Abstract
In many sexually reproducing species, individuals can gather information about potential mates by observing their mating success. This behavioral pattern, that we call mate-copying, was reported in the fruit fly Drosophila melanogaster where females choosing between 2 males of contrasting phenotypes can build a preference for males of the phenotype they previously saw being chosen by a demonstrator female. As sex ratio is known to affect mate choice, our goal was to test whether mate-copying is also affected by encountered sex ratios. Thus, we created a gradient of sex ratio during demonstrations of mate-copying experiments by changing the number of females observing from a central arena 6 simultaneous demonstrations unfolding in 6 peripheral compartments of a hexagonal device. We also tested whether the sex ratio experienced by females during demonstrations affected their choosiness (male courtship duration and double courtship rate) in subsequent mate-choice tests. Experimental male:female sex ratio during demonstrations did not affect mate-copying indices, but positively affected the proportion of both males courting the female during mate-choice tests, as well as male courtship duration, the latter potentially explaining the former relationship. As expected, the sex ratio affected female choosiness positively, and Drosophila females seem to have evolved a mate-copying ability independently of sex ratio, and a capacity to adapt their choosiness to male availability. This suggests that, as in many animal species, individuals, especially females, can adapt their mate choice depending on the current sex ratio.
Keywords: competition, Drosophila melanogaster, experimental protocol, mate-copying, social learning, sex ratio
Choosing a mate is a major fitness-affecting decision in any sexually reproducing organism. In species where females invest more than males in the production of a single offspring, females are selected to become the choosy sex (Johnstone et al. 1996; Trivers 1972). Furthermore, in males of such species, natural and sexual selection shape traits that are related to male quality, which in turn can be used by females to assess male quality because these traits reveal that they are better fathers providing better or more resources to the female or the offspring (Candolin 2003). Male mating success is thus affected by various parameters revealing their intrinsic quality (Weatherhead and Boag 1995), such as their size, ornaments, bright colors, or songs as this was documented in many animal taxa including vertebrates and invertebrates (Partridge and Farquhar 1983; Searcy 1992; Madsen et al. 1993; Andersson 1994; Aspi and Hoikkala 1995; Bateman et al. 2001; Dreher and Pröhl 2014; reviewed in Danchin and Cézilly 2008).
Alternatively but not exclusively, females can get further information about potential mates by observing their mating success (Danchin et al. 2004). We call such observational learning mate-copying. It leads females to either mate preferentially with the specific male they saw being chosen by another female (individual-based copying, Pruett-Jones 1992; Bowers et al. 2012), or with a male showing similar characteristics as the male they saw being chosen by another female (trait-based copying, Bowers et al. 2012). Trait-based mate-copying is particularly interesting because learning to prefer males of a given phenotype rather than a specific male (Witte et al. 2015) may potentially lead to the establishment of persistent local traditions in mate choice (Danchin et al., submitted for publication), which in turn may strongly affect sexual selection differentially across populations, thus setting the stage for speciation.
Mate-copying has been reported in many social and non-social species, including fish (Dugatkin and Godin 1993), birds (White and Galef 1999), humans (Waynforth 2007), and other mammals (Galef et al. 2008), as well as in 1 insect, Drosophila melanogaster (Mery et al. 2009; Loyau et al. 2012; Dagaeff et al. 2016; Germain et al. 2016; Danchin et al., submitted for publication; Nöbel et al., submitted for publication). In particular, D. melanogaster females can perform trait-based mate-copying after watching only a single live demonstration of 1 female copulating with a male of a given phenotype and 1 male of another phenotype being rejected (Dagaeff et al. 2016; Danchin et al., submitted for publication; Nöbel et al., submitted for publication). More generally, it is now accepted that many animal species from a vast array of taxa can learn from others (i.e., socially learn), particularly in the context of mate choice (Avital and Jablonka 2000; Danchin et al. 2004; Galef and Laland 2005).
Sex ratio is known to affect male–male competition in a mate-choice context in various species (Lawrence 1986; Jirotkul 1999; Weir et al. 2011), probably because it affects the availability of potential partners. For instance, in D. melanogaster, male sexual behavior is influenced by the number of rivals (Bretman et al. 2009), and male sperm depletion starts after just one copulation, so that the number of emerging offspring is divided by more than 3 after 4 consecutive copulations (Demerec and Kaufmann 1941; Lefevre and Jonsson 1962; Loyau et al. 2012). Thus, females are also expected to adapt their sexual behavior to the sex ratio (i.e., to the number of competitor females), for instance by accepting mates more readily when the male-to-female ratio is low, that is, when the number of potential female competitors is high.
Females can use 2 different sources of information to select a male partner: females might rely on their personal assessment of males’ courtship during the mate-choice test, or only rely on the social information provided by demonstrator females during the demonstration. The latter option is probably more economical in a context of high level of female competition, as mate choice is costly to females (reviewed in Reynolds and Gross 1990; Andersson 1994; Vakirtzis 2011). For instance, females invest time and energy to assess male quality, and male courtship may sometimes be harmful to them (Andersson 1994). Moreover, the risk of losing potential mates to competitors increases with the time spent in assessing males. Under high competition scenario, one could expect females to use the more easily gathered social information only, thus minimizing risks of losing potential mates to competitors. In contrast, under low female competition (i.e., in male-biased sex ratios), this risk is minor, allowing them to spend time in male assessment.
So far, no study has investigated the effect of sex ratio on mate-copying or social learning in general. As in the more general context of mate choice, we can expect females to show contrasting choosiness when being within groups of varying sex ratios (Berglund 1994; Jirotkul 1999; Passos et al. 2014). For example, being under strong competition to access males, females learning within a mixed-sex group mainly composed of females might be much more prone to accept the very first male that courts them, and thus, ignore social information. Contrastingly, females learning within a mixed-sex group essentially composed of males can be expected to be much choosier and to take the time to gather more information about the various potential males before selecting one of them.
Here, we studied mate-copying along a gradient of group sex ratio during demonstrations. All mate-copying designs in Drosophila involve a demonstration phase (or simply demonstration) followed by a mate-choice test. We manipulated the population sex ratio during demonstrations by varying the number of observer females (and thus group size) in the central arena of a hexagonal experimental device (see “Materials and Methods” section). By assuming that observer females can assess and remember the group size and sex ratio they experienced during the demonstration, we predicted that along our gradient of increasing sex ratios during demonstrations, observer females would become more and more choosy during the subsequent mate-choice test, leading them to 1) accept copulation slower (i.e., a longer delay between first courtship and copulation initiation), and thus 2) increasing the rate of replicates in which both males courted the female before copulation initiation (“double courtship rate”), a parameter with methodological implications for future mate-copying experiments.
Concerning mate-copying, we could predict at least 3 possible outcomes, according to how we envisage the group size and sex ratio effects. The group size effect can be either positive or negative: first, females might learn better in a group than alone, thus predicting a positive mate-copying to group size relationship in a form of “social social-learning.” Inversely, in large groups of females (i.e., at female-biased sex ratios) group members may start to disturb and stress each other, thus hampering proper learning and leading to a negative mate-copying to group size relationship in our system. On the other hand, sex ratio could have a negative effect: we could expect that under female-biased sex ratios during demonstration (i.e., in large groups) observer females will adopt the less costly strategy and thus favor social information use over the time-consuming assessment of both males, thus leading to a negative mate-copying to sex-ratio relationship. These potential and contradictory effects of group size and sex ratio on mate-copying, being non-exclusive, might also cancel out each other, leading to no detectable pattern in mate-copying with group size and/or sex ratio. They may also lead to an optimal group size at which mate-copying is more efficient. The mate-copying to group size and sex ratio relationship should thus depend on the relative importance of these various potential effects, so that we did not have clear predictions.
Materials and Methods
Fly maintenance
Wild-type Canton-S flies were raised in 30 ml vials containing 10 ml of a standard corn flour—agar—yeast medium. They were maintained at 25 ± 1°C, 59 ± 5% humidity in a 12 h/12 h light/dark cycle. Virgin flies were collected daily within 7 h after emergence, sexed without anesthesia, and kept in same-sex groups of 7 in a vial with medium. Experiments were conducted on 3–5 days old flies and fly manipulation was performed by gentle aspiration, using a glass pipette, tubing, and gauze.
Experimental protocol
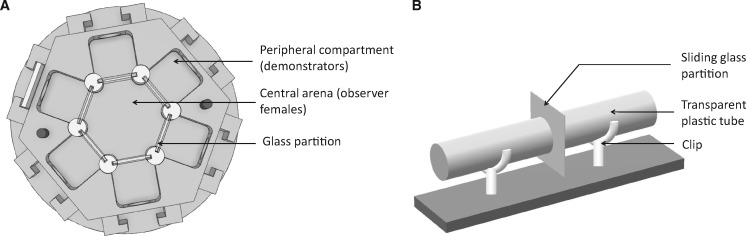
All experiments were conducted under similar conditions as fly maintenance. Air pressure at the airport Toulouse-Blagnac weather station was in the range of 1,004–1,034 hPa and was previously shown to constitute a highly reliable proxi of atmospheric pressure in the experimental room (Dagaeff et al. 2016). Contrasting male phenotypes were created by randomly dusting them with green or pink powders (Mery et al. 2009) 20–30 min prior to use them as demonstrators or potential mates, so that they could clean the excess of dust. Demonstrations were run according to the “speed learning” design (Dagaeff et al. 2016) within a hexagon device (Figure 1A) composed of 1 central arena devoted to female observers (2.7 cm × 1.5 cm, volume = 8.6 cm3) and 6 peripheral compartments devoted to demonstrators (1.5 cm × 1.5 cm × 1.5 cm each) separated from the central arena by a glass partition (0.8 mm thick). The 4 experimental groups differed in the number of observer females: 1, 6, 12, and 24 observer females in the central arena, being able to witness 6 simultaneous demonstrations each ongoing in 1 of the 6 peripheral compartments. Each demonstration involved 1 female apparently choosing the same male color as the other demonstrator females and rejecting the male of the other color. Thus, with a total of 6 demonstrator females and 12 demonstrator males, with 1, 6, 12, and 24 observer females in the central arena, the sex ratio within a hexagon ranged from 1.7 to 1, 0.67, and 0.4 males per female, respectively. Mate-choice tests were run using a device made of a double plastic tube (0.8 cm × 3 cm each) separated by a microscopy cover slide (1.6 cm × 1.6 cm, Figure 1B).
Figure 1.
Experimental set-up used for demonstrations and mate-choice tests. (A) Demonstrations took place in a hexagon device (Danchin et al., submitted for publication). Observer females were placed in the central arena and were able to observe 6 demonstrator trios of 1 female copulating with a male of 1 color plus an apparently non-preferred male of the other color placed in the 6 peripheral compartments. In all 6 demonstrations the female copulated with the male of the same color so that the social information provided was consistently favoring males of that color. The device “Hexagon” can be purchased from Toulouse Tech Transfer and Paul Sabatier University through a Material Transfer Agreement. (B) Mate-choice tests unfolded in double plastic tubes separated by a microscopy cover slide. The observer female was placed on one side, and 2 virgin males, 1 green and 1 pink, on the other side. The test began by lifting the partition, allowing males and females to meet.
Before the beginning of the demonstration, observer females were placed in the central arena. Demonstrator females were placed individually in small plastic tubes with 2 males of the color chosen for the demonstration of that hexagon. As soon as copulation started, the couple and a male of the opposite color were transferred carefully into a peripheral compartment of the hexagon in which the copulation continued. Demonstrations started when the first demonstrator trio was transferred, and ended when all 6 couples were broken, or as soon as new courtship occurred in 1 peripheral compartment after the end of copulation, despite the fact that some of the other copulations might still be ongoing. Thus, demonstration length varied from 16 to 24 min. Then, all observer females were removed by gentle aspiration and placed all together in a food vial until the mate-choice test. For the condition with 24 observer flies, only 12 randomly chosen observer females were kept together in a vial for the test. Results were obtained from 57, 16, 13, and 14 demonstration blocks (i.e., hexagons) for conditions with 1, 6, 12, and 24 observer flies, respectively.
The mate-choice tests started 45–60 min after the end of the demonstration (for practical reasons, half of the flies were tested after 45 min and the other half after 60 min). Each observer fly was placed in one side of a double plastic tube device, and a pair of males, one of each color, in the other side. The males used in the test phase came from a different vial than those used in the demonstration, and were powdered 20–30 min before the beginning of the test. After 2 min resting, the partition was removed, thus beginning the test. The first wing vibration of a male was recorded as courtship initiation, as well as, the color of the courting male, the time of the beginning of copulation, and the color of the chosen male. In trials in which both males courted the female before she chose, the median time of double courtship was 68 s, and the minimum time of double courtship was 1 s.
After the end of the experiment, flies were transferred into a vial and euthanized in a freezer. As in previous studies (Dagaeff et al. 2016; Danchin et al., submitted for publication; Nöbel et al., submitted for publication) mate-choice tests were successful if they led to a copulation and if both males courted the observer female before the initiation of the copulation, as this was the only situation when observer females were visibly in a situation of choice.
Mate-copying index
Replicates in which the observer female copulated with the male of the phenotype preferred during the demonstration (copied) were attributed a mate-copying score of 1, and 0 in the opposite case. The mate-copying index (MCI) was calculated as the mean mate-copying score for each treatment, which reveals female preference in the corresponding experimental group. MCIs significantly higher than 0.5 (random choice) reveal mate-copying.
Statistical analysis
Analyses were conducted with the R software 3.4.0 (R Core Team 2017). For each treatment, the departure from random choice was tested with a binomial test. Mate-copying scores were then analyzed in a generalized linear mixed model (GLMM) with binary logistic regression (package lme4, Bates et al. 2015). We analyzed all successful replicates, that is, replicates in which both males courted the female before she mated (172 trials out of 455). We tested the effect of potential confounding parameters in univariate tests and found no significant effect (time of the demonstration, color of the male chosen by the demonstrator female, and delay between demonstration and test: GLMM, Wald χ2-test, N = 172, χ2 = 1.516, 0.020, and 0.205, P = 0.218, 0.889, and 0.651, respectively). Starting models included sex ratio, normalized air pressure (measured air pressure minus the average air pressure in our data-set, which was 1,022 hPa), and air pressure change within 6 h before the experiment as fixed effects. Air pressure and its variations were introduced into the model because they were shown to influence mate-copying in D. melanogaster (Dagaeff et al. 2016). We also included “first-courting male” as a fixed effect: this parameter takes the value 1 if the color of the first courting male was the “preferred” color in the preceding demonstration, and 0 in the opposite case. For instance, if demonstrator females in the hexagon mated with green males, then during the mate-choice test, if the new green male was the first one to court the observer female, the value was 1. We first introduced a random block (i.e., hexagon) effect into the model in order to account for the non-independence of observer flies from the same hexagon. However, as models including this effect always had higher Akaike Information Criteria (AIC, Akaike 1969) and because this effect could potentially capture part of the main effect as treatments differed from one block to the next one we only report on models that did not include the hexagon as a random effect. Results were not qualitatively affected by the exclusion of that random effect. The significance of fixed effects was tested using Wald χ2 tests implemented in the ANOVA function of the car package (Fox and Weisberg 2011). All starting models included interactions between fixed effects. We applied a backward selection method using P-values, by dropping out non-significant effects, starting with the highest order interaction. We used AIC to determine the final model(s).
To test experimental effects on the rate of double courtship, we analyzed the number of males courting the female in a GLMM with binary logistic regression (1 vs. 2 males courted before copulation initiation). We analyzed all trials in which the female copulated after at least 1 male courted her (441 trials out of 455). The dependent variable “number of males courting” was set to 1 when both males courted, and 0 if only one of the males courted. All potential confounding parameters (time of the test, test chamber ID in the set of 6 tube designs, delay between demonstration and test) were found non-significant in univariate tests (GLMM, Wald χ2-test, N = 441, χ2 = 1.299, 3.390, and 1.738, P = 0.255, 0.640, and 0.187, respectively). Thus, the starting model included sex ratio, normalized air pressure, time when the first courtship began, and first-courting male as well as their interactions as fixed effects.
The log-transformed courtship duration was analyzed in a LMM with logistic regression. We analyzed all trials with detailed times of courtship and copulation initiation (432 trials out of 455). Log-transformation (natural log) was used to achieve a Gaussian distribution of that variable. All potential confounding parameters (time of the test, test chamber ID in the set of 6 tube designs, delay between demonstration and test) were non-significant in univariate tests (LMM, N = 432, F = 1.039, 0.997, and 0.228, P = 0.309, 0.419, and 0.633, respectively). The starting model thus included sex ratio, log-transformed time of first courtship initiation, and first-courting male as fixed effects.
Results
Mate-copying along a gradient of sex ratio
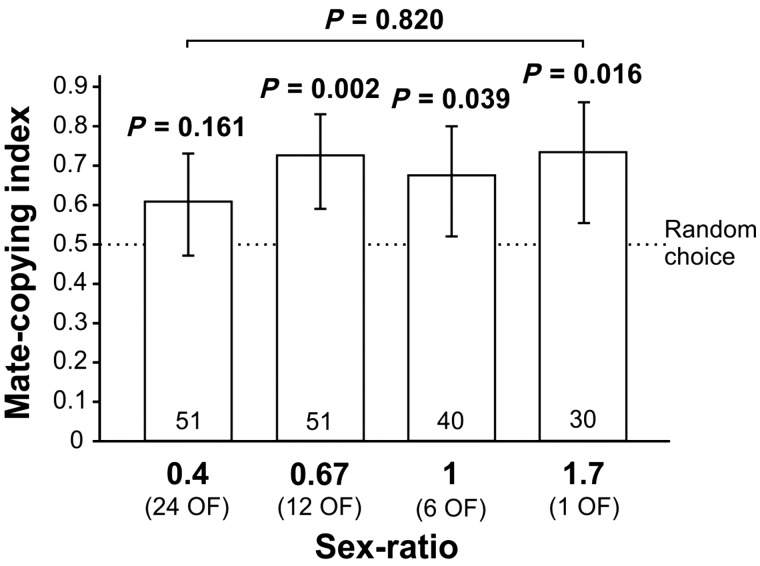
We analyzed mate-copying indices in a GLMM with binary logistic regression. The starting model included the sex ratio as a continuous variable, first-courting male, and normalized air pressure, as well as air pressure changes within 6 h before the experiment as fixed effects. None of the interactions were significant (P > 0.14 in all cases). Sex ratio had no effect on mate-copying (GLMM, Wald χ2-test, N = 172, χ2 = 0.052, P = 0.820; Figure 2), nor did air pressure changes within 6 h before the experiment (GLMM, Wald χ2 test, N = 172, χ2 = 0.25, P = 0.616). The selected model included normalized air pressure (GLMM, Wald χ2 test, N = 172, χ2 = 3.81, P = 0.051, positive effect) and first courting male (GLMM, Wald χ2 test, N = 172, χ2 = 4.13, P = 0.042, negative effect). The MCI was higher when the first male courting in the test was the one of the color that was rejected by the demonstrator females.
Figure 2.
Mate-copying indices along a sex-ratio gradient. Mate-copying indices above 0.5 indicate a preference for the male of the color chosen during the preceding demonstration. OF, observer females; vertical bars: Agresti–Coull intervals. Apart from binomial tests provided above bars, statistical analyses detected a significant negative effect of first-courting male and an almost significant positive effect of air pressure (see text). Sample sizes are provided at the bottom of each bar.
Thus, we did not find any significant relationship of mate-copying with sex ratio (Figure 2). When analyzing mate-copying indices in each group using binomial tests, we found a significant departure from random choice for the groups with sex ratios of 1.7, 1, and 0.67 males per female, but not in the group with a sex ratio of 0.4 (Figure 2), although the trend was in the same direction with a tendency to choose the male of the color selected during the demonstration.
Rate of double courtship along a sex ratio gradient
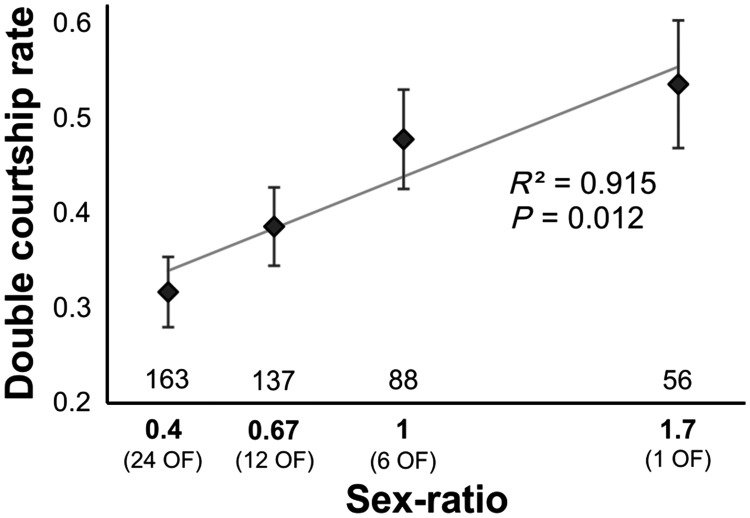
We measured the rates of both males courting the female (“double courtship rate”), that is, the proportion of trials in which both males courted the female before she initiated mating with one of them. The starting binary logistic regression model included the sex ratio as a continuous variable, first-courting male, and normalized air pressure, as well as the log-transformed time of first courtship initiation as fixed effects. None of the interactions were significant (P > 0.69 in all cases). First-courting male had no effect on the double courtship rate (GLMM, Wald χ2 test, N = 441, χ2 = 1.15, P = 0.283). The selected model included sex ratio (GLMM, Wald χ2 test, N = 441, χ2 = 6.333, P = 0.012, positive effect, Figure 3), normalized air pressure, and log-transformed time of first courtship initiation (GLMM, Wald χ2 test, N = 441, χ2 = 5.818 and 8.978, P = 0.016 and 0.003, positive and negative effects, respectively). As expected, we found that the double courtship rate increased along the sex-ratio gradient (Figure 3). Females thus appeared to be able to assess the sex ratio during demonstrations, remember it, and adapt their behavior during the subsequent mate-choice test accordingly.
Figure 3.
Female choosiness measured as double courtship rate. Rates are expressed as the number of trials in which both males courted the female on the total number of trials. OF, observer females. Error bars represent SEM. Sample sizes are provided above the X-axis.
Courtship duration along the sex ratio gradient
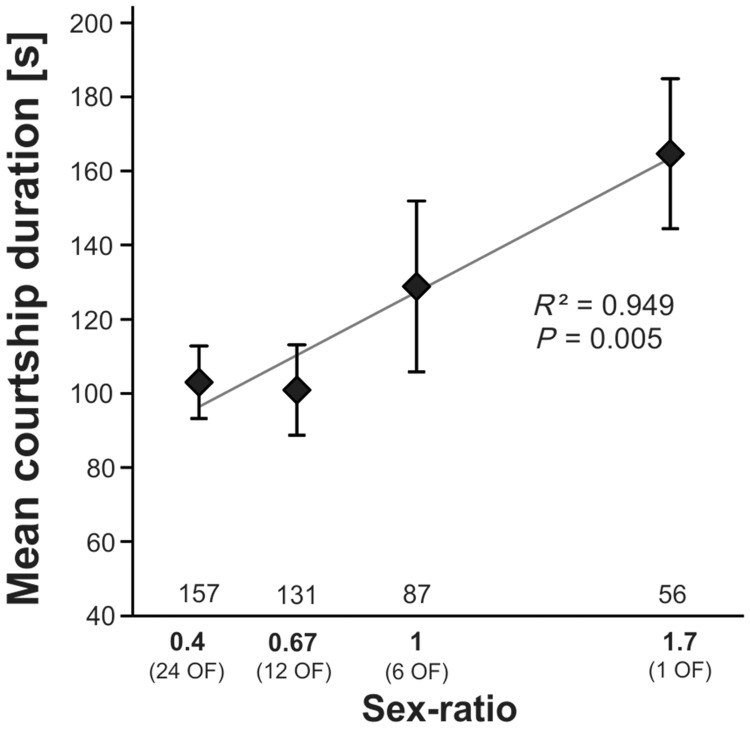
Because copulation initiation in D. melanogaster is mainly under female control (Connolly and Cook 1973; Kimura et al. 2015), we tested whether the decrease in the double courtship rate was due to faster acceptance of copulation in groups with lower sex ratios during the demonstration. In a preliminary model analyzing number of males courting as a fixed effect depending on log-transformed courtship duration, we first found that these 2 variables were highly correlated (LMM, N = 432, F = 124.3, P < 0.001, positive effect), which supports our hypothesis: in trials in which both males courted, the latency between first courtship and copulation initiation was the highest. We then analyzed the delay between first courtship initiation and copulation initiation along the sex ratio gradient (Figure 4). In a LMM with logistic regression in which the response variable was the log-transformed courtship duration, including the sex ratio as a continuous variable, first-courting male, and the log of the time of first courtship initiation, none of the interactions were significant (P > 0.32 in all cases). The first-courting male index was not associated with courtship duration (LMM, N = 432, F = 0.048, P = 0.827), while sex ratio (Figure 4) and time of first courtship initiation were (LMM, N = 432, F = 7.828 and 14.19, P = 0.005 and <0.001, positive and negative effect, respectively). Thus, as expected, copulation occurred faster in low sex ratio conditions, suggesting that observer females were much quicker in accepting copulation in such situations.
Figure 4.
Courtship duration measured as mean latency between time of first courtship initiation and copulation initiation along the sex-ratio gradient. OF, observer females. Error bars represent SEM. Sample sizes are provided above the X-axis.
Discussion
We investigated sex-ratio and group size effects during mate-choice demonstrations on the observer females’ tendency to copy the mate choice of demonstrator females for a specific male phenotype as well as their choosiness in the subsequent mate-choice tests. We expected that along a gradient of increasing sex ratio during demonstrations, observer females would accept copulation slower, thus increasing the double courtship rate. We had at least 3 contradictory predictions concerning the mate-copying to sex ratio and group size relationship, so that we had no specific expectation about the direction of the effect. The relationship of mate-copying to group size could be either (i) positive, because of some social facilitation in mate-copying or (ii) negative, as a result of decreasing disturbance by other females with decreasing group size, and the relationship of mate-copying to sex ratio could be (iii) negative, as a result of decreasing female competition for males when sex ratio increases. Finally, a combination of these effects could also produce an optimal group size and sex ratio at which social learning is maximized.
As expected, we found that the frequency of both males courting the observer female and the delay between first courtship initiation and copulation initiation increased along the increasing experimental sex-ratio gradient (and decreasing group size). This supports our hypothesis that female choosiness decreases when sex ratio gets more female biased. Interestingly, females copied on average the observed mate-choice decisions for a certain male phenotype regardless the experienced sex ratio. We can thus conclude that mate-copying seems to be similarly efficient under all tested sex ratio conditions. In accordance, none of our initial hypotheses was supported.
No detectable effect of sex ratio on mate-copying
Although non-significant the MCI of the largest group (24 observer females, sex ratio of 0.4 males per female) did not differ from those of the other treatments, suggesting that females did not learn better in a group. There seemed to be no “social social-learning” in this observational learning paradigm, which is in accordance with the fact that in a form of olfactory learning, flies tested in groups show increased memory retrieval compared with flies tested individually, but training condition (single vs. group) does not affect memory formation (Chabaud et al. 2009). Similarly, in large groups of observer females (i.e., with female-biased sex ratios), we did not detect any evidence for increased disturbance among flies that would have hampered proper learning, as mate-copying appeared relatively unchanged with the size of the group of observer females. Finally, we found no evidence that females learning in female biased groups favor social information use over the personal assessment of male’s quality. This might be explained by a lack of quality differences among presented males that led females to rely preferably on social information gathered during demonstrations. Another explanation is that several contradictory effects were ongoing simultaneously and cancelled out each other. Unfortunately, our experimental design did not allow us to disentangle group size from sex ratio effects as they co-varied in our design.
The ability to copy may be an adaptation to the naturally crowded conditions existing on rotten pieces of fruit to which females are attracted both as sources of food and egg laying site (Rodrigues et al. 2015; Keesey et al. 2016). In these aggregations, copulations are common (Danchin E, personal observation) and surrounding females have the opportunity to watch the mate choice of other females, thus setting the stage for mate-copying in natural situations.
As for methodological implications, our results suggest that it is possible to increase the number of observer females in the central arena of the hexagon during demonstrations at least up to 12 without affecting mate-copying efficiency. This could help in designing future mate-copying experiments, for instance by increasing the number of males in the peripheral compartments, and thus, increasing the sex ratio in order to increase double courtship rates without affecting mate-copying scores. This would allow us to extend the proportion of replicates in which females are visibly in a situation of choice during the mate-choice test.
All in all, our results suggest that mate-copying is quite robust to sex ratio conditions as well as group size differences in general. While this might be caused by contradictory effects of varying sex ratios and group sizes cancelling each other out, the high observed robustness in mate-copying may suggest a real importance of social information use in mate choice (see also Danchin et al. 2004; Galef and Laland 2005). In general, selection might have favored the evolution of mate-copying under varying environmental conditions. In particular, the Fisher runaway process (Fisher 1930) predicts that females should conform to the local preference because male descendants of females mating with the locally non-preferred males would inherit of the non-preferred trait, thus counter-selecting them for as long as the local preference persists (Danchin et al., submitted for publication). There is thus strong selection for conforming to the majority. In our experiments, females saw all 6 demonstrator females having apparently chosen to copulate with the same male color morph and rejecting the other, revealing a very strong local preference. In such conditions, the Fisher runaway process predicts that they should build a preference for that type of males independently of the other local conditions.
Female choosiness increased with sex ratio
From a female’s point of view, a female-biased local sex ratio is concomitant with high competition for mates, and thus, females should accept any encountered mate more readily and more quickly. Following this logic, we found that females were quicker in initiating copulations under female-biased sex ratios. Similarly, double courtship rates, which constitute a positive proxy of female choosiness in Drosophila, decreased when the sex ratio shifted from male-to-female bias. However, the rate of double courtship likely depends on both sexes as males can be more or less interested in courting the female (Eastwood and Burnet 1977; Clowney et al. 2015), and females can exhibit varying choosiness (Maklakov and Arnqvist 2009). Nonetheless, because all males used during mate-choice tests were new and naïve males, they had no information on the previous demonstration and thus could not have generated the observed pattern. They all saw a young virgin female, which should be attractive per se (Tompkins and Hall 1981). Contrastingly, observer females having been under varying sex ratios during the demonstration are more likely to have driven the observed pattern. All observer females of all experimental groups were raised in unisex groups for 3–5 days from hatching to experiment, and thus all of them experienced the same situation of acute lack of potential mates for a long period before experiments. As a consequence, the difference in choosiness observed between these groups should only come from the difference in the sex ratio and group size experienced during the ∼20 min of the demonstration. In effect, demonstrations constituted the first time since sexual maturity in which observer females were in the presence of males, and vice versa, which might have made them highly sensitive to the presence of mates, potentially explaining the fact that the short demonstration period was sufficient to elicit differential behavior in females according to the sex ratio and group size they experienced just before.
Results on double courtship rates and on courtship to copulation latency were probably not independent because the shorter the courtship, the shorter the time for the second male to court the female. However, globally our results support our hypothesis that varying levels of competition along the sex-ratio gradient affected female choosiness in Drosophila. Similarly, it was shown in several species (Berglund 1994; Passos et al. 2014; Pompilio et al. 2016), as well as in a theoretical study (Bleu et al. 2012), that females tend to maximize the chances of mating with a high-quality male in male-biased sex ratios, while minimizing the risk of remaining unmated in female-biased sex ratios. For similar reasons, the positive relationship between sex ratios and rates of both males courting the observer female during the mate-choice test appears adaptive.
Mechanisms of sex ratio detection
Concerning the mechanisms by which females detected the local sex ratio, the characteristics of our hexagon device implies that observer females could only perceive sex ratio visually, which is consistent with the fact that flies can recognize visual patterns and sense motion and color (Behnia and Desplan 2015; Liu et al. 2006; reviewed in Guo et al. 2017), and may even be able to visually recognize individual males and behave differently relative to them according to these males’ past experience (Loyau et al. 2012). Our results thus support the idea that Drosophila probably use vision in much more diverse and subtle contexts than previously thought (Loyau et al. 2012). More generally, insect cognitive capacities are being discovered as surprisingly sophisticated. For instance, honeybees Apis mellifera have been shown to be able to count visually (Chittka and Geiger 1995; Gross et al. 2009; reviewed in Dacke and Srinivasan 2008). The question of the existence of such skills in Drosophila remains open. It would be interesting to study how and for how long such specific behavior can last after the demonstration, in order to assess the dynamics of Drosophila mating behavior determinants.
Effect of first-courting male on MCI
We found the fact that the first courting male was or was not of the same color that was selected during the demonstration significantly affected mate-copying. Among the group of flies that chose after a double courtship, mate-copying indices were higher when the first courting male had the color that was rejected during the demonstration. A female has 2 options when the first male courted: accept copulation with the courting male or wait for the second male to start courting. A copier observer female would be less likely to wait for her “non-preferred” male to court her if the first male to court her is of the color that she learnt to prefer during the previous demonstration: a situation that most often would lead to a single courtship before copulation. Contrastingly, a non-copier female or a copier female first courted by the male of her non-preferred color would both be more likely to wait for the second male to court her. Because in our analyses of mate-copying indices, it was necessary to discard mate-choice tests in which only one male courted the female before the onset of the copulation to only keep situations in which females were in a real situation of choice between the 2 males (see “Materials and Methods” section), we could thus expect a lower proportion of copiers in the group “First-courting male = 1” (i.e., females that were first courted by their preferred male), than in the group “First-courting male = 0” (i.e., females that either had no preference or that were first courted by the male of their non-preferred phenotype), as we found. Thus, our measured MCI is conservative.
Effect of atmospheric pressure on Drosophila sexual behavior
As in a previous study (Dagaeff et al. 2016), we found a significant positive effect of air pressure on the rate of double courtship, and a slight positive effect on mate-copying. Mating behavior was previously found to be correlated with atmospheric pressure in D. pseudoobscura (Ankney 1984) and in D. melanogaster (Austin et al. 2014). Bad weather can mean death for small insects, and it seems adaptive for them to be able to anticipate weather variations, like air pressure changes, to find a shelter and then save energy in bad weather. Interestingly, flies from the same population were found to differ in response to air pressure: under low pressure some individual flies reduced their mating activity, while others increased it (Austin et al. 2014). Such a polymorphism might reveal phenotypic variation in relation to dispersal. Similarly, mate-copying was found to be reduced under low atmospheric pressure (Dagaeff et al. 2016). Here, we further found that D. melanogaster females seem to become much less choosy under bad weather forecast (revealed by lower double courtship rates), probably because they act as quick as possible in such cases.
In conclusion, we provide evidence that sex ratio as a proxy of female–female competition may affect female choosiness in D. melanogaster. We did not find any relationship between sex ratio and mate-copying efficiency, suggesting that mate-copying is fairly robust to variation in this environmental condition. We speculate that this may be partly because our experimental design did not allow us to separate the contradictory effects of sex ratio on the propensity to mate-copy, or because selection favors copying in general independently from sex ratio conditions. In terms of evolution, our findings suggest that females may have acquired the ability to mate-copy independently of group size and sex ratio, without hampering their capacity to adapt their choosiness to the current population sex ratio, which determines the relative availability of male partners.
Author Contributions
M.M. carried out the experiments, performed the analysis, and drafted the manuscript; S.N. contributed in the analysis and writing of the manuscript; E.D. and G.I. designed the experiment and jointly supervised all steps in the process. All authors gave final approval for the publication.
Acknowledgments
We would like to thank Nathalie Parthuisot for help in fly care. This work was supported by the “Laboratoires d’Excellence (LABEX)” TULIP (ANR-10-LABX-41), as well as ANR funded Toulouse Initiative of Excellence “IDEX UNITI” (ANR11-IDEX-0002-02). E.D. and S.N. were also supported by the Soc-H2 ANR project (ANR-13-BSV7-0007-01) to E.D. M.M.’s salary was provided by a grant from the French ministry of higher education and research. S.N.’s salary was provided by Soc-H2 and a Marie Curie PRESTIGE grant (PRESTIGE-2014-1-0005). G.I. benefited from a CNRS Excellence Chair.
References
- Akaike H, 1969. Fitting autoregressive models for prediction. Ann Instit Statist Math 21:243–247. [Google Scholar]
- Andersson M, 1994. Sexual Selection. Monographs in Behavior and Ecology. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Ankney PF, 1984. A note on barometric pressure and behavior in Drosophila pseudoobscura. Behav Genet 14:315–317. [DOI] [PubMed] [Google Scholar]
- Aspi J, Hoikkala A, 1995. Male mating success and survival in the field with respect to size and courtship song characters in Drosophila littoralis and D. montana (Diptera: drosophilidae). J Insect Behav 8:67–87. [Google Scholar]
- Austin CJ, Guglielmo CG, Moehring AJ, 2014. A direct test of the effects of changing atmospheric pressure on the mating behavior of Drosophila melanogaster. Evol Ecol 28:535–544. [Google Scholar]
- Avital E, Jablonka A, 2000. Animal Traditions: Behavioural Inheritance in Evolution. Cambridge: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- Bateman PW, Gilson LN, Ferguson JWH, 2001. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim Behav 61:631–637. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Behnia R, Desplan C, 2015. Visual circuits in flies: beginning to see the whole picture. Curr Opin Neurobiol 34:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund A, 1994. The operational sex ratio influences choosiness in a pipefish. Behav Ecol 5:254–258. [Google Scholar]
- Bleu J, Bessa-Gomes C, Laloi D, 2012. Evolution of female choosiness and mating frequency: effects of mating cost, density and sex ratio. Anim Behav 83:131–136. [Google Scholar]
- Bowers RI, Place SS, Todd PM, Penke L, Asendorpf JB, 2012. Generalization in mate-choice copying in humans. Behav Ecol 23:112–124. [Google Scholar]
- Bretman A, Fricke C, Chapman T, 2009. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc R Soc Lond B Biol Sci 276:1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U, 2003. The use of multiple cues in mate choice. Biol Rev 78:575–595. [DOI] [PubMed] [Google Scholar]
- Chabaud MA, Isabel G, Kaiser L, Preat T, 2009. Social facilitation of long-lasting memory retrieval in Drosophila. Curr Biol 19:1654–1659. [DOI] [PubMed] [Google Scholar]
- Chittka L, Geiger K, 1995. Can honey bees count landmarks? Anim Behav 49:159–164. [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V, 2015. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87:1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K, Cook R, 1973. Rejection responses by female Drosophila melanogaster: their ontogeny, causality and effects upon the behaviour of the courting male. Behaviour 44:142–165. [Google Scholar]
- Dacke M, Srinivasan MV, 2008. Evidence for counting in insects. Anim Cogn 11:683–689. [DOI] [PubMed] [Google Scholar]
- Dagaeff AC, Pocheville A, Nöbel S, Loyau A, Isabel G. et al. , 2016. Drosophila mate copying correlates with atmospheric pressure in a speed learning situation. Anim Behav 12:163–174. [Google Scholar]
- Danchin É, Cézilly F, 2008. Sexual selection: another evolutionary process In: Danchin É, Girladeau LA, Cézilly F, editors. Behavioural Ecology. Oxford: Oxford University Press, 363–426. [Google Scholar]
- Danchin É, Giraldeau LA, Valone TJ, Wagner RH, 2004. Public information: from nosy neighbors to cultural evolution. Science 305:487–491. [DOI] [PubMed] [Google Scholar]
- Demerec M, Kaufmann BP, 1941. Time required for Drosophila males to exhaust the supply of mature sperm. Am Nat 75:366–379. [Google Scholar]
- Dreher CE, Pröhl H, 2014. Multiple sexual signals: calls over colors for mate attraction in an aposematic, color-diverse poison frog. Front Ecol Evol 2:1–10. [Google Scholar]
- Dugatkin LA, Godin JGJ, 1993. Female mate copying in the guppy Poecilia reticulata: age-dependent effects. Behav Ecol 4:289–292. [Google Scholar]
- Eastwood L, Burnet B, 1977. Courtship latency in male Drosophila melanogaster. Behav Genet 7:359–372. [DOI] [PubMed] [Google Scholar]
- Fisher RA, 1930. The Genetical Theory of Natural Selection: A Complete Variorum Edition. Oxford: Clarendon Press. [Google Scholar]
- Fox J, Weisberg S, 2011. An {R} Companion to Applied Regression. 2nd edn. Thousand Oaks: Sage Publishing. [Google Scholar]
- Galef BG, Laland KN, 2005. Social learning in animals: empirical studies and theoretical models. AIBS Bull 55:489–499. [Google Scholar]
- Galef BG, Lim TC, Gilbert GS, 2008. Evidence of mate choice copying in Norway rats Rattus norvegicus. Anim Behav 75:1117–1123. [Google Scholar]
- Germain M, Blanchet S, Loyau A, Danchin É, 2016. Mate-choice copying in Drosophila melanogaster: impact of demonstration conditions and male–male competition. Behav Processes 125:76–84. [DOI] [PubMed] [Google Scholar]
- Gross HJ, Pahl M, Si A, Zhu H, Taut J. et al. , 2009. Number-based visual generalisation in the honeybee. PLoS ONE 4:e4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Li H, Li Y, Liu L, Liu Q. et al. , 2017. Vision, memory, and cognition in Drosophila In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. 2nd edn. New York: Elsevier Academic Press, 483–503. [Google Scholar]
- Jirotkul M, 1999. Operational sex ratio influences female preference and male–male competition in guppies. Anim Behav 58:287–294. [DOI] [PubMed] [Google Scholar]
- Johnstone RA, Reynolds JD, Deutsch JC, 1996. Mutual mate choice and sex differences in choosiness. Evolution 50:1382–1391. [DOI] [PubMed] [Google Scholar]
- Keesey IW, Koerte S, Retzke T, Haverkamp A, Hansson BS. et al. , 2016. Adult frass provides a pheromone signature for Drosophila feeding and aggregation. J Chem Ecol 42:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Sato C, Koganezawa M, Yamamoto D, 2015. Drosophila ovipositor extension in mating behavior and egg deposition involves distinct sets of brain interneurons. PLoS ONE 10:e0126445.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence WS, 1986. Male choice and competition in Tetraopes tetraophthalmus: effects of local sex ratio variation. Behav Ecol Sociobiol 18:289–296. [Google Scholar]
- Lefevre G, Jonsson UB, 1962. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics 47:1719–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K. et al. , 2006. Distinct memory traces for two visual features in the Drosophila brain. Nature 439:551–556. [DOI] [PubMed] [Google Scholar]
- Loyau A, Blanchet S, Van Laere P, Clobert J, Danchin E, 2012. When not to copy: female fruit flies use sophisticated public information to avoid mated males. Sci Rep 2:768.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T, Shine R, Loman J, Håkansson T, 1993. Determinants of mating success in male adders Vipera berus. Anim Behav 45:491–499. [Google Scholar]
- Maklakov AA, Arnqvist G, 2009. Testing for direct and indirect effects of mate choice by manipulating female choosiness. Curr Biol 19:1903–1906. [DOI] [PubMed] [Google Scholar]
- Mery F, Varela SA, Danchin É, Blanchet S, Parejo D. et al. , 2009. Public versus personal information for mate copying in an invertebrate. Curr Biol 19:730–734. [DOI] [PubMed] [Google Scholar]
- Partridge L, Farquhar M, 1983. Lifetime mating success of male fruitflies Drosophila melanogaster is related to their size. Anim Behav 31:871–877. [Google Scholar]
- Passos C, Tassino B, Reyes F, Rosenthal GG, 2014. Seasonal variation in female mate choice and operational sex ratio in wild populations of an annual fish Austrolebias reicherti. PLoS ONE 9:e101649.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompilio L, González Franco M, Chisari LB, Manrique G, 2016. Female choosiness and mating opportunities in the blood-sucking bug Rhodnius prolixus. Behaviour 153:1863–1878. [Google Scholar]
- Pruett-Jones S, 1992. Independent versus nonindependent mate choice: do females copy each other? Am Nat 140:1000–1009. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing RL [cited 2018 February 2]. Available at: https://www.R-project.org/.
- Reynolds JD, Gross MR, 1990. Costs and benefits of female mate choice: is there a lek paradox? Am Nat 136:230–243. [Google Scholar]
- Rodrigues MA, Martins NE, Balancé LF, Broom LN, Dias AJ. et al. , 2015. Drosophila melanogaster larvae make nutritional choices that minimize developmental time. J Insect Physiol 81:69–80. [DOI] [PubMed] [Google Scholar]
- Searcy WA, 1992. Song repertoire and mate choice in birds. Am Zool 32:71–80. [Google Scholar]
- Tompkins L, Hall JC, 1981. The different effects on courtship of volatile compounds from mated and virgin Drosophila females. J Insect Physiol 27:17–21. [Google Scholar]
- Trivers R, 1972. Parental Investment and Sexual Selection. Cambridge: Biological Laboratories, Harvard University. [Google Scholar]
- Vakirtzis A, 2011. Mate choice copying and nonindependent mate choice: a critical review. Ann Zool Fennici 48:91–107. [Google Scholar]
- Waynforth D, 2007. Mate choice copying in humans. Hum Nat 18:264–271. [DOI] [PubMed] [Google Scholar]
- Weatherhead PJ, Boag PT, 1995. Pair and extra-pair mating success relative to male quality in red-winged blackbirds. Behav Ecol Sociobiol 37:81–91. [Google Scholar]
- Weir LK, Grant JW, Hutchings JA, 2011. The influence of operational sex ratio on the intensity of competition for mates. Am Nat 177:167–176. [DOI] [PubMed] [Google Scholar]
- White DJ, Galef BG Jr,. 1999. Mate choice copying and conspecific cueing in Japanese quail Coturnix coturnix japonica. Anim Behav 57:465–473. [DOI] [PubMed] [Google Scholar]
- Witte K, Kniel N, Kureck IM, 2015. Mate-choice copying: status quo and where to go. Curr Zool 61:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]