This study estimated costs attributable to primary and recurrent Clostridium difficile infection (CDI). A total of 5.20 hospital days and $24205 are attributable to primary CDI; 1.95 days and $10580 are attributable to recurrent CDI.
Keywords: Clostridium difficile, healthcare resource utilization, recurrence, hospitalization, risk factors
Abstract
Background
The economic burden of Clostridium difficile infection (CDI), the leading cause of nosocomial infectious diarrhea, is not well understood. The objective of this study was to estimate the healthcare resource utilization (HCRU) and costs attributable to primary CDI and recurrent CDI (rCDI).
Methods
This is a database (MarketScan) study. Patients without CDI were matched 1:1 by propensity score to those with primary CDI but no recurrences to obtain HCRU and costs attributable to primary CDI. Patients with primary CDI but no recurrences were matched 1:1 by propensity score to those with primary CDI plus 1 recurrence in order to obtain HCRU and costs attributable to rCDI. Adjusted estimates for incremental cumulative hospitalized days and healthcare costs over a 6-month follow-up period were obtained by generalized linear models with a Poisson or gamma distribution and a log link. Bootstrapping was used to obtain 95% confidence intervals (CIs).
Results
A total of 55504 eligible CDI patients were identified. Approximately 25% of these CDI patients had rCDI. The cumulative hospitalized days attributable to primary CDI and rCDI over the 6-month follow-up period were 5.20 days (95% CI, 5.01–5.39) and 1.95 days (95% CI, 1.48–2.43), respectively. The healthcare costs attributable to primary CDI and rCDI over the 6-month follow-up period were $24205 (95% CI, $23436–$25013) and $10580 (95% CI, $8849–$12446), respectively.
Conclusions
The HCRU and costs attributable to primary CDI and rCDI are quite substantial. It is necessary to reduce the burden of CDI, especially rCDI.
Clostridium difficile is an anaerobic, gram-positive, spore-forming, toxin-producing bacillus. Clostridium difficile infection (CDI) is the leading cause of nosocomial infectious diarrhea in adults [1]. CDI caused 453000 new and 83000 recurrent infections and was associated with approximately 29000 deaths in the United States in 2011 [2]. The most important risk factor for CDI is disruption of the normal intestinal flora by exposure to prolonged use of antibiotics [3, 4]. Advanced age, immunosuppression, surgical procedures, increased severity of underlying illness, use of antiulcer medications, chemotherapeutic agents, and hospitalization (including stays at long-term care facilities) are risk factors for healthcare-associated CDI [3, 4].
The rate of healthcare-associated CDI has been increasing, and the diagnosis of CDI is estimated to raise the cost of a hospitalization stay by 54% in the United States [1, 5–7]. In addition, CDI has been associated with increasing morbidity and mortality, likely due to a combination of the changing virulence of C. difficile strains and the greater number of risk factors among vulnerable hospitalized patients [8–12].
A key issue with the management of CDI is recurrent infection. Recurrent CDI (rCDI) occurs due to relapse or reinfection [13]. Reports have shown that 18%–25% of patients will experience their first primary episode of CDI recurrence following the completion of treatment with vancomycin or metronidazole [14–16]. In patients with at least 1 recurrence, the risk for subsequent recurrences increases to 45%–65% [17]. A recent meta-analysis showed several risk factors for rCDI, including continuation of non– C. difficile antibiotics, advanced age, and use of antacid medications [18].
CDI places a substantial economic burden on the healthcare system. The cost of CDI was estimated at $5.4 billion in the United States, with $4.7 billion (86.7%) incurred in healthcare settings and $725 million (13.3%) incurred in the community [19]. The main driver of the economic burden is hospitalization and recurrence. rCDI is associated with excessive costs, mostly because of longer hospital stays and admittance in to intensive care units [20–22]. Despite this burden associated with rCDI, the cost attributable to rCDI in the United States is not well understood, and studies estimating these costs are limited. Dubberke et al [23] compared the average cost of all patients in an academic, urban, tertiary care hospital that had at least 1 recurrence to a matched cohort with no recurrence over a 6-month period. The estimated attributable cost of rCDI was $11631 (2010 dollars). Patients with rCDI were significantly more likely to have hospital costs compared with those without a recurrence. Other studies have assessed the cost of primary CDI and not the recurrence [24, 25].
Given the substantial burden of CDI and its association with increased hospital stay, there is a need to assess the cost and healthcare resource utilization (HCRU) that can be attributed to primary CDI (compared to having no CDI) and to rCDI (compared to having a primary CDI only). Therefore, the objectives of this study were (1) to describe patient characteristics with primary CDI and rCDI; (2) to assess the rate of rCDI after an episode of primary CDI; (3) to estimate cumulative hospitalized days and healthcare costs attributable to primary CDI, and; (4) to estimate cumulative hospitalized days and healthcare costs attributable to rCDI.
METHODS
Study Design and Data Source
This was a retrospective observational study. Two Truven Health MarketScan databases were used: (1) the Commercial Claims and Encounters database and (2) the Medicare Supplemental and Coordination of Benefits database. The commercial database represents approximately 100 employer-sponsored private health plans with coverage of an estimated 45 million members. The Medicare Supplemental database covers approximately 4.2 million retirees covered by their previous employers. Both databases record patient demographic data, health plan information, medical diagnosis/procedure codes, prescriptions, and cost data. Each member in the datasets has a unique identifier used to track patients over time across the sites of service and providers.
The Medicare Supplemental database contains the amount paid by Medicare in the field of Coordinate of Benefit. If Medicare is the primary payer and a service is 100% covered by Medicare, the Medicare Supplemental database will not capture the claim as there is no need for the service provider to send the claim to the employer-sponsored supplemental plan for further payment. Institutional review board approval was not obtained because this study was an analysis of de-identified secondary data. The study was conducted in accordance with Guidelines for Good Pharmacoepidemiology Practices [26].
Study Sample and Cohorts
Subjects were included in this study if they (1) had a CDI diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code = 008.45) from 1 July 2010 to 30 June 2014 (the earliest CDI diagnosis date was defined as the index date); (2) were continuously enrolled in a health plan for 12 months prior to and 6 months after the index date (subjects who died within 6 months after the index date were included in the analysis). Subjects were excluded from the study if they had a CDI diagnosis during the 84 days prior to the index date, had multiple primary CDI episodes during the follow-up period (ie, 6 months from the index date), or had potentially invalid payment data (eg, negative values or extremely large values).
CDI diagnoses overlapping within 14 days were collapsed and combined to build CDI episodes. This accounts for multiple diagnoses that may be due to refractoriness and longer treatment periods needed with intervening retesting. The CDI episode starting from the index date was defined as the primary episode. Those CDI episodes that occurred within 84 days of the previous episode were classified as recurrences. Details are provided in Supplementary Appendix 1.
To estimate the incremental hospitalized days and healthcare costs, 4 cohorts were further constructed for 2 comparisons. For the first comparison, patients without CDI were matched 1:1 by propensity score (non-CDI) to patients with primary CDI only (CDIPRIMARY1) to obtain hospitalized days and costs attributable to primary CDI. For the second comparison, patients with primary CDI only were matched 1:1 by propensity score (CDIPRIMARY2) to patients with 1 recurrence (CDIRECURRENT) to obtain hospitalized days and costs attributable to an rCDI. While all the patients with primary CDI were included in CDIPRIMARY1 for the first comparison, only the patients with primary CDI who matched with those having a recurrence were included in CDIPRIMARY2 for the second comparison. The index date of patients without CDI was randomly selected from the service dates of all the medical claims during 1 July 2010 to 30 June 2014. Propensity score was calculated based on covariates related to primary or rCDI.
STUDY VARIABLE MEASUREMENTS
Outcomes
The outcomes of this study are rCDI rate, cumulative hospitalized days, total healthcare costs, and healthcare costs within different settings (inpatient, outpatient, and emergency department) during the 6 months after the index date. The rCDI rate was measured as the proportion of patients with a primary CDI episode that had a recurrence. Cumulative hospitalized days were measured as total number of days admitted in the hospital during the 6-month follow-up period. The total healthcare costs were calculated as the amount paid by primary and secondary insurers and by patients (ie, copayment and deductibles) across all claims (medical and pharmacy) during the 6 months after the index date. Healthcare costs were inflation-normalized to 2014 US dollars using the Consumer Price Index All Urban Consumers for Medical Care Services in accordance with the International Society for Pharmacoeconomics and Outcomes Research recommendations [27].
Covariates
Covariates were used to describe the characteristics of CDI patients and to create propensity scores for matching the 4 comparison cohorts. Covariates included age, sex, geographic region of residence, health plan type, Charlson comorbidity index, specific comorbidities, immunocompromised status, use of certain medications, and previous 3-month HCRU. Charlson comorbidity index and specific comorbidities were identified by ICD-9-CM diagnosis codes during the 12 months prior to the index date (see Supplementary Appendix 2). Conditions used to imply immunocompromised status were identified by ICD-9-CM diagnosis/procedure codes during the 6 months prior to the index date (see Supplementary Appendix 3). Medications were identified by National Drug Codes during the 3 months prior to the index date.
Subgroup Analyses
In addition to overall CDI patients, subgroup analysis was conducted by age (<65 years vs ≥65 years), sex, immunocompromised status, and antibiotic use prior to the primary CDI episode.
Statistical Analysis
Descriptive statistics such as frequencies and percentages for categorical variables and mean (standard deviation [SD]) for continuous variables were used to describe the characteristics of the patients. Differences of characteristics between the 4 comparison cohorts (CDIPRIMARY1 vs non-CDI and CDIRECURRENT vs CDIPRIMARY2) were assessed for significance using standardized difference scores [28]. Adjusted estimates of cumulative hospitalized days and healthcare costs for the 4 comparison cohorts were obtained by generalized linear models with a Poisson (hospitalized days) or gamma (costs) distribution and a log link. This approach resolves the issue of skewed distribution that is common in claims data [29]. Furthermore, it has been demonstrated that a generalized linear model can provide more robust coefficient estimates than logged ordinary least squares regression, where the log transformation is often used to address skewed data [30]. Bootstrapping was used to obtain the 95% confidence interval (CI) of the estimates. A P value of .05 was the threshold for statistical significance. All statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, North Carolina).
RESULTS
After applying the inclusion and exclusion criteria, a total of 55504 CDI patients were identified for the study (Table 1). Full CDI patient demographics are reported in Table 2. The mean age of these patients was 61.3 years (SD, 21.1 years), and 62.0% were female. The most commonly reported health plan type was Preferred/Exclusive Provider Organization (45.9%). Approximately 31.8% and 29.9% of the patients resided in the North Central and South regions, respectively, of the United States. Approximately 64% of the CDI patients had used antibiotics prior to the primary episode. On average, the CDI patients had 0.6 (SD, 0.8) hospitalizations, 0.2 (SD, 0.5) emergency department visits, and 4.7 (SD, 4.9) doctor office visits during the 3 months prior to the primary episode.
Table 1.
Clostridium difficile Infection Cohort Construction
| Inclusion/Exclusion Criteria | No. of Subjects |
|---|---|
| Diagnosis of CDI from 1 July 2010 to 30 June 2014 (the earliest diagnosis was the index date) | 146942 |
| Continuous health plan enrollment for 12 mo before and 6 mo after the index date (subjects who died during the follow-up were included in the study) | 59837 |
| No diagnosis of CDI during 84 d before the index date | 59262 |
| No multiple primary CDI episodes during the follow-up period | 56646 |
| No invalid cost data (eg, negative values or extremely large values) | 55504 |
Abbreviation: CDI, Clostridium difficile infection.
Table 2.
Baseline Characteristics of Patients With Clostridium difficile Infection (N = 55504)
| Characteristic | No. of Subjects (%) |
|---|---|
| Age, y | |
| Mean (SD) | 61.3 (21.1) |
| <65 | 29667 (53.5) |
| ≥65 | 25837 (46.6) |
| Sex | |
| Female | 34389 (62.0) |
| Male | 21115 (38.0) |
| Health plan type [1] | |
| HMO | 7697 (13.9) |
| PPO/EPO | 25447 (45.9) |
| POS | 3386 (6.1) |
| HDHP/CDHP | 2862 (5.2) |
| Comprehensive | 14956 (27.0) |
| Unknown | 1156 (2.1) |
| Geographic region of residence | |
| Northeast | 10399 (18.7) |
| North Central | 17673 (31.8) |
| South | 16605 (29.9) |
| West | 10321 (18.6) |
| Unknown | 506 (0.9) |
| Medical conditions 12 mo prior to primary CDI | |
| Charlson comorbidity score, mean (SD) | 3.1 (3.4) |
| Diabetes | 14530 (26.2) |
| Cardiovascular disease | 11693 (21.1) |
| Renal dysfunction | 13066 (23.5) |
| Pulmonary disease | 37201 (67.0) |
| Inflammatory bowel disease | 3618 (6.5) |
| Immunocompromised | 12884 (23.2) |
| Medications 3 mo prior to primary CDI | |
| Antibiotics | 35432 (63.8) |
| Gastric acid suppression | 8332 (15.0) |
| Laxatives | 1953 (3.5) |
| NSAIDs | 22938 (41.3) |
| Healthcare resource utilization 3 mo prior to primary CDI | |
| Hospitalizations, mean (SD) | 0.6 (0.8) |
| Emergency department visits, mean (SD) | 0.2 (0.5) |
| Doctor office visits, mean (SD) | 4.7 (4.9) |
Abbreviations: CDHP, consumer-driven health plan; CDI, Clostridium difficile infection; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; NSAID, nonsteroidal anti-inflammatory drug; POS, point of service; PPO, preferred provider organization; SD, standard deviation.
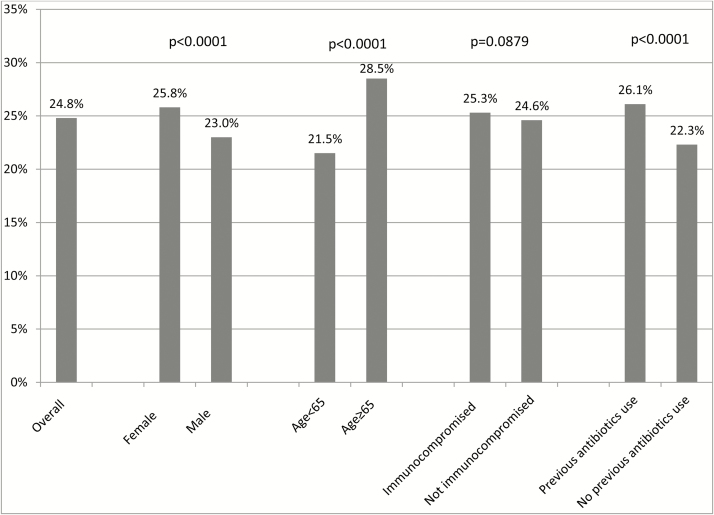
Figure 1 shows CDI recurrence rates overall and by subgroup. Among the 55504 CDI patients, 24.8% had a recurrence. The recurrence rate was significantly higher in patients who were female (25.8%), were ≥65 years old (28.5%), and used antibiotics (26.1%) prior to the primary episode than those patients who were male (23.0%), were <65 years old (21.5%), and did not use antibiotics (22.3%), respectively (all P < .0001).
Figure 1.
Clostridium difficile recurrence rates, overall and by subgroup.
Supplementary Table 1 presents the comparison of the baseline characteristics of the patients with CDI primary episode only vs the matched non-CDI patients and the CDI patients with recurrences vs the matched patients who only had CDI primary episode. The standardized differences of all the baseline characteristics across the 2 comparisons are <0.2 (small effect), suggesting that the propensity score matching algorithm was effective in minimizing these baseline differences.
Table 3 describes the cumulative hospitalized days attributable to the primary CDI, overall and by subgroups. The average hospitalized days across all primary CDI patients without recurrences is 8.01 days (95% CI, 7.83–8.17 days) vs 2.81 days (95% CI, 2.71–2.90 days) across the matched non-CDI patients. Therefore, the hospitalized days of 5.20 days (95% CI, 5.01–5.39 days) is attributable to a primary CDI episode.
Table 3.
Cumulative Hospitalized Days Attributable to Primary Clostridium difficile Infection, Overall and by Age, Sex, and Immunocompromising Status
| Characteristic | Cumulative Hospitalized Days in CDIPRIMARY1 Cohort (n = 41767) | Cumulative Hospitalized Days in Non-CDI Cohort (n = 41767) | Cumulative Hospitalized Days Attributable to Primary CDI |
|---|---|---|---|
| Overall | 8.01 (7.83–8.17) | 2.81 (2.71–2.90) | 5.20 (5.01–5.39) |
| Age <65 y | 7.99 (7.74–8.22) | 2.46 (2.34–2.59) | 5.53 (5.26–5.80) |
| Age ≥65 y | 8.05 (7.82–8.27) | 3.24 (3.10–3.38) | 4.81 (4.54–5.08) |
| Male | 9.59 (9.28–9.90) | 3.37 (3.21–3.54) | 6.22 (5.86–6.54) |
| Female | 7 (6.80–7.20) | 2.45 (2.34–2.56) | 4.55 (4.34–4.76) |
| Not immunocompromised | 7.07 (6.89–7.24) | 2.21 (2.12–2.31) | 4.86 (4.64–5.05) |
| Immunocompromised | 11.07 (10.69–11.49) | 4.74 (4.49–4.98) | 6.33 (5.87–6.84) |
Data are presented as mean (95% confidence interval).
Abbreviations: CDI, Clostridiumdifficile infection; CDIPRIMARY1, cohort with primary CDI only matched to those without CDI.
Table 4 describes the cumulative hospitalized days attributable to an rCDI, overall and by subgroups. The average cumulative hospitalized days across all rCDI patients is 9.27 days (95% CI, 8.87–9.66 days) vs 7.33 days (95% CI, 7.01–7.67 days) across the matched primary CDI patients in CDIPRIMARY2; therefore, the hospitalized days of 1.95 (95% CI, 1.48–2.43 days) is attributable to an rCDI episode.
Table 4.
Cumulative Hospitalized Days Attributable to Recurrent Clostridium difficile Infection, Overall and by Age, Sex, and Immunocompromising Status
| Characteristic | Cumulative Hospitalized Days in CDIRECURRENT Cohort (n = 8502) | Cumulative Hospitalized Days in CDIPRIMARY2 Cohort (n = 8502) | Cumulative Hospitalized Days Attributable to Recurrent CDI |
|---|---|---|---|
| Overall | 9.27 (8.87–9.66) | 7.33 (7.01–7.67) | 1.95 (1.48–2.43) |
| Age <65 y | 8.82 (8.32–9.33) | 7.32 (6.90–7.85) | 1.49 (.80–2.16) |
| Age ≥65 y | 9.72 (9.18–10.26) | 7.33 (6.88–7.79) | 2.39 (1.76–3.07) |
| Male | 11.57 (10.81–12.33) | 8.88 (8.24–9.57) | 2.68 (1.71–3.59) |
| Female | 7.98 (7.54–8.41) | 6.44 (6.10–6.84) | 1.53 (1.04–2.04) |
| Not immunocompromised | 8.18 (7.75–8.60) | 6.54 (6.16–6.92) | 1.64 (1.11–2.17) |
| Immunocompromised | 12.84 (11.93–13.70) | 9.89 (9.17–10.64) | 2.95 (1.84–3.98) |
Data are presented as mean (95% confidence interval).
Abbreviations: CDI, Clostridium difficile infection; CDIPRIMARY2, cohort with primary CDI only matched to those with recurrent CDI; CIDRECURRENT, cohort with recurrent CDI.
Table 5 describes the healthcare costs attributable to the primary CDI, overall and by subgroup. The average healthcare cost across all patients with primary CDI only is $43718 (95% CI, $43001–$44572) vs $19513 (95% CI, $19053–$20046) for those matched patients without CDI. Therefore, the healthcare cost of $24205 (95% CI, $23436–$25013) is attributable to primary CDI, compared to those without CDI.
Table 5.
Healthcare Costs Attributable to Primary Clostridium difficile Infection, Overall and by Age, Sex, and Immunocompromising Status
| Characteristic | Healthcare Costs in CDIPRIMARY1 Cohort, $ (n = 41767) | Healthcare Costs in Non-CDI Cohort, $ (n = 41767) | Healthcare Costs Attributable to Primary CDI, $ |
|---|---|---|---|
| Overall | 43718 (43001–44572) | 19513 (19053–20046) | 24205 (23436–25013) |
| Age <65 y | 44704 (43585–45861) | 18041 (17423–18652) | 26663 (25551–27846) |
| Age ≥65 y | 42497 (41513–43549) | 21337 (20646–22042) | 21160 (20016–22335) |
| Male | 53450 (51931–55105) | 22378 (21569–23351) | 31073 (29542–32700) |
| Female | 37463 (36668–38369) | 17672 (17161–18196) | 19791 (18944–20736) |
| Not immunocompromised | 33213 (32571–33900) | 12998 (12650–13334) | 20215 (19556–20913) |
| Immunocompromised | 77801 (75468–80618) | 40653 (39028–42320) | 37148 (34561–40070) |
Data are presented as mean (95% confidence interval).
Abbreviations: CDI, Clostridium difficile infection; CDIPRIMARY1, cohort with primary CDI only matched to those without CDI.
When looking at healthcare cost by settings (Supplementary Table 2), the average inpatient cost of the patients with primary CDI only is $28014 (95% CI, $26767–$29737) vs $6918 (95% CI, ($6521–$7287) for those matched non-CDI patients, suggesting that inpatient cost is the main driver of the total healthcare cost.
Table 6 describes the healthcare costs attributable to a rCDI, overall, and by subgroup. The average healthcare cost for patients with recurrence in the CDIRECURRENCE cohort is $49456 (95% CI, $47847–$50997) vs $38876 (95% CI, $37550–$40291) for those matched primary CDI patients in the CDIPRIMARY2 cohort. Therefore, the total healthcare cost attributable to a rCDI, compared to those with primary CDI only, is $10580 (95% CI, $8849–$12446).
Table 6.
Healthcare Costs Attributable to Recurrent Clostridium difficile Infection, Overall and by Age, Sex, and Immunocompromising Status
| Characteristic | Healthcare Costs in CDIRECURRENT Cohort, $ (n = 8502) | Healthcare Costs in CDI PRIMARY2 Cohort, $ (n = 8502) | Healthcare Costs Attributable to Recurrent CDI, $ |
|---|---|---|---|
| Overall | 49456 (47847–50997) | 38876 (37550–40291) | 10580 (8849–12446) |
| Age <65 y | 48342 (46013–50858) | 39597 (37595–41831) | 8745 (6039–11354) |
| Age ≥65 y | 50532 (48562–52724) | 38180 (36420–40046) | 12352 (9911–14736) |
| Male | 58552 (55540–61732) | 45528 (43160–48078) | 13024 (9624–16479) |
| Female | 44300 (42528–46019) | 35105 (33603–36772) | 9194 (7110–11166) |
| Not immunocompromised | 39316 (37915–40681) | 30484 (29334–31722) | 8832 (7218–10414) |
| Immunocompromised | 82410 (77362–87666) | 66151 (62112–70403) | 16259 (10726–22207) |
Data are presented as mean (95% confidence interval).
Abbreviations: CDI, Clostridium difficile infection; CDIPRIMARY2, cohort with primary CDI only matched to those with recurrent CDI; CIDRECURRENT, cohort with recurrent CDI.
When looking at healthcare costs by settings (Supplementary Table 3), similar to the comparison between primary CDI patients and non-CDI patients, the inpatient cost of patients with primary CDI plus 1 recurrence is $32190 (95% CI, $29983–$36110) compared with $22456 (95% CI, $21008–$25072) for those matched patients with primary CDI only.
DISCUSSION
This study is consistent with previous literature that has demonstrated a significant and substantial increase in HCRU for CDI over and above similar patients without CDI. It has also shown that having rCDI is associated with substantial healthcare resource use as compared to similar CDI patients who do not have a recurrence. The subgroup analyses comparing the incremental HCRU for those patients with rCDI compared to those with primary CDI only are consistent with the concept that more vulnerable patients, such as immunocompromised and older patients, incur even greater need for healthcare resources.
Estimating the cost of rCDI is complex because of potential confounding factors that are independently associated with both recurrence of CDI and more healthcare utilization regardless of recurrence. To estimate the true burden, it is necessary to account for this confounding. Multivariable analysis is one method, but this is generally not as robust as a carefully done propensity score matching that also allows for more statistical robustness by the matching approach [31]. Most of the literature on CDI-associated costs assesses only the cost of primary CDI. To our knowledge, there is only one other study using an appropriate approach in the United States. Dubberke and colleagues [23] used a matched cohort approach to determine the cost of rCDI. Their overall estimate of the inpatient 6-month cost of rCDI was $11631 (2010 US dollars). Most of the incremental cost over and above primary CDI only in our study is almost solely due to inpatient expenses. Moreover, our study also enables us to estimate the cost associated with primary episode compared with those without any CDI. One difference in the Dubberke et al study is that our recurrent population contains those having a single recurrence in the 84-day period. As a result, our study estimated the cost attributable to a single recurrence, while Dubberke et al’s paper includes costs of all subsequent recurrences.
The other uniqueness of this study was the ability to describe HCRU by high-risk subgroup and shows the particular importance to prevent this disease, primary or recurrent, in these groups. The subgroups of immunocompromised patients and those ≥65 years old reveal even greater incremental costs of an rCDI with payments of $16259 and $12352, compared with $8832 and $8745 for immunocompetent patients and those <65 years old, respectively. This is consistent with there being more of an adverse effect of CDI or rCDI in these more vulnerable patients. The anomaly of those <65 years old incurring more costs than those ≥65 years old for primary CDI could be because of partial reporting of costs in this database for claims that were fully adjusted by Medicare. Comparing rCDI patients to those with primary only CDI by these age groups, the situation is reversed, consistent with the other high-risk group of immunocompromised patients.
A significant strength of this study is that our data were drawn from a much broader sample of the US population, which increases its external validity. Recurrence rates by risk group were consistent with that in the published literature. The baseline comparisons of the patients with CDI primary episode only vs the matched non-CDI patients and the CDI patients with recurrences vs the matched patients with CDI primary episode only are quite similar in their characteristics, which demonstrates the effectiveness of the matching algorithm. The ability to examine the different sources of costs (eg, inpatient, outpatient, and emergency department), as well as the high-risk groups that extend the view and the consistency of these relationships, all amplify the robustness of these findings.
Several limitations inherent to administrative claims data apply to this study. First, due to unknown and/or unmeasured factors, residual confounding might still exist despite the use of the propensity score method. The propensity score method, when successfully implemented, can adjust for observed imbalances between treatment arms. However, it cannot correct imbalances that may exist between treatment arms with regard to potentially important unobserved characteristics. Second, CDI and comorbidity status were based only on claims database information (without any medical record review). The database captures only an outpatient prescription fill and does not record what was actually taken by the patients. These could likely result in some misclassification. Third, as the Medicare Supplemental database is sourced from the employer-sponsored supplemental plans, if a service is 100% covered by Medicare and there is no need for the service provider to send the claim to the employer-sponsored supplemental plan for further payment, the Medicare Supplemental database will not capture the claim. Therefore, this study may underestimate the total number of days staying in hospital and total healthcare costs for patients aged ≥65 years. Finally, as MarketScan includes only commercially insured patients, results may not be generalizable to the overall population in the United States.
In conclusion, the HCRU and economic burden associated with primary and rCDI are quite substantial. Better prevention and treatment of CDI, especially rCDI, are needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. D. Z., V. S. P., and S. W. M. designed the research and wrote the manuscript. D. Z. performed the research and analyzed the data.
Acknowledgments. The authors thank Yu Feng, MS (Merck & Co, Inc, Kenilworth, New Jersey) for extracting and cleaning the database using SAS, and Carol Zecca (Merck & Co, Inc, Kenilworth, New Jersey) for editorial/submission assistance.
Financial support. This work was supported by Merck & Co, Inc.
Potential conflicts of interest. All authors are employees of Merck & Co, Inc, Kenilworth, New Jersey, and may own stock/hold stock options in the company. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–40. [DOI] [PubMed] [Google Scholar]
- 2. Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15. [DOI] [PubMed] [Google Scholar]
- 4. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 2009; 58:403–10. [DOI] [PubMed] [Google Scholar]
- 5. Dubberke ER, Butler AM, Yokoe DS et al. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol 2010; 31:1030–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gravel D, Miller M, Simor A et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis 2009; 48:568–76. [DOI] [PubMed] [Google Scholar]
- 7. Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–53. [DOI] [PubMed] [Google Scholar]
- 8. Loo VG, Poirier L, Miller MA et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 9. Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg 2007; 142:624–31. [DOI] [PubMed] [Google Scholar]
- 10. Kotila SM, Virolainen A, Snellman M et al. Incidence, case fatality and genotypes causing Clostridium difficile infections, Finland, 2008. Clin Microbiol Infect 2011; 17:888–93. [DOI] [PubMed] [Google Scholar]
- 11. Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile–related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis 2008; 14:929–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile–related mortality rates, United States, 1999–2004. Emerg Infect Dis 2007; 13:1417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 2009; 58:403–10. [DOI] [PubMed] [Google Scholar]
- 14. Louie TJ, Miller MA, Mullane KM et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 15. Lowy I, Molrine DC, Leav BA et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362:197–205. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Pardo D, Almirante B, Bartolome RM. et al. Epidemiology of Clostridium difficile infection and risk factors for unfavorable clinical outcomes: results of a hospital-based study in Barcelona, Spain. J Clin Microbiol 2013; 51:1465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 18. Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect 2008; 70:298–304. [DOI] [PubMed] [Google Scholar]
- 19. Desai K, Gupta SB, Dubberke ER et al. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis 2016; 16:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heimann SM, Vehreschild JJ, Cornely OA et al. Economic burden of Clostridium difficile associated diarrhoea: a cost-of-illness study from a German tertiary care hospital. Infection 2015; 43:707–14. [DOI] [PubMed] [Google Scholar]
- 21. Vincent C, Miller MA, Edens TJ et al. Bloom and bust: intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 2016; 4:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dubberke ER, Carling P, Carrico R et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35:628–45. [DOI] [PubMed] [Google Scholar]
- 23. Dubberke ER, Schaefer E, Reske KA et al. Attributable inpatient costs of recurrent Clostridium difficile infections. Infect Control Hosp Epidemiol 2014; 35:1400–7. [DOI] [PubMed] [Google Scholar]
- 24. O’Brien JA, Lahue BJ, Caro JJ et al. The emerging infectious challenge of Clostridium difficile–associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007; 28:1219–27. [DOI] [PubMed] [Google Scholar]
- 25. Campbell R, Dean B, Nathanson B et al. Length of stay and hospital costs among high-risk patients with hospital-origin Clostridium difficile-associated diarrhea. J Med Econ 2013; 16:440–8. [DOI] [PubMed] [Google Scholar]
- 26. Public Policy Committee, International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf 2016; 25:2–10. [DOI] [PubMed] [Google Scholar]
- 27. Mullins CD, Seal B, Seoane-Vazquez E et al. Good research practices for measuring drug costs in cost-effectiveness analyses: Medicare, Medicaid and other US government payers perspectives: the ISPOR Drug Cost Task Force report–—Part IV. Value Health 2010; 13:18–24. [DOI] [PubMed] [Google Scholar]
- 28. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS Available at: http://support.sas.com/resources/papers/proceedings12/335–2012.pdf. Accessed 7 July 2017.
- 29. Wedderburn RWM. Quasi-likelihood functions, generalized linear models, and the Gauss-Newton method. Biometrika 1974; 61:439–47. [Google Scholar]
- 30. Manning WG, Mullahy J. Estimating log models: to transform or not to transform?J Health Econ 2001; 20:461–94. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.