Abstract
Cardiac calsequestrin (Casq2) associates with the ryanodine receptor 2 channel in the junctional sarcoplasmic reticulum to regulate Ca2+ release into the cytoplasm. Patients carrying mutations in CASQ2 display low resting heart rates under basal conditions and stress-induced polymorphic ventricular tachycardia (CPVT). In this study, we generate and characterize novel conditional deletion and conditional rescue mouse models to test the influence of developmental programs on the heart rate and CPVT phenotypes. We also compare the requirements for Casq2 function in the cardiac conduction system (CCS) and in working cardiomyocytes. Our study shows that the CPVT phenotype is dependent upon concurrent loss of Casq2 function in both the CCS and in working cardiomyocytes. Accordingly, restoration of Casq2 in only the CCS prevents CPVT. In addition, occurrence of CPVT is independent of the developmental history of Casq2-deficiency. In contrast, resting heart rate depends upon Casq2 gene activity only in the CCS and upon developmental history. Finally, our data support a model where low basal heart rate is a significant risk factor for CPVT.
Introduction
Cardiac calsequestrin (Casq2) is the most abundant Ca2+-binding protein in the junctional sarcoplasmic reticulum (jSR) and plays a critical role in maintaining cardiac Ca2+ homeostasis necessary for excitation–contraction coupling in working cardiomyocytes (1,2). Each Casq2 peptide sequesters Ca2+ in the jSR with low affinity (Kd = 1 mm) and high capacity, thus allowing 20 mm of stored Ca2+ with 1 mm in solution (3,4). Additionally, Casq2 plays an active role in regulating Ca2+ levels in the cytoplasm. As Ca2+ concentrations increase, Casq2 polymerizes and forms a quaternary complex with membrane bound co-regulatory proteins, junctin and triadin 1. This complex binds to the Ryanodine Receptor 2 (RyR2) Ca2+ channel protein and reduces RyR2 channel opening probability to limit Ca2+ leak into the cytoplasm (5–7). Accordingly, loss of function mutations in the CASQ2 gene lead to RyR2-dependent spontaneous Ca2+ leak and delayed after depolarizations that result in premature ventricular contraction (PVC).
In humans, loss of function mutations in CASQ2 are associated with catecholaminergic polymorphic ventricular tachycardia (CPVT2), a rare familial arrhythmogenic disorder within a group of diseases characterized as Sudden Arrhythmic Death (8–12). These patients display sinus bradycardia but otherwise have normal electrocardiogram (ECG) tracings, making this disease particularly vexing to diagnose and manage. However, physical or emotional stress serves as a trigger for electrical abnormalities, including PVCs or even ventricular tachycardia and death (2,13,14).
Casq2−/− mice recapitulate the human CASQ2-deficiency phenotype with sinus bradycardia under non-stress conditions and stress-induced bidirectional ventricular tachycardia (5,7). In addition to the absence of the Casq2 peptide, mice lacking Casq2 gene function display several secondary changes. At the cellular level, loss of Casq2 gene function results in changes in protein expression, including reduced levels of triadin 1 and junctin. Ultrastructural changes are also present which include increased SR surface area and myofibrillar volume (3,5,7). At the tissue level, abnormal calcium release secondary to loss of calsequestrin may contribute to increased sino-atrial node interstitial fibrosis, resulting in loss of sinus rhythm (15). The cumulative effects of these changes and their relevance to CPVT pathogenesis are not fully understood. In particular, it is not yet clear which of these secondary changes (if any) work to alleviate which act to exacerbate the CPVT phenotype. Furthermore, it is not yet understood if the animal’s ability to respond with these secondary changes (or with other secondary changes that have not yet been characterized) is limited to the developing heart or can occur in response to loss of Casq2 gene function at any developmental stage. These issues are of medical significance because any secondary changes that are significant to the CPVT phenotype are themselves potential targets for therapeutic intervention.
The impact of inherited CASQ2 mutations on normal heart development is especially relevant as recent advances in gene editing techniques may soon allow physicians to target and restore mutant genes. Specific mutations in CASQ2 that lead to life-threatening stress-induced arrhythmias have been well characterized, making rescue of CASQ2 an attractive target for gene therapy (14). Before the possibility of clinical intervention, the effects on cardiac development caused by the absence of Casq2 must be determined, considering that if there is a critical period during development where the presence of Casq2 is uniquely important in establishing normal heart function, then its restoration via gene therapy in the post-development period may be ineffective or lead to unexpected long-term consequences.
Finally, the success of genomic therapies relies on their ability to target the specific cell type and minimum number of cells responsible for maintaining the arrhythmia phenotype in CASQ2-deficient hearts. To that end, it becomes important to characterize the cell subtypes critical for ablation of the disease and the extent of rescue penetrance necessary. Significantly, the cardiac conduction system (CCS) has been implicated in CPVT as the initiating site for arrhythmogenesis (16,17). In addition, low sinus rates are a hallmark of the condition and artificially raising resting heart rate through chronotropic drugs or atrial overdrive pacing has been shown to be cardioprotective in Casq2−/− mouse models (18). Together, these results have suggested that the Casq2 function in the CCS might be of unique importance.
To understand the interplay between Casq2 mutations, bradycardia, and cardiac development and to assess the impact of CCS dysfunction in CPVT, we generated two novel Casq2 alleles: a conditional Casq2 deletion and a conditional Casq2 rescue allele. When paired with tissue-specific and temporally controlled Cre recombinases, these new models allowed us to examine and compare the effects of knocking-out or rescuing Casq2 in young adult mice in either the whole heart or in the cardiac conduction system (CCS).
Phenotypic analyses of these mice show that the CPVT phenotype is independent of developmental history. That is, the presence of arrhythmia depends on the status of Casq2 at the time of analysis with minimal influence by the heart’s developmental history in regards to Casq2 gene function. Moreover, our data indicate that CPVT phenotype is dependent upon concurrent loss of Casq2 peptide in both the CCS and in working cardiomyocytes. The practical significance of this finding is that therapies that rescue Casq2 only in the CCS may be sufficient to prevent CPVT. In contrast to the CPVT phenotype, heart rate phenotypes are dependent only on the loss of Casq2 in the CCS. More interestingly, heart rates are dependent upon CCS developmental history. That is, heart rates are determined by two factors: (1) the status of Casq2 at the time of analysis and (2) CCS developmental history in regards to Casq2 gene function. Altogether, our data indicate that the relationship between heart rate and CPVT is complex but support the idea that sino-atrial node dysfunction, perhaps in the form of reduced basal heart rate, is a central contributor to increased risk of stress-induced arrhythmias in Casq2-deficient hearts.
Results
Generation and characterization of conditional null and conditional rescue Casq2 mice
To evaluate the role of developmental programs in modulating the Casq2-deficiency phenotypes, we generated and characterized two new Casq2 alleles. Casq2Flox is a wild-type allele that is inactivated by Cre-mediated recombination, while Casq2RevFlox is a null allele that is rescued by Cre recombination (Fig. 1). To test the efficacy of each allele, we generated homozygous adult mice that also carried the Myh6-MERCreMER transgene. These mice express Cre recombinase protein in all cardiomyocytes but the recombinase is only localized to the cell nucleus upon tamoxifen treatment (19).
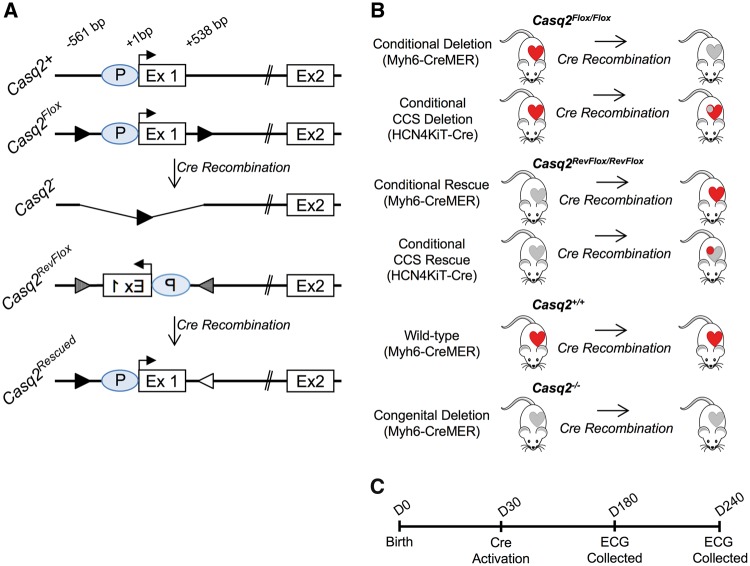
Figure 1.
Novel Casq2 alleles and experimental plan. (A) Depictions of the 5′ ends of wild-type and mutant Casq2 alleles display the Casq2 promoter (P), transcriptional start site (horizontal arrow) and exons 1 and 2. Casq2Flox carries loxP insertions (black arrowheads) at −561 and +538 bp (relative to the transcriptional start). Casq2Flox is functionally wild type but Cre recombination generates the Casq2− allele, which is functionally null (5). Casq2RevFlox inverts the Casq2 promoter and exon 1 relative to rest of the gene and flanks this inverted region with loxP66 (arrowhead with vertical stripes) and loxP71 (arrowhead with horizontal stripes) sequences. Casq2RevFlox is functionally null but Cre recombination restores gene function. (B) Combination of Casq2Flox/Casq2Flox and Casq2RevFlox/Casq2RevFlox mice with the Myh6-MERCreMER transgene (Myh6-CreMER) or with the Hcn4KiT-Cre knock-in allele will generate mice that display tamoxifen-dependent loss of Casq2 or tamoxifen-dependent restoration of Casq2 in either the whole heart or specifically in the CCS. Control mice, both wild type (+/+) and congenital deletion (−/−), carry the Myh6-CreMER transgene and are treated with tamoxifen to control for side effects. In this diagram, CCS is indicated by the circle inside the heart. Red fill denotes functional Casq2 while gray fill denotes non-functional Casq2. (C) Timeline describing the experimental procedures used for each mouse.
Inactivation of the Casq2Flox allele is rapid as demonstrated by the loss of 99% of Casq2 RNA within 5 days of tamoxifen administration (Supplementary Material, Fig. S1A). However, Casq2 protein persists for up to 14 weeks (Fig. 2A). Western blot analyses from several independent experiments indicate that Casq2 half-life is about 3–4 weeks.
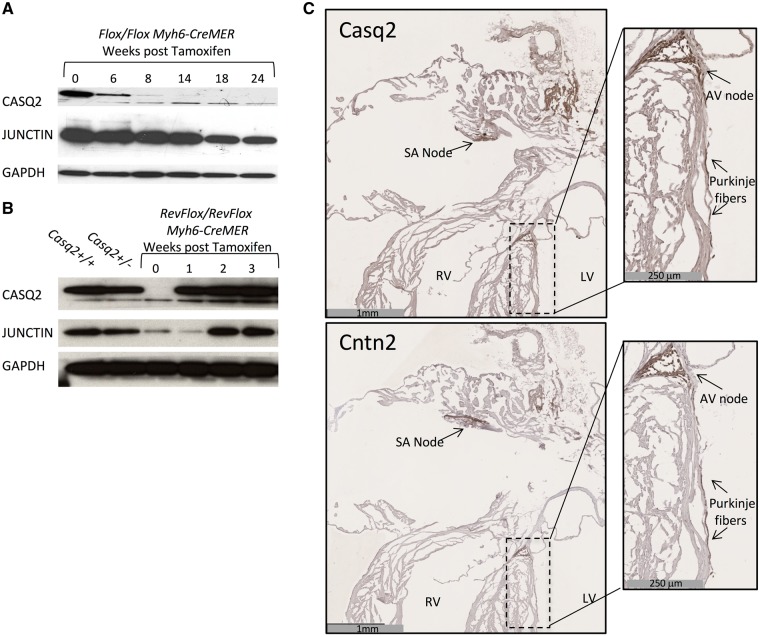
Figure 2.
Characterization of conditional deletion and rescue alleles. (A and B) Extracts prepared from adult whole hearts were analyzed by western blot for Casq2 and Junctin to detect the effects of tamoxifen induction in Casq2Flox/Casq2Flox Myh6-CreMER (conditional deletion) and Casq2RevFlox/Casq2RevFlox Myh6-CreMER (conditional rescue) models. GAPDH was analyzed as a loading control. (C) IHC staining tested CCS specificity in the Casq2RevFlox/Casq2RevFlox Hcn4KiT-Cre (conditional CCS rescue) model. Adjacent sections were either stained for Casq2 or for CCS marker Contactin2 (Cntn2). Note that Casq2 protein is detected only in Cntn2 staining regions such as the SA node. The right panels are insets shown at higher magnification to demonstrate Casq2 rescue in the AV node and Purkinje fibers. See Supplementary Material, Figure S3 for additional analyses and demonstration of antibody specificity. RV, right ventricle; LV, left ventricle.
Cre-mediated activation of the Casq2RevFlox allele is also rapid: Casq2 RNA is fully restored within 3 days of tamoxifen treatment (Supplementary Material, Fig. S1B). Restoration of Casq2 protein to wild-type levels is achieved within 2 weeks (Fig. 2B).
Note that these experiments also show that junctin peptide levels follow those of Casq2 (Fig. 2A and B). That is, with a short delay, junctin is restored when Casq2 is restored and depleted when Casq2 is depleted. Similarly, triadin 1 is restored in response to reactivation of Casq2 gene expression (data not shown). Earlier studies had already shown that junctin and triadin 1 dependence upon Casq2 occurs post-transcriptionally (5). Altogether, these results are consistent with the idea that junctin and triadin 1 peptides are unstable unless associated in a multimeric complex with Casq2 and RyR2.
Having demonstrated that the Casq2 alleles function as anticipated, we next tested the specificity of the HCN4KiT-Cre system by inducing Cre recombination in Casq2RevFlox/Casq2RevFlox Hcn4KiT-Cre/Hcn4+ mice (20). Consistent with restriction of Cre recombinase activity to the CCS, Casq2 RNA levels were only restored to 2% of those seen in wild-type mice (Supplementary Material, Fig. S1B). To directly demonstrate that Hcn4KiT-Cre-induced expression was specific to the CCS, we performed immunohistochemistry to detect Casq2 and Contactin2 (Cntn2), a marker for specialized conduction cells (21). We saw Casq2 peptide only in regions where Contactin2 is also expressed including the nodes and the Purkinje cells (Fig. 2C, Supplementary Material, Fig. S2). Thus the Hcn4KiT-Cre knock-in is an effective tool to modulate Casq2 gene function in the CCS.
Overview of experimental plan and impact of Myh6-MERCreMER toxicity
Our experimental strategy is summarized in Figure 1B and C and consists of the following steps: (1) generate the six genotypes, (2) treat each mouse with tamoxifen at 1 month to induce Cre recombination, (3) allow time for appropriate changes in protein levels and (4) obtain surface lead ECGs from anesthetized mice at age 6 and 8 months. Based on previous work with congenital deletion mice, we estimated that 10 mice would give us statistical power to distinguish between wild type and mutant phenotype. The number of mice used in the study and the male/female composition for each cohort are described in Supplementary Material, Table S1. Experimental work and data analyses were done blinded to genotype.
It is now well established that activation of Myh6-Cre transgene is associated with cardiotoxicity (22,23). Our experimental design addressed this problem in three ways. First, we used tamoxifen-inducible Cre recombinases so that exposure of the heart to high levels of recombinase and cardiotoxicity is restricted temporally to the 5-day tamoxifen administration period. Second, we identified the minimal tamoxifen dosage that induced recombination. Third, control mice (Casq2+/+ and Casq2−/− genotypes) also carried the Myh6-MERCreMER transgene and were dosed with tamoxifen.
Despite these precautions, unexpected problems with Cre toxicity were evident by increased mortality during tamoxifen treatments (Supplementary Material, Table S2). Mortality data were analyzed by logistic regression. Our analyses indicate that mortality risk is dependent on the presence of the Myh6-MERCreMER transgene (P = 0.004) but is also associated with Casq2 genotype and with sex. Specifically, Myh6-MERCreMER transgenic mice that lack Casq2 protein at the time of tamoxifen administration (Casq2−/− and Casq2RevFlox/Casq2RevFlox mice) show an increased association with mortality (P < 0.01) compared with Casq2+/+ mice. Casq2Flox/Casq2Flox mice, which have Casq2 peptide at the time of tamoxifen administration, show no statistically increased mortality risk relative to wild-type mice. Interestingly, being female is also a risk factor for Cre-dependent mortality in a Casq2-deficiency context (P = 0.002).
The implications of the Casq2-dependent Cre toxicity are 2-fold. First, these results identify a new phenotype for Casq2-deficient mice: at 1 month age, their hearts are unusually sensitive to cardiotoxicity. Second, the biased mortality likely introduced some sample bias. The implications of this bias are addressed in the Discussion section.
Post-developmental deletion of Casq2 results in CPVT
To measure the incidence and severity of ventricular arrhhythmia across our models, we collected 20 min ECGs for each mouse at 5 and at 7 months post-tamoxifen: observing 5 min of resting heart rate and then 15 min of catecholamine challenged heart activity. Heart rates are the average of the two independent readings. Individual mice were considered to have VT if they exhibited four or more consecutive PVCs at either reading. Similarly, a mouse was scored as having sustained VT if it exhibited VT for at least 15 consecutive seconds during either reading. ECG strips from a wild type (Casq2+/+ Myh6-MERCreMER) and a mutant control (Casq2−/− Myh6-MERCreMER) mouse are shown in Figure 3A and B, respectively. Data for each sample set are summarized in Table 1 while the results for individual mutant mice are described in Supplementary Material, Table S3. Wild-type mice (n = 16) never displayed VT. In contrast, 12 of 25 deletion mice (Casq2−/−) displayed VT, which is reminiscent of bi-directional VT seen in patients with CPVT2. Thus, as already reported, congenital deletion of Casq2 results in VT (chi square of proportion, P = 0.001).
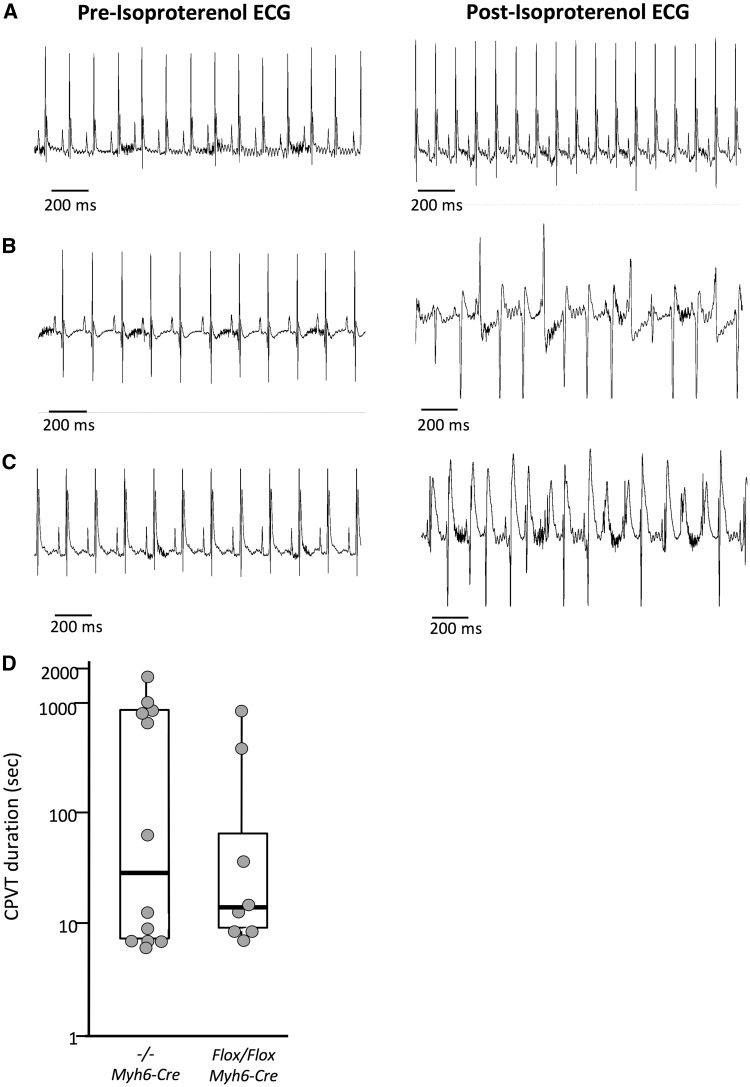
Figure 3.
Conditional Casq2 deletion leads to CPVT. Representative ECGs of from wild-type (A), congenital deletion (B) and conditional deletion (C) mice before and after administration of isoproterenol. Left column: normal ECG morphology and sinus rhythm preceding isoproterenol injection. Right column: morphologic abnormalities and loss of sinus rhythm in congenital and conditional deletion mice are consistent with polymorphic ventricular tachycardia (CPVT). (D) VT duration in congenital (n = 25) and conditional deletion (n = 24) mice are depicted by box plot. See Table 1 for methods.
Table 1.
Cardiac arrhythmias in wild-type and mutant mice
| Mouse model | N | % VT | % Sustained VT | VT Duration (s) | VT mean ± S.E.M. (s) | % Bigemini | Heart rate |
|---|---|---|---|---|---|---|---|
| Wild type | 16 | 0 | 0 | – | – | 0 | 430±10 |
| Congenital deletion | 25 | 48 | 24 | 7–1814 | 456±174 | 44 | 381±6 |
| Conditional deletion | 24 | 33 | 17 | 8–890 | 172±113 | 12 | 405±25 |
| Conditional CCS deletion | 17 | 0 | 0 | – | – | 0 | 407±14 |
| Conditional rescue | 15 | 0 | 0 | – | – | 0 | 468±11 |
| Conditional CCS rescue | 15 | 0 | 0 | – | – | 0 | 488±10 |
Data were collected from anesthetized mice at 6 and at 8 months (Fig. 1C). For calculating %VT, % Sustained VT and % bigemini, a single event in either reading was sufficient. VT durations for each mouse were calculated by summing the durations from each reading. The range of VT durations are reported in this table and described in detail in Fig. 3D. Heart rate (mean ± S.E.M.) for each mouse is the average of basal (pre-stress) heart rates from both readings.
Analysis of the conditional whole heart deletion mice (Casq2Flox/Flox Myh6-MERCreMER) revealed that adult ablation of Casq2 results in a robust VT phenotype (Fig. 3C). VT was observed in 33% of the conditional whole heart deletion mice, compared to 48% of the congenital deletion mice (Table 1, Supplementary Material, Table S4). These VT frequencies are not significantly different from each other (P = 0.29) but differ significantly from wild type (P = 0.001 and P = 0.01, respectively). Congenital deletion mice and conditional whole heart deletion mice also exhibited similar incidences of sustained VT (24% vs. 17%, P = 0.55) (Table 1). With respect to VT episode duration, there was no clear distinction between congenital and induced loss of function mice. Across the 30 min of ECGs recorded after stress induction, VT durations ranged from 7 to 1814 s in −/− controls (mean = 456 ± 174 s, median = 40 s). In whole heart deletion animals, VT durations ranged from 8 to 890 s (mean = 172 ± 113 s, median = 14 s) (Fig. 3D). The only statistically significant difference between congenital and whole heart deletion animals was bigeminy frequency, which was more frequent in congenital deletion mice (44% vs. 12%, P = 0.02).
In sum, whole heart deletion of Casq2 (either congenital or adult-induced) results in VT within 40 s of isoproterenol injection. VT episodes are characterized by rapid ventricular rate with loss of or dissociated P waves along with QRS axis and/or morphology change. The penetrance of the phenotype upon adult ablation is similar to the VT phenotype in congenital mutants.
CCS-specific Casq2 deletion alters CCS function but does not result in VT
In contrast with adult whole heart deletion, CCS-specific deletion mice (Casq2Flox/Casq2Flox Hcn4KiT-Cre/Hcn4+) mice do not exhibit VT (0% frequency) (Table 1). These data indicate that Purkinje fiber and nodal dysfunction alone is insufficient to establish CPVT.
Although CCS-specific deletion does not result in VT, ECG tracings from these animals do show subtler defects. Eight of 17 mice show sinus and/or AV-nodal dysfunction, including evidence of accelerated junctional rhythm (Supplementary Material, Fig. S3 and Table S5). (Incidence in wild-type mice = 0/16; P = 0.002 using chi square of proportions.) Two additional mice display long clusters of PVCs that never degenerate into VT or CPVT. In contrast to the VT phenotype described above, neither of these phenotypes are associated with the initiation of catecholamine stress but instead are equally likely to occur at any time during the 20 min ECG recording, including one instance where accelerated junction occurred even before dosage with isoproterenol.
Conditional rescue of Casq2 in the whole heart or in the CCS eliminates CPVT
Whole heart rescue of Casq2 at 1 month (Casq2RevFlox/Casq2RevFlox Myh6-MERCreMER mice) protected against all incidences of VT, with 0/15 mice exhibiting abnormal cardiac events after catecholamine challenge (P = 0.002 relative to VT frequency of mutant control mice, Casq2−/− Myh6-MERCreMER) (Table 1). Notably, the CCS-specific restoration of Casq2 at 1 month (Casq2RevFlox/Casq2RevFlox Hcn4-Cre/Hcn4+ cohort) equivalently protects against VT. These observations demonstrate that genomic rescue of Casq2 in either the whole heart or in only the CCS is sufficient to prevent VT phenotype. Thus, development of the heart in the absence of Casq2 does not lead to secondary effects that permanently alter the heart in a way that prevents normal electrical function once cardiac calsequestrin is restored.
Collectively, the CCS rescue and deletion models show that conditional deletion in only the CCS is insufficient to cause CPVT while restoration is enough to prevent it. That is, we observe VT only in mice that are lacking Casq2 function in both the working cardiomyocytes and in the CCS at the time of ECG analysis.
Heart rate depends upon ongoing Casq2 gene activity plus developmental history
To investigate the link between Casq2 activity and bradycardia as well as between bradycardia and VT, we analyzed resting heart rates in our experimental mice. As already reported, Casq2−/− mice show a significantly reduced heart rate relative to wild-type (+/+) controls (381 ± 6 vs. 430 ± 10 bpm, P <0.01) (Table 1 and Fig. 4A). The heart rates of the CCS-specific deletion mice (407 ± 14 bpm) and whole heart deletion mice (405 ± 25 bpm) are indistinguishable from each other but are intermediate between wild type and congenital deletion. The heart rates in these mice are not statistically different than those of wild-type mice. That is, counter to expectation, late ablation of Casq2 does not result in an atrial bradycardia that is equivalent to that observed upon congenital deletion.
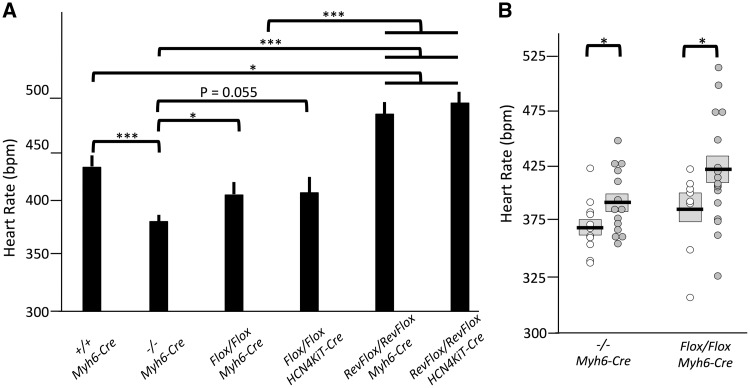
Figure 4.
Analyses of basal heart rates in wild-type and mutant mice. Heart rates were analyzed at 6 and 8 months as described in Table 1. (A) Heart rates are dependent upon Casq2 gene activity in the CCS and upon developmental history. Depicted are basal sinus heart rates (mean ± S.E.M.); N values are described in Table 1. (B) Lower heart rate is associated with subsequent VT episodes. Scatterplots depict basal sinus heart rates of congenital and conditional deletion mice. Open circles, basal sinus heart rates in mice that showed VT episodes after isoproterenol; filled circles, basal sinus heart rates in mice that did not show VT after isoproterenol. Mean and S.E.M. are indicated by horizontal line and gray box. For both A and B, significance was determined using two-tailed Student’s t-test. * P < 0.05; *** P < 0.005.
Heart rates in whole heart rescue and in CCS rescue are comparable to each other (468 ± 11 and 488 ± 10 bpm, respectively; P = 0.20) and are each increased relative to mutant controls (381 ± 6 bpm; P < 0.0001) (Fig. 4A). These data show that bradycardia in Casq2−/− animals is entirely dependent upon the loss of Casq2 in the CCS. However, adult restoration not only rescued sinus bradycardia, but also resulted in heart rates significantly higher than those observed in wild-type controls (430 ± 10 bpm; P = 0.018).
Mice with CPVT exhibited lower resting heart rates than non-CPVT mice
Heart rate analyses of our conditional deletion models were surprising because they seemed to break correlation between VT and reduced heart rates. That is, whole heart and CCS-specific Cre recombination in Casq2Flox/Casq2Flox mice result in identical heart rates but VT occurs only when Casq2 loss is across the entire heart. To further investigate the link between sinus rate and VT, we compared the resting sinus heart rates of Casq2-deficient mice that demonstrated VT to the resting sinus heart rates of Casq2-deficient mice with no VT expression (Fig. 4B). Within each genotype (Casq2−/− Myh6CreMER and Casq2Flox/Casq2Flox Myh6CreMer), average heart rate is significantly lower in animals that displayed VT, supporting a link between heart rate and arrhythmia.
Discussion
Detailed electrophysiological comparisons of four novel models of adult Casq2 deletion or rescue yielded new understanding of the influence of developmental programs on the CPVT phenotype. These findings provide insight into the development and function of the CCS, including calsequestrin’s contribution to arrhythmia mechanisms underlying sudden death in CPVT, and are consistent with an important role for reduced heart rate in the CPVT2 phenotype. Furthermore, such insights further illuminate potential gene therapy strategies for CPVT patients.
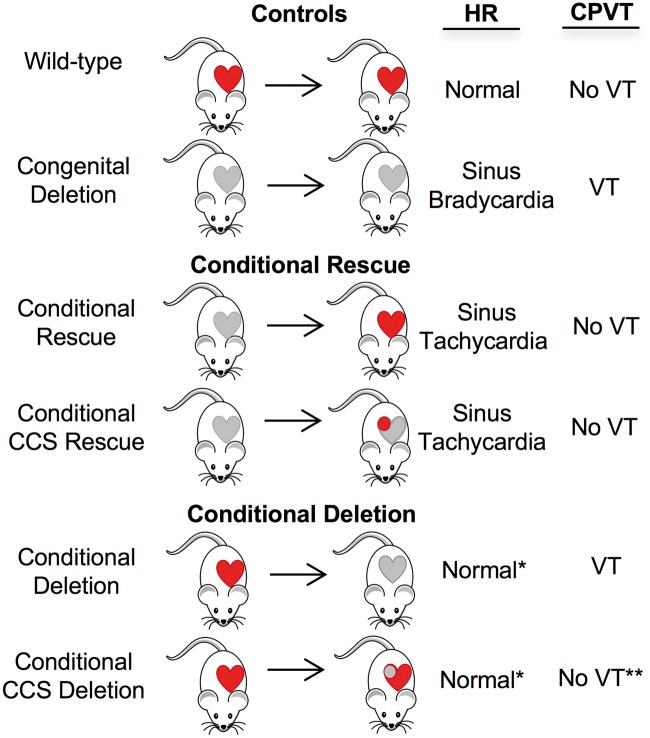
Previous studies already demonstrated that loss of Casq2 peptide results in changes in the cardiomyocyte proteome and ultrastructure and in the structure of the heart tissue (3,5,7). It is plausible that other changes occur but have just not yet been identified. However, it has been difficult to assess the significance of these changes on VT phenotype. To address this problem, we took a genetic approach and generated Casq2 alleles that allow the rescue and ablation of Casq2 gene function in specific cell types and at specific times in development. Findings are summarized in Figure 5.
Figure 5.
Summary of phenotypes associated with conditional rescue and conditional deletion mice. Casq2 gene deletion/rescue was achieved in the whole heart or in CCS only (circle) using Myh6-MERCreMRE or Hcn4KiT-Cre, respectively. Red, functional Casq2; gray, non-functional Casq2. For each study group, we depicted Casq2 gene activity before (left of the arrow) and after (right of the arrow) Cre recombinase induction. Heart rate depends upon the status of Casq2 in the CCS and upon the developmental history of the CCS. Normal*, significantly faster than the heart rate in congenital deletion mice but not quite at wild-type levels. CPVT depends upon concurrent loss of Casq2 in both the CCS and the working cardiomyocytes and is not dependent upon developmental history. However, loss of Casq2 in the CCS only does results in incidents of decoupling of atrial and ventricular contractions (no VT**).
Rescue of Casq2 gene function in young adult mice results in total elimination of the CPVT phenotype: VT frequency is 48% in Casq2−/− mice but 0% in conditional whole heart rescue animals. From these results, we conclude that all secondary changes associated with loss of Casq2 during heart development are either impermanent (and therefore, rectified by restored expression) or they are not relevant to the CPVT phenotype. In addition, these results support the idea that gene therapy can potentially be highly effective in treating VT (24–27).
Heart-wide ablation of Casq2 gene function at 1 month gives rise to a significant CPVT phenotype. VT frequency is 33% with postnatal Casq2 ablation, while congenital deletion results in 48% VT frequency. This outcome was surprising to us because we had anticipated that the embryonic/neonatal heart carried a developmental plasticity that would allow it to adapt in unique and effective ways to the loss of Casq2. Instead, our data show that either effective adaptations occur whenever Casq2 peptide is lost or alternatively that secondary changes do not significantly impact CPVT phenotype. Either way, developmentally restricted adaptations are not critical regulators of the CPVT phenotype.
In fact, overall our data are consistent with the idea that the conditional deletion phenotype is less severe. Although not statistically significant, CPVT frequency and average duration were reduced relative to those seen in congenital mutants. Bigeminy frequency is significantly lower in conditional deletion mice. Moreover, we think it is plausible that the mortality of Casq2-deficient mice during tamoxifen dosage caused us to underestimate the CPVT phenotype in Casq2−/− animals by eliminating animals where the Casq2-deficiency phenotype was most penetrant. To address this hypothesis, we analyzed ECG recordings that we had obtained from mice just prior to tamoxifen dosage. Consistent with previous experience with such young mice, we did not detect VT in animals of any genotype. However, we did notice that heart rates were significantly lower in Casq2-deficient animals that died relative to those that lived (non-survivors = 361 ± 6 bpm, survivors = 396 ± 7 bpm, P < 0.001). In contrast, heart rates were not distinguishable between Casq2-sufficient animals that lived or died during tamoxifen dosage (non-survivors = 417 ± 14 bpm, survivors = 398 ± 10 bpm, P = 0.33). In sum, the penetrance of the Casq2-deletion phenotype, at least as measured by bradycardia, predicted mortality. Thus these analyses are consistent with the notion that animals with a more penetrant phenotype were more likely to succumb to Cre toxicity. Note that an underestimate of the CPVT phenotype in the Casq2−/− cohort would not change our key conclusion that early developmental plasticity does not promote adaptations that mitigate CPVT. However, such an underestimate would prevent us from fully appreciating the degenerative effect of time spent without Casq2 as a contributor to the CPVT phenotype.
In addition to timing, our study design allowed us to investigate the relative importance of Casq2 function in the CCS and in working cardiomyocytes. Restoration of Casq2 only in the CCS was sufficient to prevent CPVT. These findings emphasize a critical role for Casq2 in CCS cells and reveal that a very targeted therapy can provide relief from cardiac arrhythmias. Equally surprising, however, was the observation that CCS Casq2 ablation alone was not sufficient to provoke the CPVT. Jalife and colleagues, among others, showed that arrhythmias in other CPVT models appeared to originate primarily in Purkinje cells (16,17). As such, one might predict that CCS Casq ablation would be sufficient to recapitulate this phenotype. While our data show that CCS Casq is necessary for the CPVT phenotype (since working myocardial Casq deletion alone did not give rise to VT), it was not sufficient to give rise to VT. Instead, CCS Casq deletion gave rise to sinus and AV nodal dysfunction as well as frequent episodes of accelerated junctional rhythm. The presence of increased junctional beats in CCS deletion mice extend earlier results by directly confirming that Casq2 sufficiency is necessary for electrical homoeostasis in the CCS but that Casq2 deficiency in ventricular cardiomyocytes is required to turn CCS disturbances into a CPVT phenotype. This implies that while the CCS calcium dysregulation is necessary to initiate arrhythmia seen in CPVT, calcium dysregulation in the working myocardium is necessary to maintain and sustain the arrhythmia.
Our study complements and extends recent papers that described rescue of Casq2 gene function using adeno-associated viral vector therapy (24,26,27). Denegri and colleagues showed that treatment of neonatal mice was sufficient to protect against CPVT for up to 1 year (24). Kurtzwald-Josefson et al. treated mice that were 3 months of age and saw strong protection against CPVT (26). Thus, our results agree that developmental history of Casq2 deficiency is not critical in predicting cardiac function once Casq2 is restored. Our study extends these results by showing that CCS rescue alone is enough to prevent CPVT.
Besides stress-induced arrhythmias, Casq2-deficient patients and mice show sinus bradycardia and sinus node dysfunction. The relationship between CPVT and heart rate is intriguing. In fact, increasing heart rate by pharmaceutical intervention or via pacemakers can reduce the frequency and severity of CPVT in Casq2−/− mice (28). Our study revealed at least three things about heart rate and Casq2. First, as expected but in contrast to VT phenotypes, heart rate is only dependent upon activity of Casq2 in the CCS. We know this because heart rates for conditional deletion and for conditional rescue mice were the same regardless of whether the deletion/rescue occurred via a CCS specific Cre or by recombination across the entire heart.
Second, again in contrast to VT phenotypes, heart rate is dependent not only on the status of Casq2 gene function at the time of analysis but also on the developmental history of the CCS. Conditional rescue of Casq2 results in a heart rate that is not only completely rescued relative to the congenital deletion but is also significantly higher than heart rates in wild-type mice (14% increase, P <0.001). Conditional deletion of Casq2 does not reduce heart rates to those of Casq2−/− animals, but instead results in an intermediate phenotype that is not actually statistically different than wild type (wild type = 430 ± 10 bpm; conditional CCS deletion = 407 ± 14 bpm; P = 0.20). These results imply that the presence or absence of Casq2 likely induces ion channel remodeling that effects postnatal sinus node function.
Recent analyses have emphasized the importance of RyR in maintenance and control of heart rhythm by establishing local calcium release events during diastole. These Ca2+ sparks then activate the Na+/Ca2+ exchanger and produce an inward current that leads to cell depolarization. Increased Ca2+ sensitivity of the RyR channel leads to decreased heart rates because loss of synchronicity of the Ca2+ sparks. (29–31, 32). Our data on heart rate decreases in Casq2−/− hearts are readily explained by this model but also emphasize an interesting new point: that the molecular clock is not completely flexible but is permanently altered by its developmental history.
Finally, our results indicate that while the relationship between heart rate and VT is complex, the two phenotypes are functionally related. In general, we see a correlation between heart rate and VT frequency. Among Casq2-deficient animals (congenital and conditional), heart rates of mice with VT are significantly lower than heart rates of mice that do not display VT (Fig. 4B). Conditional rescue of Casq2 prevents VT and raises heart rates significantly above wild-type levels. The one situation where heart rates and VT do not correlate is when we look at conditional deletion models. Casq2 deletion in CCS and the whole heart result in comparable heart rates (407 ± 14 and 405 ± 25 bpm, respectively; P = 0.92) but only the whole heart deletion results in CPVT.
Altogether, our data support a model where reduced heart rate increases VT risk by a mechanism to be determined. “Pause dependent” ventricular arrhythmia or ventricular arrhythmia following “short-long-short” R-R intervals have been observed at the initiation of ventricular arrhythmia episodes for some time, and it was thought to enhance the initiation of reentrant arrhythmia (33–35). Furthermore, this mode of arrhythmia induction was shown to dramatically increase VT incidence when directed toward altering refractoriness in the His-Purkinje system (36,37). In this regard, it is interesting to speculate that the effectiveness of CCS-specific rescue was dependent upon the increased sinus rate suppressing Casq2-related calcium mishandling. In this case, the ability of the CCS-specific rescue to prevent CPVT would be a downstream consequence of the interactions between developmental biology and Casq2-deficiency in determining contraction rates in the CCS. This notion might be tested directly using a genetic system that activates Cre recombination in Purkinje but not in nodal cells.
As a final note, we recall the unexpected observation that female Casq2−/− mice are hypersensitive to the cardiac stress caused by Myh6-CreMER transgene activity. While these results are interesting and potentially have high biomedical significance, we want to emphasize their preliminary nature. In future studies we will need to address at least two important biological issues before we can begin to interpret these results. First, an alternate source of cardiac stress should be used to ensure that the effect is not due to male/female differences in tamoxifen metabolism, which would make the phenotype of limited relevance in regards to understanding cardiac biology. Second, future study designs should address a potential confounding role for developmental biology. Specifically, it is possible that the gender differences only appear because different rates of sexual maturation put male and female one month mice at different stages of puberty at the time of tamoxifen administration (38).
Materials and Methods
Mice
The Hcn4KiT-Cre knock-in (20) and Myh6-MERCreMER transgenic lines (19) (Jackson Laboratories strain 005657) were generated as described.
Mice carrying the Casq2 null allele (Casq2−) and the Casq2Flox allele were generated as described (5). Basically, the Casq2Flox allele is functionally wild type but carries loxP insertions as direct repeats at −561 and +538 bp (relative to the major transcriptional start site) so that Cre-mediated recombination results in deletion of the Casq2 gene promoter and exon 1, thus generating the Casq2− allele (Fig. 1).
We generated mice carrying the Casq2RevFlox allele in a two-step process. In step 1, mouse embryonic stem cells (R1 line, 129SV) were transformed with linearized plasmid pKP700. pKP700 includes a 2.1 kb 5′ homology flank (−2.6 to −0.561 bp) and a 2.0 kb 3′ homology flank (+0.538 to +2.5 bp) to direct insertion of a 2.1 kb NeoR cassette (flanked with Frt elements) plus a 1.1 kb fragment that carries Casq2 sequences (from −561 to +538 bp) that are inverted relative to their normal endogenous orientation and are flanked with loxP66 and loxP71 sequences inserted in an inverted orientation relative to each other. G418 resistant colonies were isolated and scored for homologous recombination using one primer from outside the flanking sequences included in pKP700 and a second primer internal to the NeoR cassette (5). Targeted clones were injected into C57BL/6 blastocysts and chimeric founder mice were crossed with C57BL/6 females to establish the Casq2RevFlox + Neo line. In step 2, Casq2RevFlox + Neo heterozygotes were crossed to Rosa26 Flp transgenic females (Jackson Laboratories strain 003946) to remove the NeoR cassette via Flp recombinase-mediated site-specific recombination. The Casq2RevFlox line thus generated is depicted in Figure 1. Mice were backcrossed three additional times into a C57BL/6 background before crossing with mice carrying tamoxifen-inducible, cell-type specific transgenes to generate animals for this study. The Casq2RevFlox allele is functionally null but Cre-mediated recombination restores gene function by inverting the 1.1 kb Casq2 gene fragment back to its normal orientation. The recombination is unidirectional because recombination between loxP66 and loxP71 sequences generates one wild type loxP element and one that carries a double point mutation and further recombination between these two substrates is inefficient (39).
Genotypes were determined from PCR analysis of gDNAs extracted from tail snip or ear punch biopsies. For Casq2 genotyping, we used a three primer assay (5′-CCTGCGGTGACCGGTAAACTTCTC, 5′-CGAGGACAGGCACACTCTCCACATGC and 5′-CCACCTTAAGAGTTTGCCCACAG) that yields bands of 212, 253, 280 and 423 bp that represent +, Flox, − and RevFlox Casq2 alleles, respectively. For Hcn4 genotyping, we used a three-primer assay (5′-CTCACTGGCAGGCGCACCTG, 5′-CATGGACGGCGGCAGCTTGT and 5′-GCATCGACCGGTAATGCAGGC) that yields bands of 140 and 100 bp that represent the KiT-Cre and the + alleles, respectively. To determine the presence of the Myh6-MERCreMER transgene we used a three-primer assay (5′-TAGAGTCCTGGTGGGAGAGC, 5′-CTTTCGGAGGTACTGGGCTG and 5′-GCATCGACCGGTAATGCAGGC) that yields bands of 1300 and 208 bp that represent the Myh6-MERCreMER transgene and the endogenous Myh6 locus, respectively.
All mouse studies were performed according to NIH and PHS guidelines and only after protocols were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee.
RNA analysis
RNAs were extracted from frozen isolated hearts and cDNAs were prepared and analyzed as described previously (5).
Protein analysis
Frozen isolated hearts were homogenized using the Polytron tissue grinder in 50 mm Tris pH 7.5, 150 mm NaCl, 0.5% NP-40, 10 mm NaF, 1 mm Na-pyrophosphate, 10 mm Beta-glycerophosphate, Protease Inhibitor Cocktail (Sigma). Proteins (5 ug/well) were separated using NuPage® Novex™ 4–12% Bis-Tris Mini Gels and were transferred to PVDF membranes for immunoblotting using the iBlot™ Gel Transfer System (Invitrogen). Membranes were probed with 1: 2500 dilution rabbit polyclonal anti-calsequestrin (Affinity BioReagents™), 1: 2000 rabbit polyclonal IgG anti-GAPDH (Santa Cruz Biotechnology), 1: 4000 rabbit anti-Junctin (Pfeifer Laboratory), 1: 5000 rabbit anti-Triadin (Pfeifer Laboratory) and 1: 1000 mouse anti-RyR (Developmental Studies Hybridoma Bank) primary antibodies and then probed with 1: 10 000 goat anti-rabbit IgG and/or 1: 3000 goat anti-mouse IgG conjugated HRP (Santa Cruz Biotechnology) secondary antibodies.
Immunohistochemistry
Hearts from 1-year-old mice were fixed by Langendorff perfusion using 4% paraformaldehyde buffered with PBS and then embedded in Tissue-Tek O.C.T. compound. Ten micron sections were prepared via a Leica 3050 Cryostat and mounted on charged slides. Tissue sections were treated with 0.6% hydrogen peroxide in methanol (20 min) and then with 10% normal serum before incubating overnight with antibody to Contactin-2 (R&D Systems AF4439), with antibody to Calsequestrin-2 (ThermoScientific PA1–913) or with a secondary antibody only as a negative control. Primary antibody staining was visualized with horseradish perioxidase/DAB method (Vectastain Elite ABC HRP Kit, Vector Laboratories PK-6100). Hematoxylin staining was used to visualize nuclei and IHC images were collected on Whole Slide Imaging NanoZoomer (Hamamatsu) and analyzed on Nanozoomer Digital Pathology software (Hamamatsu).
Tamoxifen treatment
Tamoxifen (Sigma Aldrich T5648) was diluted to 10 mg/ml in 10% ethanol/90% corn oil, mixed for 1 h to dissolve fully, and 0.1 ml (1 mg) administered by oral gavage once daily for 5 days total. This dosage was the minimum sufficient to induce full recombination at the Casq2 locus.
ECG readings
Multi-lead ECGs were collected using PowerLab (ADInstruments) from anesthetized mice (1.5–2.0% isoflurane) kept on a warming pad at 1, 6 and 8 months of age by researchers blinded to mouse genotype (5). To be included in the study, mice must have lived through all reading time points. Baseline ECG readings were collected for 5 min before intraperitoneal injection with β-adrenergic agonist isoproterenol (Isuprel, 1.5 mg/kg). Then 15 min of additional tracings were recorded. Heart rate was calculated over minutes 1–4.5 (prior to stress administration) and averaged from the 6 and 8 month readings. ECG tracings were analyzed by at least two observers (blinded to genotype) to identify individual PVCs, VTs (four or more contiguous polymorphic PVCs), sustained VT (VT lasting >15 s) and bigeminy (40). In calculating VT frequency, a mouse was considered to display arrhythmia if an event occurred during either the 6 or 8 months reading. To calculate VT duration, we summed the total time spent by each mouse in CPVT during the two 15 min periods recorded after stress induction. In most mice, VT was one continuous event but two animals fluctuated between VT and sinus rhythm (Supplementary Material, Tables S3 and S4). In all mice, VT occurred within the first 40 s after isoproterenol injection or not at all.
Statistical analyses
Frequency of cardiac events (VT, PVC and bigeminy) were analyzed by chi square of proportions (https://www.medcalc.org/calc/comparison_of_proportions.php; date last accessed February 22, 2018). Heart rates were analyzed using a two-tailed, type 2 student’s t-test using Microsoft Excel software. Mortality data were analyzed by logistic regression as detailed in Results section.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We thank Tannia Clark and Jeanne Yimdjo for advice and support in animal care.
Conflict of Interest statement. None declared.
Funding
This work was supported by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (ZIA HD001804).
References
- 1. Berridge M.J., Bootman M.D., Roderick H.L. (2003) Calcium: calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol., 4, 517–529. [DOI] [PubMed] [Google Scholar]
- 2. Faggioni M., Kryshtal D., Knollmann B.C. (2012) Calsequestrin mutations and catecholaminergic polymorphic ventricular tachycardia. Pediatr. Cardiol., 33, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy R., Mollica J., Beard N., Knollmann B.C., Lamb G. (2011) Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2+-binding protein changes in CSQ2 knockout mice. Am. J. Physiol. Circ. Physiol., 300, H595–H604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S., Trumble W., Liao H., Wesson C., Dunker A., Kang C. (1998) Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol., 5, 476–483. [DOI] [PubMed] [Google Scholar]
- 5. Knollmann B.C., Chopra N., Hlaing T., Akin B., Yang T., Ettensohn K., Knollmann B.E., Horton K.D., Weissman N.J., Holinstat I.. et al. (2006) Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest., 116, 2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manno C., Figueroa L.C., Gillespie D., Fitts R., Kang C., Franzini-Armstrong C., Rios E. (2017) Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. U.S.A., 114, E638–E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song L., Alcalai R., Arad M., Wolf C.M., Toka O., Conner D.A., Berul C.I., Eldar M., Seidman C.E., Seidman J.G. (2007) Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest., 117, 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behr E., Casey A., Sheppard M., Wright M., Bowker T., Davies M., McKenna W., Wood D. (2007) Sudden arrhythmic death syndrome: a national survey of sudden unexplained cardiac death. Heart, 93, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lahat H., Pras E., Olender T., Avidan N., Ben-Asher E., Man O., Levy-Nissenbaum E., Khoury A., Lorber A., Goldman B.. et al. (2001) A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedoin families from Israel. Am. J. Hum. Genet., 69, 1378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postma A.V., Denjoy I., Hoorntje T., Lupoglazoff J., Da Costa A., Sebillon P., Mannens M., Wilde A., Guicheney P. (2002) Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ. Res., 91, 21e–226. [DOI] [PubMed] [Google Scholar]
- 11. Priori S.G. (2002) Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation, 106, 69–74. [DOI] [PubMed] [Google Scholar]
- 12. Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalova Z., Gyorke I., Terentyeva R., Vedamoorthyrao S., Blom N.A., Valle G.. et al. (2006) Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res., 98, 1151–1158. [DOI] [PubMed] [Google Scholar]
- 13. Chopra N., Knollmann B.C. (2009) Cardiac calsequestrin: the new kid on the block in arrhythmias. J. Cardiovasc. Electrophysiol., 20, 1179–1185. [DOI] [PubMed] [Google Scholar]
- 14. van der Werf C., Wilde A.A.M. (2014) VTs in catecholaminergic cardiomyopathy (catecholaminergic polymorphic ventricular tachycardia). In Zipes D.P., Jalife J. (eds), Clinical Cardiogenetics Elsevier, Amsterdam, pp. 895–902. [Google Scholar]
- 15. Glukhov A.V., Kalyanasundaram A., Lou Q., Hage L.T., Hansen B.J., Belevych A.E., Mohler P.J., Knollmann B.C., Periasamy M., Györke S.. et al. (2015) Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur. Heart J., 36, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerrone M., Noujaim S.F., Tolkacheva E.G., Talkachou A., O'Connell R., Berenfeld O., Anumonwo J., Pandit S.V., Vikstrom K., Napolitano C.. et al. (2007) Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ. Res., 101, 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herron T.J., Milstein M.L., Anumonwo J., Priori S.G., Jalife J. (2010) Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm., 7, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faggioni M., Hwang H.S., van der Werf C., Nederend I., Kannankeril P.J., Wilde A.A.M., Knollmann B.C. (2013) Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circ. Res., 112, 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sohal D., Nghiem M., Crackower M., Witt S., Kimball T., Tymitz K., Penninger J., Molkentin J. (2001) Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible cre protein. Circ. Res., 89, 20–25. [DOI] [PubMed] [Google Scholar]
- 20. Hoesl E., Stieber J., Herrmann S., Feil S., Tybl E., Hofmann F., Feil R., Ludwig A. (2008) Tamoxifen-inducible gene deletion in the cardiac conduction system. J. Mol. Cell. Cardiol., 45, 62–69. [DOI] [PubMed] [Google Scholar]
- 21. Pallante B., Giovannone S., Fang-Yu L., Zhang J., Liu N., Kang G., Dun W., Boyden P., Fishman G. (2010) Contactin-2 expression in the cardiac purkinje fiber network. Circ. Arrhythm. Electrophysiol., 3, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koitabashi N., Bedja D., Zaiman A.L., Pinto Y.M., Zhang M., Gabrielson K.L., Takimoto E., Kass D.A. (2009) Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MERCreMER gene deletion models. Circ. Res., 105, 12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pugach E.K., Richmond P.A., Azofeifa J.G., Dowell R.D., Leinwand L.A. (2015) Prolonged Cre expression driven by the α-myosin heavy chain promoter can be cardiotoxic. J. Mol. Cell. Cardiol., 86, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Denegri M., Bongianino R., Lodola F., Boncompagni S., De Giusti V.C., Avelino-Cruz J.E., Liu N., Persampieri S., Curcio A., Esposito F.. et al. (2014) Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation, 129, 2673–2681. [DOI] [PubMed] [Google Scholar]
- 25. Hajjar R.J., Lyon A.R. (2014) Gene therapy for the treatment of catecholaminergic polymorphic ventricular tachycardia. Circulation, 129, 2633–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurtzwald-Josefson E., Yadin D., Harun-Khun S., Waldman M., Aravot D., Shainberg A., Eldar M., Hochhauser E., Arad M. (2017) Viral delivered gene therapy to treat catecholaminergic polymorphic ventricular tachycardia (CPVT2) in mouse models. Heart Rhythm, 14, 1053–1060. [DOI] [PubMed] [Google Scholar]
- 27. Lodola F., Morone D., Denegri M., Bongianino R., Nakahama H., Rutigliano L., Gosetti R., Rizzo G., Vollero A., Buonocore M.. et al. (2016) Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis., 7, e2393.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu B., Ho H.T., Brunello L., Unudurthi S.D., Lou Q., Belevych A.E., Qian L., Kim D.H., Cho C., Janssen P.M.. et al. (2015) Ablation of HRC alleviates cardiac arrhythmia and improves abnormal Ca handling in CASQ2 knockout mice prone to CPVT. Cardiovasc. Res., 108, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lakatta E.G., Maltsev V.A., Vinogradova T.M. (2010) A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res., 106, 659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stern M.D., Maltseva L.A., Juhaszova M., Sollott S.J., Lakatta E.G., Maltsev V.A. (2014) Hierarchical clustering of ryanodine receptors enables emergence of a calcium clock in sinoatrial node cells. J. Gen. Physiol., 143, 577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yaniv Y., Lakatta E.G., Maltsev V.A. (2015) From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol., 6, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neco P., Torrente A.G., Mesirca P., Zorio E., Liu N., Priori S.G., Napolitano C., Richard S., Benitah J.P., Mangoni M.E.. et al. (2012) Paradoxical effect of increased diastolic Ca(2+) release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation, 126, 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El-Sherif N., Gough W., Restivo M. (1991) Reentrant ventricular arrhythmias in the late myocardial infarction period: mechanism by which a short-long-short cardiac sequence facilitates the induction of reentry. Circulation, 83, 268–278. [DOI] [PubMed] [Google Scholar]
- 34. Leclercq J., Maisonblanche P., Cauchemez B., Coumel P. (1988) Respective role of sympathetic tone and of cardiac pauses in the genesis of 62 cases of ventricular fibrillation recorded during Holter monitoring. Eur. Heart J., 9, 1276–1283. [DOI] [PubMed] [Google Scholar]
- 35. el-Sherif N., Caref E., Chinushi M., Restivo M. (1999) Mechanism of arrhythmogenicity of the short-long cardiac sequence that precedes ventricular tachyarrhythmias in the long QT syndrome. J. Am. Coll. Cardiol., 33, 1415–1423. [DOI] [PubMed] [Google Scholar]
- 36. Denker S., Lehmann M., Mahmud R., Akhtar M. (1984) Facilitation of macroreentry within the His-Purkinje system with abrupt changes in cycle length. Circulation, 69, 26–32. [DOI] [PubMed] [Google Scholar]
- 37. Denker S., Lehmann M., Mahmud R., Gilbert C., Akhtar M. (1983) Divergence between refractoriness of His-Purkinje system and ventricular muscle with abrupt changes in cycle length. Circulation, 68, 1212–1221. [DOI] [PubMed] [Google Scholar]
- 38. Vettner O., Hagen C., Spear L. (2012) Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol., 54, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Albert H., Dale E., Lee E., Ow D. (1995) Site-specific integration of DNA into wild-type and mutant loxP sites placed in the plant genome. Plant J., 7, 649–659. [DOI] [PubMed] [Google Scholar]
- 40. Curtis M.J., Hancox J.C., Farkas A., Wainwright C.L., Stables C.L., Saint D.A., Clements-Jewery H., Lambiase P.D., Billman G.E., Janse M.J.. et al. (2013) The Lambeth Conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther., 139, 213–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.