. Int J Occup Environ Health. 2003;9:153–63. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson's disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience. 2003;23(15) doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Long Z, Jiang YM, Li XR, Fadel W, Xu J, Yeh CL, et al. Vulnerability of welders to manganese exposure - a neuroimaging study. Neurotoxicology. 2014a;45:285–92. doi: 10.1016/j.neuro.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Li XR, Xu J, Edden RA, Qin WP, Long LL, et al. Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS One. 2014b;9:e88220. doi: 10.1371/journal.pone.0088220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, D'Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Archives of Clinical Neuropsychology. 1993;8:123–35. [PubMed] [Google Scholar]
- Melrose RJ, Harwood D, Khoo T, Mandelkern M, Sultzer DL. Association between cerebral metabolism and Rey-Osterrieth Complex Figure Test performance in Alzheimer's disease. J Clin Exp Neuropsychol. 2013;35:246–58. doi: 10.1080/13803395.2012.763113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. Rey Complex figure test and recognition trial: professional manual. Odessa, Fl.: PAR; 1995. [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of general psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K, Golnick J, Korn T, Angle C. Manganese encephalopathy: Utility of early magnetic resonance imaging. Br J Ind Med. 1993;50:510–3. doi: 10.1136/oem.50.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp Neurol. 1989;106:251–8. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Ono J, Harada K, Kodaka R, Sakurai K, Tajiri H, Takagi Y, et al. Manganese deposition in the brain during long-term total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1995;19(4):310–2. doi: 10.1177/0148607195019004310. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–38. [PubMed] [Google Scholar]
- Sabati M, Maudsley AA. Fast and high-resolution quantitative mapping of tissue water content with full brain coverage for clinically-driven studies. Magn Reson Imaging. 2013;31:1752–9. doi: 10.1016/j.mri.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. State-of-the-science-review: Does manganese exposure during welding pose a neurological risk? J Toxicol Environ Health. 2007;10:417–65. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, et al. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006;1118:222–31. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Williams C, Ault M, Guilarte TR. Chronic manganese exposure impairs visuospatial associative learning in non-human primates. Toxicol Lett. 2013;221:146–51. doi: 10.1016/j.toxlet.2013.06.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Williams C, Ault M, Guilarte TR. Effects of chronic manganese exposure on attention and working memory in non-human primates. Neurotoxicology. 2015;48:217–22. doi: 10.1016/j.neuro.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Chang Y, Jang KE, Park JW, Kim YT, Park SJ, Jeong KS, Kim A, Kim SH, Kim Y. Altered executive function in the welders: A functional magnetic resonance imaging study. Neurotoxicol Teratol. 2016;56:26–34. doi: 10.1016/j.ntt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Shin YC, Kim E, Cheong HK, Cho S, Sakong J, Kim KS, et al. High signal intensity on magnetic resonance imaging as a predictor of neurobehavioral performance of workers exposed to manganese. Neurotoxicology. 2007;28:257–62. doi: 10.1016/j.neuro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological bulletin. 1984;95:3–28. [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes and language. In: Goldberg E, editor. Contemporary neuropsychology and the legacy of Luria. Hillsdale, NJ: Erlbaum; 1990. [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–33. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Verina T, Kiihl SF, Schneider JS, Guilarte TR. Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology. 2011;32:215–26. doi: 10.1016/j.neuro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E, Nour M, Snyder S, Rosenthal F, Dydak U. Human toenails – A viable biomarker for mixed metal exposure in U.S. Welders Toxicol Sci suppl. 2015;144(1):347. [Google Scholar]
- Wechsler D. WAIS-III & WMS-III Technical Manual. The Psychological Corporation; 1997. [Google Scholar]
- Yim HW, Kim JH, Phee YG, Koo JW, Lee KS, Park CY, et al. An association between brain MRI and neurologic findings in welders exposed to manganese fume. Korean J Occup Environ Med. 1988;10:161–71. [Google Scholar]
- Zoni S, Albini E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. Am J Ind Med. 2007;50:812–30. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]


