Abstract
Objectives
We aimed at assessing whether differences among males and females in carpal tunnel syndrome (CTS) epidemiology might be attributable to segregation with respect to occupational biomechanical exposures or differential access to care by sex.
Methods
We analysed surgically treated cases of CTS occurring among non-manual workers in Tuscany between 1997 and 2000. We conducted a Monte Carlo simulation to estimate the difference in occupational biomechanical exposures between males and females necessary to explain the observed incidence rate ratios. We also accounted for the sex-specific probability of receiving surgery after the diagnosis of CTS, as women were reported to be more likely to undergo surgery in a subset of our study population. We quantified the hypothetical biomechanical overload through the hand activity level (HAL) metric proposed by the American Conference of Governmental Industrial Hygienists. To quantify the effect of HAL on CTS risk, we assumed a prior distribution based on findings from two large cohort studies of industrial workers.
Results
After adjustment for the probability of receiving surgery, women showed a 4-fold incidence of CTS as compared with men. To explain this association among non-manual workers, women should have an average value of HAL at least 5 points higher.
Conclusions
Our analysis does not support the hypothesis that the difference in CTS incidence between males and females is entirely attributable to occupational risk factors or to differential access to surgery. The causal pathway between sex and CTS might include more determinants such as hormonal factors, anthropometric characteristics, and non-occupational exposure to biomechanical overload (e.g. household tasks).
Keywords: biomechanical overload, carpal tunnel syndrome, hand activity level, non-manual workers, occupational exposures, population studies, probabilistic bias analysis, repetition, sensitivity analysis, sex
Introduction
Population studies demonstrated an important difference in carpal tunnel syndrome (CTS) incidence between males and females (Mondelli et al., 2002; Mattioli et al., 2008; English et al., 2015; Roquelaure et al., 2017). In Italy, a 4- to 5-fold increase in risk was observed for women compared with men for both CTS diagnosis and surgery (Mondelli et al., 2002; Violante et al., 2007; Mattioli et al., 2008). In the past, several epidemiological studies have been conducted to identify plausible sex-specific causes of CTS, possibly including hormonal factors, pregnancy, anthropometric characteristics, and non-occupational biomechanical exposures (Dieck and Kelsey, 1985; Padua et al., 2010; Apostoli et al., 2012; Mondelli et al., 2016a). Intriguingly, lower sex ratios were observed for CTS diagnosis in large cohort studies of industrial workers where the exposure to biomechanical risk factors was carefully assessed (Kapellusch et al., 2014; Violante et al., 2016). This fact suggests that the association between sex and CTS risk might convey information on residual confounding due to biomechanical exposures (Messing and Silverstein, 2009; Silverstein et al., 2009; Burt et al., 2013). Indeed, on the one hand, biomechanical risk factors—in particular the execution of forceful movements and high exertion frequencies—are important determinant of CTS (Kozak et al., 2015). On the other hand, available evidence suggests that women are more likely to perform repetitive task and to work at high pace, also within a specific occupation (Eng et al., 2011). Thus, sex might act as a surrogate of exposure when biomechanical risk factors are poorly measured and misclassified. Establishing whether the effect of sex on CTS risk is merely determined by the pattern of occupational exposures or not could be fundamental for preventive purposes (Messing and Silverstein, 2009). A further issue in the study of differences between males and females in CTS is the potential for differential access to surgery. Women might be more likely than men to report symptoms (Mondelli et al., 2005); in addition, an ecological comparison of diagnosis and surgery rates in the district of Siena—one of the ten provinces of Tuscany—suggested that women tended to undergo surgery more frequently than men (Mattioli et al., 2009a). Hence, the potential of ascertainment bias (i.e. a different probability of receiving surgery given the presence of CTS by sex) should be assessed in population studies of the incidence of surgically treated CTS incidence by sex.
The aim of the present study is to explore the hypothesis that differences between males and females in surgically treated idiopathic CTS registered among non-manual workers in Tuscany (Italy), between 1997 and 2000, could be explained by ascertainment bias and/or by gender segregation with respect to occupational biomechanical exposures.
Methods
Study design and population
In common epidemiological practice, researchers estimate parameters of interest (e.g. relative risk for the exposure under study) running multivariable regression models; these models consider information on measured confounders and provide ‘adjusted’ estimates. Unfortunately, this approach fails to consider (i) source of bias other than confounding and (ii) the possible effect of unknown or unmeasured confounders (Lash et al., 2014). In our analysis, we wanted to consider explicitly two possible sources of bias in the study of the association between sex and surgically treated CTS: (i) case ascertainment bias and (ii) confounding due to unmeasured occupational biomechanical risk factors. To deal with these aspects, we conducted a Monte Carlo simulation assuming probability distributions for the unmeasured parameters based on available data from the scientific literature.
The study population and the data collection were described in detail elsewhere (Mattioli et al., 2009b; Curti et al., 2014). Briefly, information on 17988 surgically treated cases of CTS among residents of Tuscany occurring during 1997–2000 was collected from obligatory discharge records of all Italian public/private hospitals. To restrict the study to idiopathic CTS, 245 cases with the following secondary (i.e. coexisting) diagnoses were excluded: hypothyroidism (ICD-9 codes 243, 244), thyroiditis (245), diabetes mellitus (250), gout (274.0), amyloidosis (277.3), overweight/obesity (278), complications of pregnancy (646.8, 646.9), connective tissue diseases (710), rheumatoid arthritis (714), osteoarthritis of the hand/forearm (715.3, 715.4), wrist fractures (813.4), shoulder/upper limb peripheral nerve injuries (955), and pregnancy (V22) (Atcheson et al., 1998; van Dijk et al., 2003; Geoghegan et al., 2004). Because of limited numbers of cases in the youngest age groups and selection bias considerations related to ‘retired’ occupational status (in Italy, women retire at a younger age than men do), workers aged below 25 (n = 222) or above 59 years (n = 6687) were not considered in our study. Full-time housewives (n = 3332) and members of armed forces (n = 18) were excluded from this analysis, along with students (n = 16), cases with undeclared/unknown employment status (n = 350), patients treated outside Tuscany (n = 303), unemployed (n = 185), retired subjects (n = 890), first job seekers (n = 22), and those with ‘other’ (unspecified) job titles (n = 236). After these exclusions, 5482 cases of idiopathic surgically treated CTS entered our analysis.
Denominators (i.e. number of at-risk subjects) were constructed based on the national census data (2001) that report the population structure by age and sex. As we excluded from the analysis the cases occurring among housewives, we also subtracted from the denominators the number of full-time housewives using information from National Institute for Statistics (ISTAT) classification of ‘non-workforce’, which includes a specific ‘housewife’ category.
In Tuscany, all discharge records report the current (i.e. at the time of the hospitalization) occupation classified as follows: managers (14 CTS cases during the study period), self-employed professionals (75), entrepreneurs (34), clerical workers (672), associate professionals (280), skilled/unskilled manual workers (1424), service workers (1905), home-based workers (155), and self-employed manual workers (923). Unfortunately, the occupational coding of the discharge records does not match the ISTAT-2001 Classification of Occupations applied to classify job titles in the 2001 census. However, both classifications allow distinguishing non-manual workers and manual workers (also including routine-service workers). Supplementary Table 1 (available at Annals of Occupational Hygiene online) presents the matching scheme of occupational grouping used in our analysis. In the present article, we focused on non-manual workers, considering manual workers only for preliminary analysis.
Analysis of sex-related ascertainment bias
Available evidence suggests that women might be more likely to undergo surgery for CTS after the diagnosis, possibly due to a more severe clinical course (Mondelli et al., 2005; Mattioli et al., 2009a); this factor might introduce a form of selection bias when evaluating the association between CTS and sex. Noteworthy, there are available data on sex-specific incidence rates of diagnosed and surgically treated CTS in the province of Siena in 1997–1998 [Table 1, adapted from Mattioli et al. (2009a)]. Siena is one of the ten provinces in Tuscany and represents 7–8% of the total population of the region. Thus, we had information on the proportion of patients with CTS that underwent surgery based on data registered during the study period in a subsample of our study population. As shown in Table 1, in 1997–1998 there were 604 diagnoses of CTS among women in Siena. The probability of receiving surgery for a woman with CTS was 44.7% [95% confidence intervals (CI) 40.7–48.8%]. Among men, there were 70 surgeries over a total of 194 diagnoses; the probability of receiving surgery after the diagnosis was 36.1% (95% CI 29.3–43.3%). Based on these sex specific probabilities, we estimated the total number of sex-specific CTS diagnoses as follows:
Table 1.
Crude incidence rates (per 100000 person-years) of carpal tunnel syndrome in the province of Siena, 1997–1998. Table adapted from Mattioli et al. 2009a. The number of cases was reconstructed based on the rates reported in the original publications and the number of subjects at-risk (Mattioli et al., 2009a; Mondelli et al., 2002).
| Women | Men | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnoseda | Surgically treated | Treated %diagnosed | Diagnoseda | Surgically treated | Treated %diagnosed | |||||||
| Year | IR | Cases | IR | Cases | % | 95% CI | IR | Cases | IR | Cases | % | 95% CI |
| 1997 | 478.6 | 296 | 244.2 | 151 | 51 | 45–57 | 161.4 | 92 | 68.4 | 38 | 41 | 31–52 |
| 1998 | 497.8 | 308 | 193.9 | 119 | 37 | 33–44 | 178.5 | 102 | 57.7 | 32 | 31 | 23–41 |
| 1997–1998 | 604 | 270 | 45 | 41–49 | 194 | 70 | 36 | 29–43 | ||||
IR, incidence rate.
aDiagnosis based on clinical symptoms and nerve conduction studies.
| (1) |
| (2) |
where P(S+|D+) is the probability of receiving surgery after the diagnosis of CTS. The estimated numbers of diagnosis were rounded to the nearest integer and modelled as the dependent variable in Poisson regression models. In the Monte Carlo simulation, at each repetition the sex-specific P(S+|D+) was drawn from a β distribution. Supplementary Figures 1 and 2 (available at Annals of Occupational Hygiene online) present the assumed distributions among women (parameter α = 269.55, parameter β = 333.45) and men (parameter α = 69.64, parameter β = 123.36).
Analysis of the role of occupational biomechanical risk factors
Biomechanical risk factors are major determinants of CTS risk and the fraction of cases attributable to occupational exposures in certain occupational groups might be above 70% (Rossignol et al., 1997; Roquelaure et al., 2009). The risk of CTS has been consistently reported to be higher among manual workers (e.g. Mattioli et al., 2009b). Thus, to reduce the potential for confounding due to occupational biomechanical exposures, we restricted our main analysis to non-manual workers, a subset of the general population that should be less exposed to biomechanical risk factors. However, available evidence suggests that occupational risk factors could be important determinants of CTS also among lower-grade white-collar workers (Roquelaure et al., 2009). According to current evidence, biomechanical risk factors of CTS include the following (Kozak et al., 2015):
force (high-quality evidence);
repetition/high exertion frequency (high-quality evidence);
vibration (moderate evidence);
wrist postures (low-quality evidence).
Differences between males and females in the exposure to forceful movements are not a major concern in our analysis, as they are likely to bias the estimates of interest (the relative risk for women compared with men) towards the null hypothesis. Indeed, previous studies reported that women tend to perform tasks with higher hand activity, but lower force (Landau et al., 1996; Eng et al., 2011). Thus, neglecting it in a sensitivity analysis aimed at assessing a positive association between female sex and CTS risk should be a conservative approach. Nevertheless, we must consider that ergonomic assessment should be based on the evaluation of force on a relative scale; as the American Conference of Governmental Industrial Hygienists (ACGIH) recommends, the ergonomists should estimate the relative level of effort rather than the absolute value of force applied (ACGIH, 2001). As females are expected to have a lower force than males, we cannot exclude that for a given level of exposure to biomechanical risk factors a female might have a higher risk than a male.
Repetition/high exertion frequency is an important determinant of CTS. A priori evidence suggests that confounding due to this exposure is likely to inflate the relative risks of CTS observed for women (Landau et al., 1996; Eng et al., 2011; Burt et al., 2013). Hence, we wanted to provide risk estimates accounting for differences in exposure to repetitive movements between males and females. The most straightforward approach to account for unmeasured confounders is to perform an indirect adjustment, possibly within a Bayesian framework that allows incorporating uncertainty (Steenland and Greenland, 2004). However, this method requires prior knowledge on the distribution of the confounder in the studied population. At present, a reliable survey on the exposure to repetitive movements by sex is not available for the population of Tuscany. Hence, we decided to reverse the approach, and conduct a target-adjustment sensitivity analysis to quantify the difference in the exposure to repetitive movements by sex that would be necessary to explain the apparent relative risk for females (Phillips, 2003).
Lin and colleagues proposed an approach to study the sensitivity of point and interval estimates of the exposure under investigation (E) to the residual confounding effect of an unmeasured variable (U) after adjusting for measured covariates (Lin et al., 1998). Assuming that U is normally distributed and that there is no interaction between E and U, the relative risk (RR) can be estimated by knowing the apparent relative risk (RR*), the relative risk of diseases associated with U (Γ), and the mean difference in the exposure to U across the E strata (δ):
| (3) |
The value of RR depends on the mean difference (δ) and not on the actual values of exposure to U. The aforementioned formula can be solved for δ:
| (4) |
where β*, β, and γ are, respectively, the regression parameters for RR*, RR, and Γ.
Estimation technique
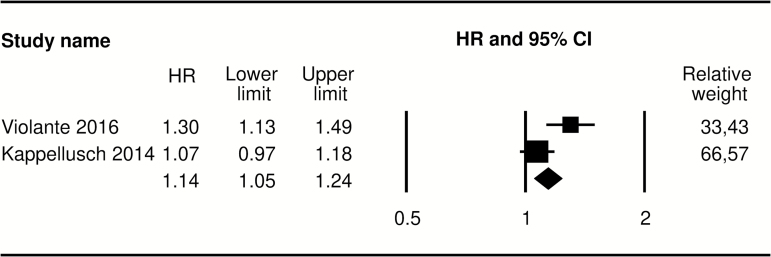
We estimated β* by fitting regression models to empirical data (RR 5.78, 95% CI 4.93–6.76; see Table 2). β, the target coefficient of the sensitivity analysis, is by definition chosen by the investigators (Phillips, 2003). Usually the target β is set to zero (absence of association). However, we performed two sets of analyses, the first with β equal to zero and the second with a β of 0.69 (RR = 2). The latter was a conservative choice made to account for further residual confounding due to occupational exposures other than repetition. We quantify the association between repetition and CTS risks (expressed by γ) with reference to the hand activity level (HAL) proposed by the ACGIH (ACGIH, 2001). HAL considers exertion frequency, recovery time, and the speed of motion and it ranges between 0 (hands idle most of the time; no regular exertions) and 10 (rapid steady motion; difficulty keeping up or continuous exertion). At present, there is prospective evidence on the risk of CTS for a unitary increase in HAL from two large prospective cohort studies of industrial workers (Kapellusch et al., 2014; Violante et al., 2016); Fig. 1 presents the estimates from the two studies and the fixed-effect pooled estimate [hazard ratio 1.14, 95% CI 1.05–1.24]. Hence, in our Monte Carlo simulation we assumed γ (the β for HAL) as normally distributed with mean 0.1327 and standard deviation 0.0408, corresponding to the hazard ratio of CTS for a unitary increase in HAL observed in the available longitudinal studies. We also performed an analysis where γ was assumed constant with a value of 0.2127 (corresponding to a hazard ratio of 1.24, the upper bound of the 95% CI for the pooled estimate presented in Fig. 1).
Table 2.
Incidence rate ratios of CTS from Poisson regression models. Estimates based on observed data on surgically treated cases and Monte Carlo simulation of CTS diagnosis. Non-manual workers, Tuscany region, Italy, 1997–2000.
| Observed surgically treated CTS cases | Simulated CTS diagnosis | |||
|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | |
| Gender | ||||
| Men | 1.00 | Ref. | 1.00 | Ref. |
| Women | 5.78 | 4.93–6.76 | 4.01 | 3.34–4.93 |
| Age (years) | ||||
| 25–29 | 1.00 | Ref. | 1.00 | Ref. |
| 30–34 | 1.66 | 1.17–2.38 | 1.71 | 1.17–2.48 |
| 35–39 | 2.29 | 1.64–3.21 | 2.27 | 1.59–3.24 |
| 40–44 | 3.71 | 2.68–5.12 | 3.65 | 2.59–5.15 |
| 45–49 | 4.44 | 3.22–6.12 | 4.49 | 3.18–6.31 |
| 50–54 | 6.38 | 4.64–8.76 | 6.33 | 4.52–8.88 |
| 55–59 | 5.68 | 4.03–8.01 | 5.69 | 3.92–8.17 |
CI, confidence interval; CTS, carpal tunnel syndrome; Ref., reference category.
Figure 1.
Meta-analysis of available cohort studies on the association between HAL and CTS incidence.
Hand-arm vibration and non-neutral wrist postures are suspected to be risk factor for CTS, but the evidence is limited (Kozak et al., 2015). Regarding hand-arm vibration, exposure is unlikely to occur among non-manual workers; thus, this factor is unlikely to bias the estimates observed for gender. On opposite, non-neutral wrist postures might be present in some non-manual occupations (e.g. those involving intense use of computers). However, there is only limited evidence of an increased risk of CTS due to hand-wrist postures and no sign of association was observed in the only large longitudinal study where a careful assessment of force and repetition was also performed (Harris-Adamson et al., 2015; Kozak et al., 2015). Moreover, even though postures might be implied in the genesis of CTS, they are extremely difficult to be quantified (Bao et al., 2009). On the balance, we decided against the direct incorporation of a possible effect of postures on CTS risk in our analysis. However, we conducted an analysis with a target RR of two, which allows for some residual confounding due to unmeasured occupational exposures, including hand-wrist postures.
We estimated the parameters of the β distribution for P(S+|D+) among women and men with the parameter solver of Multc Lean desktop v2.1 (MD Anderson Cancer Center, Houston, TX).
Statistical analysis was carried out using Stata 14.2 SE (Stata Corporation, College Station, TX). At first, we conducted a preliminary analysis of observed data; we estimated the incidence ratios (and 95% Poisson CI) of surgically treated CTS by occupation, sex, and age class. We then restricted our analysis to non-manual workers and we estimated the incidence rate ratio (IRR) of surgically treated CTS by fitting Poisson regression models with the observed counts as the dependent variable, age and sex as independent variables, and the logarithm of the number of person-years at-risk as the offset.
In our analysis, we accounted for systematic error (sex-related ascertainment bias) and random error by implementing a probabilistic bias analysis (Lash et al., 2011). For our Monte Carlo simulation, we generated 100000 simulated datasets based on observed data and assumed independent distributions for P(S+|D+) among women and men; hence, we were able to estimates the counts of CTS diagnoses as the nearest integer of the observed counts multiplied by 1/P(S+|D+).
Then, within each database, we performed the following steps:
We estimated β coefficients adjusted for systematic error by adapting a Poisson regression models with the estimated counts of diagnoses as the dependent variable, age and sex as independent variables, and the logarithm of the number of person-years at-risk as the offset.
We calculated βs adjusted for systematic and random error through the formula (Lash et al., 2011): where Z is a standard normal random variable (mean 0, SD 1) and SEobserved data is the standard error estimated for the βs in the original data.
We drew γ from a random normal distribution with mean 0.1327 and standard deviation 0.0017 and we calculated the difference in average exposure to HAL between women and men necessary to explain the effect of sex (i.e. moving the β for sex adjusted for systematic and random error to the target β).
We presented our results as point estimates (50th percentile from the simulation) and 95% CI (2.5th and 97.5th percentiles); whenever appropriate, the estimated values were exponentiated to present IRR. The Stata code written for the Monte Carlo simulation is reported in the supplementary material.
Results
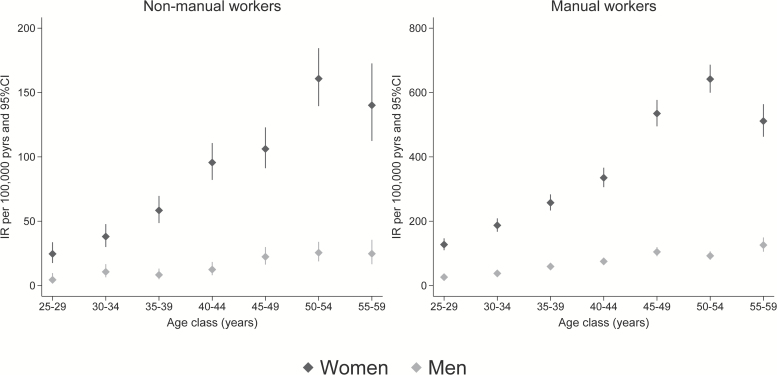
During the study period, 5482 CTS cases meeting our case definition were surgically treated in the Tuscany region. Table 3 shows the number of surgically treated CTS cases and the population at risk by occupational group, sex, and age; the corresponding incidence rates are presented in Fig. 2. As expected, non-manual workers presented lower incidence of CTS. Also, a remarkable difference between males and females was consistently observed across all age classes. Among non-manual workers, the age-specific incidence rate ratios for women compared with men ranged between 3.57 (30–34 years) and 7.69 (40–44 years).
Table 3.
Surgically treated cases of CTS and population at risk by occupational group, sex, and age. Tuscany region, Italy, 1997–2000.
| Age (years) | Non-manual workers | Manual workers | ||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
| Cases | Population | Cases | Population | Cases | Population | Cases | Population | |
| 25–29 | 39 | 158240 | 6 | 134288 | 190 | 149132 | 65 | 247904 |
| 30–34 | 75 | 196876 | 19 | 178036 | 320 | 170952 | 113 | 298668 |
| 35–39 | 124 | 212272 | 18 | 216388 | 419 | 162728 | 170 | 286732 |
| 40–44 | 178 | 186136 | 25 | 200976 | 483 | 144164 | 185 | 246052 |
| 45–49 | 179 | 168588 | 45 | 201196 | 665 | 124348 | 218 | 208228 |
| 50–54 | 203 | 126260 | 47 | 183872 | 851 | 132596 | 196 | 211760 |
| 55–59 | 88 | 62804 | 29 | 117112 | 402 | 78624 | 130 | 103244 |
Figure 2.
Incidence rates of surgically treated CTS by occupational groups, sex, and age classes.
Table 2 presents the IRR of CTS. The age-adjusted IRR of surgically treated CTS for women compared with men was 5.78 (95% CI 4.93–6.76). After accounting for sex-specific differential probability of receiving surgery through the Monte Carlo simulation, the IRR for sex declined to 4.01 (95% CI 3.34–4.93).
As shown in Table 4, only huge differences in the average exposure of HAL between males and females could bring the estimated IRR to the targets established for our sensitivity analysis. When we assumed HAL as distributed according to the best available evidence (see Fig. 1), more than five points difference was needed to reach a target IRR of two. When the target was set to an IRR of one, we estimated an impossible difference in the average of HAL of 10.51 between males and females [a score of 10 corresponds to the greatest level of repetition imaginable (ACGIH, 2001)]. As expected, estimates were lower when we assumed an effect of HAL on CTS risk equal to the upper bound of the 95% CI for the pooled estimate presented in Fig. 1; however, the difference necessary to reach a target IRR of two was still as high as 3.28 (95% CI 2.42–4.24).
Table 4.
Difference in average value of HAL necessary to explain the observed effect of sex on the risk of CTS.
| Observed IRRa |
Target IRR |
Β HALb |
ΔHALc | |
|---|---|---|---|---|
| Point estimate | 95% CI | |||
| 4.01 | 2 | Normally distributed, mean 0.1327, SD 0.0408 | 5.27 | 2.98–13.51 |
| 4.01 | 2 | Constant, 0.2127 | 3.28 | 2.42–4.24 |
| 4.01 | 1 | Normally distributed, mean 0.1327, SD 0.0408 | 10.50 | 6.38–26.40 |
| 4.01 | 1 | Constant, 0.2127 | 6.54 | 5.68–7.51 |
CI, confidence interval; HAL, hand activity level; IRR, incidence rate ratio.
aIRR from the simulation presented in Table 3.
bAssumed effect on CTS risk for a unitary increase in HAL.
cAverage value of HAL among women—average value of HAL among men.
Discussion
In the Tuscany region, between 1997 and 2000, an almost 6-fold increase in the risk of surgically treated CTS was observed for women compared with men among non-manual workers. After accounting for differential access to CTS surgery, the IRR for women (compared with men) declined to four. Occupational biomechanical risk factors might also contribute to explain some of the residual effect for sex, but they are highly unlikely to be the only determinant of the increased risk of CTS observed for females compared with males.
We believe that the differences in the average of HAL necessary to reach the target IRR in our analysis were implausible in all the simulated scenarios, as they ranged between 3.28 and the impossible value of 10.50. According to the HAL metric, six points are the difference between a task where there is no regular exertion and a task characterized by steady exertions and infrequent pauses (ACGIH, 2001). Also, a shift of three points in the HAL scale corresponds to a 4- to 8-fold variation in frequency (exertion/s) (ACGIH, 2001).
Our findings are probably not surprising given that differences between males and females in CTS incidence have been reported consistently in different countries and periods (Atroshi et al., 1999; Mondelli et al., 2002; Bland et al., 2003; Mattioli et al., 2008; Gelfman et al., 2009; English et al., 2015; Roquelaure et al., 2017). Moreover, a 2-fold increase in risk for women has also been demonstrated in a large prospective cohort study of industrial workers where biomechanical exposures where assessed according to ACGIH recommendations (Bonfiglioli et al., 2013; Violante et al., 2016). However, available prospective cohort studies with careful assessment of occupational risk factors were designed to investigate the incidence of CTS only among manual workers (Kozak et al., 2015); thus, our study provides novel evidence supporting the existence of differences between males and females in CTS incidence not attributable to occupational risk factors among non-manual workers.
The causal pathway between sex and CTS could be mediated by several factors, including anthropometric variables, pregnancy, endocrine factors, and non-occupational biomechanical exposures. In Italy, during the study period, women used to perform most of the housework (Bianchi et al., 2000; ISTAT 2007). Apostoli and colleagues evaluated the upper limb exposure to biomechanical risk factors experienced by a group of housewives; some common tasks resulted above the action limit recommended by the ACGIH (e.g. cleaning bathroom furniture) or even above the threshold limit value (e.g. floor cleaning, ironing) (Apostoli et al., 2012). Moreover, full-time housewives showed a higher incidence of surgically treated CTS compared with non-manual workers in our former analysis of Tuscany discharge records (Mattioli et al., 2009b).
Hormonal factors could also play an important role in the genesis of CTS. A systematic review by Padua and colleagues reported an incidence of CTS during pregnancy ranging from 7 to 43% and a persistence of symptoms in about 30% of women 3 years after the pregnancy (Padua et al., 2010). Alongside hormonal factors that characterized pregnancy, also those associated to menopause have been hypothesized as risk factor for CTS. In a Korean study, an up-regulation of estrogens receptors in the tenosynovial tissue was found to be associated with idiopathic CTS in postmenopausal women (Kim et al., 2010). Moreover, in a Turkish case–control study, age at menopause was a predictor of the risk of CTS (Kaplan et al., 2008). Anthropometric characteristics that differ between males and females might also be associated with CTS risk. Earlier studies suggested that the anatomical configuration of the wrist and of the carpal tunnel could be a predisposing factor for CTS (Boz et al., 2004; Moghtaderi et al., 2005). Moreover, in a study of carpal tunnel characteristics performed with magnetic resonance imaging, women turned out to have a smaller carpal tunnel area and a smaller free space in carpal tunnel (Bower et al., 2006). Recent studies confirmed that certain hand/wrist anthropometric configurations are associated with increased risk and severity of CTS (Mondelli et al., 2014, 2015, 2016a, 2016b). Among anthropometric factors, body mass index is a well-known determinant of CTS (Shiri et al., 2015); it might also increase the detrimental effect of biomechanical exposures (Burt et al., 2013; Shiri et al., 2015). However, according to the statistics released by ISTAT, in the Tuscany region, during the study period, the prevalence of overweight and obesity was lower in women than in men (ISTAT, 2007). Thus, body mass index was probably a negative confounder in our analysis and was not one of the causes of the observed increased risk of CTS among females compared with males.
On the balance, we believe that our analysis provides support to the hypothesis that the risk of CTS is higher among women independently from occupational exposures. At first sight, the study of non-modifiable risk factors such as sex might have little apparent public health value (Silverstein et al., 2009). Thus, some authors suggested stratifying analysis by sex when studying musculoskeletal disorders (Messing and Silverstein, 2009; Silverstein et al., 2009). This approach could have the beneficial effect of reducing residual confounding when sex acts as a surrogate of occupational exposures not completely captured by the measured variables. However, the study of the effect of sex (direct or mediated) may also add an important piece of information. Indeed, the association between a disease and sex does not imply the absence of potential for primary or secondary prevention. Sex is often related to specific diseases through complex pathways also involving modifiable risk factors; for instance, in the case of CTS, the risk associated with biomechanical exposures during housework should be properly addressed, as this activity might represent an important source of biomechanical overload also among workers (in Italy, particularly among women) (ISTAT, 2007). Information on differences between males and females could also be useful to plan secondary prevention strategies, including health surveillance at the workplace.
Study strength and limitations
We presented data from a large population study of CTS surgery. Available information included sex, age, a broad classification of occupations, and the presence of comorbidities at the time of the surgery. The main strength of our study is the complete availability of discharge records from every Italian public/private hospital. We were virtually able to identify all Tuscany residents that received in-hospital surgery for CTS, independently from the location of the hospital. At the time of the study, out-of-hospital CTS surgery was almost completely absent in Italy and going abroad for treatment was presumably not a widely considered option for CTS (Mattioli et al., 2009b).
The main limitation of our study is that we were not able to produce indirectly adjusted estimates due to the absence of reliable information on average levels of exposure to biomechanical risk factors among non-manual workers. Thus, we had to conduct a target-adjustment sensitivity analysis that conveyed information on how likely were unmeasured confounders to explain the observed association, but do not allowed us estimating the residual risk of CTS for females compared with males after accounting for occupational biomechanical exposures. As for any register-based study, we cannot exclude the presence of misclassification. However, age and sex should be classified almost perfectly because, for administrative purposes, all discharge records include the fiscal code that convey information of sex and birth year. On opposite, some amount of misclassification could be present for occupation, which was added to the records based on self-reported information. Thus, we cannot exclude that some of the subjects included in our main analysis were actually manual workers. This sort of bias might have slightly increased the potential for confounding due to occupational biomechanical exposures. In our analysis, we did not consider the role of forceful movements. This sort of exposure is likely to introduce negative confounding in the association between sex and CTS because is more common among males (Landau et al., 1996; Eng et al., 2011; Burt et al., 2013). Hence, we left out force with the purpose to provide more conservative estimates. Unfortunately, we did not have enough data to account for occupational risk factors other than repetition and force. To deal with this issue, we set the target IRR to two, allowing for residual confounding. To quantify the exposure to repetitive movements, we applied the HAL metric proposed by the ACGIH (ACGIH, 2001). However, in a real world setting, this method is recommended only for jobs characterized by cyclic tasks, while most non-manual workers perform a complex mixture of acyclic tasks. Although it would not be materially possible to assess the exposure among many non-manual workers, our analysis refers to theoretical average values that, in principle, exist for every job. In our simulation, we considered as null the interaction between age and the exposure to repetitive movements (different effect of HAL across age classes); this assumption is in line with available knowledge, but we cannot exclude the presence of minor biases (Kapellusch et al., 2014; Violante et al., 2016). Another limitation of our analysis is the absence of information on some putative occupational determinants of CTS that could have different distribution by sex. Among others, the exposure to low temperatures might impair the nerve conduction and increase the likelihood of receiving a diagnosis of CTS (Scalco et al., 2013); at the same time, the exposure to low temperatures might be more frequent in some industry with a high proportion of female employees. Thus, we cannot exclude that our estimates might still be confounded by minor putative occupational risk factors.
The study period ended on 31 December 2000; this decision was made because after this date the number of surgical procedures for CTS performed out-of-hospital—and not reported in discharge records—started to increase. Extending the study periods to recent years could have introduced new sources of ascertainment bias.
To restrict our case definition to idiopathic CTS, we excluded a small proportion of cases (245 out of 17988, 1.4%) with known local/systemic diseases predisposing to CTS onset. However, we could not adjust the denominators (number of workers at risk) as reliable statistics on the distribution of the selected diseases by gender. This fact might introduce a minor bias in our analysis. As most of the selected comorbidities are age-related (e.g. diabetes or arthritis), a possible bias should be stronger when studying aged workers, irrespectively of sex; thus, we believe that the possible misspecification of the denominators in our analysis did not induce any major source of bias when analyzing the IRR by sex.
Conclusions
Our study does not support the hypothesis that differences in the incidence of CTS between males and females are fully attributable to the exposure to occupational biomechanical risk factors or differential access to care by sex. The complex pathway between sex and CTS should be assessed through the conduction of studies with information on both occupational and non-occupational risk factors.
Supplementary data
Supplementary data are available at Annals of Work Exposures and Health online.
Declaration
No funding was provided for this research. The authors have no conflict of interest to declare.
References
- American Conference of Governmental Industrial Hygienists (ACGIH) (2001)TLVs and BEIs. Cincinnati: American Conference of Governmental Industrial Hygienists. ISBN 1-882417-40-2. [Google Scholar]
- Apostoli P, Sala E, Curti S et al. (2012)Loads of housework? Biomechanical assessments of the upper limbs in women performing common household tasks. Int Arch Occup Environ Health; 85: 421–5. [DOI] [PubMed] [Google Scholar]
- Atcheson SG, Ward JR, Lowe W (1998)Concurrent medical disease in work-related carpal tunnel syndrome. Arch Intern Med; 158: 1506–12. [DOI] [PubMed] [Google Scholar]
- Atroshi I, Gummesson C, Johnsson R et al. (1999)Prevalence of carpal tunnel syndrome in a general population. JAMA; 282: 153–8. [DOI] [PubMed] [Google Scholar]
- Bao S, Howard N, Spielholz P et al. (2009)Interrater reliability of posture observations. Hum Factors; 51: 292–309. [DOI] [PubMed] [Google Scholar]
- Bianchi SM, Milkie MA, Sayer LC et al. (2000)Is anyone doing the housework? Trends in the gender division of household labor. Soc Forc; 79: 191–228. [Google Scholar]
- Bland JD, Rudolfer SM (2003)Clinical surveillance of carpal tunnel syndrome in two areas of the United Kingdom, 1991-2001. J Neurol Neurosurg Psychiatry; 74: 1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglioli R, Mattioli S, Armstrong TJ et al. (2013)Validation of the ACGIH TLV for hand activity level in the OCTOPUS cohort: a two-year longitudinal study of carpal tunnel syndrome. Scand J Work Environ Health; 39: 155–63. [DOI] [PubMed] [Google Scholar]
- Bower JA, Stanisz GJ, Keir PJ (2006)An MRI evaluation of carpal tunnel dimensions in healthy wrists: implications for carpal tunnel syndrome. Clin Biomech (Bristol, Avon); 21: 816–25. [DOI] [PubMed] [Google Scholar]
- Boz C, Ozmenoglu M, Altunayoglu V et al. (2004)Individual risk factors for carpal tunnel syndrome: an evaluation of body mass index, wrist index and hand anthropometric measurements. Clin Neurol Neurosurg; 106: 294–9. [DOI] [PubMed] [Google Scholar]
- Burt S, Deddens JA, Crombie K et al. (2013)A prospective study of carpal tunnel syndrome: workplace and individual risk factors. Occup Environ Med; 70: 568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, Coggon D, Baldasseroni A et al. (2014)Incidence rates of surgically treated rhegmatogenous retinal detachment among manual workers, non-manual workers and housewives in Tuscany, Italy. Int Arch Occup Environ Health; 87: 539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieck GS, Kelsey JL (1985)An epidemiologic study of the carpal tunnel syndrome in an adult female population. Prev Med; 14: 63–9. [DOI] [PubMed] [Google Scholar]
- van Dijk MA, Reitsma JB, Fischer JC et al. (2003)Indications for requesting laboratory tests for concurrent diseases in patients with carpal tunnel syndrome: a systematic review. Clin Chem; 49: 1437–44. [DOI] [PubMed] [Google Scholar]
- Eng A, ‘t Mannetje A, McLean D, Ellison-Loschmann L, Cheng S, Pearce N (2011)Gender differences in occupational exposure patterns. Occup Environ Med; 68: 888–94. [DOI] [PubMed] [Google Scholar]
- English JH, Gwynne-Jones DP (2015)Incidence of carpal tunnel syndrome requiring surgical decompression: a 10.5-year review of 2309 patients. J Hand Surg Am; 40: 2427–34. [DOI] [PubMed] [Google Scholar]
- Gelfman R, Melton LJ III, Yawn BP et al. (2009)Long-term trends in carpal tunnel syndrome. Neurology; 72: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan JM, Clark DI, Bainbridge LC et al. (2004)Risk factors in carpal tunnel syndrome. J Hand Surg Br; 29: 315–20. [DOI] [PubMed] [Google Scholar]
- Harris-Adamson C, Eisen EA, Kapellusch J et al. (2015)Biomechanical risk factors for carpal tunnel syndrome: a pooled study of 2474 workers. Occup Environ Med; 72: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT (2007)La vita quotidiana nel 2005. Indagine multiscopo sulle famiglie. Roma: Istituto Nazionale di Statistica. ISBN 9788845813870 [Google Scholar]
- Kapellusch Jm JM, Gerr FE, Malloy EJ et al. (2014)Exposure-response relationships for the ACGIH threshold limit value for hand-activity level: results from a pooled data study of carpal tunnel syndrome. Scand J Work Environ Health; 40: 610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan Y, Kurt SG, Karaer H (2008)Carpal tunnel syndrome in postmenopausal women. J Neurol Sci; 270: 77–81. [DOI] [PubMed] [Google Scholar]
- Kim JK, Hann HJ, Kim MJ et al. (2010)The expression of estrogen receptors in the tenosynovium of postmenopausal women with idiopathic carpal tunnel syndrome. J Orthop Res; 28: 1469–74. [DOI] [PubMed] [Google Scholar]
- Kozak A, Schedlbauer G, Wirth T et al. (2015)Association between work-related biomechanical risk factors and the occurrence of carpal tunnel syndrome: an overview of systematic reviews and a meta-analysis of current research. BMC Musculoskelet Disord; 16: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau K, Imhof-Gildein B, Mucke S (1996)On the analysis of sector-related and gender-related stresses at the workplace—an analysis of the AET data bank. Int J Ind Ergon; 17:175–86. [Google Scholar]
- Lash TL, Fink AK, Fox MP (2011)Probabilistic bias analysis. In Lash TL, Fox MP, Fink AK, editors. Applying quantitative bias analysis to epidemiologic data. New York: Springer Science & Business Media; pp. 117–150. ISBN 978-0-387-87960-4. [Google Scholar]
- Lash TL, Fox MP, MacLehose RF et al. (2014)Good practices for quantitative bias analysis. Int J Epidemiol; 43: 1969–85. [DOI] [PubMed] [Google Scholar]
- Lin DY, Psaty BM, Kronmal RA (1998)Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics; 54: 948–63. [PubMed] [Google Scholar]
- Mattioli S, Baldasseroni A, Bovenzi M et al. (2009a) Risk factors for operated carpal tunnel syndrome: a multicenter population-based case-control study. BMC Public Health; 9: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli S, Baldasseroni A, Curti S et al. (2008)Incidence rates of in-hospital carpal tunnel syndrome in the general population and possible associations with marital status. BMC Public Health; 8: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli S, Baldasseroni A, Curti S et al. (2009b) Incidence rates of surgically treated idiopathic carpal tunnel syndrome in blue- and white-collar workers and housewives in Tuscany, Italy. Occup Environ Med; 66: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing K, Silverstein BA (2009)Gender and occupational health. Scand J Work Environ Health; 35: 81–3. [DOI] [PubMed] [Google Scholar]
- Moghtaderi A, Izadi S, Sharafadinzadeh N (2005)An evaluation of gender, body mass index, wrist circumference, and wrist ratio as independent risk factors for carpal tunnel syndrome. Acta Neurol Scand; 112: 375–9. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Aprile I, Ballerini M et al. (2005)Sex differences in carpal tunnel syndrome: comparison of surgical and non-surgical populations. Eur J Neurol; 12: 976–83. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Aretini A, Ginanneschi F et al. (2014)Waist circumference and waist-to-hip ratio in carpal tunnel syndrome: a case-control study. J Neurol Sci; 338: 207–13. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Curti S, Farioli A et al. (2015)Anthropometric measurements as a screening test for carpal tunnel syndrome: receiver operating characteristic curves and accuracy. Arthritis Care Res (Hoboken); 67: 691–700. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Curti S, Mattioli S et al. (2016)Associations between body anthropometric measures and severity of carpal tunnel syndrome. Arch Phys Med Rehabil; 97: 1456–64. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Farioli A, Mattioli S et al. (2016)Severity of carpal tunnel syndrome and diagnostic accuracy of hand and body anthropometric measures. PLoS One; 11: e0164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli M, Giannini F, Giacchi M (2002)Carpal tunnel syndrome incidence in a general population. Neurology; 58: 289–94. [DOI] [PubMed] [Google Scholar]
- Padua L, Di Pasquale A, Pazzaglia C et al. (2010)Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve; 42: 697–702. [DOI] [PubMed] [Google Scholar]
- Phillips CV. (2003)Quantifying and reporting uncertainty from systematic errors. Epidemiology; 14: 459–66. [DOI] [PubMed] [Google Scholar]
- Roquelaure Y, Chazelle E, Gautier L et al. (2017)Time trends in incidence and prevalence of carpal tunnel syndrome over eight years according to multiple data sources: pays de la loire study. Scand J Work Environ Health; 43: 75–85. [DOI] [PubMed] [Google Scholar]
- Roquelaure Y, Ha C, Fouquet N et al. (2009)Attributable risk of carpal tunnel syndrome in the general population: implications for intervention programs in the workplace. Scand J Work Environ Health; 35: 342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M, Stock S, Patry L et al. (1997)Carpal tunnel syndrome: what is attributable to work? the Montreal study. Occup Environ Med; 54: 519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalco RS, Pietroski F, Celli LF et al. (2013)Seasonal variation in prevalence of carpal tunnel syndrome. Muscle Nerve; 47: 925–7. [DOI] [PubMed] [Google Scholar]
- Shiri R, Pourmemari MH, Falah-Hassani K et al. (2015)The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev; 16: 1094–104. [DOI] [PubMed] [Google Scholar]
- Silverstein B, Fan ZJ, Smith CK et al. (2009)Gender adjustment or stratification in discerning upper extremity musculoskeletal disorder risk?Scand J Work Environ Health; 35: 113–26. [DOI] [PubMed] [Google Scholar]
- Steenland K, Greenland S (2004)Monte Carlo sensitivity analysis and Bayesian analysis of smoking as an unmeasured confounder in a study of silica and lung cancer. Am J Epidemiol; 160: 384–92. [DOI] [PubMed] [Google Scholar]
- Violante FS, Armstrong TJ, Fiorentini C et al. (2007)Carpal tunnel syndrome and manual work: a longitudinal study. J Occup Environ Med; 49: 1189–96. [DOI] [PubMed] [Google Scholar]
- Violante FS, Farioli A, Graziosi F et al. (2016)Carpal tunnel syndrome and manual work: the OCTOPUS cohort, results of a ten-year longitudinal study. Scand J Work Environ Health; 42: 280–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.