Abstract
Background
An important decision with accelerometry is the threshold in counts per minute (CPM) used to define moderate to vigorous physical activity (MVPA). We explore the ability of different thresholds to track changes in MVPA due to a physical activity (PA) intervention among older adults with compromised function: 760 CPM, 1,041 CPM, and an individualized threshold. We also evaluate the ability of change in accelerometry and self-reported PA to attenuate treatment effects on major mobility disability (MMD).
Methods
Data from a week of hip worn accelerometers and self-reported PA data (30-day recall) were examined from baseline, 6-, 12-, and 24-months of follow-up on 1,528 older adults. Participants were randomized to either PA or Health Education (HE). MMD was objectively defined by loss of ability to walk 400 m during the follow-up.
Results
The three thresholds yielded similar and higher levels of MVPA for PA than HE (p < .001), however, this difference was significantly attenuated in participants with lower levels of physical function. Self-reported PA that captured both walking and strength training totally attenuated the intervention effect for MMD, an 18% reduction to a 3% increase. Accelerometer CPMs showed less attenuation of the intervention effect.
Conclusions
Accelerometry assessment within the LIFE study was not sensitive to change in level in physical activity for older adults with very low levels of physical function. A combination of self-report and objective measures are recommended for use in physical activity intervention studies of the elderly; limitations of accelerometry deserve closer attention.
Keywords: Older Adults, Accelerometry, Thresholds, Mobility Disability, LIFE-study
Introduction
Physical activity (PA) can range in intensity from simple postural shifts (1) to extremely vigorous forms of movement that are now common to high intensity training regimens (2). Whereas PA guidelines recommend prescribing moderate to vigorous levels of physical activity (MVPA) for older adults that is relative to each individual (3), it is very difficult to objectively do so. Thus, from a clinical perspective, these guidelines recommend establishing relative intensity by using Ratings of Perceived Exertion (3). The current study explored the implications of defining MVPA within the Lifestyle Interventions and Independence for Elders (LIFE) study using two different objective thresholds, along with a method developed by Rejeski and colleagues (4) that individualizes thresholds using variables known to correlate with fitness (age and 400 m walk time).
The most commonly cited adult threshold applied to the Actigraph acclerometer is 1952 counts per minute (CPM) published by Freedson and colleagues (5), although in the NHANES data the threshold was increased to 2020 (6). Copeland and colleagues (7) used a similar approach as Freedson et al. on a healthy cohort of older men and women (N = 38, mean age of 69.7), who were not taking any medications and were able to walk on a treadmill at a fast pace. They identified the threshold to define MVPA in older, healthy adults as 1,041 CPM. To complicate matters further, Matthew and colleagues (8) conducted secondary analyses on data from participants with a mean age in their 40’s (range from 19 to 74 y) who had performed activities of daily living in a real world setting that were known to elicit either a light or moderate metabolic demand. Based on their findings, the authors identified a threshold of 760 CPM, a value that was later applied to the NHANES data set by Evenson and colleagues (9).
The problem of applying a single threshold for MVPA when studying older adults is clearly illustrated in a recent publication on ancillary data collected in conjunction with the LIFE study. Rejeski and colleagues (4) examined accelerometry data collected during a bout of supervised exercise in a stratified random subset of LIFE participants and found that, while the median threshold was 1,220 CPM (not far from the 1,041 CPM reported by Copeland and colleagues (7)), the 25th and 75th percentiles encompassed a wide range from 715 CPM to 1,930 CPM. In fact, there are a number of studies with various populations that have come to a similar conclusion regarding the limitations of using a single threshold to define MVPA (10–12).
Considering the confusion surrounding the choice of a threshold for use in defining MVPA in older adults and interest in PA within the fields of gerontology and geriatric medicine, the current study had three aims. First, we provide descriptive data and tests of group differences on time spent in MVPA for participants in either the PA or Health Education (HE) arms of the LIFE study over the 24 months of the trial using three different thresholds: individualized based on a predictive model using age and 400 m walk time (4), 760 CPM (8), and 1,041 CPM (7). Based on data from the LIFE main outcomes study, we expected to find statistically significant higher minutes per week for each threshold among those in the PA group as compared to HE. Second, we then partitioned these data by baseline quartiles for 400 m walk time to determine whether the effects of the thresholds on patterns of MVPA differed on the basis of participants’ level of physical function. And third, we examined how differences between PA and HE in both accelerometry and on a brief self-report measure of PA (the CHAMPS-5) (13), influenced differences in incident major mobility disability (MMD) that were observed between PA and HE across the 24 months of the trial (13). This third aim was exploratory and designed to evaluate the degree to which the intervention effect on MMD may have been associated with the effect of either activity measure (accelerometry or self-report) on MMD.
Methods
Participants
From February 2010 to December 2011, 14,831 participants were screened for the LIFE study at eight different field centers (see Acknowledgments); 1,635 of these potential participants were eligible and randomized to intervention, 818 to PA and 817 to HE. Details regarding screening, recruitment yields and baseline characteristics have been published (14) as has the CONSORT Diagram and the main outcomes of the trial (13). The study protocol was approved by the institutional review boards at all participating sites and the trial is registered at ClinicalsTrials.gov with the identifier NCT01072500.
Measures
Accelerometry
Participants were asked to wear a GT3X+ accelerometer for a period of 7 days at each assessment visit—baseline, 6-, 12-, and 24-months. The device was worn on the right hip and produced output that was digitized by a twelve-bit analog to digital convertor at a rate of 30 Hz. Once digitized, the signal passed through a digital filter limiting the frequency range from 0.25 to 2.5 Hz. Data were collected once per second (1 Hz) over the 7 days of data collection. To be considered valid, accelerometry data for any single assessment had to include ≥5 days where the device had been worn ≥10 hours each day. We followed standard guidelines for detecting non-wear time by calculating an amplitude threshold of zero for a period of a consecutive 90-min time window with an allowance of a 2-min interval of nonzero counts (15).
400 m Walk
The 400-m walk test is a modified version of a fast-pace mobility walking test originally developed by investigators within the Health ABC study (16). Participants walk at their usual walking pace for 400 m (10 laps of a 20 m course). At baseline, all participants had to be able to complete the 400-m walk test in 15 minutes to qualify for inclusion into the trial. Failure to complete the 400-m walk test in 15 minutes constituted the primary outcome for the LIFE study, MMD (13).
CHAMPS-5
The CHAMPS-5 is a brief, interview administered, self-report index of PA that is based on five items collected as part of the CHAMPS (17) that were directly related to the LIFE intervention; ie, it assessed both walking and strength training. Participants were asked to indicate whether they did any of the following activities in the past month: (a) walk leisurely for exercise or pleasure; (b) walk fast or briskly for exercise; (c) walk or hike uphill; (d) do light strength training, or (e) do moderate to heavy strength training. If they answered yes to any item, then they were asked how much time each week they spent on that activity. The total time in minutes for the five items was used as a measure of self-reported involvement in the LIFE PA intervention activities. The CHAMPS-5 was found to be sensitive change to the LIFE study PA intervention (13).
Procedures
As noted above, we applied three different thresholds for defining the lower boundary of MVPA for the accelerometry data: (a) an individualized threshold based on a predictive model developed by Rejeski and colleagues (4), (b) 760 CPM (8), and (c) 1,041 CPM (7). We did not include 1952 (5) or 2020 (6) because most participants in LIFE were unable to achieve these levels of intensity during training bouts. A cap of 1952 was placed on the individualized threshold since this is the standard threshold used for MVPA in adults. A total of 1,528 participants of the 1,635 that were randomized to the LIFE study had valid accelerometry data at baseline or at least one follow-up visit. For the interested reader, Supplementary Figure 1 provides frequency distributions for MVPA in minutes per week for the individualized threshold by intervention group.
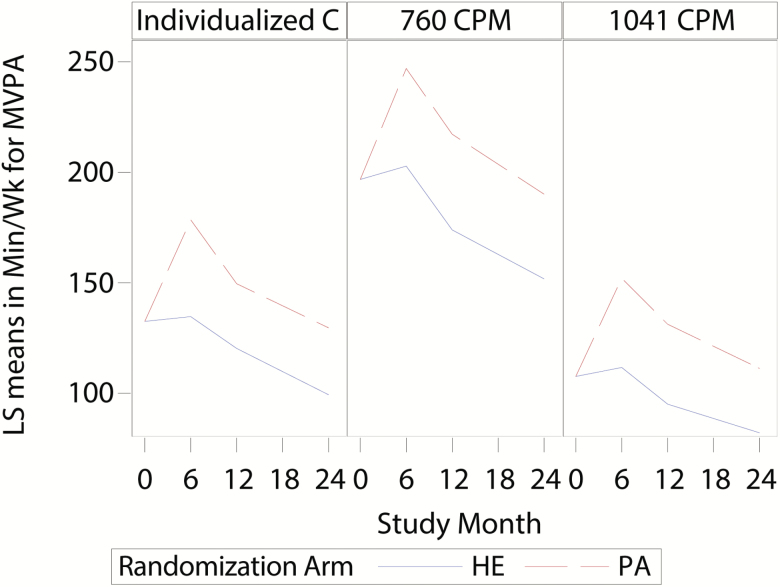
Figure 1.
Minutes of MVPA per week using an individualized threshold, 760 CPM and 1,041 CPM.
Statistical Methods
Descriptive statistics, t-tests, and chi-square tests were used to characterize and evaluate the balance between intervention groups. Constrained mixed models (18) were used to evaluate follow-up differences for each MVPA and self-reported PA outcomes between randomized groups. For randomized trials, constrained mixed models can provide more efficient estimates of post-randomization intervention differences when either baseline or post-randomization measures are missing (18). Models contained terms allowing for separate intervention effects at each time point. Linear combination of parameter estimates were used to obtain estimates of visit specific means (95% CI) and changes between visits; contrasts of these combinations were used to test for equality of visit specific and average intervention effects. Analyses also were performed on log transformed MVPA outcomes to explore the sensitivity of conclusions to the normality assumption. Prior to log transformation, 0.5 min/week was added to the median CPM to permit taking logs for participants that had 0 min/week of MVPA. Estimated means obtained from the linear combination of parameter estimates were interpreted as arithmetic means for untransformed outcomes and, after exponentiation, as geometric means for models on transformed outcomes. To explore the above results by baseline quartile of 400 m walk time, terms representing quartile and higher order interactions with the intervention and visit effects were added to the models. Tests of average intervention effects across follow-up between 1st and 4th quartiles were performed using contrasts. For analysis of time until the initial MMD event or last follow-up, censoring time was defined as the time from randomization until the last ascertainment of MMD. To explore intervention effects overall, and within quartiles of baseline function, we estimated hazard ratios (HR) with 95% confidence intervals (CI) using Cox regression models. Models used sex as a stratifying factor for the underlying hazards. Time-dependent measures of self-reported PA and MVPA from accelerometry were added to these models to evaluate whether post-randomization associations of these variables attenuated the intervention effects on MMD. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) with hypotheses evaluated at the 0.05 significance level.
Results
Table 1 provides descriptive data for the analysis sample by intervention group. As expected, the age of the sample was close to 80 years (M = 78.9; SD = 5.23), 66.9% were women, 16.8% were African American and there was considerable socioeconomic diversity. Despite their advanced age, the average BMI was 30.15 (SD = 5.98) kg/m2. The most prevalent comorbidity was diabetes at 25.0%.
Table 1.
Baseline Characteristics of Subjects by Intervention Group
| Health Education (N = 771)* | Physical Activity (N = 757) | p value** | |
|---|---|---|---|
| Age | 79.07 (5.23) | 78.74 (5.21) | .22 |
| Sex | .43 | ||
| Female | 523 (67.8) | 499 (65.9) | |
| Male | 248 (32.2) | 258 (34.1) | |
| Race/ethnicity | .13 | ||
| White | 604 (78.3) | 567 (74.9) | |
| African American/Black | 113 (14.7) | 143 (18.9) | |
| Hispanic | 29 (3.8) | 29 (3.8) | |
| Other | 25 (3.2) | 18 (2.4) | |
| Education | .90 | ||
| No formal education (00) | 6 (0.8) | 7 (0.9) | |
| Elementary school (K-08) | 13 (1.7) | 14 (1.8) | |
| High school/equivalent (09–12) | 228 (29.6) | 229 (30.3) | |
| College (13–16) | 315 (40.9) | 322 (42.5) | |
| Post graduate | 205 (26.6) | 182 (24.0) | |
| Other | 4 (0.5) | 3 (0.4) | |
| Health-related variables | |||
| Body mass index | 30.25 (6.22) | 30.04 (5.71) | .48 |
| Arthritis | 153/771 (19.8) | 139/757 (18.4) | .46 |
| Diabetes | 203/767 (26.5) | 178/754 (23.6) | .20 |
| Heart failure/congestive heart failure | 42/764 (5.5) | 25/752 (3.3) | .04 |
| MI | 63/766 (8.2) | 56/754 (7.4) | .56 |
| Stroke | 51/768 (6.6) | 50/753 (6.6) | .99 |
| Pain/stiffness in the knees | 105/175 (60.0) | 106/158 (67.1) | .18 |
| Pain and/or stiffness in the hips | 67/172 (39.0) | 65/159 (40.9) | .72 |
| Pain and/or stiffness in the back/spine | 96/175 (54.9) | 101/160 (63.1) | .12 |
| Physical function measurements | |||
| SPPB total score | 7.33 (1.60) | 7.46 (1.58) | .11 |
| 400 m walk time (seconds) | 507.53 (111.84) | 503.31 (112.73) | .46 |
| Executive function | |||
| DSST Score | 46.89 (12.62) | 46.06 (12.92) | .21 |
Notes: *Mean (SD) or N (%).
**Note that tests between groups at baseline were performed because those with accelerometry data comprised a subset of all randomized participants. In the absence of exclusions for missing accelerometry data, randomization between groups would typically provide good balance and tests would not be performed according to CONSORT recommendations.
Description and Group Differences in Minutes of Mvpa per Week Using the three Thresholds
Table 2 provides the expected means (95% CIs) for untransformed MVPA by intervention group across time by the three CPM thresholds. These data are also presented graphically in Figure 1. First, as shown in Figure 1 and confirmed by the data in Table 2, for all three MVPA thresholds there were significant differences between PA and HE at each follow-up visit as well as for the average effect across all follow-up visits (all p < .001). Inspection of the intervention group differences across time for the different thresholds reveal that the greatest differences occurred at 6 months, with the PA group engaging in roughly 45 minutes more MVPA each week than HE (see Table 2). Second, irrespective of the threshold considered, both intervention groups declined in volume of MVPA from the 6th to the 24th month follow-up visit—p < .001. And third, the main difference in patterns of MVPA for the different thresholds was in the absolute total minutes of MVPA each week. For example, the largest average volume of MVPA at 6 months in the PA group was 247.0 minutes/week (95% CI = 233.4, 260.6) when using 760 CPM and the lowest when using 1,041 CPM (152.1 minutes/week; 95% CI = 142.8, 161.4). The individualized threshold yielded an average value that was intermediate between the two, 178.5 minutes/week; 95% CI = 167.6, 189.4). Analysis of log transformed outcomes (see Supplementary Table 1) provided similar conclusions.
Table 2.
Estimated Means (95% CI) in Minutes/Week for MVPA by Intervention Group for Assessment Visits for Different Thresholds Using Constrained Mixed Effects Models
| MVPA Threshold | Time Point | Estimated Mean (95% CI) for Intervention Groups | Intervention Effect (95% CI); p value |
|
|---|---|---|---|---|
| HE | PA | |||
| Individualized | Adjusted baseline | 132.5 (124.7, 140.4) | ||
| 6 months | 134.7 (123.8, 145.6) | 178.5 (167.6, 189.4) | 43.8 (30.6, 57.0); <.001 | |
| 12 months | 120.3 (111.4, 129.2) | 149.6 (140.6, 158.6) | 29.3 (18.9, 39.6); <.001 | |
| 24 months | 99.3 (90.0, 108.6) | 129.6 (120.2, 139.0) | 30.3 (19.0, 41.6); <.001 | |
| Average FU effect | 118.1 (109.8, 126.4) | 152.6 (144.2, 160.9) | 34.5 (25.3, 43.7); <.001 | |
| Decline from 6 to 24 months | –35.4 (–45.1, –25.6) | –48.9 (–58.7, –39.1) | 13.5 (–0.3, 27.3); .055 | |
| 760 | Baseline visit | 196.8 (187.8, 205.8) | ||
| 6-month visit | 202.8 (189.2, 216.4) | 247.0 (233.4, 260.6) | 44.2 (26.8, 61.6); <.001 | |
| 12-month visit | 174.0 (163.7, 184.2) | 217.3 (206.9, 227.6) | 43.3 (31.0, 55.6); <.001 | |
| 24-month visit | 151.8 (141.3, 162.3) | 190.1 (179.5, 200.7) | 38.3 (25.2, 51.5); <.001 | |
| Average FU effect | 176.2 (166.6, 185.8) | 218.1 (208.5, 227.8) | 42.0 (30.7, 53.2); <.001 | |
| Decline from 6 to 24 months | –51.0 (–63.4, –38.7) | –56.9 (–69.3, –44.5) | 5.9 (–11.6, 23.4); .511 | |
| 1041 | Baseline visit | 107.6 (101.4, 113.8) | ||
| 6-month visit | 111.7 (102.4, 121.0) | 152.1 (142.8, 161.4) | 40.4 (28.3, 52.5); <.001 | |
| 12-month visit | 95.1 (87.8, 102.3) | 131.3 (123.9, 138.6) | 36.2 (27.2, 45.2); <.001 | |
| 24-month visit | 82.1 (74.9, 89.3) | 111.2 (104.0, 118.5) | 29.2 (19.9, 38.4); <.001 | |
| Average FU effect | 96.3 (89.6, 102.9) | 131.5 (124.8, 138.2) | 35.3 (27.2, 43.3); <.001 | |
| Decline from 6 to 24 months | –29.6 (–37.9, –21.2) | –40.8 (–49.2, –32.4) | 11.2 (–0.6, 23.1); .062 | |
Notes: FU = follow-up visit; HE = health education; PA = physical activity.
Baseline Physical Function and Patterns in MVPA by Thresholds for PA and HE
A second aim of this investigation was to partition the data in Table 2 by baseline quartiles for the 400-m walk time to determine whether the effect of the thresholds on patterns of MVPA between PA and HE differed by participants’ level of physical function. Figure 2 provides a graphic display of the expected means (95% CIs) from models on untransformed outcomes and the data associated with this figure and results using the log transformation can be found in Supplementary Tables 2 and 3.
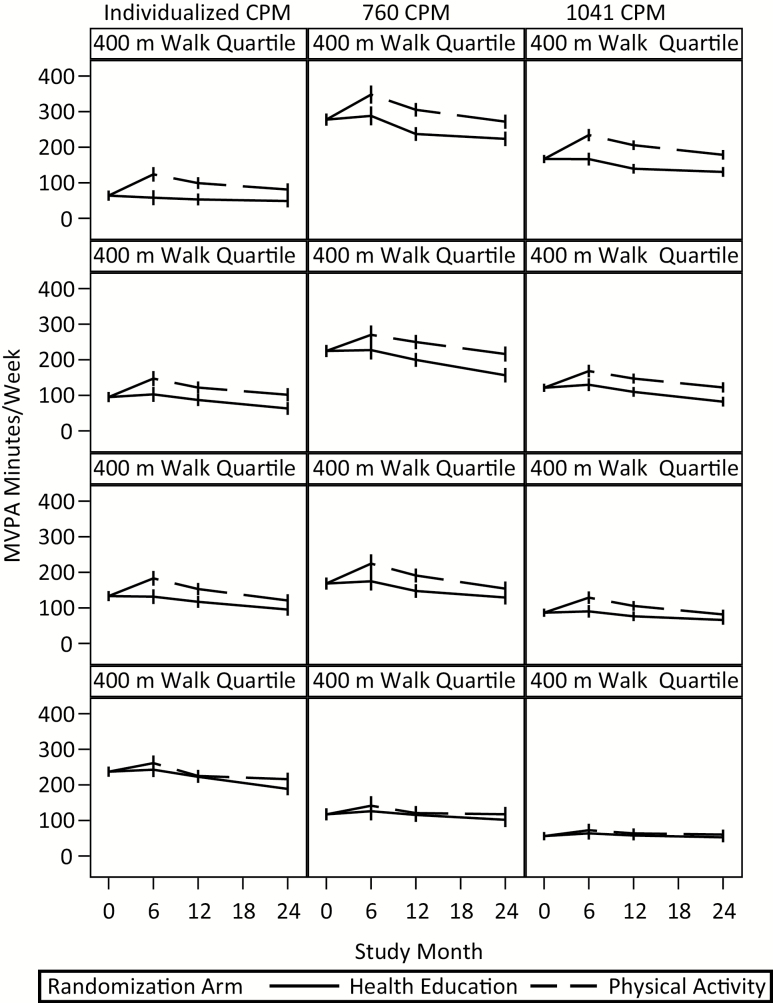
Figure 2.
Minutes of MVPA per week for different thresholds by quartiles of physical function (1st is fastest and 4th is slowest) of physical function (ie, 400 m walk time).
There are four interesting points to glean from this figure. First, irrespective of the threshold considered, there was a decrease in MVPA from month 6 to month 24 in the first three quartiles, irrespective of whether examining the data for either PA or HE (p < .0001 within both PA and HE; see Table 2). Second, ignoring the 4th quartile (ie, the slowest) due to the absence of an intervention effect, the difference in total volume of MVPA for the individualized threshold between the 1st and 3rd quartile for the PA group was less than the difference in MVPA using either 760 CPM or 1,041 CPM. To underscore this point, using the data represented in Figure 2, we computed the difference in average volume of weekly MVPA for those in the 1st versus 3rd quartile of function in the PA group at the 6-month time assessment. For the individualized threshold, the difference in weekly volume of MVPA between the 1st and 3rd quartile was –59.40 minutes; it was –123.4 minutes and –105.9 minutes for 760 CPM and 1,041 CPM, respectively. Third, in sharp contrast to the 1st quartile in Figure 2, note that for the 4th quartile, there were minimal differences in MVPA between PA and HE for any of the three thresholds at any of the follow-up visits, with the largest intervention difference being 27 minutes per week (95% CI 5, 50) for the individualized threshold at month 24 and the remaining differences being 15 minutes per week or less. In addition, for each threshold, tests of whether the average intervention effect across follow-up was the same between 1st and 4th quartiles identified significant differences for individualized and 1,041 CPM on both transformed and untransformed outcomes (p < .05) and for untransformed outcomes for 760 CPM (p = .003). And fourth, within the fourth quartile it is evident that the individualized CPM provides higher estimates of PA than either 760 CPM or 1,041 CPM. Despite the fact that the individualized CPM should be more sensitive to differences in physical activity between PA and HE, levels are essentially identical for these two groups emphasizing the fact that accelerometry, as operationalized in this investigation, has limitations when used with low functioning older adults.
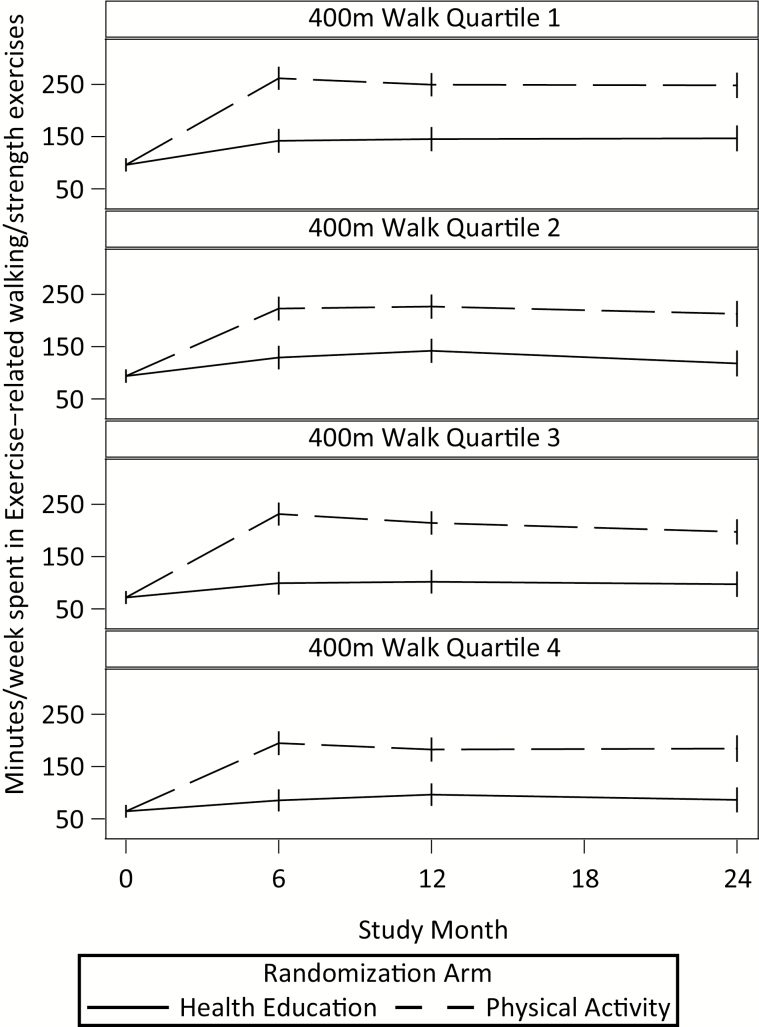
Figure 3 provides a graphic display of the self-reported PA estimated mean minutes/week by intervention group for each quartile. In contrast to the accelerometry data, participants in the PA arm self-reported greater levels of PA than HE across all four quartiles of physical function. For the interested reader, Supplementary Table 4 provides the correlations between the different accelerometry thresholds and the CHAMPS-5 at the different assessment visits. As one might expect, there was a strong relationship between the two fixed thresholds at each assessment visit (r = 0.97), whereas the individual threshold was modestly correlated with the two fixed thresholds with r values in the range of 0.37–0.46. The relationships between all accelerometry thresholds and PA as assessed by the CHAMPS-5 were weak with r values ranging from 0.02 to 0.23.
Figure 3.
Minutes of MVPA per week by quartiles of physical function.
In is important to note that participants in the 4th quartile had a more debilitating clinical profile than participants in quartiles 1–3. They had the highest BMI as compared to the average of Q1–Q3 (p < .0001) [means (±SD) by quartile from Q1 to Q4 were 28.45 (4.82) kg/m2, 30.00 (5.40) kg/m2, 30.31 (5.70) kg/m2, and 31.83 (7.25) kg/m2], and the lowest score on the DSST (p < .0001) [49.16 (12.52), 46.88 (12.82), 46.23 (12.34), 43.62 (12.82)]. Compared to the average of Q1–Q3, participants in Q4 also had a higher frequency of heart failure/congestive heart disease (p = .0003) [4.5%, 2.9%, 3.2%, 7.1%], a higher frequency of diabetes (p = .01) [24.1%, 23.2%, 23.2%, 29.7%], and a higher frequency of arthritis (p = .02) [14.1%, 19.4%, 19.9%, 23.0%]. See Supplementary Table 5 for all baseline characteristics by quartiles.
Finally, using Cox regression with accelerometry and self-reported levels of PA as time-dependent variables, Table 3 compares the effect that both the accelerometry and self-reported PA measures have on the hazard ratio for incident MMD overall and within quartiles of 400 m walk time. Note that the main intervention effect was within the two slowest quartiles (Q3 and Q4), with estimated reductions of at least 20% in those quartiles, but equal to 1% in the two fastest quartiles. Inclusion of the self-report PA measure in the model attenuates the intervention effect on MMD from a 34% reduction to a 12% reduction in Q3 and from 21 to 12% in Q4. There was less attenuation of the intervention effect when using any of the three different thresholds of MVPA from the accelerometry data.
Table 3.
Hazard Ratio (95% CI) for Initial MMD Among Participants with at Least Baseline Champs and Accelerometry Data*
| Walk Time Quartile† | Intervention Group Alone | Intervention Group + CHAMPS-5 | Intervention Group + Individualized CPM | Intervention Group + 760 CPM | Intervention Group + 1041 CPM |
|---|---|---|---|---|---|
| All | 0.82 (0.68, 0.99) | 1.03 (0.84, 1.26) | 0.78 (0.64, 0.94) | 0.94 (0.78, 1.13) | 0.93 (0.77, 1.13) |
| 1 | 0.99 (0.58, 1.70) | 1.15 (0.64, 2.04) | 1.23 (0.71, 2.15) | 1.28 (0.73, 2.23) | 1.35 (0.77, 2.36) |
| 2 | 0.99 (0.63, 1.56) | 1.21 (0.74, 1.97) | 1.04 (0.65, 1.65) | 1.08 (0.68, 1.71) | 1.06 (0.67, 1.68) |
| 3 | 0.66 (0.45, 0.95) | 0.88 (0.58, 1.34) | 0.65 (0.45, 0.95) | 0.70 (0.48, 1.02) | 0.68 (0.47, 1.00) |
| 4 | 0.79 (0.59, 1.04) | 0.88 (0.65, 1.19) | 0.80 (0.60, 1.07) | 0.83 (0.63, 1.11) | 0.82 (0.62, 1.09) |
*These analysis were based on a log transformation of the accelerometry measures with 0.5 being added to all scores to remove negative values.
†The 1st quartile represents participants with the best function whereas those in the 4th quartile had the poorest function. The time (gait speed) for each quartile for the 400-m walk was as follows: quartile 1 = ≤428s (≥0.934 m/s; quartile 2 = >428 s to ≤484 s (<0.934 m/s to ≥0.826 m/s); quartile 3 = >484 s to ≤568 s (<0.826 m/s to ≥0.704 m/s); quartile 4 = >568 s (<0.704 m/s).
Discussion
The results of this investigation support the position that defining MVPA using different absolute thresholds—760 CPM (8) or 1,041 CPM (7)—or an individualized threshold based on age and 400 m walk time (4) did not alter interpretation that the PA intervention in the LIFE study led to greater change in MVPA than HE. Additionally, irrespective of the threshold used, the greatest difference between PA and HE was observed at the 6-month follow-up visit, with a gradual decline in MVPA for both PA and HE from 6 to 24months. However, the use of different thresholds did result in different absolute values of MVPA with the fixed thresholds being particularly problematic when partitioning the cohort by baseline level of physical function. For example, at 6 months, the average weekly minutes of MVPA for the PA group was 178.5 minutes/week using the individualized threshold, 247 minutes/week using 760 CPM, and 152.2 minutes/week using 1,041 CPM (see Table 2). At 6 months, the difference in total minutes of MVPA between the 1st and 3rd quartiles of function was –59.40 minutes using the individualized (relative) threshold, whereas it was –123.4 minutes and –105.9 minutes for 760 CPM and 1,041 CPM, respectively. This finding reinforces existing research showing that thresholds for MVPA are highly influenced by the fitness levels of participants, supporting the case for using individualized (relative) thresholds (10–12).
In previous research investigating individualized thresholds for accelerometry, researchers have relied upon a comparison of accelerometry counts relative to the percentage of VO2max during treadmill exercise (10–12). An exception is work by Pruitt and colleagues (19) as part of the LIFE-pilot study. Whereas, Pruitt and colleagues used accelerometer counts assessed during a 400-m self-paced walk test to determine individualized thresholds, the current study used a formula published by Rejeski and colleagues (4) that enabled prediction of individualized thresholds for MVPA based on median CPM achieved with the Actigraph accelerometer during actual training bouts of MVPA. Whereas investigators interested in using accelerometry in older adult populations may continue to use fixed thresholds to define MVPA, it is worth re-emphasizing that in older adults similar to LIFE or when studying older adults that are sedentary, it is inappropriate to use thresholds of 1952 CPM (5) or 2020 CPM (6) to define MVPA since approximately only 20% of the LIFE cohort was capable of achieving such intensities during structured bouts of activity (4). This raises concern regarding conclusions reached on the PA behavior of older adults in recent large epidemiological studies that employ 2020 CPM as the threshold for MVPA (6,20). Additionally, one can expect significant over- and under-estimation of MVPA among older adults using fixed thresholds due to the wide variability in their individual levels of physical functioning and fitness.
Irrespective of the threshold used, the lack of meaningful group differences in MVPA between PA and HE within the 4th (lowest) quartile of physical function was unexpected since, as reported in the LIFE study main outcomes paper (13), there was a trend for those lower in physical function to derive the most benefit in terms of the prevention of MMD. In fact, as shown in Table 3, the effect for the LIFE PA intervention on MMD can be attributed to the benefit derived by participants in the 3rd and 4th quartiles of baseline 400 m walk time. Interesting, when using the CHAMPS-5 self-report measures which captured both walking and strength training, participants in the 4th quartile of baseline physical functioning of the PA group reported activity levels that were considerably higher than those in the HE group. Also, the difference in self-reported activity levels between PA and HE attenuated the between group difference in incident MMD both in the overall analysis and in 3rd and 4th quartiles, suggesting that data from the CHAMPS-5 may be particularly valuable when examining dose–response effects in the LIFE study. There was less attenuation when using the accelerometry data, an effect that was most evident within the 3rd quartile with a similar pattern, albeit less marked, for the 4th quartile. Parenthetically, participants in the 4th quartile were more clinically disadvantaged than participants in Q1–Q3. Further examination of this subgroup is warranted to better understand the challenges they face and the content of an intervention that may best serve their needs.
The pattern of accelerometry data between the PA and HE groups in the 4th quartile, and the fact that there was less attenuation of group differences in MMD with accelerometry, have led us to offer the following explanations. First, these data cast doubt on whether the Actigraph accelerometer when worn at the hip is sufficiently precise for capturing levels of MVPA among older adults with low levels of physical function. Second, standing and sitting exercises that involve balance and/or resistance training in the LIFE protocol could well be misclassified as sedentary behavior via accelerometry, yet be pivotal in preventing MMD. It is important to underscore the fact that in the current study the CHAMPS-5 self-report measure of PA included items related to both walking and strength training; hence, the content of this measure had improved sensitivity to the LIFE PA intervention. Also, one could also argue that self-report measures of PA such as the CHAMPS-5 capture activity relative to an individual’s capacities and may be more sensitive to the heterogeneity in function observed in the 4th quartile of this cohort. For example, Zisko and colleagues (12) recently reported that among older men aged 70–77 years, the threshold for MVPA can go as low as 205 CPM. The finding that the correlations between the CHAMPS-5 and the various measures of accelerometry defined MVPA were so low supports the conclusion that these measures are capturing different behaviors.
And third, software algorithms and protocols for evaluating accelerometry data in physically compromised older adults is an area that deserves attention given the growing interest in assessing PA under free-living conditions across the lifespan and the importance of identifying interventions to prevent MMD in our aging population. In subsequent research with the elderly, investigators using accelerometry would be well advised to include intervention-specific self-report markers of PA behavior.
Limitations
This study is not without limitations. First, typical of most studies in the accelerometry literature, our data analyses were limited to the assessment of accelerations in the vertical axis. There is at least one study on older men suggesting that vector magnitude, which accounts for accelerations in all three axes, is a more sensitive measure (12). Also, it is possible that the use of machine learning methodology or other advanced analytic methods of processing data from all three axes of the accelerometer would enable researchers to capture the unique movement patterns of participants with very low levels of function. Notwithstanding this criticism, there still exists the problem of what threshold to use in defining MVPA behavior for this population. In fact, individuals who have severely compromised physical functioning may only employ a single gait speed when ambulating. In this sense, perhaps all movement should be classified as MVPA. Second, accelerometry data was collected prospectively for 7 days whereas self-reported PA was collected retrospectively for the past month. Despite this methodological difference, it still remains that self-reported PA for those in the 4th quartile of function was higher for participants in PA than HE and self-reported PA attenuated the intervention effect on MMD. Neither effect was observed when using the three different accelerometry thresholds for MVPA. Our intent is not to discourage the use of accelerometry with older adults who have severely compromised physical function; rather, it is to motivate researchers to consider alternative methods for better understanding patterns of PA in this important and quickly expanding population.
Summary
The use of three different accelerometry thresholds—individualized, 760 CPM or 1,041 CPM—to define the threshold for MVPA in this study of older adults resulted in similar differences between PA and HE at all follow-up visits; however, when examining these data by quartile of baseline physical function, there were no meaningful differences in MVPA between PA and HE for the 4th quartile. Furthermore, irrespective of the threshold used or the quartile of baseline function considered, there was less attenuation of the intervention effect on MMD using accelerometry than when using self-reported differences in PA. This was true in both the overall analysis and in the analysis of participants in the 3rd and 4th quartiles of baseline physical function, the two quartiles in which most incident MMD events occurred. Thus, we conclude that a combination of self-report and objective measures are warranted for charting the PA behavior change with intervention studies in the elderly.
Supplementary Material
Supplementary Material is available at The Journals of Gerontology Series A: Biological Sciences and Medical Sciences online.
Funding
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. The first author was supported, in part, from the following mechanisms: (a) National Heart, Lung, and Blood Institute grant R18 HL076441, and (b) National Institutes for Aging grant P30 AG021332.
Conflict of Interest
None of the authors declare any conflict of interest related to this manuscript.
Supplementary Material
References
- 1. Healy GN, Dunstan DW, Salmon J et al. . Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–666. doi:10.2337/dc07-2046 [DOI] [PubMed] [Google Scholar]
- 2. Azevedo LF, Dos Santos MR. High-intensity intermittent exercise training for cardiovascular disease. J Novel Physiother. 2014;4:1–8. doi:10.4172/2165-7025.1000199 [Google Scholar]
- 3. Nelson ME, Rejeski WJ, Blair SN et al. ; American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi:10.1161/CIRCULATIONAHA.107.185650 [DOI] [PubMed] [Google Scholar]
- 4. Rejeski WJ, Marsh AP, Brubaker PH et al. ; LIFE Study Investigators. Analysis and interpretation of accelerometry data in older adults: The LIFE study. J Gerontol A Biol Sci Med Sci. 2016;71:521–528. doi:10.1093/gerona/glv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi:10.1097/00005768-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 6. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi:10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 7. Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Act. 2009;17:17–30. doi:10.1123/japa.17.1.17 [DOI] [PubMed] [Google Scholar]
- 8. Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11 Suppl):S512–S522. doi:10.1249/01.mss.0000185659.11982.3d [DOI] [PubMed] [Google Scholar]
- 9. Evenson KR, Wen F, Metzger JS, Herring AH. Physical activity and sedentary behavior patterns using accelerometry from a national sample of United States adults. Int J Behav Nutr Phys Act. 2015;12:20. doi:10.1186/s12966-015-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller NE, Strath SJ, Swartz AM, Cashin SE. Estimating absolute and relative physical activity intensity across age via accelerometry in adults. J Aging Phys Act. 2010;18:158–170. doi:10.1123/japa.18.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ozemek C, Cochran HL, Strath SJ, Byun W, Kaminsky LA. Estimating relative intensity using individualized accelerometer cutpoints: the importance of fitness level. BMC Med Res Methodol. 2013;13:53. doi:10.1186/1471-2288-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zisko N, Carlsen T, Salvesen Ø et al. . New relative intensity ambulatory accelerometer thresholds for elderly men and women: the Generation 100 study. BMC Geriatr. 2015;15:97. doi:10.1186/s12877-015-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pahor M, Guralnik JM, Ambrosius WT et al. ; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi:10.1001/jama.2014.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marsh AP, Lovato LC, Glynn NW et al. ; LIFE Study Research Group. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi:10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. doi:10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman AB, Simonsick EM, Naydeck BL et al. . Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi:10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 17. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 18. Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2010;66:891–896. doi:10.1111/j.1541-0420.2009.01332.x [DOI] [PubMed] [Google Scholar]
- 19. Pruitt LA, Glynn NW, King AC et al. . Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Act. 2008;16:416–434. doi:10.1123/japa.16.4.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pate RR, Taverno Ross SE, Liese AD, Dowda M. Associations among physical activity, diet quality, and weight status in US adults. Med Sci Sports Exerc. 2015;47:743–750. doi:10.1249/MSS.0000000000000456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.