Abstract
Pemetrexed-based chemotherapy regimens (pem regimens) are the standard first-line treatment option in patients with non-squamous non–small cell lung cancer (NSCLC). The objective of this systematic review was to assess the efficacy of pemetrexed in the context of epidermal growth factor receptor (EGFR) mutation-positive NSCLC following the failure of EGFR–tyrosine kinase inhibitor (TKI) treatment. We searched biomedical literature databases (PubMed, EMBASE, and the Cochrane library) and conference proceedings for studies evaluating the efficacy of pemetrexed monotherapy or pemetrexed combined with platinum or any other chemotherapeutic agent in EGFR–mutation-positive NSCLC after EGFR-TKI failure. We extracted data of primary outcomes of interest (progression-free survival [PFS], overall survival [OS], and overall response rate [ORR]). The weighted median PFS, OS, and ORR were then calculated. Of 83 potentially relevant studies, eight (three randomized studies and five retrospective studies) were identified (involving 1,193 patients) and included in this systematic review, with 640 patients receiving pem regimens. The weighted median PFS, median OS, and ORR for patients treated with pem regimens were 5.09 months, 15.91 months, and 30.19%, respectively. Our systematic review results showed a favorable efficacy profile of pem regimens in NSCLC patients with EGFR mutation after EGFR–TKI failure.
Keywords: pemetrexed, advanced non–small cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitor
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide for both men and women.1,2 Non–small cell lung cancer (NSCLC) is the most common subtype, accounting for approximately 80%–85% of all lung cancers.1,2 Most patients with NSCLC are diagnosed at advanced stages (IIIb and IV) of the disease, with only 16%–30% diagnosed at early stages.1–3 Patients diagnosed with advanced NSCLC generally have a poor prognosis, with a median survival of 8–10 months as well as 2- and 5-year survival rates of approximately 20% and 15%, respectively.1,3,4
The standard treatment for NSCLC includes platinum-based doublet chemotherapy, which increases the survival time and quality of life of patients with advanced-stage disease.5 However, the discovery of activating mutations in the kinase domain of the epidermal growth factor receptor (EGFR) gene in a subset of NSCLC cases has led to the development of new targeted therapeutic approaches. EGFR-tyrosine kinase inhibitors (TKIs) are now the preferred first-line therapy for patients with advanced non- squamous NSCLC with activating EGFR mutations, with randomized clinical studies confirming the superiority of EGFR-TKIs to traditional chemotherapy regimens in terms of the overall response rate (ORR), progression-free survival (PFS), and quality of life score.1,5,6 Several clinical studies have indicated that EGFR-TKIs are not recommended for patients with EGFR–mutation-negative NSCLC.7 In addition, although EGFR-TKIs are associated with an initially high tumor response rate in EGFR–mutation-positive NSCLC, the development of resistance to TKIs is inevitable and the majority of patients receiving EGFR-TKIs experience disease progression after 9–13 months of treatment.8,9
The most common cause of acquired resistance to EGFR-TKIs is the development of a secondary mutation in EGFR, threonine 790 to methionine (T790M), which accounts for approximately 50% of cases that progress after EGFR-TKI treatment.10 The presence of the T790M variant prevents the binding of EGFR-TKIs to EGFR, which results in impairment of EGFR-TKI–mediated inhibition.8–10 A third-generation EGFR-TKI, osimertinib, has been shown to be an effective treatment for patients with the EGFR T790M mutation.8 Chemotherapy is the primary treatment option for patients who do not have the T790M mutation and experience progression after first-line EGFR-TKI.2 However, the optimal chemotherapeutic regimens for EGFR–mutation-positive cases after EGFR-TKI failure are not well understood.
Pemetrexed – an anti-folate cytotoxic agent – is effective in the treatment of patients with advanced non-squamous NSCLC and has a favorable safety profile.11,12 Pemetrexed is indicated for use in the first-line, maintenance, and second- line settings for patients with non-squamous NSCLC;11–13 in addition, it was found to be effective and well tolerated in patients with NSCLC after EGFR-TKI failure.14 Among many chemotherapeutic drugs that are widely used for lung cancer, pemetrexed is the preferred candidate in patients with non-squamous NSCLC who have EGFR mutations because of its low toxicity and relatively good efficacy.15 To better understand the efficacy of pemetrexed in this latter setting, we conducted a systematic review, searching the relevant literature and assessing the evidence supporting pemetrexed- based regimens (pem regimens) in NSCLC patients after EGFR-TKI failure. The objective of this systematic review was to assess the efficacy of pemetrexed in the context of EGFR–mutation-positive NSCLC following the failure of EGFR-TKI treatment.
Materials and methods
Search strategy
A comprehensive literature search was undertaken to identify published studies that evaluated pemetrexed monotherapy or combination therapy with pemetrexed and platinum or another chemotherapeutic agent in EGFR–mutation- positive NSCLC after EGFR-TKI failure. The PubMed, EMBASE, and Cochrane databases were searched for relevant trials. The search strategy included the following term combination, without restrictions on language and sex: “pemetrexed,” “NSCLC,” “non–small cell lung cancer,” “TKI failure,” “gefitinib failure,” “afatinib failure,” “erlotinib failure,” and “icotinib failure.” Additional searches through Google Scholar were conducted. Moreover, bibliographies and citation sections of retrieved articles were reviewed for additional pertinent studies. An initial review of the title and abstract of these studies was conducted to exclude irrelevant studies. The full texts of the remaining articles were read to extract information on the topic of interest. Abstracts of research presented at related conferences (American Society of Clinical Oncology [ASCO], European Society for Medical Oncology [ESMO], and American Association for Cancer Research [AACR]) were also searched.
Two of the authors conducted the search independently, with no language or date restrictions set. These two authors – who were not blinded to the names of original researchers, journals, or institutions – independently checked the titles, abstracts, and keywords from the searches to identify potentially eligible studies. Upon obtaining the full texts of potentially eligible studies, the same two authors conducted an independent study selection; disagreements were resolved by consensus and, if necessary, by consultation with a third reviewer.
Selection criteria
To be eligible for inclusion, studies had to meet all of the following criteria: 1) the study population included patients with advanced non-squamous NSCLC who have activating EGFR mutations; 2) the study population included NSCLC patients with acquired resistance or progression after EGFR-TKIs; 3) studies evaluated the efficacy of pemetrexed monotherapy or pemetrexed combined with platinum/other chemotherapeutic agent(s); and 4) studies reported outcomes of interest (PFS, overall survival [OS], and ORR).
Data extraction
Data were extracted using a standardized collection process. The following information was extracted from each selected study: 1) first author’s last name, year of publication, and nationality of the population studied; 2) study design; 3) intervention type/treatment arms; 4) number of patients per treatment arm/group; and 5) outcomes of interest (PFS, OS, and ORR).
Statistical analysis
The median PFS, median OS, and ORR for each regimen were extracted and classified for the pem regimen group and the non-pemetrexed–based chemotherapy (non-pem) regimen group. Pooled median PFS, median OS, and ORR were calculated with sample size as weight for each group. However, no formal statistical comparison was conducted to compare regimen groups due to lack of sufficient data.
Results
Search results
Figure 1 represents the selection process for clinical studies included in this systematic review. A total of 83 potentially relevant studies were identified through database searches, of which 75 were considered ineligible because they did not meet the specified inclusion criteria. Eight studies1,5,6,9,16–19 (published between 2014 and 2017) met the eligibility criteria for this systematic review and were subjected to data evaluation. Of the eight eligible studies, one was published in abstract form at the ASCO and the remaining seven were published as full-text articles. Three were randomized controlled trials (two Phase III studies and one Phase II study), and five were retrospective observational studies. All studies included in the systematic review are summarized in Table 1. In all of the eight studies, the majority of patients with EGFR–mutation-positive NSCLC had been previously treated with gefitinib and erlotinib and had subsequently developed resistance. Of the eight studies, two were conducted globally, three were in Taiwan, and three in Korea. A total of 1,193 patients participated in the eight studies. Of the patients with EGFR–mutation-positive NSCLC who experienced resistance or disease progression after EGFR-TKI treatment, 640 received pem regimens and 97 received non-pem regimens. For the pem regimen group, data from 640 patients (eight studies), 343 patients (five studies), and 606 patients (seven studies) were available to evaluate PFS (Table 2), OS (Table 3), and ORR (Table 4), respectively. For the non-pem regimen group, data for 97 patients (three studies) are included to evaluate PFS (Table 2) and OS (Table 3), and data for 71 patients (two studies) are included to evaluate ORR (Table 4).
Figure 1.
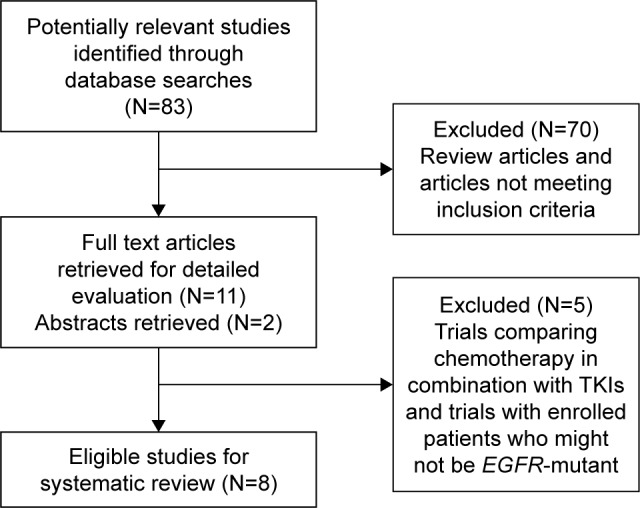
Flowchart representing the selection process of clinical studies in the systematic review.
Abbreviations: EGFR, epidermal growth factor receptor; N, number of studies; TKI, tyrosine kinase inhibitor.
Table 1.
Characteristics of studies included in the systematic review
| Study | Study design | Previous EGFR TKIs | Intervention type | Sample size (EA, CA) | |
|---|---|---|---|---|---|
|
| |||||
| EA | CA | ||||
| Yang et al16 | Retrospective | Gefitinib, erlotinib | Pemetrexed + platinum | Non-pem platinum doublet | 60 (34, 26) |
| Park et al1 | Retrospective | Gefitinib, erlotinib | Pemetrexed | Non-pem platinum doublet | 83 (37, 46) |
| Soria et al6 | Randomized Phase III trial | Gefitinib | Gefitinib + pemetrexed + cisplatin (maximum of six cycles) | Pemetrexed + cisplatin (maximum of six cycles) | 265 (133, 132) |
| Yoo et al19 | Randomized Phase II trial | EGFR TKI (not specified) | Pemetrexed + cisplatin (four cycles) - pemetrexed maintenance | Pemetrexed (until progressive disease) | 96 (48, 48) |
| Tseng et al18 | Retrospective | Gefitinib, erlotinib, and afatinib | Pemetrexed + platinum | Pemetrexed + platinum as first-line therapy | 105 (61, 44) |
| Tseng et al17 | Retrospective | Gefitinib, erlotinib, and afatinib | Pem chemotherapy - pemetrexed + platinum | Non-pem platinum doublet or single agent | 102 (77, 25) |
| Mok et al9 | Randomized Phase III trial | Gefitinib, erlotinib, and afatinib | Osimertinib | Platinum - pemetrexed + pemetrexed maintenance | 419 (279, 140) |
| Lee et al5 | Retrospective | Gefitinib, erlotinib, and afatinib | Pemetrexed + platinum (maintenance pemetrexed after four cycles of pemetrexed + platinum) | Pemetrexed maintenance | 63 (34, 29) |
Abbreviations: CA, control arm; EA, experimental arm; EGFR, epidermal growth factor receptor; Pem, pemetrexed-based; TKI, tyrosine kinase inhibitor.
Table 2.
Median PFS of pemetrexed-based regimens and non-pemetrexed-based regimens in published studies
| Study | Pem regimens (number of patients) N=640 | Non-pem regimens (number of patients) N=97 | Median PFS (months) | HR (95% CI), p-value (pem vs non-pem regimens) | |
|---|---|---|---|---|---|
|
| |||||
| Pem regimens | Non-pem regimens | ||||
| Soria et al6 | Pemetrexed + cisplatin (132) | – | 5.4 | – | – |
| Yoo et al19 | Pemetrexed + cisplatin (48) | – | 5.4 | – | – |
| Yoo et al19 | Pemetrexed (48) | – | 6.4 | – | – |
| Tseng et al18 | Pemetrexed + platinum (61) | – | 6.1 | – | – |
| Park et al1 | Pemetrexed (37) | Non-pem platinum doublet (46) | 4.2 | 2.7 | 0.54 (0.34–0.86), p=0.009 |
| Yang et al16 | Pemetrexed + platinum (34) | Non-pem platinum doublet (26) | 6.4 | 4.1 | 0.47 (0.26–0.84), p=0.0101 |
| Tseng et al17 | Pemetrexed + platinum (77) | Non-pem platinum doublet (25) | 4.7 | 3.3 | p=0.62a |
| Lee et al5 | Pemetrexed + platinum (34) | – | 5.2 | – | – |
| Lee et al5 | Pemetrexed (29) | – | 2.7 | – | – |
| Mok et al9 | Platinum + pemetrexed (140) | – | 4.4 | – | – |
| Weighted median PFS (months) | 5.09 | 3.23 | |||
Notes: p-value was calculated based on log-rank test. “–” indicates data not available in included studies.
HR and CI are not available in the full-text article.
Abbreviations: CI, confidence interval; HR, hazard ratio; N, total number of patients; Pem, pemetrexed-based; PFS, progression-free survival.
Table 3.
Median OS of pemetrexed-based regimens and non-pemetrexed-based regimens in published studies
| Study | Pem regimens (number of patients) N=343 | Non-pem regimens (number of patients) N=97 | Median OS (months) | HR (95% CI), p-value (pem vs non-pem regimens) | |
|---|---|---|---|---|---|
|
| |||||
| Pem regimens | Non-pem regimens | ||||
| Soria et al6 | Pemetrexed + cisplatin (132) | – | 17.2 | – | – |
| Park et al1 | Pemetrexed (37) | Non-pem platinum doublet (46) | 15.1 | 11.0 | 0.92 (0.50–1.68) p=0.785 |
| Yang et al16 | Pemetrexed + platinum (34) | Non-pem platinum doublet (26) | 19.2 | 14.1 | 0.50 (0.22–1.13), p=0.0972 |
| Tseng et al17 | Pemetrexed + platinum (77) | Non-pem platinum doublet (25) | 15.1 | 8.1 | p=0.168a |
| Lee et al5 | Pemetrexed + platinum (34) | – | 15.1 | – | – |
| Lee et al5 | Pemetrexed (29) | – | 10.3 | – | – |
| Weighted median OS (months) | 15.91 | 11.08 | |||
Notes: p-value was calculated based on log-rank test. “–” indicates data not available in included studies.
HR and CI are not available in full-text article.
Abbreviations: CI, confidence interval; HR, hazard ratio; N, total number of patients; OS, overall survival; Pem, pemetrexed-based.
Table 4.
Overall response rates of pemetrexed-based regimens and non-pemetrexed-based regimens in published studies
| Study | Pem regimens (number of patients) N=606 | Non-pem regimens (number of patients) N=71 | Overall response rate (%) | p-value (pem vs non-pem regimens) | |
|---|---|---|---|---|---|
|
| |||||
| Pem regimens | Non-pem regimens | ||||
| Soria et al6 | Pemetrexed + cisplatin (132) | – | 34.00 | – | – |
| Yoo et al19 | Pemetrexed + cisplatin (48) | – | 34.80 | – | – |
| Yoo et al19 | Pemetrexed (48) | – | 17.80 | – | – |
| Tseng et al18 | Pemetrexed + platinum (61) | – | 24.60 | – | – |
| Park et al1 | Pemetrexed (37) | Non-pem platinum doublet (46) | 32.40 | 17.40 | 0.111 |
| Tseng et al17 | Pemetrexed + platinum (77) | Non-pem platinum doublet (25) | 26.00 | 20.00 | 0.799 |
| Lee et al5 | Pemetrexed + platinum (34) | – | 43.80 | – | – |
| Lee et al5 | Pemetrexed (29) | – | 25.90 | – | – |
| Mok et al9 | Platinum + pemetrexed (140) | – | 31.00 | – | – |
| Weighted ORR (%) | 30.19 | 18.32 | |||
Notes: p-value was calculated based on log-rank test. “–” indicates data not available in included studies.
Abbreviations: N, total number of patients; ORR, overall response rate; Pem, pemetrexed-based regimen.
Progression-free survival analysis
All eight studies reported PFS in patients treated with pemetrexed monotherapy or a combination of pemetrexed with platinum-based regimens. Only three studies1,5,19 evaluated pemetrexed monotherapy (n=114), with PFS ranging from 2.7 to 6.4 months. Seven studies5,6,9,16–19 evaluated the combination of pemetrexed with platinum-based regimens (n=526), with PFS ranging from 4.4 to 6.4 months. Weighted median PFS for patients treated with pemetrexed monotherapy and those treated with a combination of pemetrexed with platinum-based regimens was between 4.75 and 5.16 months, respectively. Pemetrexed – either alone or in combination with other chemotherapy regimens – was effective among NSCLC patients with EGFR mutations who experienced resistance or disease progression after failure of EGFR-TKI treatment, with PFS ranging from 2.7 to 6.4 months and a weighted median PFS of 5.09 months (Table 2). Three studies1,16,17 directly compared the PFS data of pem regimens (n=148) versus non-pem regimens (n=97) in NSCLC patients with EGFR mutations who experienced resistance or disease progression after failure of EGFR-TKI treatment (Table 2). In all three studies, median PFS was longer for the pem regimens as compared to the non-pem regimens (Table 2). In two studies,1,16 pemetrexed-based chemotherapy significantly extended PFS in EGFR-mutant NSCLC patients who failed first-line treatment with EGFR-TKIs (Park et al1: hazard ratio [HR] [95% CI] = 0.54 [0.34, 0.86], p=0.009; Yang et al16: 0.47 [0.26, 0.84], p=0.0101). Furthermore, Tseng et al17 showed numerically longer PFS in NSCLC patients treated with pem regimens (median 4.7 months) compared to non-pem regimens (median 3.3 months). Overall, the weighted median PFS was numerically longer in patients treated with pemetrexed (5.09 months) than in patients receiving non-pem regimens (3.23 months; Table 2).
Overall survival analysis
Similar results to those of PFS were reported for OS. Five studies1,5,6,16,17 reported OS in patients treated with pemetrexed monotherapy or a combination of pemetrexed with platinum-based regimens. Only two studies1,5 evaluated pemetrexed monotherapy (n=66), with OS ranging from 10.3 to 15.1 months. Four studies5,6,16,17 evaluated the combination of pemetrexed with platinum-based regimens (n=277), with OS ranging from 15.1 to 19.2 months. Weighted median OS for patients treated with pemetrexed monotherapy and those treated with a combination of pemetrexed with platinum-based regimens was between 12.99 and 16.60 months, respectively. Pemetrexed – either alone or in combination with other chemotherapy regimens – was effective among NSCLC patients with EGFR mutations who experienced resistance or disease progression after failure of EGFR-TKI treatment, with median OS ranging from 10.3 to 19.2 months and a weighted median OS of 15.91 months (Table 3).
Three studies1,16,17 compared the OS data of pem regimens (n=148) versus non-pem regimens (n=97) in EGFR– mutation-positive NSCLC patients who experienced disease progression after EGFR-TKI treatment. In all three studies, OS was longer following pemetrexed chemotherapy; however, the difference was not statistically significant (p>0.05) in any of the studies (Table 3). Overall, the weighted median OS was numerically longer in patients treated with pem regimens (15.91 months) than in patients receiving non-pem regimens (11.08 months; Table 3).
Overall response rate analysis
Similar results to those of PFS and OS were also reported for ORR. Seven studies1,5,6,9,17–19 reported ORR in patients treated with pemetrexed monotherapy or a combination of pemetrexed with platinum-based regimens. Three studies1,5,19 evaluated pemetrexed monotherapy (n=114 patients), with ORR ranging from 17.8% to 32.4%. Six studies5,6,9,17–19 evaluated the combination of pemetrexed with platinum-based regimens involving 492 patients, with ORR ranging from 24.6% to 43.8%. Weighted ORR for patients treated with pemetrexed monotherapy and the combination of pemetrexed with platinum-based regimens was 24.6% and 31.5%, respectively. Pemetrexed – either alone or in combination with other chemotherapy regimens – was effective among NSCLC patients with EGFR mutations who experienced resistance or disease progression after failure of EGFR-TKI treatment, with ORR ranging from 17.8% to 43.8% and a weighted ORR of 30.19% (Table 4).
Two studies1,17 compared the ORR data of pem regimens (n=114) versus non-pem regimens (n=71) in EGFR– mutation-positive NSCLC patients who experienced disease progression after EGFR-TKI treatment. In both studies, the ORR was higher in patients receiving pemetrexed chemotherapy; however, the difference was not statistically significant (p>0.05) in either study (Table 4). Overall, the weighted ORR was higher in patients treated with pem regimens (30.19%) than in patients receiving non-pem regimens (18.32%; Table 4).
Discussion
Recently, EGFR mutation has emerged as an important target in the treatment of patients with advanced NSCLC. Several randomized controlled clinical trials established the superiority of first-generation EGFR-TKIs (gefitinib or erlotinib) over chemotherapy in terms of PFS and ORR in patients with EGFR–mutation-positive NSCLC, with PFS ranging from 9.2 to 13.1 months and ORR ranging from 58% to 83%.20–24 Unfortunately, patients who initially respond to first-generation EGFR-TKIs inevitably experience acquired resistance within 1–2 years.25 The possible mechanisms of resistance have been investigated in several studies and include second-site mutation (such as EGFR T790M mutation),26 EGFR amplification, activation of parallel pathways (eg, MET amplification), and downstream signaling pathways (eg, PI3K/AKT/mTOR).27–29 The most common mechanism of acquired resistance to the first- and second-generation EGFR-TKIs involves the development of an EGFR T790M mutation,26,27 accounting for approximately 50% of EGFR-mutant resistance cases.26 Clinical trials have been conducted for several T790M-targeting third-generation EGFR-TKIs. AURA3 is the first randomized, Phase III study of third-generation, oral, irreversible EGFR-TKI (osimertinib) versus a platinum-based pemetrexed regimen in patients with the EGFR T790M mutation.9 In the AURA3 study, median PFS was significantly longer in patients treated with osimertinib compared to those treated with a platinum-based pemetrexed regimen (10.1 vs 4.4 months, respectively; HR [95% CI] = 0.30 [0.23–0.41]; p<0.001); a significantly higher response was observed in patients treated with osimertinib compared to those treated with a platinum- based pemetrexed regimen (71% vs 31%, respectively; OR [95% CI] = 5.39 [3.47–8.48]; p<0.001).9
Osimertinib was approved by the US Food and Drug Administration in November 2015 for the treatment of patients with metastatic EGFR T790M mutation-positive NSCLC. However, the C797S mutation is the most commonly acquired mutation that confers resistance to third- generation EGFR TKIs.28–30 EAI045 is a fourth-generation EGFR inhibitor that has recently been reported to be an allosteric EGFR inhibitor that overcomes T790M- and C797S-mediated resistance.28–30 For patients without the T790M mutation, chemotherapy is still the primary treatment. A few studies have explored the efficacy of chemotherapy regimens in patients after TKI failure. Of these, pemetrexed is the most frequently reported regimen. Pemetrexed acts as an anti-folate, inhibiting three enzymes in the folate metabolic pathway that are essential for cell replication: thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase.18,31 A series of randomized Phase II and III clinical trials have shown that pemetrexed is effective and safe for the treatment of advanced non- squamous NSCLC, confirming its role in the treatment of advanced NSCLC in both first- and second-line settings. Moreover, pemetrexed has a significant role in maintenance therapy for NSCLC.11
The JMDB study32 investigated the efficacy of first-line pemetrexed plus cisplatin without maintenance therapy, whereas the PARAMOUNT study33 investigated efficacy of first-line pemetrexed-cisplatin therapy followed by pemetrexed maintenance therapy. In the current review, we found that the PFS and ORR results are similar to that of the JMDB study32 for patients treated with a pem regimen (PFS: between 5.09 and 4.80 months, respectively; ORR: 30.19% and 30.6%, respectively). In addition, we found that in EGFR–mutation-positive NSCLC, the OS (15.9 months) with a pem regimen is numerically longer than that in the JMDB study32 (11.8 months in non-squamous carcinoma) and is comparable with that in the PARAMOUNT study33 (15.9 vs 16.9 months, respectively). In the current systematic review, only three studies specified that patients received pemetrexed maintenance therapy; other studies did not clarify whether patients received pemetrexed maintenance therapy. This may be one of the reasons why OS is numerically longer in the current review than that in the JMDB study, which indicates that pemetrexed is also efficacious in NSCLC patients after EGFR-TKI failure. When used as the first-line treatment, TKI may not impair the efficacy of pemetrexed. This result is consistent with a study that analyzed the efficacy of pemetrexed plus platinum as first- versus second-line treatment in chemotherapy-naïve patients with advanced EGFR–mutation-positive lung adenocarcinoma, which suggested that prior EGFR-TKI treatment would not influence the efficacy of subsequent pemetrexed plus platinum therapy in chemotherapy-naïve patients with advanced EGFR–mutation-positive lung adenocarcinoma.18 However, Zeng et al34 reported that front-line EGFR-TKI treatment significantly reduced the sensitivity of subsequent chemotherapy compared with that of TKI-naïve front-line chemotherapy in EGFR–mutation-positive patients; more prospective studies are needed to clarify this finding.
In the current review, the results favor pem regimens (15.91 months) compared to non-pem regimens (11.08 months), with numerically longer median OS after EGFR TKI failure. This is also consistent with treatment outcome in the first-line setting in non-squamous NSCLC. A meta- analysis showed that pemetrexed alone or in combination with other chemotherapeutic agents was superior to other chemotherapy regimens in patients with non-squamous NSCLC (HR [95% CI] = 0.89 [0.80, 0.99]) and was associated with significantly longer OS and less toxicity.12 However, until now, only one prospective randomized study reported the comparison of single-agent pemetrexed and pemetrexed/ platinum doublets in EGFR–mutation-positive NSCLC after front-line EGFR-TKI failure.19 This study concluded that the pemetrexed with platinum-based regimens showed a higher response rate than pemetrexed monotherapy; however, no significant difference was observed in PFS between treatment groups.19 Another prospective, multicenter, open- label, randomized, Phase II ongoing clinical study was designed to evaluate the efficacy of pemetrexed versus pemetrexed plus cisplatin in EGFR–mutant-positive NSCLC patients after failure of first-line EGFR TKIs, in the People’s Republic of China (NCT02725918). More prospective studies are needed to determine the optimal chemotherapy regimens for EGFR-mutant NSCLC in the post-EGFR-TKI failure setting.
Checkpoint blockade antibodies targeting programmed cell-death protein 1 (PD-1) have shown promising clinical responses and offer survival benefits with acceptable safety profile in patients with advanced NSCLC.35–37 Results of KEYNOTE-02138 – a randomized, open-label, Phase II cohort of a multicohort study – showed that the addition of pembrolizumab to carboplatin and pemetrexed improved efficacy, with manageable safety profiles in patients with chemotherapy-naïve, advanced non-squamous NSCLC.38 Recent findings from a retrospective study suggested that the efficacy of nivolumab, a PD-1 antibody, tended to be greater in patients with EGFR–mutation-positive NSCLC who develop resistance to TKIs due to mechanisms other than acquisition of the secondary T790M mutation of EGFR than in the patients who had T790M-positive mutation;39 the difference in efficacy of nivolumab may be due to a higher level of expression of the PD-1 ligand in the patients with T790M-negative NSCLC. To confirm this, a randomized, Phase II trial (WJOG8515L) comparing nivolumab with a combination of carboplatin and pemetrexed in patients with EGFR–mutation-positive non-squamous NSCLC who acquire resistance to EGFR-TKIs due to mechanisms other than T790M was conducted and is currently ongoing.40 Due to heterogeneity in resistance mechanisms,41 a combination of EGFR TKIs with other agents, such as immune checkpoint inhibitors,42–44 cMET inhibitors,45 and chemotherapeutic agents (eg, pem regimens), should be considered as future therapeutic modalities to overcome the acquired mutation among NSCLC patients.
This is the first systematic review to assess the evidence supporting pem regimens in NSCLC patients after EGFR-TKI failure. However, this systematic review has some limitations: 1) due to the unavailability of sufficient data, no formal statistical inference was achieved; 2) we only searched the PubMed, EMBASE, Cochrane library, ASCO, ESMO, and AACR databases and, therefore, other potentially relevant articles that were published in other databases were not identified; 3) for the direct comparison of pem regimens to non-pem regimens, we only included studies that compared pem regimens and non-pem regimens; and 4) a limited number of both randomized and retrospective studies with heterogeneity of treatment regimens (with and without maintenance therapy) were available.
Conclusion
The results of our systematic review showed a favorable efficacy profile of pem regimens in NSCLC patients with EGFR mutation after EGFR TKI failure.
Acknowledgments
This work was supported by Eli Lilly and Company. Medical writing and editorial assistance were provided by Rakesh Ojha, PhD, and Joseph Durrant from Syneos Health (funded by Eli Lilly and Company).
Footnotes
Author contributions
All authors were involved in design of the study, data analysis and interpretation, and critical revision of the manuscript. All authors reviewed and approved the final manuscript draft.
Disclosure
LLY, XW, and LDY are employees of Eli Lilly and Company. HBH was involved in the design and conduct of the systematic literature review, funded by Eli Lilly and Company. The authors report no other conflicts of interest in this work.
References
- 1.Park S, Keam B, Kim SH, et al. Pemetrexed singlet versus nonpemetrexed-based platinum doublet as second-line chemotherapy after first- line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat. 2015;47(4):630–637. doi: 10.4143/crt.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masuda T, Imai H, Kuwako T, et al. Efficacy of platinum combination chemotherapy after first-line gefitinib treatment in non-small cell lung cancer patients harboring sensitive EGFR mutations. Clin Transl Oncol. 2015;17(9):702–709. doi: 10.1007/s12094-015-1297-8. [DOI] [PubMed] [Google Scholar]
- 3.Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska M. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:1023–1028. doi: 10.2147/OTT.S100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non- small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20(4):e300–e306. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Pemetrexed plus platinum versus pemetrexed alone in non-small cell lung cancer patients who have progressed after first-line EGFR TKIs. Lung Cancer. 2015;90(2):261–266. doi: 10.1016/j.lungcan.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhou F, Zhou CC. Targeted therapies for patients with advanced NSCLC harboring wild-type EGFR: what’s new and what’s enough. Chin J Cancer. 2015;34(7):310–319. doi: 10.1186/s40880-015-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura N, Okishio K, Mitsuoka S, et al. Prospective assessment of continuation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of pemetrexed. J Thorac Oncol. 2013;8(1):96–101. doi: 10.1097/JTO.0b013e3182762bfb. [DOI] [PubMed] [Google Scholar]
- 9.Mok TS, Wu YL, Ahn MJ, et al. AURA3 Investigators Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart EL, Tan SZ, Liu G, Tsao MS. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015;4(1):67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuld AD, Dragnev KH, Rigas JR. Pemetrexed in advanced non-small- cell lung cancer. Expert Opin Pharmacother. 2010;11(8):1387–1402. doi: 10.1517/14656566.2010.482560. [DOI] [PubMed] [Google Scholar]
- 12.Al-Saleh K, Quinton C, Ellis PM. Role of pemetrexed in advanced non- small-cell lung cancer: meta-analysis of randomized controlled trials, with histology subgroup analysis. Curr Oncol. 2012;19(1):e9–e15. doi: 10.3747/co.19.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhi XY, Wu YL, Bu H, et al. Lung Cancer Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health Chinese guidelines on the diagnosis and treatment of primary lung cancer (2011) J Thorac Dis. 2012;4(1):88–101. doi: 10.3978/j.issn.2072-1439.2010.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L, Han ZF, Feng ZH, Jia ZY. Comparison of pemetrexed and docetaxel as salvage chemotherapy for the treatment for nonsmall-cell lung cancer after the failure of epidermal growth factor receptor-tyrosine kinase inhibitors. J Int Med Res. 2014;42(1):191–197. doi: 10.1177/0300060513505808. [DOI] [PubMed] [Google Scholar]
- 15.Wu SG, Yang CH, Yu CJ, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011;72(3):333–339. doi: 10.1016/j.lungcan.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Yang CJ, Tsai MJ, Hung JY, et al. Pemetrexed had significantly better clinical efficacy in patients with stage IV lung adenocarcinoma with susceptible EGFR mutations receiving platinum-based chemotherapy after developing resistance to the first-line gefitinib treatment. Onco Targets Ther. 2016;9:1579–1587. doi: 10.2147/OTT.S100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng YH, Hung HY, Sung YC, et al. Efficacy of chemotherapy in epidermal growth factor receptor (EGFR) mutated metastatic pulmonary adenocarcinoma patients who had acquired resistance to first-line EGFR tyrosine kinase inhibitor (TKI) J Chemother. 2016;28(1):50–58. doi: 10.1179/1973947815Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 18.Tseng JS, Yang TY, Chen KC, et al. Prior EGFR tyrosine-kinase inhibitor therapy did not influence the efficacy of subsequent pemetrexed plus platinum in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma. Onco Targets Ther. 2014;7:799–805. doi: 10.2147/OTT.S62639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo KH, Cho J, Lee KH, et al. A randomized, open label, phase II study comparing pemetrexed plus cisplatin followed by pemetrexed versus pemetrexed alone in EGFR mutant NSCLC patients who have failed first-line EGFR TKI: KCSG-LU12-13. J Clin Oncol. 2016;34(Suppl 15) Abstract 9043. [Google Scholar]
- 20.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin– paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 21.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 22.Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 23.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 24.Rosell R, Gervais R, Vergnenegre A, et al. for Spanish Lung Cancer Group Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. J Clin Oncol. 2011;29(Suppl 15) Abstract 7503. [Google Scholar]
- 25.Xu M, Xie Y, Ni S, Liu H. The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC) Ann Transl Med. 2015;3(7):96. doi: 10.3978/j.issn.2305-5839.2015.03.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 27.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi A, Remick S, Tse W. EGFR inhibition in non-small cell lung cancer: current evidence and future directions. Biomark Res. 2013;1(1):2. doi: 10.1186/2050-7771-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol. 2016;9(1):59. doi: 10.1186/s13045-016-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanauske AR, Chen V, Paoletti P, Niyikiza C. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist. 2001;6(4):363–373. doi: 10.1634/theoncologist.6-4-363. [DOI] [PubMed] [Google Scholar]
- 32.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 33.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Z, Yan HH, Zhang XC, et al. Reduced chemotherapy sensitivity in EGFR-mutant lung cancer patient with frontline EGFR tyrosine kinase inhibitor. Lung Cancer. 2014;86(2):219–224. doi: 10.1016/j.lungcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbone DP, Reck M, Paz-Ares L, et al. CheckMate 026 Investigators First-line novolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reck M, Rodríguez-Abreu D, Robinson AG, et al. KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell Lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 38.Langer CJ, Gadgeel SM, Borghaei H, et al. KEYNOTE-021 investigators Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haratani K, Hayashi H, Tanaka T, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi H, Chiba Y, Sakai K, et al. A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for patients with EGFR mutation-positive nonsquamous non-small-cell lung cancer who acquire resistance to tyrosine kinase inhibitors not due to a secondary T790M mutation: rationale and protocol design for the WJOG8515L Study. Clin Lung Cancer. 2017;18(6):719–723. doi: 10.1016/j.cllc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Alexander PB, Wang XF. Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies. Front Med. 2015;9(2):134–138. doi: 10.1007/s11684-015-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davar D, Socinski MA, Dacic S, Burns TF. Near complete response after single dose of nivolumab in patient with advanced heavily pretreated KRAS mutant pulmonary adenocarcinoma. Exp Hematol Oncol. 2015;4:34. doi: 10.1186/s40164-015-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi-Long W, Dong-Wan K, Enriqueta F, et al. Phase (Ph) II safety and efficacy results of a single-arm ph ib/II study of capmatinib (INC280) + gefitinib in patients (pts) with EGFR-mutated (mut), cMET-positive (cMET+) non-small cell lung cancer (NSCLC) J Clin Oncol. 2016;34(15) Abstract 9020. [Google Scholar]


