Abstract
Background/Objectives
Accelerometry measures older adult (in)activity with high resolution. Most studies summarize activity over the entire wear time. We extend prior work by analyzing hourly activity data to determine how frailty and other characteristics relate to activity among older adults.
Methods
Using wrist accelerometry data collected from the National Social Life, Health and Aging Project (n = 651), a nationally-representative probability sample of older adults, we used mixed effects linear regression to model the logarithm of hourly counts per minute as a function of an adapted phenotypic frailty score, adjusting for demographic and health characteristics, season, day of week and time of day.
Results
Higher frailty scores were associated with modestly lower activity; each frailty point (0–4) corresponded to a 7% lower mean hourly counts per minute. Older age, more comorbidities, male gender, and higher BMI were also associated with lower activity, though the latter was not evident among frail respondents. After adjusting for differences associated with frailty and other covariates, a substantial amount of between-individual variability in activity remained, as well as within-individual variability across days.
Conclusion
Our findings indicate that frail elders, men, those who are older, overweight or have multiple comorbidities are most likely to have low activity. However, residual differences between individuals remain larger than the differences associated with frailty and other covariates. We suggest defining individual-specific activity goals and further research to identify the sources of between-individual variability to better understand how activity reflects health status and to permit the development of more effective interventions.
Keywords: Physical activity, Accelerometry, Frailty, NSHAP
The advent and broad adoption of accelerometry-based activity monitors raises the possibility of using objective activity measures as health indicators among frail older adults for whom maintaining mobility is essential for independent living (1,2). The traditional brief (15–30 minutes) clinical assessment of physical activity relies on self-report of past events and “typical” activity. While this face-to-face encounter is the primary vehicle for medical care delivery, it may not alone provide an ideal assessment of actual patient health behaviors, function or mobility.
Accelerometry permits an objective, continuous activity assessment in the free-living environment (3). Most accelerometry research has used summary measures, averaged over the entire wear time (eg, average daily activity counts per minute (CPM), average proportion of the day spent in various activity intensities, etc.) (4). However, accelerometry data are recorded at much higher resolution (eg, once per minute or higher), and there is growing interest in determining whether more detailed mobility patterns inform health (5–7). Several investigators have examined the activity of older adults across the day using smoothing functions (6) and spline regression (8). Others have described accelerometry-based patterns of falls in adults with progressive supra-nuclear palsy using wavelet analysis (9). This work highlights the utility of analyzing high resolution accelerometry data and of accounting for systematic changes in activity level throughout the day.
The increasing use of accelerometers raises several questions about the relevance of objective activity specifically to older adults. For example, how are specific health conditions associated with activity level? How does activity level vary among demographic and clinical subgroups? How do differences in average activity level between subgroups compare to variability between individuals within those subgroups or across days within the same individual? Throughout the life span, men and women have differing levels and types of activity (10,11), and the circadian activity patterns in older adults change with age (6). Few studies, however, have examined demographic and health-related predictors of activity in the general population of older adults while considering other sources of variability in activity level, such as the systematic tendency of all older adults to be most active in the morning and the tendency of individuals to follow specific routines (5,11).
Identifying factors associated with activity level among clinically relevant subgroups is needed to establish appropriate therapeutic targets. Frailty status is increasingly being used to risk-stratify older patients (12), and inactivity is both a defining feature of frailty (13) and a common target of frailty interventional studies (14). Physical activity is considered the model older adult health intervention given its relevance to most if not all older adults, its low side effect profile, and its global physiologic health benefits (15). Understanding how objectively measured activity relates to clinical frailty criteria would lead to improved frailty assessment and better tailored interventions, thereby advancing the field.
Using wrist accelerometry data from the National Social Life, Health and Aging Project (NSHAP), we analyzed hourly activity among a U.S. nationally-representative sample of older, home-dwelling adults. We used mixed effects linear regression with penalized splines to model the log10 mean CPM (calculated hourly) as a function of frailty and other demographic and clinical characteristics, and to estimate the residual variability between individuals and within individuals across days, adjusting for time of day, day of week and season. Our primary aims were: (i) to determine how hourly activity level is related to clinical frailty criteria in older adults; (ii) to identify demographic and clinical characteristics associated with differences in hourly activity level adjusting for frailty; and (iii) to estimate the magnitude of variation in activity level between individuals. Our secondary aim was to explore whether the effects of frailty and other covariates on hourly activity varied across the day.
Methods
Study Design
NSHAP is a U.S. national, longitudinal survey study that collects extensive information on physical, mental, cognitive, and social health (16). The study began in 2005–2006 (Wave 1) with a nationally-representative probability sample of community-dwelling adults born between 1920 and 1947 (aged 57–85 years), including oversamples of African Americans, Hispanics, and men. Three-thousand and five respondents participated, corresponding to a weighted response rate of 75.5%. Five years later (Wave 2, 2010–2011), respondents remaining alive were reinterviewed as were their cohabiting spouse or partner, for a total Wave 2 sample size of 3,377. Respondents were interviewed in their homes by professional interviewers from the National Opinion Research Center (NORC). The study was approved by NORC’s Institutional Review Board and secondary analysis of deidentified data was deemed exempt from further review from the University of Chicago Institutional Review Board. The NSHAP data are publically available and can be obtained from the National Archive of Computerized Data on Aging (http://www.icpsr.umich.edu/icpsrweb/NACDA/studies/34921) after completing a Data Use Agreement. The Stata code for this analysis has been posted on GitHub at https://github.com/mhuisingh/frailty-hourly-accel.
Wrist Accelerometry Substudy
Wrist accelerometry data were collected from a randomly-selected subset of 793 Wave 2 respondents; of these, 55 were spouses or partners not born between 1920 and 1947 and were excluded from this analysis (17). Black/African American respondents and those with more comorbidities were less likely to agree to participate in the accelerometry sub-study. Respondents were asked to wear an ActiWatch Spectrum® on their nondominant wrist continuously for 72 consecutive hours (including during bathing or swimming) (17). The Actiwatch Spectrum is a validated, omnidirectional, piezo-electric accelerometer used to measure sleep and activity (18,19). The filtered accelerometer signal is sampled at a frequency of 32Hz, with the maximum absolute value within each second summed over 15-second epochs to yield a count. Data were preprocessed using the Actiware® software available from the manufacturer (19). Wear time was recorded using a highly sensitive, built-in galvanic heat sensor that identified when the device was being worn; nonwear periods were excluded. Once put on, the devices were rarely removed (only 0.17% of epochs across all respondents during the days analyzed here were classified as off-wrist), so missing data were not imputed. Only days with at least 10 hours of daytime monitoring were included. Sundays were also excluded due to routinely lower activity on this day compared to other days among older adults (20). The primary rest intervals (one per 24-hour period) were identified using event markers, changes in ambient light data, sleep logs, and independent review by at least two study investigators and were excluded from all analyses (21). To further standardize measurement across respondents, we excluded hours of the day having wake time activity data from fewer than 45% of the sample. Hours between 11:00 pm and 6:59 am included wake time data from fewer than 293 (range 7–193) individuals per hour as most study participants were still asleep, so these hours were excluded. Hours between 7:00 am and 10:59 pm included wake time data from at least 308 (range 308–650) individuals per hour, so these hours were included. Of note, older respondents were more likely to have three valid days of accelerometry wear (as opposed to only one or two), indicating that our strategy of including all available days for all respondents may if anything overweight our analysis toward the oldest respondents (as opposed to under-representing them).
Hourly Activity
For each respondent, we calculated hourly mean CPM by summing the activity counts separately for each hour of the day and dividing by 60 minutes per hour. Hours with less than 60 minutes of accelerometry measurements were excluded (~13% of the hours, typically those in which a respondent went to bed or awoke midway through the hour).
Frailty
We used an adapted 4-point phenotypic frailty scale for which respondents received one point for each of four criteria (13,17). Weakness was identified using performance on a timed chair stands exercise. Respondents were asked to stand up and sit-down from a chair five times as quickly as possible, and were considered weak if they required ≥16.7 seconds to complete the exercise, were wheelchair bound or could not complete the task safely (17). Slow gait was measured directly by asking respondents to walk 3 meters at their “usual” pace; those requiring ≥5.7 seconds (or 0.53 m/s) to complete the faster of two timed walks—as well as those who were wheelchair bound or could not complete the exercise safely—were determined to have slow gait (17). The chair stand and timed gait cut-points were determined from the original Short Physical Performance Battery scoring (22). Exhaustion was determined using two modified Center for Epidemiologic Studies Depression (CESD) scale questions (23). Respondents were asked how often over the last week they felt that everything was an effort and how often they felt that they could not get going. Answer categories for both questions were: (i) rarely or none of the time, (ii) some of the time, (iii) occasionally, or (iv) most of the time. A point for exhaustion was given if respondents answered “occasionally” or “most of the time” to either question (17). Finally, low physical activity was assessed by asking respondents the following question: “On average over the last 12 months, how often have you participated in vigorous physical activity or exercise? By vigorous physical activity, we mean 30 minutes or more of things like sports, exercise classes, heavy housework, or a job that involves physical labor.” Answer categories were: (i) five or more times per week, (ii) three or four times per week, (iii) one to two times per week, (iv) one to three times per month, (v) Less than one time per month, or (vi) Never. Respondents were given a point for low physical activity if they reported engaging in three or fewer vigorous activities per month (answers iv–vi) (17). Respondents were identified as frail if they scored ≥3 points, prefrail if they score 1–2 points, and nonfrail if they scored 0 points. A sensitivity analysis exploring a 5-point frailty scale including weight loss (Wave 1–Wave 2 weight) did not significantly change results but did reduce sample size by 205 respondents. Therefore, we present results for the 4-point frailty scale to maintain sample size.
Covariates
Age on the date of the interview was calculated using reported date of birth. Gender (women versus men), race (white/Caucasian, black/African American, other), Hispanic ethnicity, and employment status (currently working versus not working) were self-reported. Measured weight and height were used to calculate body mass index (BMI) (24). Cognitive function was assessed using the survey-adapted Montreal Cognitive Assessment (MoCA-SA) as previously described in detail. MoCA scores (range 0–30) are estimated from the 18-item MoCA-SA using a linear prediction model (25,26). A modified Charlson Comorbidity Index (range 0–16) was constructed using self-reported comorbidity data in Wave 2. Respondents were asked whether they had ever been told by a doctor that they had the following conditions (number of points given in parentheses): congestive heart failure (1), heart attack (1), coronary procedure (1), stroke (1), diabetes (1), rheumatoid arthritis (1), asthma, emphysema, chronic obstructive pulmonary disease, or chronic bronchitis (1), dementia (1), non-metastatic cancer excluding skin-cancer (2), or metastatic cancer excluding skin cancer (6) (27).
Statistical Analysis
A mixed effects linear regression model was fit to the log10 of the hourly mean CPM (28); the logarithmic transformation was used to reduce skew and stabilize the variance. In addition to covariates measuring respondent attributes, we also included hour of day, day of week (Monday through Saturday) and month, all modeled as discrete factors, to account for possible systematic differences in activity level throughout the day and week, as well as differences by season. Random effects representing overall differences in activity level between respondents and between individual days within respondent were included, under the usual assumptions that they are normally distributed and uncorrelated. The model was fit to the overall sample and separately to subgroups based on frailty status, gender, and age. To determine whether covariate effects differed depending on time of day, we also fit the model separately to the data for three time-of-day segments: morning (7:00 am–12:00 pm), afternoon (12:00 pm–5:00 pm), and evening (5:00 pm–10:59 pm). Tests of the differences in covariate effects across subgroups and time of day were performed by fitting a model to the entire sample containing interaction terms between each covariate and the subgroup (or time of day) indicator; the interaction terms involving a given covariate were then jointly tested using a Wald test.
To visualize changes in activity level across the day, we refit the regression models described above replacing the hour of day factor variable with a penalized spline (29). We then plotted the estimated spline terms after adding the overall marginal mean for the sample (or subgroup). The resulting plots show the average change in mean activity from 7:00 am to 10:59 pm, adjusting for the covariates.
All analyses (including the descriptive statistics in Table 1) were weighted using the sampling weights distributed with the data set, and may therefore be used to make inferences about the U.S. population of adults born between 1920 and 1947. These weights account for differences in the probability of selection as well as differential nonresponse by age and race/ethnicity. Standard errors were obtained for all analyses using the sandwich variance estimator to account for nonindependence within Primary Sampling Units (PSUs) (this variance estimator also has the benefit of being robust to certain departures from model assumptions) (30). Statistical analyses were conducted with Stata 15 (StataCorp. 2015. Stata Statistical Software: Release 15. College Station, TX: StataCorp LP).
Table 1.
Descriptive Statistics (n = 651)
|
Women
Participants: N = 341 Hours: N = 10,197 |
Men
Participants: N = 310 Hours: N = 9,066 |
|||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Age | ||||
| Mean (years) | 71.3 | 70.5–72.1 | 72.5 | 71.6–73.3 |
| 62–70 (%) | 55.1 | 48.4–61.6 | 47.7 | 41.7–53.8 |
| 71–80 (%) | 32.2 | 26.5–38.6 | 36.2 | 30.6–42.2 |
| 81+ (%) | 12.7 | 9.3–17.0 | 16.1 | 11.6–22.1 |
| Race/Ethnicity (%) | ||||
| Black/African American | 7.4 | 4.8–11.4 | 6.0 | 3.7–9.7 |
| White/Caucasian | 84.0 | 78.5–88.3 | 83.9 | 78.8–88.0 |
| Hispanic (non-Black) | 5.1 | 3.1–8.2 | 6.3 | 3.6–11.0 |
| Other | 3.5 | 1.5–7.8 | 3.7 | 2.0–6.7 |
| Employment (%) | ||||
| Working | 23.9 | 18.5–30.2 | 28.4 | 21.9–35.9 |
| Body Mass Index | ||||
| Mean (kg/m2) | 28.9 | 28.2–29.5 | 29.1 | 28.4–29.8 |
| 18–24 (%) | 24.3 | 19.9–29.4 | 19.4 | 14.7–25.1 |
| 25–30 (%) | 42.0 | 36.2–48.0 | 45.5.0 | 39.2–51.9 |
| 31+ (%) | 33.7 | 28.7–39.1 | 35.1 | 28.8–42.0 |
| Modified Charlson Comorbidity Index * | 0.8 | 0.6–0.9 | 1.2 | 1.0–1.4 |
| Montreal Cognitive Assessment—Survey Adapted ** | 23.9 | 23.3–24.4 | 22.8 | 22.1–23.4 |
| Hourly Counts Per Minute During Wake Hours Between 7:00 am and 10:59 pm (CPM) | ||||
| Hourly CPM (mean) | 244.2 | 231.7–256.7 | 199.6 | 186.3–213.0 |
| Log-Transformed Hourly CPM (mean) | 2.3 | 2.2–2.3 | 2.2 | 2.2–2.2 |
| Frailty*** | ||||
| Nonfrail | 36.5 | 31.0–42.4 | 37.1 | 30.3–44.5 |
| Prefrail | 47.0 | 41.0–53.0 | 50.7 | 43.7–57.7 |
| Frail | 16.5 | 12.6–21.3 | 12.1 | 8.6–16.9 |
| Frailty (mean) | 1.2 | 1.1–1.4 | 1.1 | 0.9–1.3 |
Note: *Modified Charlson Comorbidity Index: Range 0–16. 1 point each: congestive heart failure, prior heart attack, prior coronary procedure, stroke, diabetes, rheumatoid arthritis, asthma/emphysema/chronic obstructive pulmonary disease/chronic bronchitis, dementia; 2 points: nonmetastatic cancer (nonskin); 6 points: metastatic cancer (nonskin). **Survey-Adapted Montreal Cognitive Assessment (MoCA-SA): An 18-item version of the original 28-item Montreal Cognitive Assessment (MoCA), adapted for use in a largescale, survey-based studies. MoCA scores are predicted from MoCA-SA scores, range 0–30. ***Adapted Phenotypic Frailty Scale: Range 0–4, point assigned for slow gait, slow chair stands, self-reported exhaustion, low self-reported physical activity.
Results
After the exclusions described above, there were 10,197 hours from 341 women and 9,066 hours from 310 men available for the regression analysis. The demographic characteristics and distribution of frailty among our analytic subsample is noted in Table 1. Although men and women were roughly comparable in terms of frailty, the mean hourly CPM during awake hours was significantly lower among men than women (199.6 vs 244.2, p < .001). This subsample of accelerometry participants had fewer frail elders, particularly among women, compared to the entire age-eligible Wave 2 NSHAP sample though the mean frailty scores were similar (Women: frail prevalence: 16.5%, mean frailty score 1.2; Men: frail prevalence: 12.1%, mean frailty score 1.1).
Mixed Effects Regression Models
After adjusting for demographic and health characteristics, season, day of week and time of day, frailty was associated with lower mean log10 hourly CPM, with each frailty point corresponding approximately to 7% (10−0.03 = 0.93) lower hourly activity (Table 2). Men were less active than women, with a difference approximately equal to 3 frailty points or an additional 14 years of age. Comorbidities and higher BMI were also associated with lower activity. Quadratic terms for BMI, CCI, and age were examined, and suggested a nonlinear (ie, a decreasing marginal effect with increasing BMI) effect of BMI (BMI2: p = .004, CCI2: p = .22, age2: p = .11). Race/ethnicity, working status, and cognitive function (linear or quadratic terms) did not have statistically significant associations with activity.
Table 2.
Adjusted Mixed Effects Linear Regression: Predictors of Log10-Transformed Hourly Counts Per Minute (Wrist Accelerometry, 7:00 am–10:59 pm) Among Older, U.S. Adults in the Overall Sample and by Frailty Status*
| Total Sample N = 651 | Nonfrail N = 225 | Prefrail N = 330 | Frail N = 96 | |
|---|---|---|---|---|
| Covariate | β (p value) | β (p value) | β (p value) | β (p value) |
| Frailty** | –0.03 (p ≤ .001) | NA | NA | NA |
| Age (per decade) | –0.06 (p < .001) | –0.09 (p < .001) | –0.05 (p = .004) | –0.06 (p = .09) |
| Female Gender | 0.08 (p < .001) | 0.07 (p < .001) | 0.09 (p < .001) | –0.004 (p = .93) |
| Body Mass Index | –0.004 (p = .004) | –0.01 (p = .007) | –0.005 (p < .001) | 0.001 (p = .55) |
| Race/Ethnicity | ||||
| Black | –0.03 (p = .23) | –0.03 (p = .44) | –0.02 (p = .60) | –0.05 (p = 0.43) |
| Hispanic | 0.02 (p = .50) | –0.007 (p = .87) | 0.02 (p = .66) | –0.004 (p = .96) |
| Other | 0.03 (p = .31) | 0.11 (p = .001) | 0.03 (p = .51) | –0.46 (p < .001) |
| White | Ref | Ref | Ref | Ref |
| Working | 0.03 (p = .14) | –0.002 (p = .93) | 0.05 (p = .047) | 0.02 (p = .81) |
| MoCA-SA*** | –0.002 (p = .23) | –0.001 (p = .69) | –0.004 (p = .13) | 0.001 (p = .85) |
| Modified CCI**** | –0.02 (p < .001) | –0.02 (p = .03) | –0.03 (p < .001) | –0.02 (p = .08) |
| Standard Deviation of Variance Components | ||||
| Individual | 0.16 | 0.14 | 0.16 | 0.19 |
| Day of Week Within Individual | 0.07 | 0.06 | 0.08 | 0.07 |
| Residual | 0.30 | 0.29 | 0.30 | 0.33 |
Notes: CCI = Modified Charlson Comorbidity Index; SD = standard deviation; NA = not applicable. The bolded values and p-values indicate the findings are statistically significant. *Models adjusted for hour of day, month of wear, day of week, and survey design. Hours with less than 60 minutes, Sundays, and the 11:00 pm–6:59 am interval were excluded from analyses. **Adapted Phenotypic Frailty Scale: Range 0–4, point assigned for slow gait, slow chair stands, self-reported exhaustion, low self-reported physical activity. ***Survey-Adapted Montreal Cognitive Assessment (MoCA-SA): An 18-item version of the original 28-item Montreal Cognitive Assessment (MoCA), adapted for use in a largescale, survey-based studies. MoCA scores are predicted from MoCA-SA scores, range 0–30. ****Modified Charlson Comorbidity Index: Range 0–16. 1 point each: congestive heart failure, prior heart attack, prior coronary procedure, stroke, diabetes, rheumatoid arthritis, asthma/emphysema/chronic obstructive pulmonary disease/chronic bronchitis, dementia; 2 points: nonmetastatic cancer (nonskin); 6 points: metastatic cancer (nonskin).
Refitting the model separately by frailty group yielded the largest estimated effect of age among the nonfrail cohort, however a test of the interaction between age and frailty group was not statistically significant (p = .15). The effects of gender and comorbidities on hourly activity did not appear to vary based on frailty status, however the effect of BMI decreased with worsening frailty status (p = .005 for a test of the interaction between BMI and frailty group).
Separate models fit to gender and age subgroups (and tests of differences in effects across models) did not provide any evidence for differences in the association between frailty and activity (across gender models: p = .44; across age models: p = .98), and the same was true for age (across gender models: p = .42), BMI (across gender models: p = .86; across age models: p = .88) and comorbidities (across gender models: p = .27; across age models: p = .73) (Supplementary Table 1).
The estimated standard deviation of the individual-level random effects was 0.16 for the model fit to the entire sample (Table 2)—roughly five times the estimated magnitude of the decrease in activity level associated with each additional frailty point. This estimated variability of the random effects increased slightly with increasing frailty. The standard deviation of the within individual day-of-week effect was 0.07 in the overall sample—roughly twice the estimated magnitude of an additional frailty point—and was similar across subgroups. This variability between individuals, as well as the variability within individuals across days of wear, is further illustrated in Supplementary Figure 1 which plots the hourly CPM across days for four selected respondents. There is substantial variability in hourly activity between and within individuals.
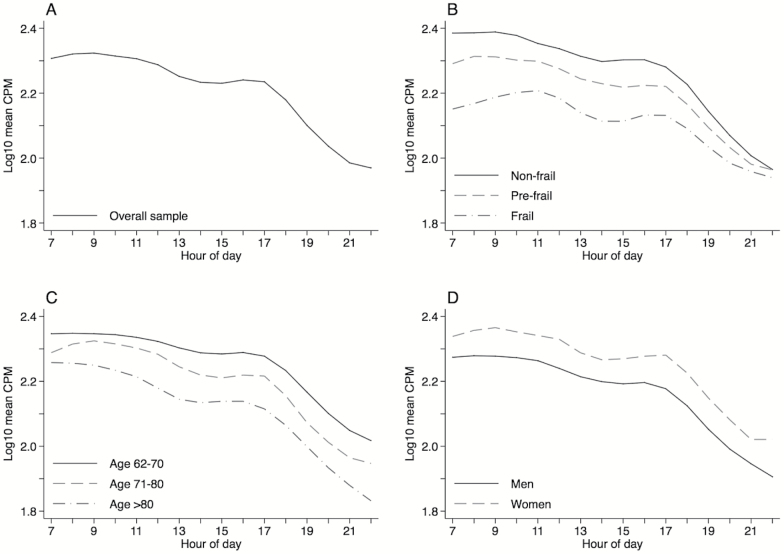
Adjusted hourly activity plotted across the day (7:00 am–10:59 pm) for the overall sample and for frailty, age, and gender subgroups is shown in Figure 1A–D. Compared to nonfrail adults, frail adults reach their peak activity later in the morning, and all three frailty groups converge toward the end of the day (Figure 1B). This is consistent with the regression models stratified by time of day (Supplementary Table 1), which show the estimated effect of frailty to be smallest in the evening (p = .023 for a test of the interaction between frailty and time-of-day). In contrast, the effect of age appears to be slightly smaller in the morning (Figure 1C and Supplementary Table 1; p = .039 for a test of the interaction between age and time-of-day), while the curves for men and women are largely parallel (Figure 1D).
Figure 1.
Multivariate mixed effects linear regression models fit with a penalized spline for hour of day and adjusted for frailty, age, gender, body mass index, race/ethnicity, working status, cognitive function, comorbidity burden, month of wear, day of week, hour of day, and survey design were used to plot the average change in mean hourly CPM during wake hours between 7:00 am and 10:59 pm among older U.S. adults. Activity curves are plotted separately for the overall sample (A) and by frailty status (B), age category (C), and gender (D).
Discussion
These results demonstrate an association between clinical frailty and objectively-measured hourly activity level in the general population of older adults that is uniformly present across gender and age subgroups, a new contribution to the literature. This association persists despite adjusting for additional potential confounders such as BMI and comorbidity burden. We further found that the greatest difference in hourly activity among frailty subgroups was in the morning hours of the day, and frail adults tend to have delayed peak morning activity. This delay in activity may reflect symptoms of fatigue and exhaustion, also commonly present in this syndrome. Understanding this frailty-associated activity pattern may provide new insight into detecting frailty. However, the magnitude of the association between frailty and hourly activity is modest relative to associations between activity level and demographic characteristics and is similar in magnitude to having an additional comorbidity. Thus, although frailty is in part defined by the presence of low activity, empirically it is by no means synonymous with it. While individuals clinically identified as frail are indeed at higher risk of having low activity, other characteristics (including perhaps some not studied here) predict objective low activity as well or better.
Characteristics associated with lower physical activity include male gender, older age, higher BMI, and a higher comorbidity burden (8,31). Current guidelines provide general physical activity targets for the entire older adult population ≥ 65 (32). We found that hourly activity was substantially lower at older ages regardless of the subgroup analyzed (we estimate an approximate 13% decrease in hourly activity for each additional decade of age). While activity was lower across the day with advancing age (Figure 1C), regression models stratified by time of day suggest advancing age may have a bigger impact in the afternoon and evening compared to the morning hours as others have previously described among older adults (6). Using the same activity targets for all older adults may thus be inappropriate, and age-adjusted goals may provide more achievable benchmarks that can still improve health. Supporting this idea, recent work in older osteoarthritis patients found that maintenance of high functioning was obtained with as few as 45 minutes (compared to the recommended 150 minutes) of moderate-vigorous activity per week (33). Furthermore, age-associated conditions (eg, gait impairment) typically increase the energy cost of activity, suggesting that moderate to vigorous activity may be achieved at lower thresholds for older adults (34). The drop in afternoon activity suggests an intervention for older adults targeting these hours could be particularly fruitful.
Although higher BMI was associated with lower activity overall, the magnitude of the effect decreased with worsening frailty. While this finding needs to be confirmed through replication, it is possible that frail elders with unintentional weight loss, exhaustion, and sarcopenia are unable to maintain activity regardless of their weight. In fact, both low and high BMI are considered risk factors for frailty, which might help to explain the lack of association between BMI and activity among frail respondents (35). Consequently, simultaneous efforts to reduce excessive weight and increase activity may be most effective among nonfrail and prefrail individuals, regardless of age.
Aging researchers have been perplexed by the apparent “frailty gender paradox” referring to the finding that while frailty is typically more common among women they nonetheless live longer than men (36). We found only a small increase in the proportion frail among women relative to men (16.5% vs 12.1%) while mean hourly activity level among the nonfrail and prefrail was actually higher for women. This apparent discrepancy may be due in part to the difference between moderate-to-vigorous exercise (which is the focus of clinical frailty activity assessment and of our frailty scale) and either light activity and/or a reduction in sedentary behavior (both of which are reflected in accelerometry-based activity measures). Both lower activity and time spent in sedentary behavior increase risk of mortality (37). Thus, one possible explanation for the frailty-gender paradox is that the maintenance of even light activity among older women may extend their survival while a disproportionate increase in sedentary behavior among men may decrease theirs. Indeed, others have noted an increase in sedentary behavior among men compared to women 60 years of age and older while older women maintain their light activity (10). While our results are consistent with this explanation, further work is required to confirm this hypothesis.
Our results reveal considerable individual-level variability in activity level exceeding the average differences associated with demographics, frailty status and health characteristics, another important contribution from this study. We further found substantial variability within an individual across days of the week. These findings have two important implications for public health and clinical practice. First, it raises the possibility that there may be other individual characteristics that distinguish between those who are more versus less active, and that identifying such characteristics could improve our ability to predict which individuals are at greatest risk of having low activity. Second, clinicians and those designing interventions should consider customizing activity targets to an individual’s existing level of activity and day-of-week patterns in order to maximize their effectiveness.
Hourly activity analyses have been conducted in populations other than older adults, providing many avenues for future directions. Hourly activity plotted across the day, in particular hourly activity calculated from wrist accelerometry, detected activity recovery among malnourished children hospitalized in Ethiopia (38). Others have merged sleep and activity analytic approaches to describe how childhood diseases affect both sleep and activity (39). This novel approach created new and unique measures calculated from the full-day hourly activity (eg, the most and least active periods of the day, the difference in activity between these two segments, the frequency with which an individual transitions between rest and activity, the stability of the circadian pattern between days, and the time of the day most highly correlated with expected circadian rhythms.) (39) More sophisticated methods, such as functional data analysis, have been applied to study relationships between multiple independent variables and dependent circadian activity patterns using function-on-scalar regression and accelerometry data from children (40). These novel approaches to studying activity patterns across the day offer promising new directions for studying older adult activity patterns.
When comparing these results to those of other studies, it is important to keep the following in mind. Although we constructed our phenotypic frailty scale and comorbidity index to be as comparable as possible to those used in previous literature, unavoidable differences may alter the estimated distribution of frailty in the population and/or its association with physical activity. In addition, NSHAP used wrist rather than hip accelerometry, the latter often used in studies of daytime physical activity (41). Wrist accelerometry may measure more light activity, such as the activity associated with activities of daily living or instrumental activities of daily living, while hip accelerometry may better measure and differentiate a wide range of activity intensities (sedentary behavior through vigorous activity). Due to these measurement differences, our study may have underestimated the impact of frailty on activity, particularly at the extremes of activity intensity (sedentary behavior and vigorous activity). However, hourly CPM calculated for each hour across the full day or wake periods from the wrist Actiwatch is significantly associated with the hip Actigraph in multilevel mixed models (42), and Actiwatch output is significantly related to important older adult health and functional outcomes (1,43). The population-based nature of this study is a strength; however, the overall sample size is somewhat limited causing the cell sizes for less prevalent conditions (eg, frailty, “other” race/ethnicity) to be small. The small cell sizes limited our power to detect significant relationships.
Mobility is a critical indicator of health among older adults. In this study, we focused on differences in overall activity level among the general population of older adults. However, as hinted at in Supplementary Figure 1, there is considerable variation between individuals in their patterns of activity and even within individuals between days. As a result, patterns in mean activity averaged over individuals—as appearing in the literature (6,8) and in this paper—may not adequately capture important variation in activity patterns across individuals. Such individual activity patterns, including patterns in napping and sedentary behavior, may reveal additional, important information about an individual’s health, and may provide information that can be used to tailor behavior modifications to increase their effectiveness.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by National Institute on Aging (1K23AG049106 to M.H.-S.). The National Social Life, Health and Aging Project is supported by the National Institute on Aging and the National Institutes of Health (R37AG030481; R01AG033903). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Huisingh-Scheetz MJ, Kocherginsky M, Magett E, Rush P, Dale W, Waite L. Relating wrist accelerometry measures to disability in older adults. Arch Gerontol Geriatr. 2016;62:68–74. doi:10.1016/j.archger.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dunlop DD, Song J, Arnston EK et al. . Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. J Phys Act Health. 2015;12:93–101. doi:10.1123/jpah.2013-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atienza AA, Moser RP, Perna F et al. . Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc. 2011;43:815–821. doi:10.1249/MSS.0b013e3181fdfc32 [DOI] [PubMed] [Google Scholar]
- 4. Manns P, Ezeugwu V, Armijo-Olivo S, Vallance J, Healy GN. Accelerometer-derived pattern of sedentary and physical activity time in persons with mobility disability: National Health and Nutrition Examination Survey 2003 to 2006. J Am Geriatr Soc. 2015;63:1314–23. doi:10.1111/jgs.13490 [DOI] [PubMed] [Google Scholar]
- 5. Goldsmith J, Zipunnikov V, Schrack J. Generalized multilevel function-on-scalar regression and principal component analysis. Biometrics. 2015;71:344–353. doi:10.1111/biom.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrack JA, Zipunnikov V, Goldsmith J et al. . Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69:973–9. doi:10.1093/gerona/glt199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao L, Huang L, Schrack JA, Ferrucci L, Zipunnikov V, Crainiceanu CM. Quantifying the lifetime circadian rhythm of physical activity: a covariate-dependent functional approach. Biostatistics. 2015;16:352–367. doi:10.1093/biostatistics/kxu045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80:187–191. doi:10.1016/j.maturitas.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 9. Palmerini L, Bagalà F, Zanetti A, Klenk J, Becker C, Cappello A. A wavelet-based approach to fall detection. Sensors (Basel). 2015;15:11575–11586. doi:10.3390/s150511575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin KR, Koster A, Murphy RA et al. . Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003-04 and 2005-06. J Am Geriatr Soc. 2014;62:1263–1271. doi:10.1111/jgs.12893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomes TN, Hedeker D, dos Santos FK, Pereira S, Katzmarzyk PT, Maia JA. Why are children different in their daily sedentariness? An approach based on the mixed-effects location scale model. PLoS One. 2015;10:e0132192. doi:10.1371/journal.pone.0132192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makary MA, Segev DL, Pronovost PJ et al. . Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi:10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 14. Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi:10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 15. Fried LP. Interventions for human frailty: physical activity as a model. Cold Spring Harb Perspect Med. 2016;6 http://perspectivesinmedicine.cshlp.org.proxy.uchicago.edu/content/6/6/a025916.full.pdf+html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Muircheartaigh C, English N, Pedlow S, Kwok PK. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S15–S26. doi:10.1093/geronb/gbu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huisingh-Scheetz M, Kocherginsky M, Schumm PL et al. . Geriatric syndromes and functional status in NSHAP: rationale, measurement, and preliminary findings. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S177–S190. doi:10.1093/geronb/gbu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen KY, Acra SA, Majchrzak K et al. . Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technol Ther. 2003;5:1023–1033. doi:10.1089/152091503322641088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. PhilipsRespironics. Actiwatch: Koninklijke Philips Electronics N.V; 2013. (2/19/2013). http://www.healthcare.philips.com/main/homehealth/sleep/actiwatch/default.wpd#&&/wEXAQUOY3VycmVudFRhYlBhdGgFCUVkdWNhdGlvbrs7D4d8dwFrxbRmM0TsUP60b3xr.
- 20. Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L. Measuring physical activity with hip accelerometry among U.S. older adults: how many days are enough?PLoS One. 2017;12:e0170082. doi:10.1371/journal.pone.0170082 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lauderdale DS, Philip Schumm L, Kurina LM et al. . Assessment of sleep in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S125–S133. doi:10.1093/geronb/gbu092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guralnik JM, Simonsick EM, Ferrucci L et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 23. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1: 385–401. [Google Scholar]
- 24. WorldHealthOrganization. WHO: Global Database on Body Mass Index (July 28, 2011). http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 25. Kotwal AA, Schumm P, Kern DW et al. . Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29:317–324. doi:10.1097/WAD.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shega JW, Sunkara PD, Kotwal A et al. . Measuring cognition: the Chicago Cognitive Function Measure in the National Social Life, Health and Aging Project, Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S166–S176. doi:10.1093/geronb/gbu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vasilopoulos T, Kotwal A, Huisingh-Scheetz MJ, Waite LJ, McClintock MK, Dale W. Comorbidity and chronic conditions in the National Social Life, Health and Aging Project (NSHAP), Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S154–S165. doi:10.1093/geronb/gbu025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skrondal A, Rabe-Hesketh S.. Generalized Latent Variable Modeling. New York: Chapman and Hall/CRC; 2004. [Google Scholar]
- 29. Ruppert D, Wand MP, Carrol RJ.. Semiparametric Regression. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 30. Rabe-Hesketh S, Skrondal A. Multilevel modelling of complex survey data. J R Stat Soc Series A. 2006;169:805–27. [Google Scholar]
- 31. Bellettiere J, Carlson JA, Rosenberg D et al. . Gender and age differences in hourly and daily patterns of sedentary time in older adults living in retirement communities. PLoS One. 2015;10:e0136161. doi:10.1371/journal.pone.0136161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Center for Disease Control and Prevention. Physical Activity for Everyone: CDC; 2011. (cited September 16, 2011). http://www.cdc.gov/physicalactivity/everyone/guidelines/index.html.
- 33. Dunlop DD, Song J, Lee J et al. . Physical activity minimum threshold predicting improved function in adults with lower limb symptoms. Arthritis Care Res (Hoboken). 2016. doi: 10.1002/acr.23181 [doi]. PMID: 28029748; PMCID: PMC5521176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knaggs JD, Larkin KA, Manini TM. Metabolic cost of daily activities and effect of mobility impairment in older adults. J Am Geriatr Soc. 2011;59:2118–2123. doi:10.1111/j.1532-5415.2011.03655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi:10.1111/j.1532-5415.2005.53300.x [DOI] [PubMed] [Google Scholar]
- 36. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol. 2017;89:30–40. doi:10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 37. Evenson KR, Herring AH, Wen F. Accelerometry-assessed latent class patterns of physical activity and sedentary behavior with mortality. Am J Prev Med. 2017;52:135–143. doi:10.1016/j.amepre.2016.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faurholt-Jepsen D, Hansen KB, van Hees VT et al. . Children treated for severe acute malnutrition experience a rapid increase in physical activity a few days after admission. J Pediatr. 2014;164:1421–1424. doi:10.1016/j.jpeds.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 39. Mumford RA, Mahon LV, Jones S, Bigger B, Canal M, Hare DJ. Actigraphic investigation of circadian rhythm functioning and activity levels in children with mucopolysaccharidosis type III (Sanfilippo syndrome). J Neurodev Disord. 2015;7:31. doi:10.1186/s11689-015-9126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldsmith J, Liu X, Jacobson JS, Rundle A. New insights into activity patterns in children, found using functional data analyses. Med Sci Sports Exerc. 2016;48:1723–1729. doi:10.1249/MSS.0000000000000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc. 2013;45:964–975. doi:10.1249/MSS.0b013e31827f0d9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lambiase MJ, Gabriel KP, Chang YF, Kuller LH, Matthews KA. Utility of actiwatch sleep monitor to assess waking movement behavior in older women. Med Sci Sports Exerc. 2014;46:2301–2307. doi:10.1249/MSS.0000000000000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huisingh-Scheetz M, Kocherginsky M, Dugas L et al. . Wrist accelerometry in the health, functional, and social assessment of older adults. J Am Geriatr Soc. 2016;64:889–891. doi:10.1111/jgs.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.