Abstract
In the era of antibiotic resistance, alternative treatment options for multidrug-resistant bacterial infections are being explored. We present a case of multidrug-resistant Acinetobacter baumannii infection treated with bacteriophages. Clinical trials are needed to further investigate bacteriophage therapy as an option to treat multidrug-resistant bacterial infections.
Keywords: bacteriophage, multidrug resistance, phage
Antibiotics have revolutionized treatment for infectious diseases, prolonged life, and may improve quality of life; however, with the globally increasing prevalence of antibiotic resistance, we continue to observe the limitations of antibiotics. At least two million people become infected with bacteria that are resistant to some antibiotics each year in the United States, and at least 23,000 patients die because of their infection [1]. Multidrug-resistant (MDR) bacteria are a result of overuse of antibiotics in the medical setting, availability of antibiotics without prescriptions in some countries, and mass administration of antibiotics to livestock creating selective pressure [2]. Antibiotic production has not matched the rates of antibiotic resistance, and, therefore, the medical community has turned towards alternative treatment for MDR infections. Lytic bacteriophage therapy may be an opportunity to combat the rapidly growing number of MDR bacteria.
Bacteriophages are viruses that are abundant in the environment, and they have been studied for the treatment of bacterial infections for approximately 100 years. They invade and kill target bacteria by lysis and do not attack mammalian cells. Phages are specific to different bacteria, and they bind to receptors on bacterial cell walls to inject deoxyribonucleic acid into the cell and ultimately lyse the cell in the lytic phase [3]. During the lysogenic cycle, phages integrate into their host genome or exist in the cell as plasmids, evolving to coexist with bacteria.
Bacteriophages were first discovered by Twort and d’Herelle in 1919 and used briefly in the early 1900s, but they fell out of favor in Western Europe and the United States after the development of antibiotics. Bacteriophage research and use continued in Eastern Europe, predominately in Russia, Georgia, and Poland; however, no randomized controlled trials were conducted [4].
The US Army studied phages in the 1940s in animal models, which showed promise for the treatment of Gram-negative infections with Shigella dysenteriae [5]. Little phage research ensued until animal models re-emerged in the 1980s. A randomized control trial (RCT) with topical phage treatment of venous leg ulcers in 2009 showed that this therapy was not associated with any adverse events [6]. Wright et al [7] performed an RCT to evaluate the efficacy and safety of bacteriophages in patients who had chronic otitis externa infections with antibiotic-resistant Pseudomonas aeruginosa. Patients who received phage therapy had improved symptoms and lower colony counts of P aeruginosa from external ear culture. More recently, phages were used to treat a patient suffering from necrotizing pancreatitis and MDR Acinetobacter baumannii pancreatic pseudocyst infection. Two 4-phage cocktails were administered intravenously and into 3 intra-abdominal drains, resulting in cure of the infection and complete clinical recovery [8].
In this report, we describe a patient with MDR A baumannii infection who was treated with bacteriophages. The man was a previously healthy 77-year-old, who suffered assault, subdural hematoma, and traumatic brain injury. He underwent craniectomy complicated by postoperative infection with cerebritis, subdural and epidural empyema, requiring debridement. A subdural drain was left in place. Intraoperative cultures grew MDR A baumannii. His isolate was resistant to all antibiotics; however, some isolates were sensitive to colistin. Susceptibility testing included amikacin (minimum inhibitory concentration [MIC] >32 mcg/mL), ampicillin/sulbactam (MIC >16/8 mcg/mL), cefepime (MIC >16 mcg/mL), ceftazidime (MIC = 16 mcg/mL), ciprofloxacin (MIC > 2 mcg/mL), colistin ([COL] MIC = mcg/mL), doripenem (MIC > 32 mcg/mL), gentamicin (MIC = 8 mcg/mL), imipenem (>32 mcg/mL), levofloxacin (>4 mcg/mL), meropenem (MIC >8 mcg/mL), minocycline (MIC = 16 mcg/mL), tetracycline (>8 mcg/mL), tigecycline (4 mcg/mL), tobramycin (>8 mcg/mL), and trimethoprim/sulfamethoxazole (>2/38 mcg/mL). Broth microdilution antimicrobial susceptibility testing and checkerboard assays were performed in cation-adjusted Mueller-Hinton broth and in accordance with Clinical and Laboratory Standards Institute guidelines using COL, rifampin (RIF), azithromycin (AZM), chloramphenicol (CM), and combinations of COL with RIF, AZM, and CM (Table 1). The isolate exhibited resistance to RIF (MIC >32 mcg/mL), AZM (MIC >32 mcg/mL), and CM (MIC >32 mcg/mL) individually. Combinational antimicrobial synergy, additivity, and antagonism identified using checkerboard assays were defined using the fractional inhibitory concentration index (FICI): FICI ≤0.5 defined synergy, >0.5 to ≤1 additivity, >1 to <4 indifference, and ≥4 antagonism. Ultimately, additivity was noted for all combinations (COL + RIF, COL + AZM, COL + CM) studied. However, FICI values for COL + RIF (FICI = 0.53) and COL + AZM (FICI = 0.56) neared synergy. Based on available antimicrobial susceptibility testing, the patient was placed on COL + AZM + RIF but without clinical improvement. An emergency investigational new drug application to use bacteriophage therapy was requested and approved by the US Food and Drug Administration (FDA). The patient could not provide informed consent because of poor mental status, and informed consent was obtained from his next of kin.
Table 1.
MIC90 (µg/mL) of Antibiotic Against Acinetobacter baumannii Clinical Isolate and Corresponding Combinational Therapy (COL+RIF, COL+AZM, COL+CM) Assessed by Checkerboard Assays Sing CA-MHB
| MIC (mg/L) | Checkerboard FIC Interpretation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| COL | RIF | AZM | CM | COL+RIF | COL+AZM | COL+CM | FICICOL+RIF | FICICOL+AZM | FICICOL+CM |
| 1 (S) | >32 (R) | >32 (R) | >32 (R) | 0.5 + 1 | 0.5 + 2 | 0.5 + 16 | 0.53125 (S) | 0.5625 (S) | 1 (A) |
Abbreviations: A, additivity; AZM, azithromycin; CA-MHB, cation-adjusted Mueller-Hinton broth; COL, colistin; CM, chloramphenicol; FICI, fractional inhibitory concentration index; I, indifference; I, intermediate; MIC, minimum inhibitory concentration; R, resistant; RIF, rifampin; S, susceptible; S, synergy.
MATERIALS AND METHODS
The A baumannii strain cultured intraoperatively was sent to the Naval Medical Research Center-Frederick ([NMRC] Fort Detrick, MD) and was grown in tryptic soy broth. A library with 104 A baumanii bacteriophages from the NMRC’s phage-Biolog system were screened for activity against the patient’s isolate. Five phages had activity against the patient’s strain of A baumanii. High-titer phage lysate stocks of the phage with highest virulence was propagated and amplified in corresponding host bacteria using standard procedures described in Sambrook et al [9]. After amplification, the phage was treated with DNase (final concentration, 1 µg/mL) and precipitated from the media using polyethylene glycol. The polyethylene glycol was removed using chloroform. The phages were purified in a cesium chloride density gradient and removed from the gradient and then dialyzed using a Slide-A-Lyzer in phosphate-buffered saline. Phages were then sterilized through 0.22-µ filters. The phages were titrated and evaluated for endotoxin.
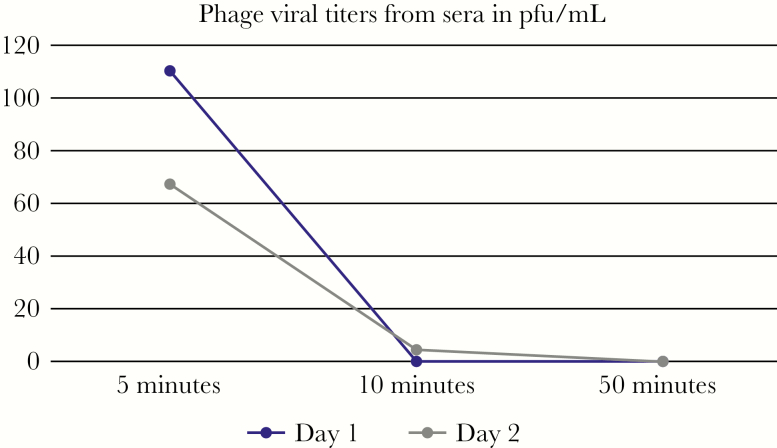
The first dose of bacteriophage was administered intravenously on hospital day 12. The concentration of the phage was 2 × 1010 plaque-forming units (PFU)/mL, with an endotoxin level of 3.5 × 105 endotoxin units (eu)/mL. The phage dose given was 2.14 × 107 PFU/mL, in accordance with the FDA-recommended endotoxin lipopolysaccharide limit of 5 eu/kg body weight per hour. The phage was administered intravenously through a peripherally inserted central catheter line every 2 hours, in 4 mL Lactated Ringer’s, for 8 days. A total of 98 doses were administered. The patient had a skin flap over the prior craniectomy site with a subdural drain placed intraoperatively at the time of debridement, and the option of administration of phage through the drain to provide direct topical bacteriophage was discussed. This was not done due to concern that the integrity of the flap would be impaired. Intrathecal administration was proposed to the family; however, the family declined lumbar drain placement. Phage levels were monitored by collecting blood samples at 5, 20, and 50 minutes after the phage was given, on days 1, 2, 3, 7, and 14. An aliquot of each heparinized blood sample was centrifuged at 2000 ×g for 2 minutes, and plasma was collected in sterile vials. One hundred microliters of actively growing A baumannii culture was inoculated with 100 µl of whole blood or plasma and incubated at 37°C for 18 minutes before plating on an agar plate after conventional soft agar overlay technique. Plates were incubated at 37°C inside of a humidified chamber overnight, and visible plaques were counted the next day. One hundred plaque-forming units per milliter was observed from whole blood, and 110 PFU/mL was observed from sera in samples obtained 5 minutes after phage administration. No plaques were observed from whole blood or sera in samples obtained 10 or 50 minutes after phage administration. This was repeated on day 2 of phage treatment. Sixty-seven plaque-forming units per milliter was observed from whole blood, and 165 PFU/mL was observed from plasma obtained 5 minutes after phage administration. Both whole blood and plasma samples obtained 10 minutes after phage administration had 4 PFU/mL when plated, and no plaques were observed from samples obtained 50 minutes after phage administration (Figure 1). Cerebrospinal fluid collection through a lumbar drain to determine central nervous system penetration of phage therapy was proposed to the patient’s family; however, they declined the procedure.
Figure 1.
Phage viral titers from sera measured in plaque-forming units (pfu)/mL, at time points of 5 minutes, 10 minutes, and 50 minutes after a single dose of intravenous phage was administered on days 1 and 2 of phage therapy.
The patient was in the intensive care unit at the time of phage administration and was frequently monitored for hemodynamic or neurologic changes. The patient tolerated the first dose well with no change in vital signs. One hundred and fifteen minutes after his first dose, he became briefly hypotensive but did not require vasopressors, and there were no further hemodynamic changes. Complete blood count, liver function tests, and comprehensive metabolic panel were measured daily and remained stable, except for a creatinine rise, which was attributed to colistin use. Antibiotics were discontinued on hospital day 13 due to decrease in glomerular filtration rate to 34.
RESULTS
After phage administration, the patient initially seemed to be more alert, but he continued to be unresponsive. His fevers and leukocytosis persisted, although the craniotomy site and skin flap healed well. There were no further signs of infection at the craniotomy site after surgical debridement, and no purulence to send for repeat culture. Respiratory, blood, and urine cultures were obtained before phage administration, and all were negative; therefore, we had little data to monitor for phage efficacy beyond the patient’s clinical status. Before the receipt of the second phage cocktail, the patient’s family decided to withdraw care including extubation. The patient died on hospital day 20. He received 8 days of phage therapy, which was discontinued on hospital day 19.
DISCUSSION
As morbidity and mortality from MDR organisms increase, the interest in bacteriophage therapy has reemerged, and its importance for further research has been elevated. Benefits of phage therapy include tolerability and lower rates of resistance [10]. In addition, phages are highly specific to their target microbes, in contrast to broad-spectrum antibiotics, which kill normal bacterial flora and disturb the healthy human microbiome [11]. Although our patient did not recover with bacteriophage therapy, other patients have benefited from bacteriophage therapy. We cannot conclude that the lack of response to bacteriophage therapy in our patient was due to bacteriophage failure because of the severity of his underlying assault injury, which could have contributed to his stagnant recovery. In addition, low to nil PFUs were observed from plated blood samples as described above, and we consider that a higher dose of phage may have been more effective. Continued investigation is required to better evaluate the efficacy of phages, and many questions remain unanswered regarding this treatment modality. We suggest that future studies continue to investigate dosage of phages as well as route of administration of phages. For isolated infections, such as in our patient, we question whether localized phage administration would have been beneficial. Administration of bacteriophage through the surgical drain and intrathecally in our patient likely would have had more benefit than parenteral administration. Given that individualized phage preparation is more time consuming than antibiotic administration, we question whether less individualized phages with broader activity would be as efficacious or whether personalized phage therapy could be developed more rapidly and administered earlier in the course of infection. More information is needed on the bacterial response to phages and what kind of mutations may occur when bacteria are exposed to phages.
CONCLUSIONS
We hope our experience with this case can assist clinicians who consider phage therapy for MDR bacterial infections. Clinical trials with phage therapy to determine efficacy and feasibility will become increasingly important, especially as the emergence of MDR bacteria continues to grow.
Acknowledgments
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Navy, Department of Defense, nor the US Government.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance Available at: cdc.gov/drugresistance/index.html. Accessed 11 October 2017.
- 2. Zaman SB, Hussain MA, Nye R, et al. . A review on antibiotic resistance: alarm bells are ringing. Cureus 2017; 9:e1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage 2011; 1:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sulakvelidze A, Alavidze Z, Morris JG Jr. Bacteriophage therapy. Antimicrob Agents Chemother 2001; 45:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubos RJ, Straus JH, Pierce C. The multiplication of bacteriophage in vivo and its protective effect against an experimental infection with shigella dysenteriae. J Exp Med 1943; 78:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhoads DD, Wolcott RD, Kuskowski MA, et al. . Bacteriophages therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 2009; 18:237–8; 240–83. [DOI] [PubMed] [Google Scholar]
- 7. Wright A, Hawkins CH, Anggård EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 2009; 34:349–57. [DOI] [PubMed] [Google Scholar]
- 8. Schooley RT, Biswas B, Gill JJ, et al. . Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 2017; 61:e00954–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sambrook J, Fritch EF, Maniatis T.. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 10. Ormälä AM, Jalasvuori M. Phage therapy: Should bacterial resistance to phages be a concern, even in the long run?Bacteriophage 2013; 3:e24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelfrene E, Willebrand E, Cavaleiro Sanches A, et al. . Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother 2016; 71:2071–4. [DOI] [PubMed] [Google Scholar]