Abstract
Aims
We aimed to determine whether treatment with sildenafil improves outcomes of patients with persistent pulmonary hypertension (PH) after correction of valvular heart disease (VHD).
Methods and results
The sildenafil for improving outcomes after valvular correction (SIOVAC) study was a multricentric, randomized, parallel, and placebo-controlled trial that enrolled stable adults with mean pulmonary artery pressure ≥ 30 mmHg who had undergone a successful valve replacement or repair procedure at least 1 year before inclusion. We assigned 200 patients to receive sildenafil (40 mg three times daily, n = 104) or placebo (n = 96) for 6 months. The primary endpoint was the composite clinical score combining death, hospital admission for heart failure (HF), change in functional class, and patient global self-assessment. Only 27 patients receiving sildenafil improved their composite clinical score, as compared with 44 patients receiving placebo; in contrast 33 patients in the sildenafil group worsened their composite score, as compared with 14 in the placebo group [odds ratio 0.39; 95% confidence interval (CI) 0.22–0.67; P < 0.001]. The Kaplan–Meier estimates for survival without admission due to HF were 0.76 and 0.86 in the sildenafil and placebo groups, respectively (hazard ratio 2.0, 95% CI = 1.0–4.0; log-rank P = 0.044). Changes in 6-min walk test distance, natriuretic peptides, and Doppler-derived systolic pulmonary pressure were similar in both groups.
Conclusion
Treatment with sildenafil in patients with persistent PH after successfully corrected VHD is associated to worse clinical outcomes than placebo. Off-label usage of sildenafil for treating this source of left heart disease PH should be avoided.
The trial is registered with ClinicalTrials.gov, number NCT00862043.
Keywords: Sildenafil, Pulmonary hypertension, Valvular heart disease, Heart failure
Introduction
The most common cause of pulmonary hypertension (PH) worldwide is left heart disease (LHD),1 and valvular heart disease (VHD) is amongst the leading causes of this type of secondary PH.2 Pulmonary hypertension affects virtually all patients with severe symptomatic mitral valve disease and up to 65% of those with symptomatic aortic stenosis.3 Mitral and aortic valve diseases increase left atrial pressure which, in turn, leads to an initially passive and potentially reversible increase in pulmonary pressures. Vascular injury then triggers a cascade of venous and small artery remodelling, non-reversible arterial PH, and eventually, right ventricular dysfunction.4 Regression of PH is frequently incomplete after the correction of the valvular lesion,5,6 persisting in up to 75% of patients with moderate or severe preoperative PH.7 Furthermore, PH sometimes develops lately in patients who did not show PH before valve surgery.8 Once established, PH in corrected VHD is an untreatable risk-factor of mortality and disability in the long-term.6,8–10
5-phosphodiesterase (PDE5) inhibitors have proven clinical efficacy in pulmonary arterial hypertension,11 but have shown discordant results in the field of LHD-PH.12–16 Nonetheless, sildenafil is frequently used off-label for treating this condition.17 In the setting of VHD, short-term studies have shown favourable effects of the drug in the immediate phases after surgery.18 To our knowledge no clinical trial has yet addressed the chronic effects of PDE5 inhibitors aimed specifically at treating persistent PH after correction of VHD. The sildenafil for improving outcomes after valvular correction (SIOVAC) trial was designed to test the hypothesis that, as compared with placebo, long-term therapy with the PDE5-inhibitor sildenafil improves clinical outcomes of patients with persistent PH after successful correction of the underlying VHD.
Methods
Study design
SIOVAC is an investigator-driven, academically sponsored, multicentric, randomized, double-blind, placebo-controlled, and parallel clinical trial. The study was performed in 18 academic hospitals in Spain, and the Fundación de Investigación Biomédica Hospital Gregorio Marañón served as the co-ordinating centre. The trial protocol (see Supplementary Material S2) was authorized by the Spanish Agency of Medicinal Products and Medical Devices and approved by the Reference Ethic Committee and the Local Ethic Committees of all participant institutions. All patients provided written informed consent. Randomization and clinical monitoring were performed by Chiltern International Ltd which also acted as the Data and Co-ordinating Centre in terms of study drug distribution and centralized data collection. An external adjudication and data safety monitoring board (ADSMB) reviewed all major adverse events and adjudicated clinical outcomes.
Patients
Patients were screened in outpatient clinics and imaging laboratories of the participating institutions (see Supplementary Material S1, Figure S1). Inclusion criteria for randomization were: (i) age older than 18 years, (ii) unequivocal demonstration of PH (a mean pulmonary arterial pressure ≥ 30 mmHg by catheterization within the 30 days prior to randomization), (iii) a successful surgical or percutaneous valvular replacement or repair procedure (leading to a complete correction of left heart valve disease and performed at least 1 year before inclusion), and (iv) a stable clinical condition [no changes in concomitant medication or hospital admissions for heart failure (HF) in the previous month]. Major exclusion criteria were: (i) haemodynamically significant residual valvular or prosthesis dysfunction (patient-prosthesis mismatch or more than mild valvular or prosthetic valve stenosis or regurgitation, as assessed by the investigators according to current practice guidelines),19 (ii) systolic blood pressure < 90 mmHg, (iii) myocardial infarction, stroke, or life-threatening arrhythmia within the last 6 months, (iv) severe renal impairment (creatinine clearance <30 mL/min) or hepatic dysfunction, (v) life expectancy <2 years, or (vi) any established contraindication for sildenafil (see Supplementary Material S1, Table S1).
Randomization and masking
Patients were randomly assigned (1:1) to receive either sildenafil or placebo. Randomization was balanced using randomly permuted blocks of size four. Sites received the Investigational Product Kits containing two bottles of 550 tablets of the study drug with the patient's treatment allocation codes. Investigators and patients were masked to treatment assignment. Active treatment was re-bottled sildenafil (20 mg Revatio tablets, Pfizer), whereas the placebo manufacturing process ensured identical appearance to the active drug (see Supplementary Material S1).
Procedures
In patients in whom recent catheterization data was unavailable (88 patients, 44%) but showed a systolic pulmonary artery pressure ≥ 50 mmHg in a screening echocardiographic study, a per-protocol right-heart catheterization procedure was performed. An acute vasoreactivity test with open-label sublingual sildenafil (100 mg)20 was performed for patients undergoing per-protocol catheterization. For patients who underwent catheterization prior to enrolment, vasoreactivity results were registered retrospectively whenever available. The diastolic and (mean) transpulmonary pressure gradients were calculated subtracting the mean pulmonary wedge pressure from the diastolic and mean pulmonary artery pressures, respectively.
Although the recommended dose of sildenafil for pulmonary arterial hypertension is 20 mg three times daily (t.i.d.), most clinical trials of sildenafil in LHD-PH have used higher doses.12–16,21,22 Accordingly, we chose a target study dose of 40 mg orally t.i.d. Patients with a low body-mass index, hypotension, or showing severe hypotension during the vasoreactivity test initiated a 20 mg t.i.d. for 2 weeks.
Randomized patients underwent clinical assessment, 6-min walk test, and Doppler-echocardiography examinations at baseline, 3 and 6 months. Blood sampling for brain natriuretric peptide (BNP) measurements and magnetic resonance examinations (selected sites, in patients without contraindications) were performed at baseline and 6 months. All explorations were performed at least 4 h (half-life of sildenafil) after taking the study drug. Concomitant medication was recorded during clinical revisions and adherence was monitored by pill-counts at the 3 and 6 month visits. Blinded core laboratories analysed cardiac imaging and BNP concentrations.
Outcomes
The primary endpoint was based on the composite clinical score at 6 months. This score has demonstrated good sensitivity in clinical trials in the field of HF23 and fulfils the requirements for PH trials.24 The composite clinical score combines three elements (i) major clinical events, defined as occurrence of death (of any cause) or hospital admission for HF requiring intravenous diuretic treatment with or without overnight stay, which is objective evidence of change in clinical status, (ii) World Health Organisation (WHO) functional classification, which relies on the physician assessment, and (iii) the patient global self-assessment, which relies on patient’s criteria. The self-assessment score is obtained interviewing the patient for his/her perception of change from his/her baseline clinical condition at enrollment.23 The composite clinical score classifies patient’s outcome in three categories: (i) worsened, if he/she presents a major clinical event, increases his/her WHO functional class, or self-reports a moderately or markedly worse category in the global-self-assessment, (ii) improved, if he/she has not suffered a major clinical event and his/her functional class has improved or reports moderate or marked improvement in global self-assessment or (iii) unchanged (otherwise). In case of discordant information, most objective events (death or HF admission) prevail over the change in functional class; the latter, in turn prevails over patient’s self-assessment. The ADSMB blindly adjudicated the composite clinical score in every patient.
Secondary endpoints were (i) the composite clinical score adjusted by co-variables (gender, age, and baseline WHO functional class), (ii) all-cause mortality, (iii) cardiovascular mortality, (iv) Kaplan–Meier analysis of major clinical events (as defined above), and (v) number of hospital admissions because of HF requiring intravenous diuretics. Other secondary endpoints were changes from entry to 6-month follow-up in (vi) WHO functional capacity, (vii) 6-min walk test distance, and (viii) plasma BNP levels. Imaging secondary endpoints were the change in systolic pulmonary pressure and in ventricular volumes at 6 months, by Doppler-echocardiography and magnetic resonance, respectively. Interactions between the primary endpoint and a number of baseline variables were pre-specified as exploratory analyses (see Supplementary Material S2).
Statistical analysis
The null hypothesis was that at the end of the 6-month follow-up period there is no difference between patients treated with placebo and sildenafil in the distribution of the three categories of the composite clinical score. The alternative hypothesis was that compared to placebo, sildenafil increases the proportion of patients who improve and decreases the proportion of patients who worsen their composite score. We used the mathematical formulation established for ordinal outcomes to calculate sample size.25 We initially estimated proportions of improved, worsened, and unchanged categories to be 15%, 20%, and 65%, respectively in the placebo group. We assumed an absolute 10% increase in the proportion of improvement in the sildenafil group [odds ratio (OR) for improvement = 1.90]. Using a two-sided level of significance of α = 0.05, these assumptions resulted in 322 patients needed for an 80% power to reject the null hypothesis. Estimating a 10% attrition rate, the initial sample size was 354 patients. Sample-size recalculation without unblinding was pre-specified after completing the follow-up of the first 100 patients. This analysis showed a higher than expected incidence of the worsened category in the global study population. After confirming no significant differences in the number of major clinical events between blinded groups, the ADSMB authorized to continue the study and requested to recalculate sample size. Using the observed proportions of the first 100 patients, the power to reject the null hypothesis was re-estimated in 190 analysable patients; based on the 4% attrition rate observed in the first 100 patients, the final sample size was re-adjusted to 198 patients.
The safety analysis set included all randomized patients who received at least one dose of the study drug. The full-analysis set (modified-intention-to treat set) included all randomized patients who took at least one dose of the study drug, and on whom it was possible to evaluate the composite clinical score in at least one time-point. The per-protocol set excluded all patients with major protocol deviations.
We used an ordinal logistic regression model to calculate ORs for the primary endpoint under the proportionality assumption. Patients with an undetermined composite clinical score were excluded from the primary endpoint. However, sensitivity analyses were also pre-specified in which these patients’ outcomes were imputed as either ‘unchanged’ or using monotone logistic regression from baseline and 3 months variables. Odds ratios adjusted by age, sex, and WHO functional class were calculated as secondary endpoints. Time-to-event data was analysed using the Kaplan–Meier method, the log-rank test, and Cox regression. Quantitative secondary-endpoints were analysed using linear mixed-models for longitudinal data accounting for the fixed-effects of the visit, the treatment group and their interaction. Changes in functional class were analysed using a cumulative-link mixed-model for ordinal responses. Interaction analyses with baseline co-variables were performed using a logistic-regression model accounting for their interaction with the treatment group either continuously or by binary categorization. Signification was established as P-value <0.05 (two-sided). Data analysis was performed by Chiltern International Ltd and the investigators using SAS software, version 9.2 (SAS Institute, Inc.) and R version 3.3.2. The study is registered with ClinicalTrials.gov NCT00862043 and EudraCT 2007-007033-40.
Results
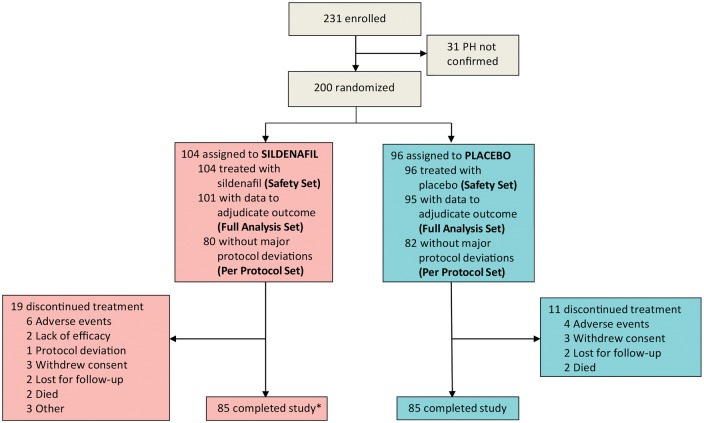
From May 2009 to December 2015, 231 patients were enrolled, but 31 did not meet the mean pulmonary arterial pressure inclusion criterion (Figure 1). Thus, 200 patients were randomized to receive either sildenafil (n = 104) or placebo (n = 96). Three patients in the sildenafil and one in the placebo group abandoned the study without undergoing follow-up visits or reporting clinical events. Thus, the full analysis set consisted of 196 patients, 101 in the sildenafil, and 95 in the placebo group. The per-protocol set consisted of 162 patients, 80 receiving sildenafil, and 82 placebo. The study was completed by 170 patients (85 in the placebo group and 85 in the control group). Nine patients took 20 mg t.i.d. throughout the full study period, two of them due to a body surface area <1.6 m2, another two due to persistent hypotension, and the other five upon investigators preferences.
Figure 1.
Flow of patients. Asterisk indicates one patient died due to heart failure 20 days after the 6-month visit and was adjudicated as death.
Characteristics of the study patients
Most baseline clinical characteristics of randomized patients were not different between groups (Table 1). Median (IQR) age was 72 (66–77) years old, 154 were women, 154 were in atrial fibrillation, and 85 were in WHO functional Class III. Most patients (n = 182) had undergone mitral valve surgery (valve replacement in 160 and valve repair in 122), 91 had undergone aortic valve replacement, and 78 had undergone tricuspid valve surgery. Valvular interventions had been surgical in all patients except in 3 cases of transcatheter aortic valve replacement. Approximately one-third of patients had undergone repeated interventions. Baseline haemodynamic (Table 1) and imaging characteristics (see Supplementary Material S2, Table S2) were not significantly different between groups.
Table 1.
Baseline characteristics
| Sildenafil (n = 104) | Placebo (n = 96) | P-value | |
|---|---|---|---|
| Age (years), median (IQR) | 70 (65, 77) | 73 (67, 77) | 0.23 |
| Women, n (%) | 76 (73) | 78 (81) | 0.23 |
| Weight (Kg), median (IQR) | 66 (59, 78) | 72 (62, 80) | 0.13 |
| Body mass index (Kg·m−2), median (IQR) | 26.5 (24.0, 30.0) | 28.4 (25.3, 32.4) | 0.04 |
| Systolic blood pressure (mmHg), median (IQR) | 131 (119, 144) | 140 (127, 154) | 0.02 |
| Diastolic pressure (mmHg), median (IQR) | 70 (64, 80) | 70 (63, 80) | 0.94 |
| Heart rate (beats·min−1), median (IQR) | 72 (67, 79) | 70 (65, 82) | 0.71 |
| Heart valve procedures | |||
| Time from last valvular surgery (years), median (IQR) | 7.5 (4.2, 13.1) | 5.8 (3.0, 12.3) | 0.12 |
| Isolated mitral valve surgery, n (%) | 27 (26) | 33 (34) | 0.22 |
| Isolated aortic valve replacement, n (%) | 8 (8) | 9 (9) | 0.80 |
| Mitral and aortic valve surgery, n (%) | 29 (28) | 16 (17) | 0.06 |
| Mitral and tricuspid valve surgery, n (%) | 26 (25) | 23 (24) | 0.87 |
| Aortic and tricuspid valve surgery, n (%) | 0 (0) | 1 (1) | 0.48 |
| Mitral, aortic and tricuspid valve surgery, n (%) | 14 (14) | 14 (15) | 0.84 |
| Patients with re-interventions, n (%) | 39 (38) | 24 (25) | 0.07 |
| Coronary artery revascularization | |||
| Coronary artery bypass graft, n (%) | 3 (3) | 10 (10) | 0.06 |
| Percutaneous coronary intervention, n (%) | 5 (5) | 7 (7) | 0.66 |
| Cardiovascular risk factors | |||
| Hypertension, n (%) | 59 (57) | 69 (72) | 0.04 |
| Hyperlipidaemia, n (%) | 51 (49) | 34 (35) | 0.07 |
| Diabetes, n (%) | 31 (30) | 27 (28) | 0.91 |
| Smoking, n (%) | 7 (7) | 6 (6) | 1.00 |
| Other comorbidities | |||
| Atrial fibrillation, n (%) | 77 (74) | 77 (80) | 0.39 |
| WHO functional classification | 0.84 | ||
| I, n (%) | 8 (8) | 8 (8) | |
| II, n (%) | 51 (51) | 44 (46) | |
| III, n (%) | 42 (42) | 43 (45) | |
| 6-min walk test distance (m), median (IQR) | 361 (285, 418) | 342 (250, 382) | 0.07 |
| Concomitant medications | |||
| Acenocoumarol or warfarin, n (%) | 97 (93) | 81 (84) | 0.70 |
| Aspirin, n (%) | 11 (11) | 11 (12) | 1.00 |
| Diuretics, n (%) | 89 (86) | 84 (88) | 0.99 |
| Aldosterone receptor antagonist, n (%) | 46 (44) | 38 (40) | 0.77 |
| Digoxin, n (%) | 43 (41) | 41 (43) | 1.00 |
| ACE inhibitors, n (%) | 45 (43) | 33 (34) | 0.47 |
| Angiotensin II receptor blocker, n (%) | 22 (21) | 20 (21) | 1.00 |
| Beta-blocker, n (%) | 53 (51) | 43 (45) | 0.69 |
| Calcium antagonist, n (%) | 11 (11) | 22 (23) | 0.07 |
| Core laboratory biomarker data | |||
| BNP (pg·mL−1), median (IQR) | 63 (28, 166) | 54 (25, 118) | 0.40 |
| Cardiac catheterization data | |||
| Right atrial pressure (mmHg), median (IQR) | 12 (9, 16) | 12 (10, 17) | 0.51 |
| Pulmonary artery oxygen saturation (%), median (IQR) | 64 (60, 70) | 64 (57, 69) | 0.38 |
| Mean pulmonary artery pressure (mmHg), median (IQR) | 39 (34, 46) | 37 (34, 44) | 0.25 |
| Mean wedge pulmonary pressure (mmHg), median (IQR) | 23 (19, 26) | 22 (19, 26) | 0.92 |
| Cardiac index (L·s−1·m−2), median (IQR) | 2.8 (2.4, 3.2) | 2.8 (2.3, 3.4) | 0.80 |
| Mean transpulmonary pressure gradient (mmHg), median (IQR) | 16.0 (13.0, 22.0) | 15.0 (12.0, 20.0) | 0.35 |
| Diastolic transpulmonary pressure gradient (mmHg), median (IQR) | 2.0 (0.0, 6.0) | 3.0 (0.0, 7.0) | 0.44 |
| Pulmonary vascular resistance (Wood units), median (IQR) | 3.4 (2.4, 4.6) | 3.1 (2.2, 4.9) | 0.33 |
ACE, angiotensin-converting enzyme; BNP, brain natriuretic peptide; IQR, interquartile range; WHO, World Health Organisation.
Primary endpoint
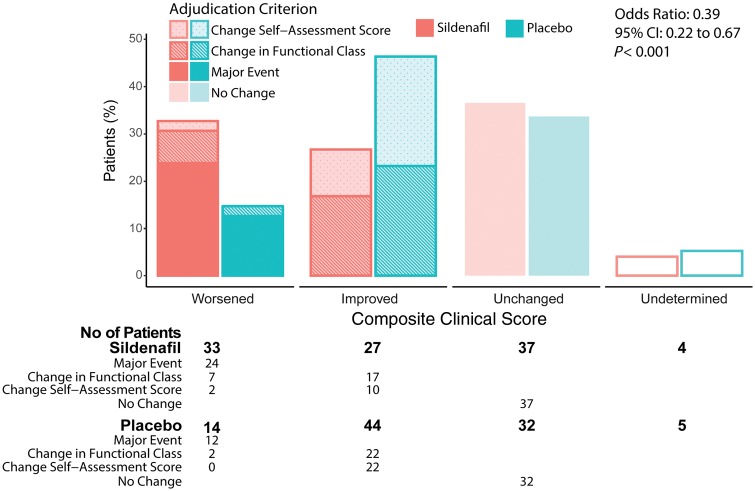
In the sildenafil group, only 27 patients improved their composite clinical score at 6 months as compared with 44 patients in the placebo group. By contrast, 33 patients in the sildenafil group worsened their primary outcome, as compared with 14 patients in the placebo group [OR for improvement 0.39; 95% confidence interval (CI) 0.22–0.67; P < 0.001] (Figure 2). These unfavourable outcomes of patients taking sildenafil were confirmed in the per-protocol set (OR 0.42; 95% CI 0.24–0.76; P = 0.004), as well as in the two sensitivity analyses in which the five patients with undetermined classifications were imputed, either as ‘unchanged’ (OR 0.40; 95% CI 0.23–0.68; P < 0.001), or using monotonic logistic regression (OR 0.41; 95% CI 0.24–0.71; P = 0.001).
Figure 2.
Primary endpoint. The composite clinical score accounts for the combination of death due to any cause, hospitalization due to heart failure requiring intravenous diuretic treatment, change in the World Health Organisation (WHO) functional class or relevant changes in the patient global self-assessment. Total bars show the proportion of patients in each category, stacked bars show the criterion used to adjudication to each category, and the table shows the number of patients. Data are shown for the full analysis set. Odds ratio calculated using ordinal logistic regression under the proportionality assumption.
Secondary endpoints
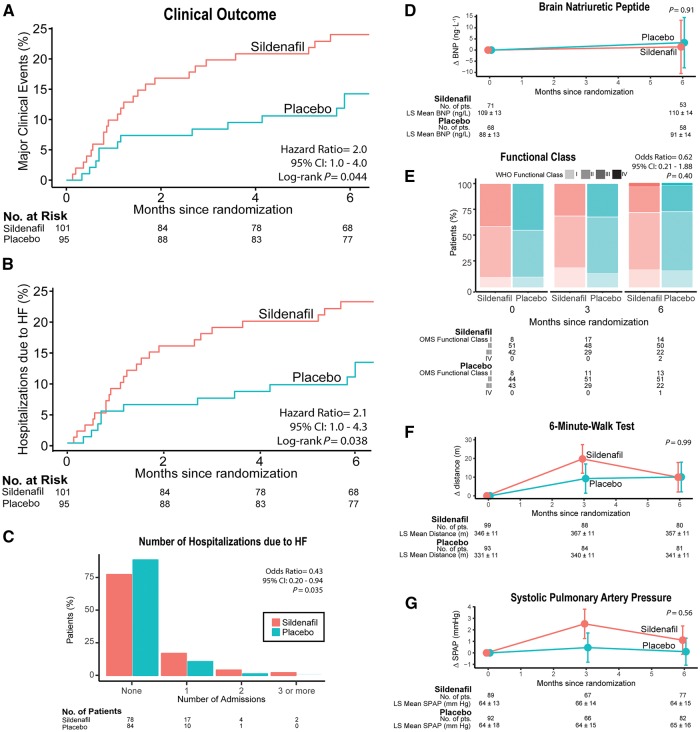
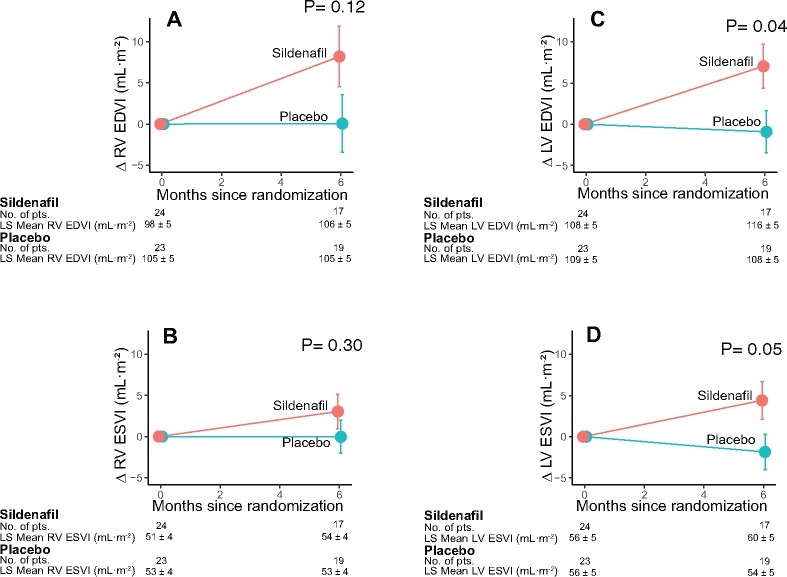
Unfavourable composite clinical scores in the sildenafil group were also confirmed when adjusting for co-variables such as age (OR 0.39; 95% CI 0.21–0.62; P < 0.001), sex (OR 0.39; 95% CI 0.22–0.67; P < 0.001), and baseline WHO functional class (OR 0.38; 95% CI 0.22–0.67; P < 0.001). There were five deaths during the study, three in the sildenafil group (two of cardiac origin; one abdominal haemorrhage) and two in the placebo group (one cardiac; one pulmonary haemorrhage; log-rank P = 0.72). The three cardiac deaths were due to HF (log-rank test P = 0.63 for sildenafil vs. placebo). The Kaplan–Meier estimates of survival at 6 months without major clinical events (death or hospitalization due to HF) were 0.76 (95% CI 0.68–0.85) and 0.86 (95% CI 0.78–0.94), respectively (hazard ratio 2.0, 95% CI = 1.0–4.0; log-rank test P = 0.044; Figure 3A). There were 31 HF hospital admissions requiring intravenous diuretics in the sildenafil group vs. 22 in the placebo group (OR 0.43, 95% CI 0.20–0.94; P = 0.035; Figure 3B). There were no significant differences between groups in the changes from baseline to 6 months in functional capacity, 6-min walk distance, BNP levels, or systolic pulmonary artery pressure (Figure 3C–F; see Supplementary Material S1, Tables S3 and S4). Magnetic resonance data showed LV dilatation from baseline to month 6 only in the sildenafil group, resulting in significant differences in the changes of LV end-diastolic and end-systolic volumes between groups (P = 0.04 and 0.05, respectively; see Supplementary material online, Figure S1).
Figure 3.
Key secondary endpoints. (A) Kaplan–Meier analysis for the incidence of major clinical events (any-cause mortality or admission for heart failure requiring intravenous diuretics). Hazard ratio calculated by proportional-hazard Cox model. P-value calculated by the log-rank test. (B) Identical analysis for the incidence of admission for heart failure. (C) Number of hospitalizations due to heart failure. Odds ratio and P-value calculated using ordinal logistic regression. (D) Changes from baseline to 6 months in the plasma levels of brain natriuretic peptide. (E) Proportion of patients in each category of World Health Organisation (WHO) functional class. Odds ratio and P-value calculated using a cumulative link mixed model for ordinal responses. (F) Changes from baseline in the 6-min walk test distance. (G) Changes in systolic pulmonary artery pressure as measured using Doppler-echocardiography. In (D, F, and G) the dots represent the least-square adjusted means, the bars represent their standard error, and P-values are shown for the effect of the interaction between visit (baseline or 6-month) and treatment group in a repeated-measure mixed-model design.
Pre-specified subgroup analyses
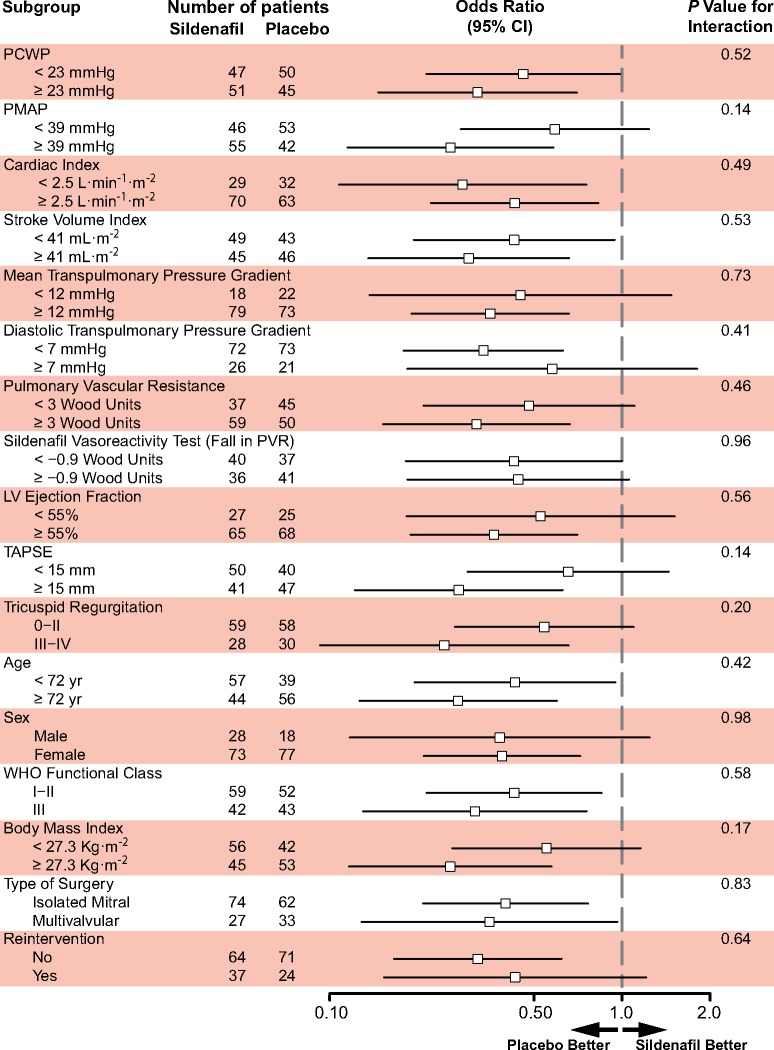
Binary interaction analyses did not identify any pre-specified baseline co-variable suggesting a benefit for the active treatment (Figure 4). Furthermore, no predictive value for response was identified for acute vasoreactivity data. Quantitative interactions analyses also failed to suggest a potential benefit of treatment in any range of the tested haemodynamic variables (Figure 5).
Figure 4.
Interaction analysis. Odds ratios and P-values calculated using logistic-regression accounting for their interaction with the treatment group after binary categorization. PCWP, pulmonary capillary wedge pressure; PMAP, pulmonary mean arterial pressure; LV, left ventricle; PVR, pulmonary vascular resistance; TAPSE, tricuspid annular plane systolic excursion.
Figure 5.
Secondary magnetic resonance imaging endpoints. Changes in end-diastolic volume index (EDVI; A and C) and end-systolic volume index (ESVI; B and D) for the right ventricle (RV; A and B) and the left ventricle (LV; C and D). Least-square (LS) adjusted means and standard error are shown for patients undergoing magnetic resonance imaging (n = 47 at baseline; n = 36 at follow-up). Symbols as in Figure 3D, F, and G.
Adverse events
The sildenafil group showed a non-significant trend towards more frequent investigator-reported serious adverse events than the placebo group, in particular related to the study drug (Table 2). More frequent infectious adverse events were observed in the placebo group (P = 0.05). No significant changes were observed in vital signs in either group (see Supplementary material online, Table S5).
Table 2.
Adverse events
| Sildenafil (n = 104) |
Placebo (n = 96) |
Odds ratio | 95% CI | P-value | |||
|---|---|---|---|---|---|---|---|
| End point or event | Number of endpoints or SAEs | Number of patients with endpoint or AE (%) | Number of endpoints or SAEs | Number of patients with endpoint or AE (%) | |||
| Safety endpoint | |||||||
| Death | 3 | 2 | — | ||||
| Cardiac death due to worsening heart failure | 2 | 1 | — | ||||
| Hospitalization due to heart failure | 31 | 23 (22) | 12 | 11 (11) | 2.18 | 0.95–5.30 | 0.06 |
| Investigator-reported adverse event | |||||||
| Serious adverse events | 68 | 42 (40) | 53 | 28 (29) | 1.64 | 0.88–3.10 | 0.10 |
| SAE possibly related to study drug | 23 | 16 (15) | 13 | 7 (7) | 2.30 | 0.85–6.96 | 0.08 |
| Any adverse event | 120 | 62 (60) | 92 | 47 (49) | 1.53 | 0.85–2.80 | 0.15 |
| Cardiac | 46 | 36 (35) | 28 | 21 (22) | 1.88 | 0.96–3.75 | 0.06 |
| Heart failure or dyspnoea | 45 | 35 (34) | 25 | 19 (20) | 2.05 | 1.03–4.17 | 0.04 |
| Atrial fibrillation/atrial flutter or tachycardia | 1 | 1 (1) | 3 | 3 (3) | 0.30 | 0.00–3.84 | 0.35 |
| Gastrointestinal | 17 | 14 (14) | 6 | 5 (5) | 2.82 | 0.91–10.41 | 0.06 |
| Diarrhea | 7 | 5 (5) | 2 | 2 (2) | |||
| Gastrointestinal/rectal or mouth haemorrhage | 7 | 7 (7) | 6 | 3 (3) | |||
| Nervous system | 13 | 11 (11) | 10 | 8 (8) | 1.30 | 0.45–3.91 | 0.64 |
| Headache | 9 | 7 (7) | 4 | 4 (4) | |||
| Injury, poisoning, or procedural complications | 7 | 7 (7) | 5 | 5 (5) | 1.31 | 0.34–5.44 | 0.77 |
| Drug overdose | 3 | 3 (3) | 1 | 1 (1) | |||
| Vascular disorders | 4 | 4 (4) | 7 | 7 (7) | 0.51 | 0.11–2.09 | 0.36 |
| Infections | 2 | 2 (2) | 12 | 8 (8) | 0.22 | 0.02–1.13 | 0.05 |
| Blood and lymphatic system disorders | 4 | 4 (4) | 4 | 4 (4) | 0.92 | 0.17–5.09 | 1.00 |
Adverse events (AE) and serious adverse events (SAE) that were reported in more than 4% of all the study patients are listed. The number of events and the number of patients experiencing AEs are summarized per system organ class, high level term and preferred term, according to the Medical Dictionary for Regulatory Activities. P-values calculated using two-sided Fisher’s exact tests.
Discussion
To the best of our knowledge this is the first clinical trial targeted to persistent PH in patients with corrected VHD. Contrary to our alternative hypothesis, long-term treatment with oral sildenafil negatively impacted outcome compared with placebo. These data confirm the recommendation of current practice guidelines1 against using PDE5 inhibitors and other drugs approved for pulmonary arterial hypertension in patients with LHD-PH.
Because it is believed to be safe and well tolerated, sildenafil is frequently used off-label to treat LHD-PH.17,26 The striking acute effects of the drug described in patients with native aortic valve stenosis,22 and after left-side valvular surgery18 have probably further expanded its use in VHD. Noticeably, our vasoreactivity test also lowered pulmonary pressures and increased cardiac output (Supplementary material online, Table S1, Table 1), in identical direction to these acute studies. Thus, the favourable acute haemodynamic effects of sildenafil may not be predictive of long-term outcome after prolonged administration in patients with LHD-PH.
Previous evidence of chronic treatment with PDE5 inhibitors in LHD-PH is controversial. Small clinical trials have suggested that sildenafil may improve the haemodynamic profile, overall exercise performance,13–15 and quality of life14 of patients with chronic LHD-PH of non-valvular etiology. Favourable evidence is highest in patients with HF and reduced ejection fraction,13,15,21 but also positive results have been reported in selected patients with HF and preserved ejection fraction.14 More recent clinical trials have questioned the clinical efficacy of sildenafil in patients with HF,12,16,21,27 and neutral findings have been reproduced with riociguat, another drug targeted to the nitric oxide signalling pathway.28
Most clinical trials of sildenafil in the field of LHD-PH have excluded elderly patients, and women have been frequently under-represented,13,14,21,27 or formally excluded.15 In fact, sildenafil resulted in a higher pulmonary capillary pressure than placebo in the only HF clinical trial showing demographic and comorbidity patterns comparable to our study.16 In our trial, neutral changes in 6-min walk test distance and natriuretic peptides did not match the incidence of hard events (readmission for HF), underscoring the need of using clinical outcomes in future trials in the field.
Although the mechanisms leading to worse outcomes of patients taking sildenafil in our study are necessarily speculative, a chronic increase in pulmonary capillary pressure is the most plausible explanation. The combination of advanced age, prevalent atrial fibrillation, and long-standing atrial overload, reduces atrial compliance in patients with VHD.4 In this context, pulmonary arterial vasodilation may rise capillary pressure because the left heart is not able to accommodate the increase in right ventricular output.29 This would render capillary pressure particularly sensitive to an increase in flow, predisposing to HF decompensation. Remarkably, our imaging findings support a certain degree of volume overload induced by sildenafil. Further analyses are need to clarify why drug-induced ventricular dilatation was higher for the LV than the RV.
As in other trials with sildenafil in HF,16 we did not exclude patients based on specific haemodynamic patterns of PH. It can be speculated that according to current guidelines,1 no benefit of sildenafil would to be expected if the SIOVAC population was mostly comprised by patients with isolated post-capillary PH. However, 57% of patients included in our trial showed a pulmonary vascular resistance > 3 Wood units, compatible with combined post- and pre-capillary PH.1 Although limited due to sample size, interaction analyses showed no evidence of potential benefit in any specific baseline or vasoreactivity haemodynamic profile. Thus, we believe our study adds clarifying information on the negative role of pulmonary vasodilators in LHD-PH.
Take home figure.
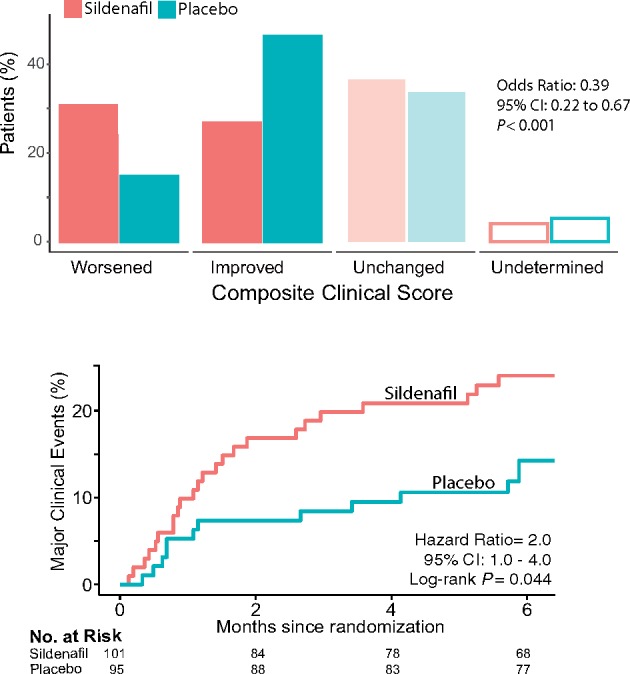
Sildenafil is associated to impaired clinical outcomes, as demonstrated by a higher proportion of patients who worsen their clinical status (upper panel) mainly due to a higher risk of readmission due to heart failure in the following six months (lower panel).
Equivalent mortality rate in our study was 5% per year. This figure is similar to the expected mortality of patients with pulmonary arterial hypertension who share the functional, biomarker, and haemodynamic profiles of patients in our study.1 Thus, VHD-PH should not be conceived as a benign condition and further basic and clinical research should continue to explore alternative therapies in this field.
Study limitations
The study group was heterogeneous in terms of VHD primary lesions, but sample size did not allow for a more detailed subgroup comparison of treated valves or type of surgery for example repair or replacement. The study was also underpowered to obtain significant results in most of the secondary endpoints. The composite clinical score on which we based our primary endpoint merges outcomes of diverse significance and may seem subjective. However composite scores meet current consensus of incorporating multiple outcome measures, circumvent the need of an allocation for testing multiple hypothesis, avoid the problems of competing risks and, most importantly, allow for lowering the cost of clinical trials by reducing required sample sizes.23 Furthermore, composite clinical scores which include self-assessment scales have been useful to demonstrate the efficacy of pharmacological30 and non-pharmacological31,32 therapies in HF. In addition, disaggregated analyses demonstrated that differences in the primary endpoint were due to an increase of the risk of HF decompensation in patients taking sildenafil. Thus, we believe the study provides reliable evidence on the impact of sildenafil on clinical outcomes of patients with LHD-PH due to VHD.
Conclusions
Treatment with oral sildenafil 40 mg t.i.d. for 6 months in patients with persistent PH after successful correction of VHD is associated to unfavourable clinical outcomes as compared to placebo. In the light this study, open label use of sildenafil in PH due to VHD should be discouraged, in agreement with current PH practice guidelines. Further efforts to identify novel therapeutic targets in this particular source of PH are needed.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgments
We thank all patients participating in this trial. We also acknowledge Margarita Rodriguez and Igor Martin from Chiltern International Ltd for their help conducting the trial, and all the personnel from the Cardiology Department of the H.G.U. Gregorio Marañon and other participating institutions for their selfless participation. We are in debt to Ana Fernández-Baza for her kind assistance in all administrative issues.
Funding
This study was supported by the Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Spain, and the EU –European Regional Development Fund (EC07/90772) as well as by the Red de Investigación Cardiovascular and CIBERCV.
Conflict of interest: none declared.
Contributor Information
Sildenafil for Improving Outcomes after VAlvular Correction (SIOVAC) investigators:
Javier Bermejo, Raquel Yotti, Teresa Mombiela, Ana Gónzález-Mansilla, Jaime Elízaga, José A García-Robles, Esther Pérez-David, Candelas Pérez del Villar, Ricardo Sanz, Enrique Gutierrez-Ibanes, María E Vázquez, Ana Mur, Yolanda Benito, Pablo Martínez-Legazpi, Alicia Barrio, Alexandra Vázquez, Rocío García-Orta, Inés Uribe, Mercedes González, Pedro Luis Sánchez, José M González-Santos, Javier Martín-Moreiras, Antonio Arribas, M Milagros Clemente Lorenzo, Alejandro Diego Nieto, Mario Castaño, Armando Pérez de Prado, David Alonso, Javier Segovia-Cubero, Manuel Gómez-Bueno, Inés Sayago Silva, Miguel Ángel Cavero, Pilar Escribano-Subias, Laura Domínguez, Rocío Tello de Meneses, M José Ruiz Cano, Carmen Jiménez López-Guarch, J Alberto San Román, Pedro Mota, Xavier Borrás, Carmen Amorós Galitó, Angel Alonso-Gómez, M Concepción Belló Mora, Dolores Mesa Rubio, Javier Botas, Raquel Campuzano, María G Crespo-Leiro, Raquel Marzoa, José Cuenca, Sonia Velasco, Roberto Muñoz, Verónica Suberviola, Cristina Beltrán Herrera, Laura Mora, M Mar Sarrión, David Vaqueriza, Antoni Bayes-Genís, Elena Ferrer, José R González-Juanatey, Belén Cid, Amparo Martínez Monzonís, Amador López, José M Arizón de Prado, Marta Santisteban, Dolores Mesa Rubio, Arturo Evangelista, David García-Dorado, Eduardo de Teresa, Manuel Jiménez-Navarro, Fernando Carrasco Chinchilla, Eduardo Moreno-Escobar, and Joaquín Alonso
References
- 1. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barbera J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis Zamorano J.. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119.26320113 [Google Scholar]
- 2. Weitsman T, Weisz G, Farkash R, Klutstein M, Butnaru A, Rosenmann D, Hasin T.. Pulmonary hypertension with left heart disease: prevalence, temporal shifts in etiologies and outcome. Am J Med 2017;130:1272–1279. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; Group ESCSD. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 4. Magne J, Pibarot P, Sengupta PP, Donal E, Rosenhek R, Lancellotti P.. Pulmonary hypertension in valvular disease: a comprehensive review on pathophysiology to therapy from the HAVEC Group. JACC Cardiovasc Imaging 2015;8:83–99. [DOI] [PubMed] [Google Scholar]
- 5. Li M, Dumesnil JG, Mathieu P, Pibarot P.. Impact of valve prosthesis-patient mismatch on pulmonary arterial pressure after mitral valve replacement. J Am Coll Cardiol 2005;45:1034–1040. [DOI] [PubMed] [Google Scholar]
- 6. Kainuma S, Taniguchi K, Toda K, Funatsu T, Kondoh H, Nishino M, Daimon T, Sawa Y.. Pulmonary hypertension predicts adverse cardiac events after restrictive mitral annuloplasty for severe functional mitral regurgitation. J Thorac Cardiovasc Surg 2011;142:783–792. [DOI] [PubMed] [Google Scholar]
- 7. Barbash IM, Escarcega RO, Minha S, Ben-Dor I, Torguson R, Goldstein SA, Wang Z, Okubagzi P, Satler LF, Pichard AD, Waksman R.. Prevalence and impact of pulmonary hypertension on patients with aortic stenosis who underwent transcatheter aortic valve replacement. Am J Cardiol 2015;115:1435–1442. [DOI] [PubMed] [Google Scholar]
- 8. Murashita T, Okada Y, Kanemitsu H, Fukunaga N, Konishi Y, Nakamura K, Koyama T.. The impact of preoperative and postoperative pulmonary hypertension on long-term surgical outcome after mitral valve repair for degenerative mitral regurgitation. Ann Thorac Cardiovasc Surg 2015;21:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magne J, Mathieu P, Dumesnil JG, Tanne D, Dagenais F, Doyle D, Pibarot P.. Impact of prosthesis-patient mismatch on survival after mitral valve replacement. Circulation 2007;115:1417–1425. [DOI] [PubMed] [Google Scholar]
- 10. Chen Y, Liu JH, Chan D, Sit KY, Wong CK, Ho KL, Ho LM, Zhen Z, Lam YM, Lau CP, Au WK, Tse HF, Yiu KH.. Prevalence, predictors and clinical outcome of residual pulmonary hypertension following tricuspid annuloplasty. J Am Heart Assoc 2016;5:e003353.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G.. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 12. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ.. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007;115:59–66. [DOI] [PubMed] [Google Scholar]
- 14. Guazzi M, Vicenzi M, Arena R.. Phosphodiesterase 5 inhibition with sildenafil reverses exercise oscillatory breathing in chronic heart failure: a long-term cardiopulmonary exercise testing placebo-controlled study. Eur J Heart Fail 2012;14:82–90. [DOI] [PubMed] [Google Scholar]
- 15. Cooper TJ, Guazzi M, Al-Mohammad A, Amir O, Bengal T, Cleland JG, Dickstein K.. Sildenafil in Heart failure (SilHF). An investigator-initiated multinational randomized controlled clinical trial: rationale and design. Eur J Heart Fail 2013;15:119–122. [DOI] [PubMed] [Google Scholar]
- 16. Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, van Veldhuisen DJ, Voors AA.. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 2015;36:2565–2573. [DOI] [PubMed] [Google Scholar]
- 17. Opitz CF, Hoeper MM, Gibbs JSR, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grünig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S.. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016;68:368–378. [DOI] [PubMed] [Google Scholar]
- 18. Jiang G, Li B, Zhang G, Xu E, Liu Y, Xu Z.. Effects of sildenafil on prognosis in patients with pulmonary hypertension after left-sided valvular surgery. Heart Lung Circ 2014;23:680–685. [DOI] [PubMed] [Google Scholar]
- 19. Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, Pepi M, Cosyns B, Dweck MR, Garbi M, Magne J, Nieman K, Rosenhek R, Bernard A, Lowenstein J, Vieira ML, Rabischoffsky A, Vyhmeister RH, Zhou X, Zhang Y, Zamorano JL, Habib G.. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589–590. [DOI] [PubMed] [Google Scholar]
- 20. Gómez-Sánchez MA, de la Calzada CS, Subías PE, Jiménez JFD, Salvador ML, González AA, Calvo LC.. Pilot assessment of the response of several pulmonary hemodynamic variables to sublingual sildenafil in candidates for heart transplantation. Eur J Heart Fail 2004;6:615–617. [DOI] [PubMed] [Google Scholar]
- 21. Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD.. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol 2007;50:2136–2144. [DOI] [PubMed] [Google Scholar]
- 22. Lindman BR, Zajarias A, Madrazo JA, Shah J, Gage BF, Novak E, Johnson SN, Chakinala MM, Hohn TA, Saghir M, Mann DL.. Effects of phosphodiesterase type 5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation 2012;125:2353–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001;7:176–182. [DOI] [PubMed] [Google Scholar]
- 24. Hoeper MM, Oudiz RJ, Peacock A, Tapson VF, Haworth SG, Frost AE, Torbicki A.. End points and clinical trial designs in pulmonary arterial hypertension: clinical and regulatory perspectives. J Am Coll Cardiol 2004;43:48S–55S. [DOI] [PubMed] [Google Scholar]
- 25. Bolland K, Sooriyarachchi MR, Whitehead J.. Sample size review in a head injury trial with ordered categorical responses. Stat Med 1998;17:2835–2847. [DOI] [PubMed] [Google Scholar]
- 26. Reddy YN, Borlaug BA.. Sildenafil, unbridled optimism, and heart failure with preserved ejection fraction. Eur J Heart Fail 2017;19:126–128. [DOI] [PubMed] [Google Scholar]
- 27. Amin A, Mahmoudi E, Navid H, Chitsazan M.. Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congest Heart Fail 2013;19:99–103. [DOI] [PubMed] [Google Scholar]
- 28. Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, Oudiz RJ, Boateng F, Scalise AV, Roessig L, Semigran MJ; Left Ventricular Systolic Dysfunction Associated with Pulmonary Hypertension Riociguat Trial Study Group. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013;128:502–511. [DOI] [PubMed] [Google Scholar]
- 29. Boilson BA, Schirger JA, Borlaug BA.. Caveat medicus! Pulmonary hypertension in the elderly: a word of caution. Eur J Heart Fail 2010;12:89–93. [DOI] [PubMed] [Google Scholar]
- 30. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 31. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P; IN-TIME study group. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 2014;384:583–590. [DOI] [PubMed] [Google Scholar]
- 32. Krum H, Forbes A, Yallop J, Driscoll A, Croucher J, Chan B, Clark R, Davidson P, Huynh L, Kasper EK, Hunt D, Egan H, Stewart S, Piterman L, Tonkin A.. Telephone support to rural and remote patients with heart failure: the Chronic Heart Failure Assessment by Telephone (CHAT) study. Cardiovasc Ther 2013;31:230–237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.