Abstract
Background
Previous studies suggested that single-nucleotide polymorphisms in dopamine receptor D2 (DRD2) are the susceptibility loci for migraine. This study was aimed at evaluating the contribution of DRD2 rs1800497 and its expression to migraine risk in Han Chinese subjects.
Methods
In total, 250 patients with migraine and 250 age- and sex-matched control subjects were included in this study. TaqMan allelic discrimination assay was used for DRD2 rs1800497 genotyping. Plasma DRD2 concentration was determined using enzyme-linked immunosorbent assay.
Results
Significant associations were observed for the rs1800497 genotype (c2=6.37, p=0.041) and allele (c2=4.69, p=0.03; odds ratio [OR]=1.33, 95% CI=1.03–1.72, power=58%) frequencies between the migraine and control groups. Sex analysis indicated a positive association for rs1800497 between female patients with migraine and control individuals (genotype: c2=7.84, p=0.019; allele: c2=6.60, p=0.010; OR=1.61, 95% CI=1.12–2.30, power=73.4%). Furthermore, a significant association was observed only in female patients with migraine without aura (MO) (genotype: c2=6.88, p=0.032; allele: c2=5.65, p=0.017; OR=1.59, 95% CI=1.08–2.36, power=65.1%). The mean plasma DRD2 levels in the control group (mean±SD: 24.20±2.78) were significantly lower than those in the migraine with aura (MA) (30.86±3.69, p<0.0001) and MO groups (31.88±4.99, p<0.0001). Additionally, there was a sex-based difference in DRD2 expression in the MA (male vs female: 29.46±3.59 vs 32.27±3.27, p<0.01) and MO groups (male vs female: 29.18±3.50 vs 34.58±4.84, p<0.0001). Moreover, plasma DRD2 levels in patients were significantly different among the three genotypes (CC vs CT vs TT: 24.76±3.76 vs 30.93±3.85 vs 37.06±3.95, p<0.0001). Similar results were observed both in the MA (CC vs CT vs TT: 25.09±3.84 vs 28.57±2.84 vs 33.37±1.58, p<0.0001) and MO groups (CC vs CT vs TT: 24.65±3.79 vs 31.65±3.86 vs 38.29±3.74, p<0.0001).
Conclusion
Our case–control study suggested that the DRD2 polymorphism rs1800497 was significantly associated with the risk of migraine in Han Chinese females. Additionally, the plasma DRD2 level was high in patients with migraine. Females with migraine had considerably higher DRD2 levels than males with migraine. DRD2 expression may be regulated by DRD2 rs1800497 genotype in patients with migraine.
Keywords: dopamine receptor D2, female, rs1800497, ELISA, migraine
Introduction
Migraine is a common neurologic disorder that has become a major public health concern worldwide.1 This disorder is characterized by recurrent, moderate-to-severe headaches. Clinical manifestations typically include unilateral headache with nausea, emesis, phonophobia, and visual sensory disturbance.2 The two major types of migraine are migraine with aura (MA) and migraine without aura (MO), as defined by the International Headache Society.3 Migraine is a complex disorder, and involves the influence of various environmental and genetic factors.4,5 While scientists have obtained valuable information concerning the development and clinical treatment of migraine, the mechanisms underlying the pathogenesis of migraine remain unclear.6
Dopamine has been suggested to be the second putative protagonist in headache.7 Among the proposed mechanisms, the dopaminergic system is responsible for maintaining the dopamine–norepinephrine ratio.8 An imbalance in this ratio makes individuals susceptible to migraine.9 Dopamine receptors are believed to play specific roles in the pathogenesis of migraine.10 Dopamine receptor D2 (DRD2) has been widely studied in central nervous system disorders, including schizophrenia, posttraumatic stress disorder, movement disorders, and migraine.11 Both DRD2 genotyping and monitoring of plasma drug concentrations may be useful for alleviating clinically dominant symptoms in patients.12 Previous studies suggested that single-nucleotide polymorphisms (SNPs) in DRD2 are susceptibility loci for migraine.13 DRD2 polymorphism was observed to be significantly associated with the risk of migraine in an Indian population.14 DRD2 haplotype may be a risk factor in migraine susceptibility. In Japanese subjects, Onaya et al showed that DRD2 polymorphism significantly and independently contributed to the development of migraine from medication overuse headache.15 However, other studies did not support the contribution of DRD2 toward the genetic predisposition to migraine in a Spanish population and a North Indian population.13,16 The SNP rs1800497 on chromosome 11q23.2 is located in exon 8 of the ankyrin repeat domain containing one gene downstream of DRD2.17 DRD2 rs1800497 was observed to be associated with different conditions such as autism,18 cramps,19 migraine,14 memory loss,20 and schizophrenia.21 DRD2 rs1800497-T-carriers faced a significantly reduced risk of schizophrenia susceptibility in Asian populations.22 Gluskin and Mickey suggested that DRD2 rs1800497 probably contributes to important individual differences in human striatal function, neuropsychiatric disease risk, and pharmacologic response.23 DRD2 rs1800497 has been consistently reported to be linked with reduced striatal D2 receptor expression in both postmortem expression studies and in vivo radioligand binding studies.24,25 However, this link between rs1800497 and D2 receptor expression was not apparent in patients with neuropsychiatric disorders.23 The D2 receptor expression in these subjects tended to be higher among rs1800497-T-carriers than among C homozygotes.23 Significant associations between DRD2 rs1800497 and migraine risk were observed in a primary cohort study (208 patients with migraine and 200 healthy control subjects).14 However, no significant associations were observed in a secondary cohort (127 patients with migraine and 200 healthy control individuals) of Indian subjects.14 Importantly, no studies have investigated the putative relationship between DRD2 rs1800497 and migraine risk in Han Chinese populations.
In this study, we enrolled 500 age- and sex-matched volunteers and conducted a case–control study to validate the relationship between the DRD2 polymorphism rs1800497 and migraine risk in a Chinese Han population. Additionally, we examined the role of DRD2 in the pathogenesis of migraine by determining plasma DRD2 levels.
Materials and methods
Case–control subjects
This study was approved by the Ethics Committees of the Affiliated Hospital of Ningbo University, and written informed consent was obtained from all participants. This study included 250 unrelated patients with migraine and 250 age- and sex-matched healthy control subjects. All subjects were enrolled randomly between January 2014 and October 2017 from the Affiliated Hospital of Ningbo University, Ningbo city, Zhejiang province, China. Patients were diagnosed by at least two independent pain specialists and neurologists based on their responses provided in a validated medical questionnaire prepared according to the International Headache Society.26 Patients with migraine were assigned to two subgroups: MA and MO. The control subjects had no history of personal or familial migraine, and were selected randomly while undergoing a health examination in the hospital. Individuals with obesity, hypertension, diabetes, and other neurologic or psychiatric disorders were excluded from this study.
Laboratory analysis
A venous blood sample was collected from each subject within 12 h after fasting in an EDTA-coated vacutainer. Biochemical indices, including levels of triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apolipoprotein A-I, and apolipoprotein B, were measured using an automated clinical chemistry analyzer (Beckman Coulter, Brea, CA, USA). Plasma was separated from the blood samples by centrifugation at 1000×g for 10 min. Totally, 168 sex- and age-matched individuals, including 56 patients with MA, 56 patients with MO, and 56 control individuals, were enrolled for protein expression analysis. Plasma DRD2 concentration was determined using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA).
Genotype analysis
Lymphocyte DNA was isolated from peripheral blood using the salting-out procedure. The TaqMan Allelic Discrimination Assay System (assay ID: C_7486676_10) was used for DRD2 rs1800497 genotyping. All assays were optimized to a total volume of 10 μL, containing 10 ng of genomic DNA, 0.125 μL of primer-TaqMan Probe mixture, and 5 μL of TaqMan Universal PCR Master Mix 2X (Thermo Fisher Scientific, Waltham, MA, USA). Polymerase chain reaction amplification was conducted using a 7500 Real-Time PCR System (Thermo Fisher Scientific). Thermal cycling conditions included 95°C for 10 min, followed by 50 cycles at 95°C for 15 s, and 60°C for 1 min. Genotypes were determined using the algorithm and software supplied by the manufacturer (Thermo Fisher Scientific). To determine the random genotyping error rate, 5% DNA samples were examined in duplicate, showing 100% consistency in genotype.
Statistical analysis
Continuous variables were expressed as the mean and SD and compared using the independent samples t-test. Categorical variables were compared using Pearson’s chi-squared test. Quantitative data were compared using one-way analysis of variance or the Kruskal–Wallis test. The odds ratio (OR) and 95% CI were calculated by logistic regression to determine the relative risk of rs1800497 genotypes for migraine development. All statistical analyses were conducted with SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA). Power analysis was performed using Power and Sample Size Calculation software (version 3.0.43).27 Statistical significance was defined as a p-value <0.05.
Results
Clinical characteristics of subjects
The clinical characteristics of both groups are listed in Table 1. The case group comprised 250 patients (125 males and 125 females) experiencing migraine with a mean age of 43.02±16.70 years, while the control group comprised 250 healthy subjects (125 males and 125 females) with a mean age of 41.98±11.23 years. Among the patients, 68 had MA (39 males and 29 females) with a mean age of 44.00±17.33 years and 182 had MO (86 males and 96 females) with a mean age of 41.04±9.70 years. No significant differences were observed in the biochemical indices (levels of TG, TC, high-density lipoprotein, low-density lipoprotein, apolipoprotein A-I, and apolipoprotein B) between the patients with migraine and controls (p>0.05).
Table 1.
Comparison of characteristics between cases and controls
| Characteristics | Control (250) | Migraine (250) | MA (68) | MO (182) | p1 | p2 | p3 |
|---|---|---|---|---|---|---|---|
| Age, years, mean±SD | 41.98±11.23 | 43.02±16.70 | 44.00±17.33 | 41.04±9.70 | 0.414 | 0.248 | 0.364 |
| Male (n) | 125 | 125 | 39 | 86 | 1.000 | 0.549 | 0.741 |
| TG (mmol/L), mean±SD | 1.48±0.88 | 1.50±1.11 | 1.58±0.99 | 1.48±1.04 | 0.823 | 0.419 | 1.000 |
| TC (mmol/L), mean±SD | 4.38±1.01 | 4.41±1.18 | 4.49±1.08 | 4.39±1.04 | 0.760 | 0.433 | 0.920 |
| HDL (mmol/L), mean±SD | 1.12±0.31 | 1.16±0.69 | 1.10±0.78 | 1.18±0.39 | 0.403 | 0.747 | 0.076 |
| LDL (mmol/L), mean±SD | 2.55±0.86 | 2.44±0.89 | 2.49±0.91 | 2.39±0.99 | 0.160 | 0.615 | 0.074 |
| ApoA (g/L), mean±SD | 1.09±0.21 | 1.07±0.22 | 1.09±0.52 | 1.06±0.72 | 0.299 | 1.000 | 0.533 |
| ApoB (g/L), mean±SD | 0.81±0.41 | 0.79±0.25 | 0.89±0.23 | 0.75±0.45 | 0.511 | 0.124 | 0.150 |
Note: p1, the p-value for control vs migraine; p2, the p-value for control vs MA; p3, the p-value for control vs MO.
Abbreviations: ApoA, apolipoprotein A; ApoB, apolipoprotein B; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MA, migraine with aura; MO, migraine without aura; TC, total cholesterol; TG, triglyceride.
Comparison of genotype and allelic distributions
The genotype distributions of DRD2 rs1800497 in the control subjects and patients were within the Hardy–Weinberg equilibrium model (p>0.05). As shown in Table 2, significant differences were observed in the genotype (c2=6.37, p=0.041) and allele (c2=4.69, p=0.03; OR=1.33, 95% CI=1.03–1.72, power=58%) frequencies between the migraine and the control groups. Sex analysis indicated a positive association for rs1800497 between female patients with migraine and control subjects (genotype: c2=7.84, p=0.019; allele: c2=6.60, p=0.010; OR=1.61, 95% CI=1.12–2.30, power=73.4%). Furthermore, a significant association was observed only in female patients with MO (genotype: c2=6.88, p=0.032; allele: c2=5.65, p=0.017; OR=1.59, 95% CI=1.08–2.36, power=65.1%). However, no significant difference was observed in the male groups (p>0.05). The genetic models analyzed are listed in Table 3, and an increased risk was observed between the case and control groups under the recessive model (CC+CT vs TT: c2=4.69, p=0.03; OR=1.99, 95% CI=1.06–3.76, power=57.9%). In the sex analysis, positive associations were observed in female patients with migraine under the dominant (c2=5.15, p=0.023; OR=1.84, 95% CI=1.08–3.14, power=61.9%) and recessive models (c2=4.72, p=0.029; OR=2.45, 95% CI=1.07–5.63, power=58.3%). Significant collections were likely to be present in the patients with MO under the dominant (c2=3.82, p=0.05; OR=1.76, 95% CI=1.00–3.11, power=49.7%) and recessive models (c2=4.85, p=0.027; OR=2.58, 95% CI=1.09–6.12, power=59.3%).
Table 2.
Distribution of rs1800497 genotypes and alleles in both groups
| Gender | Group | Genotype
|
c2 | p (df=2) | Allele
|
c2 | p (df=1) | OR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC, n (%) | CT, n (%) | TT, n (%) | C, n (%) | T, n (%) | |||||||
| All | Control | 99 (0.40) | 135 (0.54) | 16 (0.06) | 333 (0.67) | 167 (0.33) | |||||
| Case | 80 (0.32) | 140 (0.56) | 30 (0.12) | 6.37 | 0.041 | 300 (0.60) | 200 (0.40) | 4.69 | 0.03 | 1.33 (1.03–1.72) | |
| MO | 59 (0.32) | 102 (0.56) | 21 (0.12) | 4.81 | 0.09 | 220 (0.60) | 144 (0.40) | 3.47 | 0.062 | 1.31 (0.98–1.73) | |
| MA | 21 (0.31) | 38 (0.56) | 9 (0.13) | 4.29 | 0.117 | 80 (0.59) | 56 (0.41) | 2.84 | 0.092 | 1.39 (0.95–2.06) | |
| Male | Control | 48 (0.38) | 70 (0.56) | 7 (0.06) | 166 (0.66) | 84 (0.34) | |||||
| Case | 46 (0.37) | 69 (0.55) | 10 (0.08) | 0.58 | 0.748 | 161 (0.64) | 89 (0.36) | 0.22 | 0.639 | 1.09 (0.76–1.58) | |
| MO | 32 (0.37) | 49 (0.57) | 5 (0.06) | 0.03 | 0.984 | 113 (0.66) | 59 (0.34) | 0.02 | 0.887 | 1.03 (0.68–1.55) | |
| MA | 14 (0.36) | 20 (0.51) | 5 (0.13) | 2.29 | 0.318 | 48 (0.62) | 30 (0.39) | 0.62 | 0.431 | 1.23 (0.73–2.09) | |
| Female | Control | 51 (0.41) | 65 (0.52) | 9 (0.07) | 167 (0.67) | 83 (0.33) | |||||
| Case | 34 (0.27) | 71 (0.57) | 20 (0.16) | 7.84 | 0.019 | 139 (0.56) | 111 (0.44) | 6.60 | 0.01 | 1.61 (1.12–2.30) | |
| MO | 27 (0.28) | 53 (0.55) | 16 (0.17) | 6.88 | 0.032 | 107 (0.56) | 85 (0.44) | 5.65 | 0.017 | 1.59 (1.08–2.36) | |
| MA | 7 (0.24) | 18 (0.62) | 4 (0.14) | 3.39 | 0.184 | 32 (0.55) | 26 (0.45) | 2.78 | 0.095 | 1.63 (0.91–2.92) | |
Note: Results with p-values less than 0.05 are shown in bold font. The minor allele frequency of rs1800497-T was 0.19 in HapMap-CEU; 0.41 in -JPT; 0.35 in -CHB; 0.44 in -CHD.
Abbreviations: df, degrees of freedom; MA, migraine with aura; MO, migraine without aura; OR, odds ratio.
Table 3.
Comparison of the dominant model and recessive model in rs1800497 between cases and controls by gender
| Gender | Group | Dominant model
|
c2 | p | OR (95% CI) | Recessive model
|
c2 | p | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC, n (%) | CT+TT, n (%) | CC+CT, n (%) | TT, n (%) | ||||||||
| All | Control | 99 (0.40) | 151 (0.60) | 234 (0.94) | 16 (0.06) | ||||||
| Case | 80 (0.32) | 170 (0.68) | 3.14 | 0.076 | 1.39 (0.97–2.01) | 220 (0.88) | 30 (0.12) | 4.69 | 0.03 | 1.99 (1.06–3.76) | |
| MO | 59 (0.32) | 123 (0.68) | 2.34 | 0.126 | 1.37 (0.92–2.04) | 161 (0.88) | 21 (0.12) | 3.55 | 0.059 | 1.91 (0.96–3.77) | |
| MA | 21 (0.31) | 47 (0.69) | 1.73 | 0.188 | 1.47 (0.83–2.60) | 59 (0.87) | 9 (0.13) | 3.45 | 0.063 | 2.23 (0.94–5.29) | |
| Male | Control | 48 (0.38) | 77 (0.62) | 118 (0.94) | 7 (0.06) | ||||||
| Case | 46 (0.37) | 79 (0.63) | 0.07 | 0.791 | 1.07 (6.64–1.78) | 115 (0.92) | 10 (0.08) | 0.57 | 0.45 | 1.47 (0.54–3.98) | |
| MO | 32 (0.37) | 54 (0.63) | 0.03 | 0.862 | 1.05 (0.59–1.85) | 81 (0.94) | 5 (0.06) | NA | NA | 1.04 (0.32–3.39) | |
| MA | 14 (0.36) | 25 (0.64) | 0.08 | 0.777 | 1.11 (0.53–2.35) | 34 (0.87) | 5 (0.13) | NA | NA | 2.48 (0.74–8.31) | |
| Female | Control | 51 (0.41) | 74 (0.59) | 116 (0.92) | 9 (0.08) | ||||||
| Case | 34 (0.27) | 91 (0.73) | 5.15 | 0.023 | 1.84 (1.08–3.14) | 105 (0.84) | 20 (0.16) | 4.72 | 0.029 | 2.45 (1.07–5.63) | |
| MO | 27 (0.28) | 69 (0.62) | 3.82 | 0.05 | 1.76 (1.00–3.11) | 80 (0.83) | 16 (0.17) | 4.85 | 0.027 | 2.58 (1.09–6.12) | |
| MA | 7 (0.24) | 22 (0.76) | 2.78 | 0.095 | 2.17 (0.86–5.45) | 25 (0.86) | 4 (0.14) | NA | NA | 2.06 (0.59–7.23) | |
Abbreviations: MA, migraine with aura; MO, migraine without aura; OR, odds ratio; NA, not available.
DRD2 expression and genotype
The mean plasma DRD2 levels in the control individuals (mean±SD: 24.20±2.78 ng/L) were significantly lower than those in the patients with MA (30.86±3.69 ng/L, p<0.0001) and MO (31.88±4.99 ng/L, p<0.0001; Figure 1). Additionally, there was a sex-based difference in DRD2 expression in the patients with MA (male vs female: 29.46±3.59 vs 32.27±3.27 ng/L, p<0.01) and MO (male vs female: 29.18±3.50 vs 34.58±4.84 ng/L, p<0.0001; Figure 1). No significant difference was observed between the male (24.45±3.14 ng/L) and female (23.96±2.39 ng/L, p>0.05; Figure 1) control individuals. Plasma DRD2 levels for different genotypes are shown in Figure 2. There were no differences in plasma DRD2 levels among three genotypes in the control group (CC vs CT vs TT: 23.41±2.40 vs 24.51±2.69 vs 25.52±3.99 ng/L, p>0.05). The DRD2 concentration in patients with migraine with genotype CC (24.76±3.76 ng/L) was much lower than that in patients with genotypes CT (30.93±3.85 ng/L, p<0.0001) and TT (37.06±3.95 ng/L, p<0.0001). In the subsequent subgroup analysis, similar results were obtained for the patients with MA (CC vs CT vs TT: 25.09±3.84 vs 28.57±2.84 vs 33.37±1.58 ng/L, p<0.0001) and MO (CC vs CT vs TT: 24.65±3.79 vs 31.65±3.86 vs 38.29±3.74 ng/L, p<0.0001).
Figure 1.
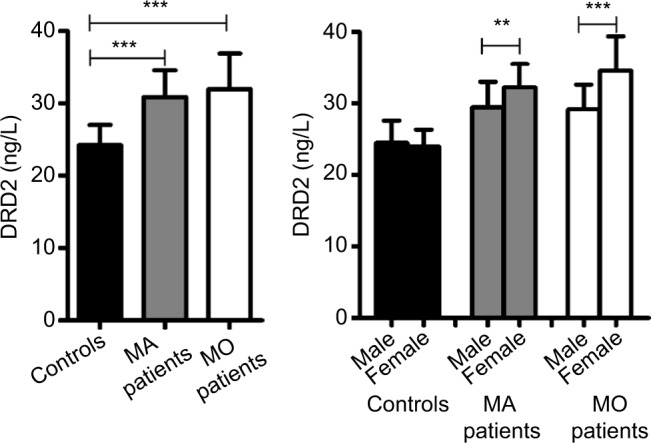
Comparison of DRD2 expression between cases and controls.
Notes: The plasma level of DRD2 (mean±SD): controls (24.20±2.78 ng/L), MA patients (30.86±3.69 ng/L), MO patients (31.88±4.99 ng/L), male controls (24.45±3.14 ng/L), female controls (23.96±2.39 ng/L), male MA patients (29.46±3.59 ng/L), female MA patients (32.27±3.27 ng/L), male MO patients (29.18±3.50 ng/L), female MO patients (34.58±4.84 ng/L). **p<0.01, ***p<0.0001.
Abbreviations: DRD2, dopamine receptor D2; MA, migraine with aura; MO, migraine without aura.
Figure 2.
Comparison of DRD2 expression among three genotypes in different groups.
Notes: The plasma level of DRD2 (mean±SD): controls CC (23.41±2.40 ng/L), controls CT (24.51±2.69 ng/L), controls TT (25.52±3.99 ng/L), migraines CC (24.76±3.76 ng/L), migraines CT (30.93±3.85 ng/L), migraines TT (37.06±3.95 ng/L), MA patients CC (25.09±3.84 ng/L), MA patients CT (28.57±2.84 ng/L), MA patients TT (33.37±1.58 ng/L), MO patients CC (24.65±3.79 ng/L), MO patients CT (31.65±3.86 ng/L), MO patients TT (38.29±3.74 ng/L). *p<0.05, ***p<0.0001.
Abbreviations: DRD2, dopamine receptor D2; MA, migraine with aura; MO, migraine without aura.
Discussion
In this case–control study, 250 patients with migraine (68 patients with MA and 182 with MO) and 250 unrelated healthy control individuals were analyzed. Our results showed that the DRD2 polymorphism rs1800497 was strongly related to the risk of MO in Han Chinese subjects. Furthermore, through sex- stratified comparison, rs1800497 was observed to be associated with the risk of MO in females. DRD2 was highly expressed in the patients with MA and MO, and in the patients with migraine, its expression was regulated by the DRD2 rs1800497 genotypes.
Growing evidence suggests that high lipid concentration constitutes a risk factor for the development of migraine.28,29 Migraine frequency was shown to be associated with dyslipidemia in women.30,31 Plasma TC and TG levels may influence migraine severity.30 Cinzia et al suggested that lipoprotein (a) levels differed significantly between female Italian patients and female Italian control subjects.32 However, Goulart et al reported that different lipid and lipoprotein subfractions were positively associated with MO in both sexes in Brazilians.33 Rist et al showed that TG and TC levels were associated with the risk of MA development, although not with other forms of headache in the French elderly.29 Chorazka et al observed that the levels of TC and low-density lipoprotein cholesterol may contribute to an increased risk of migraine in Polish subjects.34 In this study, we observed no differences in lipid concentrations between the patients with migraine and control individuals. These conflicting findings may be related to the different dietary habits of the Chinese population.
Genome-wide association studies have revealed numerous genetic variants responsible for the risk factors for migraine development.35,36 Dopaminergic gene variants are the most studied entities in migraine studies.37 For example, the dopamine beta-hydroxylase gene polymorphism rs6271 was suggested to be a genetic factor influencing migraine susceptibility in the Turkish population.38 The dopamine trans- porter gene variant SLC6A3 rs40184 contributed to the risk of MA development in Germans.13 An imbalance in dopamine receptors D2/D3 was shown to be associated with migraine attacks and ictal cutaneous allodynia.39 DRD2 polymorphisms may be the genetic determinants of detoxification outcome in patients with medication overuse headache.15,40 Although several studies reported that DRD2 polymorphisms are associated with the risk of migraine, many other studies showed both positive and negative relationships with DRD2. Significant associations of a functional polymorphism at the dopamine beta-hydroxylase locus with migraine risk were observed in Australian and German subjects,13,41 although not in Spanish subjects.42 The variant rs1800497 was located 9.5 kb downstream of DRD2. Ghosh et al observed a significant association between rs1800497 and migraine risk in a meta-analysis of an Indian population.14 However, the results of the meta-analysis involved in the two independent studies were contradictory.14 Our study including 500 age- and sex-matched individuals revealed that DRD2 rs1800497 contributed to the risk of migraine in Han Chinese. This could be partly explained by the specific genetic background and dietary habits of the Chinese.
The SNP rs1800497 was reported to mediate protein–protein interactions.17 Previous studies suggested that rs1800497 robustly influenced the availability of striatal D2 dopamine receptor.23 rs1800497 is located in the 3′ untranslated region of DRD2, near the promoter, which is an inhibitory domain for the binding of nuclear factor-kappa B (NF-κB)-regulated genes. There are two NF-κB binding sites in the DRD2 promoter, and DRD2 expression is regulated by NF-κB.43 rs1800497 variants may influence NF-κB–mediated regulation of DRD2 and upregulate DRD2 transcription.14 DRD2 expression may be involved in the mechanism of migraine pathogenesis, and DRD2 antagonists have been proposed as antimigraine drugs.44 Our study showed similar results. Plasma DRD2 levels were significantly higher in the patients with migraine than in the control subjects. Additionally, the plasma DRD2 level was regulated by different rs1800497 genotypes.
Sexual dimorphism has been observed to influence the prevalence and severity of neurologic disorders.45 Females show a much higher prevalence of migraine worldwide than males.46 Previous studies revealed that female sex hormones are the major factors determining the risk and characteristics of migraine, accounting for the sex-based differences observed in this study; however, there was evidence supporting the underlying genetic variance as well.19 Sazci et al reported a significant association of the NNMT rs694539 variant with female patients with migraine, but no association with male patients.47 CoSkun et al suggested that the genotypes rs2229741-GG and rs726281-GG are responsible for reduced risk of migraine in female Turkish patients.48 Dopamine signaling was demonstrated to be linked to the female hormones, namely, progesterone and prolactin, in the migraineurs.49 Serum prolactin level was considerably high in the female migraineurs.50 Our results showed that rs1800497 was associated with the risk of migraine in female patients among the three genotypes. Plasma DRD2 levels were significantly higher in female patients than in male patients. Our findings provide insight into the prediction of the risk of headache in females.
Conclusion
Our case–control study showed that the DRD2 polymorphism rs1800497 was significantly associated with the risk of migraine in Han Chinese females. Additionally, plasma DRD2 level was higher in patients with migraine than in control subjects. Female patients with migraine had much higher DRD2 levels than male patients with migraine. DRD2 expression may be regulated by DRD2 rs1800497 genotypes in patients with migraine.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Raval AD, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain. 2017;18(1):96–107. doi: 10.1016/j.jpain.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs B, Dussor G. Neurovascular contributions to migraine: moving beyond vasodilation. Neuroscience. 2016;338:130–144. doi: 10.1016/j.neuroscience.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14:1. doi: 10.1186/1129-2377-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparini CF, Smith RA, Griffiths LR. Genetic and biochemical changes of the serotonergic system in migraine pathobiology. J Headache Pain. 2017;18(1):20. doi: 10.1186/s10194-016-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalska M, Prendecki M, Kozubski W, Lianeri M, Dorszewska J. Molecular factors in migraine. Oncotarget. 2016;7(31):50708–50718. doi: 10.18632/oncotarget.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eising E, Huisman SM, Mahfouz A, et al. Gene co-expression analysis identifies brain regions and cell types involved in migraine pathophysiology: a GWAS-based study using the Allen Human Brain Atlas. Hum Genet. 2016;135(4):425–439. doi: 10.1007/s00439-016-1638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicuteri F. Dopamine, the second putative protagonist in headache. Headache. 1977;17(3):129–131. doi: 10.1111/j.1526-4610.1977.hed1703129.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbanti P, Fofi L, Aurilia C, Egeo G. Dopaminergic symptoms in migraine. Neurol Sci. 2013;34(Suppl 1):S67–S70. doi: 10.1007/s10072-013-1415-8. [DOI] [PubMed] [Google Scholar]
- 9.Gasparini CF, Sutherland HG, Griffiths LR. Studies on the pathophysiology and genetic basis of migraine. Curr Genomics. 2013;14(5):300–315. doi: 10.2174/13892029113149990007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14(1):27–35. doi: 10.1007/s11940-011-0150-9. [DOI] [PubMed] [Google Scholar]
- 11.Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2003;116B(1):103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 12.Yasui-Furukori N, Tsuchimine S, Saito M, et al. Comparing the influence of dopamine D(2) polymorphisms and plasma drug concentrations on the clinical response to risperidone. J Clin Psychopharmacol. 2011;31(5):633–637. doi: 10.1097/JCP.0b013e31822c09a7. [DOI] [PubMed] [Google Scholar]
- 13.Todt U, Netzer C, Toliat M, et al. New genetic evidence for involvement of the dopamine system in migraine with aura. Hum Genet. 2009;125(3):265–279. doi: 10.1007/s00439-009-0623-z. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh J, Pradhan S, Mittal B. Identification of a novel ANKK1 and other dopaminergic (DRD2 and DBH) gene variants in migraine susceptibility. Neuromol Med. 2013;15(1):61–73. doi: 10.1007/s12017-012-8195-9. [DOI] [PubMed] [Google Scholar]
- 15.Onaya T, Ishii M, Katoh H, et al. Predictive index for the onset of medication overuse headache in migraine patients. Neurol Sci. 2013;34(1):85–92. doi: 10.1007/s10072-012-0955-7. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh J, Pradhan S, Mittal B. Role of dopaminergic gene polymorphisms (DBH 19 bp indel and DRD2 Nco I) in genetic susceptibility to migraine in North Indian population. Pain Med. 2011;12(7):1109–1111. doi: 10.1111/j.1526-4637.2011.01153.x. [DOI] [PubMed] [Google Scholar]
- 17.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23(6):540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 18.Nuntamool N, Ngamsamut N, Vanwong N, et al. Pharmacogenomics and efficacy of risperidone long-term treatment in thai autistic children and adolescents. Basic Clin Pharmacol Toxicol. 2017;121(4):316–324. doi: 10.1111/bcpt.12803. [DOI] [PubMed] [Google Scholar]
- 19.Zeuner KE, Acewicz A, Knutzen A, Dressler D, Lohmann K, Witt K. Dopamine DRD2 polymorphism (DRD2/ANNK1-Taq1A) is not a significant risk factor in writer’s cramp. J Neurogenet. 2016;30(3–4):276–279. doi: 10.1080/01677063.2016.1238916. [DOI] [PubMed] [Google Scholar]
- 20.Papenberg G, Becker N, Ferencz B, et al. Dopamine receptor genes modulate associative memory in old age. J Cogn Neurosci. 2017;29(2):245–253. doi: 10.1162/jocn_a_01048. [DOI] [PubMed] [Google Scholar]
- 21.Miura I, Zhang JP, Hagi K, et al. Variants in the DRD2 locus and antipsychotic-related prolactin levels: a meta-analysis. Psychoneuroendocrinology. 2016;72:1–10. doi: 10.1016/j.psyneuen.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Liu L, Xin L, et al. The -141C Ins/Del and Taq1A polymorphism in the dopamine D2 receptor gene may confer susceptibility to schizophrenia in Asian populations. J Clin Neurosci. 2016;30:1–7. doi: 10.1016/j.jocn.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies. Transl Psychiatry. 2016;6:e747. doi: 10.1038/tp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28(1):73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 25.Hirvonen MM, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse. 2009;63(10):907–912. doi: 10.1002/syn.20672. [DOI] [PubMed] [Google Scholar]
- 26.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Ye H, Hong Q, et al. Association of CDKN2BAS polymorphism rs4977574 with coronary heart disease: a case-control study and a meta-analysis. Int J Mol Sci. 2014;15(10):17478–17492. doi: 10.3390/ijms151017478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teber S, Bektas O, Yilmaz A, Aksoy E, Akar N, Deda G. Lipoprotein a levels in pediatric migraine. Pediatr Neurol. 2011;45(4):225–228. doi: 10.1016/j.pediatrneurol.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia. 2011;31(14):1459–1465. doi: 10.1177/0333102411421682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janoska M, Chorazka K, Domitrz I. Migraine frequency and its association with dyslipidemia in women. Neurol Neurochir Pol. 2015;49(2):95–98. doi: 10.1016/j.pjnns.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Kurth T, Ridker PM, Buring JE. Migraine and biomarkers of cardiovascular disease in women. Cephalalgia. 2008;28(1):49–56. doi: 10.1111/j.1468-2982.2007.01467.x. [DOI] [PubMed] [Google Scholar]
- 32.Cinzia F, Daniela P, Elena S, et al. Lipoprotein (a) [Lp(a)]: a possible link between migraine and stroke. Transl Res. 2009;153(1):44–47. doi: 10.1016/j.trsl.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Goulart AC, Lotufo PA, Santos IS, et al. The relationship between migraine and lipid sub-fractions among individuals without cardiovascular disease: a cross-sectional evaluation in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Cephalalgia. 2017 Jan 1; doi: 10.1177/0333102417699181. Epub. [DOI] [PubMed] [Google Scholar]
- 34.Chorazka K, Janoska M, Swic P, Domitrz I. Body mass index and serum lipid levels in effect on the incidence and course of migraine. Neurol Neurochir Pol. 2013;47(6):572–576. doi: 10.5114/ninp.2013.39075. [DOI] [PubMed] [Google Scholar]
- 35.Freilinger T, Anttila V, de Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44(7):777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet. 2016;48(8):856–866. doi: 10.1038/ng.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ducros A. Genetics of migraine. Rev Neurol. 2013;169(5):360–371. doi: 10.1016/j.neurol.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Sezer S, Kurt S, Ates O. Analysis of dopamine beta hydroxylase gene polymorphisms in migraine. Clin Neurol Neurosurg. 2016;145:96–100. doi: 10.1016/j.clineuro.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 39.DaSilva AF, Nascimento TD, Jassar H, et al. Dopamine D2/D3 imbalance during migraine attack and allodynia in vivo. Neurology. 2017;88(17):1634–1641. doi: 10.1212/WNL.0000000000003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cargnin S, Viana M, Sances G, et al. Combined effect of common gene variants on response to drug withdrawal therapy in medication overuse headache. Eur J Clin Pharmacol. 2014;70(10):1195–1202. doi: 10.1007/s00228-014-1726-6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez F, Colson N, Quinlan S, MacMillan J, Lea RA, Griffiths LR. Association between migraine and a functional polymorphism at the dopamine beta-hydroxylase locus. Neurogenetics. 2009;10(3):199–208. doi: 10.1007/s10048-009-0176-2. [DOI] [PubMed] [Google Scholar]
- 42.Corominas R, Ribases M, Camina M, et al. Two-stage case-control association study of dopamine-related genes and migraine. BMC Med Genet. 2009;10:95. doi: 10.1186/1471-2350-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bontempi S, Fiorentini C, Busi C, Guerra N, Spano P, Missale C. Identification and characterization of two nuclear factor-kappaB sites in the regulatory region of the dopamine D2 receptor. Endocrinology. 2007;148(5):2563–2570. doi: 10.1210/en.2006-1618. [DOI] [PubMed] [Google Scholar]
- 44.Peroutka SJ. Dopamine and migraine. Neurology. 1997;49(3):650–656. doi: 10.1212/wnl.49.3.650. [DOI] [PubMed] [Google Scholar]
- 45.Clayton JA. Sex influences in neurological disorders: case studies and perspectives. Dialogues Clin Neurosci. 2016;18(4):357–360. doi: 10.31887/DCNS.2016.18.4/jclayton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people world- wide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 2017;372:307–315. doi: 10.1016/j.jns.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 47.Sazci A, Sazci G, Sazci B, Ergul E, Idrisoglu HA. Nicotinamide-N- Methyltransferase gene rs694539 variant and migraine risk. J Headache Pain. 2016;17(1):93. doi: 10.1186/s10194-016-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CoSkun S, Yucel Y, Cim A, et al. Contribution of polymorphisms in ESR1, ESR2, FSHR, CYP19A1, SHBG, and NRIP1 genes to migraine susceptibility in Turkish population. J Genet. 2016;95(1):131–140. doi: 10.1007/s12041-016-0625-2. [DOI] [PubMed] [Google Scholar]
- 49.Charbit AR, Akerman S, Goadsby PJ. Dopamine: what’s new in migraine? Curr Opin Neurol. 2010;23(3):275–281. doi: 10.1097/WCO.0b013e3283378d5c. [DOI] [PubMed] [Google Scholar]
- 50.Cavestro C, Rosatello A, Marino MP, Micca G, Asteggiano G. High prolactin levels as a worsening factor for migraine. J Headache Pain. 2006;7(2):83–89. doi: 10.1007/s10194-006-0272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



