Abstract
A number of controversies and challenges exist for the management of OA in health care. This paper describes the challenges and gaps in OA care, particularly in relation to population health management, complex interventions and outcomes. It sets this in the context of competing health priorities and multimorbidity, access to high quality conservative care, non-pharmacological therapies, resource limitations and models of care. The overuse of some therapies and neglect of others are discussed, as well as the potential for self-management. The roles of patient and public involvement and the healthcare team are highlighted in enhancing best care for OA and providing solutions for closing the evidence-to-practice gap. Implementation of models of care offer one solution to the challenges and progress of such implementation is described. Areas for further research are highlighted.
Keywords: controversies, challenges, osteoarthritis management, roles, Rheumatology, models of care
Rheumatology key messages
-
There are significant evidence-to-practice gaps in osteoarthritis care.
Algorithms and models of care are available as examples for rheumatologists and clinics to standardized osteoarthritis management.
The healthcare team can play key leadership roles in musculoskeletal education and championing excellent osteoarthritis management.
Introduction
The Bone and Joint Decade (2000–10) initiated the first international drive to prioritize OA and joint pain in older adults and its impact on the Western world [1, 2]. The diagnostic label of OA is ranked 11th in terms of its impact on years lived with disability. However, joint pain in those of 45 years and over is ranked as the number one cause of years with disability worldwide. According to health leaders and epidemiologists, the population trends are clear for the next 30 years, and with the ageing population and increased obesity and physical inactivity, joint pain and OA are set to rise.
Joint pain and OA are predominantly managed in primary care, and while there are national and international guidelines for the care and management of OA in adults [such as the National Institute of Health and Care Excellence (NICE), EULAR, Osteoarthritis Research Society International, ACR), there is a gap between what we know and what we do [e.g. 3–5]. This paper describes the challenges and gaps in OA care, particularly in relation to population health management, interventions and outcomes. It is set in the context of competing health priorities and multimorbidity, access to high quality care, resource limitations and models of care. The overuse of some therapies and neglect of others will be discussed, as well as the potential for self-management. Many of the challenges are predominantly taken from experiences in the National Health Service (NHS), UK. The role of the healthcare team will be highlighted in enhancing best care for OA and providing solutions for closing the evidence practice gap.
Challenges and gaps in care
Controversies in care
Data from a systematic review and meta-synthesis of qualitative studies exploring barriers and facilitators to offering best OA care, for example guideline recommendations for OA, identified only barriers and no enablers [6]. The findings addressed system-related barriers, disease-related barriers and patient-related barriers from which four distinct themes emerged from eight studies [6]: OA is not that serious, clinicians perceive they are under-prepared, to personal beliefs (e.g. negativity about OA), and dissonance in patient expectations. Such findings begin to explain some of the challenges in offering the range of treatment options recommended and described in the following sections.
Making and giving a diagnosis of OA
In primary care adults 45 years and over consulting with joint pain and limitations in everyday activity are more likely to receive NICE recommendations if a diagnosis of OA has been recorded in the medical electronic record [7]. Delay in diagnosis can lead to inappropriate treatment and suboptimal care. Overuse of imaging has prompted NICE to highlight the recommendation for diagnosing OA on clinical grounds and set a quality standard to audit this approach [8–10].
Diagnosis and subsequent treatment are often focused on a single, most painful joint rather than multisite joint problems [11]. Yet multisite joint pain is the most common presentation in consultations in primary care, and having more joint sites affected leads to more health care consultations irrespective of specialty or site [11]. As summarized in a recent systematic review, there is a paucity of evidence to guide the practitioner regarding treatment of multisite joint pain, with few studies describing interventions for considering OA in all affected joints [12]. Comorbidities also often go under-recognized in primary care and the community [13–16], which further hampers the holistic assessment recommended by NICE [8–10].
Reports have highlighted the impact of language when giving the diagnosis of OA. Health Care Professionals’ views can be perceived to be negative, for example, ‘nothing can be done’ and ‘it’s your age’ [17–20]. Unhelpful descriptions and terminology can easily transfer from the X-ray report into the consultation, with people with OA concerned for their ‘degenerative meniscal tear’. Use of language to talk about OA that offers more positive and supportive messages, such as ‘wear’ and ‘remodelling’, can enhance understanding of prognosis when verbal messages are backed up by written patient information [21]. Explaining that OA is part of a process of repair rather than degeneration can introduce a sense of optimism and reassurance because it offers a more positive outlook to life with OA, and can change patients’ and carers’ perceptions that OA inevitably leads to persistent pain, disability and joint replacement [21].
Self-management
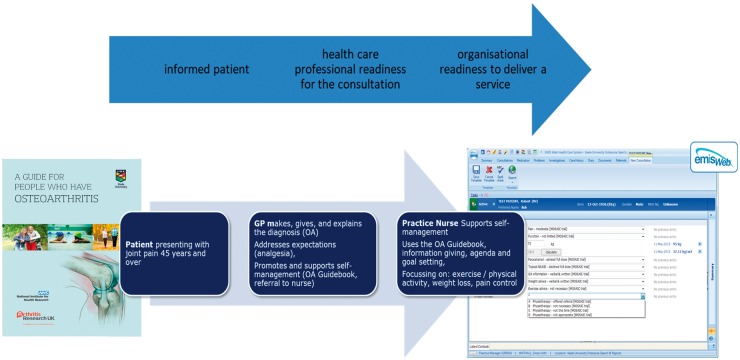
One of the most complex interventions for the core management OA is self-management support. Delivering this well through a systematic, consistent approach across the pathway is a challenge. Ways to enhance self-management support within consultations for OA have been studied [22]. A Whole Systems Informing Self-Management Engagement model [23] for guided self-management of OA, including provision of patient information (e.g. OA guidebook) [24], care responsive to patient needs [25] and good access to follow-up care (practice nurse consultations), has been proposed [26] (see Fig. 1).
Fig. 1.
A model OA consultation developed using the WISE model and tested in the MOSAICS studyMOSAICS: Managing OSteoArthritis in ConsultationS.
There is still limited knowledge on how to join up a system of care so that Quality Standards are delivered and assessed at each point in the pathway and the roles of the patient and carer are maximized. Patients with OA are clear that they want help and support to self-manage their condition, and that they want this to be health care professional-led [27–31]. Short-term changes are often not maintained in the long term, particularly for self-management programmes that require sustained lifestyle and behaviour changes [32–37].
The beneficial effects found in systematic reviews of OA self-management programmes, while small, demonstrate the need for ongoing self-management support throughout the course of the disease [38–40].
The use of digital platforms to enhance service delivery has been variable in health settings, but concerted efforts to redesigned musculoskeletal pathways and commission the use of digital platforms to enable patient information to transfer across systems and organisations are growing.
Exercise and physical activity
Since 2002 we have known the benefits of exercise as analgesia for OA pain [41, 42], but despite the substantial evidence for clinical and cost effectiveness of exercise, research funders still invest in underpowered studies looking at exercise vs no exercise [41]. More attention is needed on adherence to exercise, which is a major obstacle in exercise programmes. Currently the OA Trial Bank [43] is supporting work to determine subgroups who may respond better to exercise [44]. As of 2002 sufficient evidence had accumulated to show significant benefit of exercise over no exercise in patients with OA, and further trials were deemed unlikely to overturn this result [41]. However, the review highlighted that studies continued to be funded and published after this date. Furthermore, additional studies continue to be underpowered; in a Cochrane review, 35% of eligible studies recruited fewer than 25 participants in one or both allocation groups [45].
Topical NSAIDs and paracetamol
Topical NSAIDs have retained their role as the first line analgesia for peripheral joint OA, and electronic templates in general practice are known to enhance their uptake [7]. The role of paracetamol as first line analgesia has diminished following the evidence of poorer efficacy and increased side effects [46], but despite the concerns over paracetamol clinicians are unaware of the benefits of other therapies. Stopping ineffective therapies presents an implementation challenge. Clinicians continue with paracetamol when an alternative pharmacological or non-pharmacological approach such as topical NSAIDs and exercise might offer similar analgesic effects with fewer side effects [7, 37].
Opioids
Opioids are frequently used for OA pain [7]. Long-term opioids may benefit those with chronic pain but they have been shown to have adverse effects. In the UK there is evidence of an increase in prescribing of the more potent controlled and long-acting, long-term opioids [47]. Bedson et al. [47] showed that whilst primary care physicians had acted on national guidelines to reduce their use of new opioids, in cases in which opioids were already being prescribed, the shift towards using the more potent controlled and long-acting opioids continued. Those on more potent controlled opioids, either short-acting or long-acting, are of the greatest concern in relation to prescription opioid drug abuse and addiction [47].
Surgical approaches
Surgical treatments are offered for progressive pain and disability. The benefits of arthroscopic surgery on quality of life over the long term are minimal, and those with knee disease experience very small improvements in pain and function when compared with others who receive conservative management [48]. As the evidence fails to support a persistence of benefit over the long term, there is a trade-off between the marginal short-term benefits against the burden of the surgical procedure [48]. Many international guidelines do not recommend arthroscopy unless there is true mechanical locking of the knee [e.g. 8, 49]. The use of arthroscopy for knee OA has decreased; however, it is still prevalent [50]. Pressure from patients to do something, the perception that other options are limited, that surgeons want to meet patients’ expectations, and time pressures in clinic, all appear to influence the choice to undertake arthroscopic procedures [50]. Winter et al. [51] evaluated the risk of total knee replacement (TKA) in patients undergoing knee arthroscopy. They found that those undergoing arthroscopy might anticipate an annual rate of TKA in the order of 2%, with higher rates among older patients and those with more advanced OA [51]. Clinicians and patients considering knee arthroscopy should discuss the likelihood of subsequent TKA as they weigh risks and benefits of surgery.
Knee OA can be managed well non-surgically, but many patients and providers still consider total joint arthroplasty (TJA) the only option, especially at later stages of disease [50, 52]. Many patients have good results with TJA [49], but there is still a subset who have sub-optimal results, and all the factors that predict good outcome are unknown. Data from the Osteoarthritis Initiative on patients who had undergone TKA were used to determine the prevalence rates of TKA surgery classified as appropriate, inconclusive and inappropriate [53]. Approximately one-third of TKA surgeries were judged to be inappropriate.
Surgeons have recognized the need for support tools for making the decision for TJA [54]. Canadian stakeholders have identified several potential criteria for TJA: evidence of arthritis on joint examination; patient-reported symptoms negatively impacting quality of life; an adequate trial of appropriate non-surgical treatment; realistic patient expectations of surgery; mental and physical readiness of the individual for surgery; and patient-surgeon agreement that potential benefits exceed risks [55]. However, there remains a need for validated tools to adequately assess and communicate appropriateness criteria for TJA.
For patients with no previous history of knee repair surgery and with very minimal OA changes, autologous chondrocyte implantation may offer a treatment option for those with persistent symptoms after conservative therapy and with cartilage defects over 2 cm2 [56]. However, there remains a need for evidence on long-term effectiveness of this procedure.
Training gap
Consensus work and surveys highlight the need for health care professionals’ training in the skills of making and giving the diagnosis of OA, supporting self-management and delivering care in line with international guideline recommendations for OA [e.g. 57]. Audit of educational needs of health care professionals and patients shows the mismatch between educational need and training delivered [58]. Education and training packages for primary health care professionals now offer accredited online musculoskeletal modules [e.g. 59, 60].
Maximizing the use of transferable skills by health care professionals has been neglected. For example, general practice nurses have expertise in running chronic disease clinics, and many of the techniques for supporting self-management, for example, keeping active and weight management, can be used across long term conditions. Unfortunately, general practice nurses are given few training opportunities to enhance their skills in supporting self-management for OA.
Outcomes of care
The whole research cycle takes research from priority setting right through to implementation of best evidence. Whether the same outcomes used to demonstrate clinical effectiveness in trials are the same as those needed for evaluation of services remains unclear. Allen et al. [4] in their evaluation of models of care listed commonly used outcomes that included disease-specific measures for OA as well as generic health measures and OA quality indicators of care [e.g. 61, 62]. New measures, such as the Musculoskeletal Health Questionnaire (MSK-HQ), may also be useful for assessing outcomes of care [63] and offer an opportunity to embed outcome measures in routinely recorded medical record data. Big data and aggregated, anonymized medical record data will provide the means for understanding variations in care at an organization level [e.g. 64]. The challenges of information governance, consent, anonymization and aggregation of data are very difficult issues to resolve and are often tackled only at a local level.
Closing the evidence-to-practice gap
Given the current challenges in offering guideline recommendations, closing the evidence-to-practice gap is key. From 2002 the Arthritis and Musculoskeletal Alliance in the UK developed standards of care for OA, building on what a person with OA should expect to receive but their implementation has been lacking [65]. With the advent of NICE OA guidelines [8, 9] and NICE Quality Standards [10] we now have a set of recommendations that can be adopted, but as yet there has been no UK audit of these.
These gaps in knowledge are recognized, and yet closing the gap is complex [66]. The evidence we produce regarding how to deliver best care has its own limitations. The individual studies themselves can be methodologically outstanding and serve to increase knowledge, but transferring this knowledge to real world settings is difficult. Evidence underpinning the recommendations for clinical guidelines is predominantly derived from studies of knee OA, with fewer studies for the hip and even fewer for the hand and foot. Single treatment approaches are often studied in isolation and there is a lack of studies of integrated packages of care.
Lau et al. [66] described the causes of the evidence-to-practice gap in primary care and the ways in which the evidence gap could be addressed, although even the most effective interventions such as clinical opinion leaders show at best only small effects, and there is no certainty that multiple approaches work better [66]. What Lau and colleagues did highlight was the importance of context and the role of organizations in influencing the uptake of best practice. Context means policy such as NICE guidelines, public awareness of OA and its care, economic climate and funding, stakeholder buy-in (e.g. Sustainability Transformation Partnerships), technological advances and infrastructure to deliver best care.
For health services, Clinical Networks can offer an approach to knowledge mobilization between ‘what we know’ and ‘what we do’. The National Clinical Director for Long Term Conditions with NHS England and the Arthritis and Musculoskeletal Alliance have made musculoskeletal health a priority across the four key regions in England with the development of musculoskeletal knowledge networks to embody the potential for sharing models of care and good practice with trusted partners within a geographic boundary [67].
OA care models and pathways to enhance coordination
Many of the specific gaps in OA care can be at least partly attributed to a lack of care coordination and a purposeful management approach. Without a model for how, when and by whom specific OA-related therapies are provided, there is a high risk that some components of care will be neglected. This can be particularly challenging since OA treatments are delivered by different types of providers, yet a point person is not always apparent. Another challenge is that although OA treatment guidelines provide information about therapies that should be delivered, they are largely silent regarding when specific treatments are appropriate and how various therapies may best fit together in a comprehensive treatment approach. Unfortunately, research to date has provided little evidence regarding the optimal timing, integration or criteria for different OA therapies. However, a number of efforts have applied practical, clinical experience to OA treatment guidelines, developing treatment algorithms and care models that can serve as guides and examples for rheumatologists and other clinicians.
Development of OA treatment algorithms
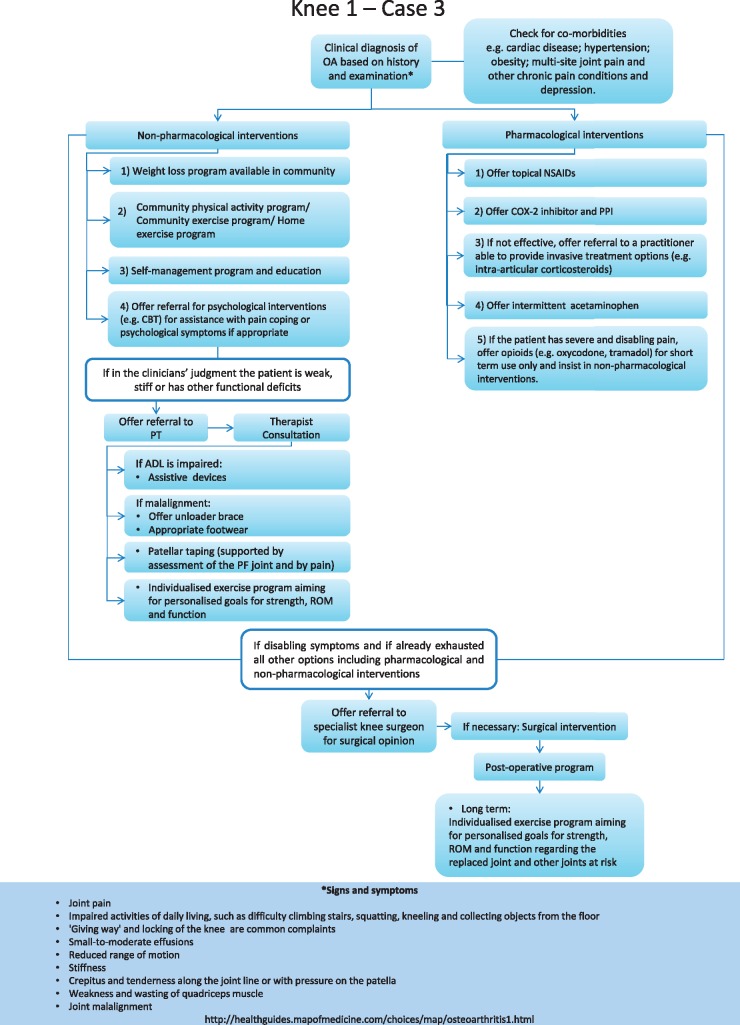
Two recent international efforts developed clinical algorithms for OA treatment [68–70]. Meneses et al. [69] performed a systematic review of OA treatment guidelines and then used an iterative expert panel process to derive clinical algorithms for the treatment of hand, hip and knee OA. These algorithms consider key issues such as comorbid health conditions and offer a step-wise approach for delivery of pharmacological and non-pharmacological therapies. The algorithms also provide general decision rules for when more intensive or different therapies should be considered. An example algorithm for a patient with knee OA and several comorbidities is shown in Fig. 2; four different algorithms are available and can provide a practical approach for rheumatologists and clinics to operationalize treatment of OA. Bruyére and colleagues [68] also developed algorithms that focus on pharmacotherapy for knee OA.
Fig. 2.
Clinical algorithms for OA treatment of the knee with several co-morbidities
Reprinted from Osteoarthritis and Cartilage, Volume 24, Meneses SR, Goode AP, Nelson AE, Lin J, Jordan JM, Allen KD et al. Clinical algorithms to aid osteoarthritis guideline dissemination, Pages 1487–99, Copyright 2016, with permission from the Osteoarthritis Research Society International, published by Elsevier Ltd [69].
Example models of OA care
There have also been efforts internationally to develop models for delivering recommended OA therapies within health systems. A selection of these models is described briefly here and in greater detail elsewhere [4]. These models vary in terms of the range of OA treatments included (e.g. some focusing on a specific area such as weight management or physical activity and some with a more comprehensive approach), types of providers involved and reimbursement model. Therefore, these programmes provide a range of examples that rheumatologists can consider with respect to feasibility of implementation in their clinical context.
Amsterdam Osteoarthritis Cohort—Netherlands
Individuals from the Amsterdam Osteoarthritis Cohort are eligible for an OA management programme if they have hip and/or knee OA, and if pain is non-traumatic, sufficient to seek care and attributed by a clinician to a hip or knee joint. This programme offers coordinated multidisciplinary management that includes supervised exercise according to a knee joint stabilization programme [71], occupational therapy, psychological support and medical management. Funding for this programme is from various sources, including the health care system and trials conducted within the cohort.
Better management of patients with OA—Sweden
Better Management of Patients with Osteoarthritis [72] is a programme for individuals with hip, knee, hand or shoulder OA [73] who have non-traumatic pain, sufficient to seek care and attributed by a clinician to their joint. In this programme physiotherapists, occupational therapists and expert patients (OA-communicators) provide education, self-management support, exercise recommendations, an optional individualized exercise programme and optional supervised exercise group sessions.
There is also an online version, Joint Academy [74], which includes recommended exercises, interactive lessons, reports and tracking tools. In addition, patients can communicate with a physical therapist in the context of the online programme. Participants from anywhere in the world can sign up for Joint Academy, and the current cost for the 6-week core programme is $45USD.
Enabling Self-management and Coping with Arthritic Pain Using Exercise—UK
Enabling Self-management and Coping with Arthritic Pain using Exercise (ESCAPE-pain) [75] is a rehabilitation programme for people with joint pain, including OA [42]. It integrates self-management and coping strategies with and individualized exercise programme. ESCAPE-pain is typically delivered by physiotherapists but can be administered by other qualified healthcare workers in various settings. The delivery format is in small groups, with meetings twice per week for 6 weeks. There is also an ESCAPE-pain app that mirrors the in-person programme.
Good Life with Arthritis in Denmark
Good Life with Arthritis in Denmark (GLA:D) is programme for individuals with hip and/or knee OA; similar to Better Management of Patients with Osteoarthritis, patients must have non-traumatic pain sufficient to seek care and attributed by a clinician to OA of the hip or knee joint. GLA: D focuses on self-management and exercise components of OA treatment [76]. There are three patient education sessions provided over the course of 2 weeks; the first two sessions are delivered by a physiotherapist and the third by an expert patient who previously participated in GLA:D. These education sessions are followed by 12 sessions of supervised neuromuscular exercise sessions based on the NEuroMuscular Exercise programme [77]. GLA:D has now been disseminated in many countries, and more information (as well as contact information for the developers) can be found on the GLA: D website [78].
Joint Implementation of Osteoarthritis Guidelines—UK
Joint Implementation of Osteoarthritis Guidelines [79] is a comprehensive OA management programme based on evidence from the MOSAICS trial [26]. This programme is offered to individuals of 45 years and over who are consulting in general practice, have knee, hip, hand and/or foot OA and have joint pain that limits function. The programme is initiated with a model OA consultation with a general practitioner and a practice nurse, including making, giving and explaining the OA diagnosis, giving an OA guidebook, offering analgesia and referral to a practice nurse (Fig. 1). The practice nurse then provides up to four sessions supporting self-management; these include exercise and physical activity advice using Arthritis Research UK booklets, weight management and support for pain relief. An electronic template is used to measure key quality indicators of OA care within the programme. Further implementation of this model is being tested in the Netherlands, Norway, Denmark and Portugal (European Institute of Innovation & Technology (EIT)-Health funded Joint Implementation of oSteoArthritis Guidelines in Western Europe (JIGSAW-E )) [80].
Osteoarthritis Chronic Care Programme—Australia
Osteoarthritis Chronic Care Programme is a programme for patients with doctor-diagnosed knee and/or hip OA, along with pain in the affected joint on most days of the past month (pain visual analogue scale ⩾ 4 out of 10) [81, 82]. This programme involves multidisciplinary, individually tailored, physiotherapy-led OA management. Treatments include exercise, diet, psychological support, occupational therapy, orthotics and medical management. Patients can be referred to the programme by any health care provider, and it was initially funded through the public hospital system. (The Osteoarthritis Chronic Care Programme model of care and other relevant documents can be accessed at [83].)
Osteoarthritis Healthy Weight for Life—Australia
The Osteoarthritis Healthy Weight For Life programme [84] is offered to individuals with knee or hip OA diagnosed by radiological evidence who are overweight (BMI ⩾ 28) and have significant joint symptoms [85]. This 18-week programme focuses on behavioural aspects of OA management, including weight loss and improved nutrition, a physical activity plan and physiotherapist-delivered exercises (strength, balance and mobility), personalized online symptom, progress and satisfaction tracking (with phone or mail options) and personal motivation via phone or other tools. This programme is available in Australia at no charge via some health insurance providers, but for those without private health insurance (a substantial proportion of the population) there is a cost involved.
Roles of the multidisciplinary team
The multidisciplinary team of health care professionals, for example, rheumatology nurse, physiotherapist, community pharmacist, dietician and rheumatologist, can assist with transferable skills and increase confidence in primary care in delivering quality care, reduce overuse of X-ray in the diagnosis of OA, reduce inappropriate referral to orthopaedic surgery and increase the uptake of core non-pharmacological treatment with confidence in the safety of exercise and its use as an analgesic [86].
The role of rheumatologist in OA management
OA is most often managed in primary care, with referral to secondary care typically only in more advanced stages or complex presentations. However, rheumatologists have a key role and opportunity for leadership in OA management [59]. First, rheumatologists have content expertise in the management of OA and can therefore be leaders in health systems, driving appropriate models of care and quality improvement. Leadership in this area is greatly needed, considering the gaps in quality of OA care and often a lack of a champion for treatment of this health condition. Second, OA commonly co-occurs with other rheumatic conditions, particularly among older adults. Therefore, rheumatologists can set an example of delivering the highest quality of OA care, incorporating both pharmacological and non-pharmacological therapies. The following are specific practical recommendations for ways rheumatologist can lead the way in optimizing OA care:
Provide education and support in OA management to primary care colleagues
Because primary care providers are responsible for managing a wide range of health conditions, it is not realistic to expect they will typically be experts in musculoskeletal medicine. Rheumatologists can bridge this gap by providing periodic educational sessions on OA care for primary care providers, covering evidence-based therapies and specific challenging clinical situations. In some health care settings, individual rheumatologists or groups of rheumatology clinicians may be able to provide virtual or electronic consults to provide input on specific patients or scenarios. This may be particularly useful for rural primary care providers who do not have ready in-person access to musculoskeletal expertise.
Provide leadership in developing an OA patient pathway or model of care within the healthcare system
The example algorithms and models of care described above can be an excellent starting place for developing a context-appropriate OA pathway. Because of content area expertise, rheumatologists are equipped to lead multidisciplinary efforts (involving primary care, orthopaedics and rehabilitation) to develop and implement pathways that facilitate evidence-based and comprehensive OA care.
Connect with community organizations and resources
Behavioural treatments such as exercise and weight loss are key components of managing OA. Resources to support patients in these behaviours are often not available within the healthcare system. However, many services are available within the community. Rheumatologists can have a significant impact on patients’ OA management by connecting them with evidence-based, reputable community resources to support healthy behaviours.
Involving the patients and the public
Rheumatology has developed a successful track record of involving patients and the public in shaping OA care pathways. Patients and the public have an increasing and influential role in shaping services and supporting changes in practice in primary care, and their role in secondary care has been established for some time.
Key areas for future research
Algorithms and models of care are available as examples for rheumatologists and clinics to standardize OA management and the healthcare team can play key leadership roles in musculoskeletal education and championing excellent OA management. However, there are significant evidence-to-practice gaps in OA care. The following are research areas and methodological considerations that would significantly improve the evidence base underlying optimal OA management: research participants should represent the range of patients seen for OA, including those with multi-joint disease, those with multiple comorbidities and the oldest old. This will enhance the generalizability of findings so that they can inform real-world clinical practices; studies should systematically examine heterogeneity of treatment effects to identify patient characteristics that predict response to therapies. This will help to inform tailoring of treatment regimens in clinical settings; although studies of individual treatments or interventions are still needed in some areas, there is a great need to study more novel, complex and integrated approaches to OA management that mirror clinical scenarios, consider the whole person and engage with caregivers and other support systems; there is a need for a greater focus on implementation research in OA, identifying models of care that can be successfully delivered, as well as considerations for cost effectiveness.
Acknowledgements
We would like to acknowledge Professor David Hunter and our reviewers for their helpful comments.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: K.D. was a member of the NICE Osteoarthritis Guidelines Development Group CG 59 (2008) and CG 177 (2014) and a member of the NICE Quality Standards Group for Osteoarthritis, has been an invited speaker at Bone and Joint Decade 2015 Conference in Oslo and Osteoarthritis Research Society International, received a grant from European Institute of Innovation & Technology (EIT)-Health for implementation and is part-funded by a Knowledge Mobilisation Research Fellowship (KMRF-2014-03-002) from the National Institute for Health Research (NIHR) and by NIHR Collaborations for Leadership in Applied Health Research and Care West Midlands. K.A. is supported by the Center for Health Services Research in Primary Care at the Durham VA Healthcare System (CIN 13-410) and a National Institute of Arthritis and Musculoskeletal and Skin Diseases Multidisciplinary Clinical Research Center P60 AR062760, received grant support (through her institutions) from the Patient Centered Outcomes Research Institute, Department of Veterans Affairs, National Institutes of Health, Department of Defence, and Centers for Disease Control an Prevention, related to clinical osteoarthritis and musculoskeletal research, is an Associate Editor for Osteoarthritis and Cartilage and has received honoraria from the American College of Rheumatology for conference presentations.
References
- 1. Cross M, Smith E, Hoy D. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Porcheret M, Jordan K, Jinks C, Croft P.. Primary care treatment of knee pain—a survey in older adults. Rheumatology 2007;46:1694–700. [DOI] [PubMed] [Google Scholar]
- 4. Allen KD, Choong PF, Davis AM. et al. Osteoarthritis: models for appropriate care across the disease continuum. Best Pract Res Clin Rheumatol 2016;30:503–35. [DOI] [PubMed] [Google Scholar]
- 5. Dziedzic KS, French S, Davis AM, Geelhoed E, Porcheret M.. Implementation of musculoskeletal Models of Care in primary care settings: theory, practice, evaluation and outcomes for musculoskeletal health in high-income economies. Best Pract Res Clin Rheumatol 2016;30:375–97. [DOI] [PubMed] [Google Scholar]
- 6. Egerton T, Diamond LE, Buchbinder R, Bennell KL, Slade SC.. A systematic review and evidence synthesis of qualitative studies to identify primary care clinicians’ barriers and enablers to the management of osteoarthritis. Osteoarthritis Cartilage 2017;25:625–38. [DOI] [PubMed] [Google Scholar]
- 7. Edwards JJ, Jordan KP, Peat G. et al. Quality of care for OA: the effect of a point-of-care consultation recording template. Rheumatology 2015;54:844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health & Clinical Excellence (NICE). Osteoarthritis: the Care and Management of Osteoarthritis in Adults. Clinical guideline [CG59]. London: NICE, 2008.
- 9.National Institute for Health & Care Excellence (NICE). Osteoarthritis: Care and Management in Adults. Clinical guideline [CG177]. London: NICE, 2014.
- 10.National Institute for Health & Care Excellence (NICE). Osteoarthritis. Quality standard 87. Manchester: NICE, 2015.
- 11. Finney AG, Dziedzic KS, Lewis MA, Healey EL.. Multisite peripheral joint pain: a cross-sectional study of prevalence and impact on general health, quality of life, pain intensity and consultation behaviour. BMC Musculoskelet Disord 2017;18:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finney A, Healey E, Jordan JL, Ryan S, Dziedzic KS.. Multidisciplinary approaches to managing osteoarthritis in multiple joint sites: a systematic review. BMC Musculoskelet Disord 2016;17:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gureje O, Von Korff M, Simon GE, Gater R.. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA 1998;280:147–51. [DOI] [PubMed] [Google Scholar]
- 14. Arnow BA, Hunkeler EM, Blasey CM. et al. Comorbid depression, chronic pain, and disability in primary care. Psychosomatic Med 2006;68:262–8. [DOI] [PubMed] [Google Scholar]
- 15. Rosemann T, Backenstrass M, Joest K. et al. Predictors of depression in a sample of 1,021 primary care patients with osteoarthritis. Arthritis Rheum 2007;57:415–22. [DOI] [PubMed] [Google Scholar]
- 16. Axford J, Heron C, Ross F, Victor CR.. Management of knee osteoarthritis in primary care: pain and depression are the major obstacles. J Psychosomatic Res 2008;64:461–7. [DOI] [PubMed] [Google Scholar]
- 17. McHugh GA, Silman AJ, Luker KA.. Quality of care for people with osteoarthritis: a qualitative study. J Clin Nursing 2007;16:168–76. [DOI] [PubMed] [Google Scholar]
- 18. Gignac MAM, Davis AM, Hawker G. et al. “What do you expect? You’re just getting older”: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthrit Rheum 2006;55:905–12. [DOI] [PubMed] [Google Scholar]
- 19. Ong BN, Jinks C, Morden A.. The hard work of self-management: living with chronic knee pain. Int J Qual Stud Heal 2011;6:10.3402/qhw.v6i3.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hill S, Dziedzic K, Thomas E, Baker SR, Croft P.. The illness perceptions associated with health and behavioural outcomes in people with musculoskeletal hand problems: findings from the North Staffordshire Osteoarthritis Project (NorStOP). Rheumatology 2007;46:944–51. [DOI] [PubMed] [Google Scholar]
- 21. Morden A, Jinks C, Ong BN, Porcheret M, Dziedzic KS.. Acceptability of a ‘guidebook’ for the management of Osteoarthritis: a qualitative study of patient and clinician’s perspectives. BMC Musculoskelet Disord 2014;15:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paskins Z, Sanders T, Croft PR, Hassell AB.. The identity crisis of osteoarthritis in general practice: a qualitative study using video-stimulated recall. Ann Fam Med 2015;13:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kennedy A, Bower P, Reeves D. et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346:f2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grime J, Dudley B.. Developing written information on osteoarthritis for patients: facilitating user involvement by exposure to qualitative research. Health Expect 2014;17:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porcheret M, Main C, Croft P. et al. Development of a behaviour change intervention: a case study on the practical application of theory. Implement Sci 2014;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dziedzic KS, Healey EL, Porcheret M. et al. Implementing the NICE osteoarthritis guidelines: a mixed methods study and cluster randomised trial of a model osteoarthritis consultation in primary care—the Management of OsteoArthritis In Consultations (MOSAICS) study protocol. Implement Sci 2014;9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mann C, Gooberman-Hill R.. Health care provision for osteoarthritis: concordance between what patients would like and what health professionals think they should have. Arthritis Care Res 2011;63:963–72. [DOI] [PubMed] [Google Scholar]
- 28. Jinks C, Ong BN, Richardson J.. A mixed methods study to investigate needs assessment for knee pain and disability: population and individual perspectives. BMC Musculoskelet Disord 2007;8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Department of Health. The NHS Improvement Plan: Putting People at the Heart of Public Services. London: Department of Health, 2004. [Google Scholar]
- 30. Kralik D, Koch T, Price K, Howard N.. Chronic illness self-management: taking action to create order. J Clin Nursing 2004;13:259–67. [DOI] [PubMed] [Google Scholar]
- 31. Townsend A, Wyke S, Hunt K.. Self-managing and managing self: practical and moral dilemmas in accounts of living with chronic illness. Chronic Illness 2006;2:185–94. [DOI] [PubMed] [Google Scholar]
- 32. Robison JI, Rogers MA.. Adherence to exercise programmes. Recommendations. Sports Med 1994;17:39–52. [DOI] [PubMed] [Google Scholar]
- 33. Sullivan T, Allegrante JP, Peterson MG, Kovar PA, MacKenzie CR.. One-year followup of patients with osteoarthritis of the knee who participated in a program of supervised fitness walking and supportive patient education. Arthritis Care Res 1998;11:228–33. [DOI] [PubMed] [Google Scholar]
- 34. Williams NH, Hendry M, France B, Lewis R, Wilkinson C.. Effectiveness of exercise-referral schemes to promote physical activity in adults: systematic review. Br J Gen Pract 2007;57:979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roddy E, Zhang W, Doherty M. et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee—the MOVE consensus. Rheumatology 2005;44:67–73. [DOI] [PubMed] [Google Scholar]
- 36. Fransen M, McConnell S., Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015; Issue 1. Art. No.: CD004376; doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hay EM, Foster NE, Thomas E. et al. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. BMJ 2006;333:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coulter A, Entwistle VA, Eccles A. et al. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev 2015;(3)CD010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du S, Yuan C, Xiao X. et al. Self-management programs for chronic musculoskeletal pain conditions: a systematic review and meta-analysis. Patient Educ Couns 2011;85:e299–310. [DOI] [PubMed] [Google Scholar]
- 40. Kroon FP, van der Burg LR, Buchbinder R. et al. Self-management education programmes for osteoarthritis. Cochrane Database Syst Rev 2014;(1)CD008963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uthman OA, van der Windt DA, Jordan JL. et al. Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ 2013;347:f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurley MV, Walsh NE, Mitchell H, Nicholas J, Patel A.. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial. Arthritis Care Res 2012;64:238–47. [DOI] [PubMed] [Google Scholar]
- 43. van Middelkoop M, Dziedzic KS, Doherty M. et al. Individual patient data meta-analysis of trials investigating the effectiveness of intra-articular glucocorticoid injections in patients with knee or hip osteoarthritis: an OA Trial Bank protocol for a systematic review. Syst Rev 2013;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holden MA, Burke DL, Runhaar J. et al. OA Trial Bank. Subgrouping and TargetEd Exercise pRogrammes for knee and hip OsteoArthritis (STEER OA): a systematic review update and individual participant data meta-analysis protocol. BMJ Open 2017;7:e018971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fransen M, McConnell S, Harmer AR. et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med 2015;49:1554–7. [DOI] [PubMed] [Google Scholar]
- 46. Roberts E, Delgado Nunes V, Buckner S. et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2016;75:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bedson J, Chen Y, Hayward RA. et al. Trends in long-term opioid prescribing in primary care patients with musculoskeletal conditions: an observational database study. Pain 2016;157:1525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hammett T, Simonian A, Austin M. et al. Changes in physical activity after total hip or knee arthroplasty: a systematic review and meta-analysis of 6 and 12 month outcomes. Arthritis Care Res 2018, Advance Access published 12 September 2017, doi: 10.1002/acr.23415. [DOI] [PubMed] [Google Scholar]
- 49. Brignardello-Petersen R, Guyatt GH, Buchbinder R. et al. Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review. BMJ Open 2017;7:e016114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barlow T, Plant CE.. Why we still perform arthroscopy in knee osteoarthritis: a multi-methods study. BMC Musculoskelet Disord 2015;16:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Winter AR, Collins JE, Katz JN.. The likelihood of total knee arthroplasty following arthroscopic surgery for osteoarthritis: a systematic review. BMC Musculoskelet Disord 2017;18:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Skou ST, Roos EM, Laursen MB. et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597–606. [DOI] [PubMed] [Google Scholar]
- 53. Riddle DL, Jiranek WA, Hayes CW.. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol 2014;66:2134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frankel L, Sanmartin C, Hawker G. et al. Perspectives of orthopaedic surgeons on patients’ appropriateness for total joint arthroplasty: a qualitative study. J Eval Clin Pract 2016;22:164–70. [DOI] [PubMed] [Google Scholar]
- 55. Hawker G, Bohm ER, Conner-Spady B. et al. Perspectives of Canadian stakeholders on criteria for appropriateness for total joint arthroplasty in patients with hip and knee osteoarthritis. Arthritis Rheumatol 2015;67:1806–15. [DOI] [PubMed] [Google Scholar]
- 56. National Institute of Health and Care Excellence (NICE). Autologous chondrocyte implantation for treating symptomatic articular cartilage defects of the knee. Technology appraisal guidance [TA477], 2017. www.nice.org.uk/guidance/ta477 (2 March 2018, date last accessed).
- 57. Holden MA, Nicholls EE, Hay EM, Foster NE.. Physical therapists’ use of therapeutic exercise for patients with clinical knee osteoarthritis in the United kingdom: in line with current recommendations? Phys Therapy 2008;88:1109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ryan S, Lillie K, Thwaites C, Adams J.. ‘What I want clinicians to know’—experiences of people with arthritis. Br J Nurs 2013;22:808–12. [DOI] [PubMed] [Google Scholar]
- 59.EULAR. On-line course on rheumatic diseases. http://www.eular-onlinecourse.org (2 March 2018, date last accessed).
- 60.Royal College of General Practitioners (RCGP). RCGP Learning. Core Skills in Musculoskeletal Care. http://elearning.rcgp.org.uk/course/info.php? popup=0&id=206 (2 March 2018, date last accessed).
- 61. Blackburn S, Higginbottom A, Taylor R. et al. Patient-reported quality indicators for osteoarthritis: a patient and public generated self-report measure for primary care. Res Involv Engagem 2016;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Osteras N, Garratt A, Grotle M. et al. Patient-reported quality of care for osteoarthritis: development and testing of the osteoarthritis quality indicator questionnaire. Arthritis Care Res 2013;65:1043–51. [DOI] [PubMed] [Google Scholar]
- 63. Hill JC, Kang S, Benedetto E, et al.Development and initial cohort validation of the Arthritis Research UK Musculoskeletal Health Questionnaire (MSK-HQ) for use across musculoskeletal care pathways. BMJ Open 2016;6:e012331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jordan KP, Joud A, Bergknut C. et al. International comparisons of the consultation prevalence of musculoskeletal conditions using population-based healthcare data from England and Sweden. Ann Rheum Dis 2014;73:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kingsbury SR, Conaghan PG.. Current osteoarthritis treatment, prescribing influences and barriers to implementation in primary care. Prim Health Care Res Dev 2012;13:373–81. [DOI] [PubMed] [Google Scholar]
- 66. Lau R, Stevenson F, Ong BN. et al. Achieving change in primary care—effectiveness of strategies for improving implementation of complex interventions: systematic review of reviews. BMJ Open 2015;5:e009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arthritis and Musculoskeletal Alliance (ARMA). MSK Knowledge Network. http://arma.uk.net/msk-clinical-networks-project/arma-associated-ccg-networks/ (2 March 2018, date last accessed).
- 68. Bruyere O, Cooper C, Pelletier JP. et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—From evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016;45(4 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- 69. Meneses SR, Goode AP, Nelson AE. et al. Clinical algorithms to aid osteoarthritis guideline dissemination. Osteoarthritis Cartilage 2016;24:1487–99. [DOI] [PubMed] [Google Scholar]
- 70. Bruyere O, Cooper C, Pelletier JP. et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2014;44:253–63. [DOI] [PubMed] [Google Scholar]
- 71. Knoop J, Dekker J, van der Leeden M. et al. Knee joint stabilization therapy in patients with osteoarthritis of the knee: a randomized, controlled trial. Osteoarthritis Cartilage 2013;21:1025–34. [DOI] [PubMed] [Google Scholar]
- 72.Bättre Omhändertagande av patienter med Artros (BOA). Träning gör att Stanley lever ett rikt och aktivt liv trots artros. www.boaregistret.se (2 March 2018, date last accessed).
- 73. Thorstensson CA, Garellick G, Rystedt H, Dahlberg LE.. Better management of patients with osteoarthritis: development and Nationwide implementation of an evidence-based supported osteoarthritis self-management programme. Musculoskelet Care 2015;13:67–75. [DOI] [PubMed] [Google Scholar]
- 74.Joint Academy. www.jointacademy.com (2 March 2018, date last accessed).
- 75.Enabling Self-management and Coping with Arthritic Pain using Exercise (ESCAPE). ESCAPE-pain. www.escape-pain.org/ (2 March 2018, date last accessed).
- 76. Skou ST, Roos EM.. Good Life with osteoArthritis in Denmark (GLA:DTM): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord 2017;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ageberg E, Link A, Roos EM.. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord 2010;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Good Life with osteoaArthritis in Denmark. https://www.glaid.dk/english.html (2 March 2018, date last accessed).
- 79.Joint Implementation of Guidelines for oSteoArthritis in the West Midlands (JIGSAW). www.keele.ac.uk/pchs/implementingourresearch/makinganimpact/osteoarthritisandosteoporosis/jigsaw/ (2 March 2018, date last accessed).
- 80.EIT Health. JIGSAW-E: Joint Implementation of Guidelines for oSteoArthritis in Western Europe. www.eithealth.eu/jigsaw-e (2 March 2018, date last accessed).
- 81. Eyles JP, Lucas BR, Patterson JA. et al. Does clinical presentation predict response to a nonsurgical chronic disease management program for endstage hip and knee osteoarthritis? J Rheumatol 2014;41:2223–31. [DOI] [PubMed] [Google Scholar]
- 82. Eyles JP, Mills K, Lucas BR. et al. ‘Can we predict those with OA who worsen following a chronic disease management program?’ Arthritis Care Res 2016;68:1268–77. [DOI] [PubMed] [Google Scholar]
- 83.Agency for Clinical Innovation (ACI). Osteoarthritis Chronic Care Program Model of Care. www.aci.health.nsw.gov.au/resources/musculoskeletal/osteoarthritis_chronic_care_program/osteoarthritis-chronic-care-program (2 March 2018, date last accessed).
- 84.Osteoarthritis Healthy Weight For Life. https://oa.hwfl.com.au/ (2 March 2018, date last accessed).
- 85. Atukorala I, Makovey J, Lawler L. et al. Is there a dose response relationship between weight loss and symptom improvement in persons with knee osteoarthritis? Arthritis Care Res 2016;68:1106–14. [DOI] [PubMed] [Google Scholar]
- 86. Quicke JG, Foster NE, Thomas MJ, Holden MA.. Is long-term physical activity safe for older adults with knee pain?: a systematic review. Osteoarthritis Cartilage 2015;23:1445–56. [DOI] [PubMed] [Google Scholar]