Abstract
BACKGROUND
Recently, we reported that angiotensin II (Ang II)-induced hypertension is mediated by group IV cytosolic phospholipase A2α (cPLA2α) via production of prohypertensive eicosanoids. Since Ang II increases blood pressure (BP) via its action in the subfornical organ (SFO), it led us to investigate the expression and possible contribution of cPLA2α to oxidative stress and development of hypertension in this brain area.
METHODS
Adenovirus (Ad)-green fluorescence protein (GFP) cPLA2α short hairpin (sh) RNA (Ad-cPLA2α shRNA) and its control Ad-scrambled shRNA (Ad-Scr shRNA) or Ad-enhanced cyan fluorescence protein cPLA2α DNA (Ad-cPLA2α DNA) and its control Ad-GFP DNA were transduced into SFO of cPLA2α+/+ and cPLA2α−/− male mice, respectively. Ang II (700 ng/kg/min) was infused for 14 days in these mice, and BP was measured by tail-cuff and radio telemetry. cPLA2 activity, reactive oxygen species production, and endoplasmic reticulum stress were measured in the SFO.
RESULTS
Transduction of SFO with Ad-cPLA2α shRNA, but not Ad-Scr shRNA in cPLA2α+/+ mice, minimized expression of cPLA2α, Ang II-induced cPLA2α activity and oxidative stress in the SFO, BP, and cardiac and renal fibrosis. In contrast, Ad-cPLA2α DNA, but not its control Ad-GFP DNA in cPLA2α−/− mice, restored the expression of cPLA2α, and Ang II-induced increase in cPLA2 activity and oxidative stress in the SFO, BP, cardiac, and renal fibrosis.
CONCLUSIONS
These data suggest that cPLA2α in the SFO is crucial in mediating Ang II-induced hypertension and associated pathogenesis. Therefore, development of selective cPLA2α inhibitors could be useful in treating hypertension and its pathogenesis.
Keywords: angiotensin II, blood pressure, cytosolic phospholipase A2α, hypertension, cPLA2α+/+, cPLA2α−/−, cPLA2α+/− mice, subfornical organ
Angiotensin (Ang) II, the main component of the renin–angiotensin system, plays an important role in the pathogenesis of cardiovascular diseases including hypertension.1 Ang II-induced hypertension is due to its action in the subfornical organ (SFO) of circumventricular organs resulting in increased oxidative and endoplasmic reticulum (ER) stress and activity of the sympathetic nervous system.2–4 There is a substantial body of evidence that increased reactive oxygen species (ROS) production and activation of immune cells mediate Ang II-induced hypertension and associated pathogenesis.5–8 Ang II also increases the activity of cytosolic phospholipase A2 (cPLA2) resulting in arachidonic acid (AA) release from tissue phospholipids.9,10 AA is metabolized by cyclooxygenase (COX), lipoxygenase, and cytochrome P450A into various eicosanoids with prohypertensive and antihypertensive effects.11–13 Prostaglandin (PG) E2, by stimulating EP1 and EP3 receptors,14 and thromboxane A2,11 12-, and 20-HETE,12,13,15,16 by their vascular actions, exert prohypertensive effects. On the other hand, PGE2 through stimulation of EP2 and EP4 receptors,14,17 PGI2,11 and epoxyeicosatrienoic acids13,18 produce vasodepressor effects. One or more of the eicosanoids contribute to Ang II-induced hypertension.19–22 Ang II-salt hypertension is also dependent on COX-1 activity.23 Intracerebroventricular administration of PGE2 increases sympathetic activity, vasopressin release, and blood pressure (BP),24 and the hypothalamic paraventricular excitation and sympathetic activation, via EP3 receptors.25 Injection of PGE2 into the rostral ventrolateral medulla also causes sympathoexcitation and pressor response via the EP3 receptor.26 These observations suggest that the release of AA by cPLA2, the rate-limiting step in the synthesis of eicosanoids, could be critical for Ang II-induced ROS production and hypertension.
Several types of mammalian cPLA2 enzymes have been identified;27 however, group IV cPLA2 shows high selectivity for AA-containing phospholipids.27,28 cPLA2 consists of six isoforms (cPLA2α, -β, -γ, -δ, -ε, and -ζ) with only 30% homology, tissue distribution, and enzymatic activity.28 In a previous study, we showed that the selective cPLA2α gene disruption prevented Ang II-induced increase in urinary levels of eicosanoids, hypertension, and associated cardiovascular, renal dysfunction and inflammation, suggesting that prohypertensive eicosanoids generated from AA mediate Ang II-induced hypertension.29,30 However, the site of eicosanoids produced by group IV cPLA2α, which mediate Ang II-induced hypertension, is not known. Since numerous tissues including cardiovascular, renal, brain, and immune cells produce eicosanoids that exert their effect locally, these should be formed from AA released by cPLA2α and act at the site of action of Ang II.
PLA2 is distributed in several regions of the brain,31 and Ang II increases expression of PLA2 in the organum vasculosum of the lamina terminalis, paraventricular nucleus (PVN), nucleus of the solitary tract, and middle cerebral artery.32 The demonstration that Ang II-induced oxidative stress and hypertension is mediated via the COX-1-derived metabolite PGE2 via EP1 receptor in the SFO33 raises the possibility that cPLA2α in the SFO might be critical for the action of Ang II to increase oxidative stress and BP. To test this hypothesis, we examined the localization and the effect of cPLA2α depletion in the SFO by transduction with adenovirus (Ad)-green fluorescence protein (GFP)-cPLA2α short hairpin (sh) RNA (Ad-cPLA2α shRNA). We also examined its reconstitution in knockout (cPLA2α−/−) mice by transduction with Ad-enhanced cyan fluorescence protein (ECFP)-cPLA2α DNA (Ad-cPLA2α DNA) in the SFO. We then examined the effect of these probes on Ang II-induced hypertension and associated pathogenesis in mice. Our results show that depletion of cPLA2α in the SFO prevents Ang II-induced hypertension, ROS and ER stress, and associated pathogenesis, while expression of cPLA2α in cPLA2α−/− mice restores these deleterious effects of Ang II.
MATERIALS AND METHODS
Details for Materials and Methods section are in the online-only Data Supplement.
Animal experiments
All animal experiments were performed using protocols approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were conducted in 8- to 10-week-old, 20- to 25-g body weight, wild-type (cPLA2α+/+), and cPLA2α gene disrupted homozygous (cPLA2α−/−) male mice on BALB/c background. Ang II (700 ng/kg/min) or saline (vehicle) was infused for 14 days with micro-osmotic pumps implanted subcutaneously. Systolic BP (SBP) was measured by the noninvasive tail-cuff method, or mean arterial pressure (MAP) daily by radio telemetry. However, 2 to 3 out of 6 cPLA2α−/− BALB/c mice implanted with radio transmitters did not survive more than 8 to 10 days. We did not encounter this problem in male C57BL/6 mice. Therefore, we first confirmed the BP measurements recorded by the tail-cuff method in the male C57BL/6 mice to that obtained in BALB/c mice and then used cPLA2α−/− mice on the C57BL/6 background to further confirm BP measurements by radio telemetry.
Statistical analysis
One or 2-way analysis of variance was used to analyze the data, Tukey’s post hoc test for multiple comparisons, and student’s t-test to compare the difference between 2 groups. The values obtained from at least 3 to 5 different experiments were expressed as the mean ± SEM, P <0.05 was considered statistically significant.
RESULTS
cPLA2α gene disruption in SFO of cPLA2α+/+ mice with Ad-cPLA2α shRNA attenuated Ang II-induced increase in BP and cPLA2 activity, but not expression of cPLA2α, and reduced collagen accumulation in the heart and kidney
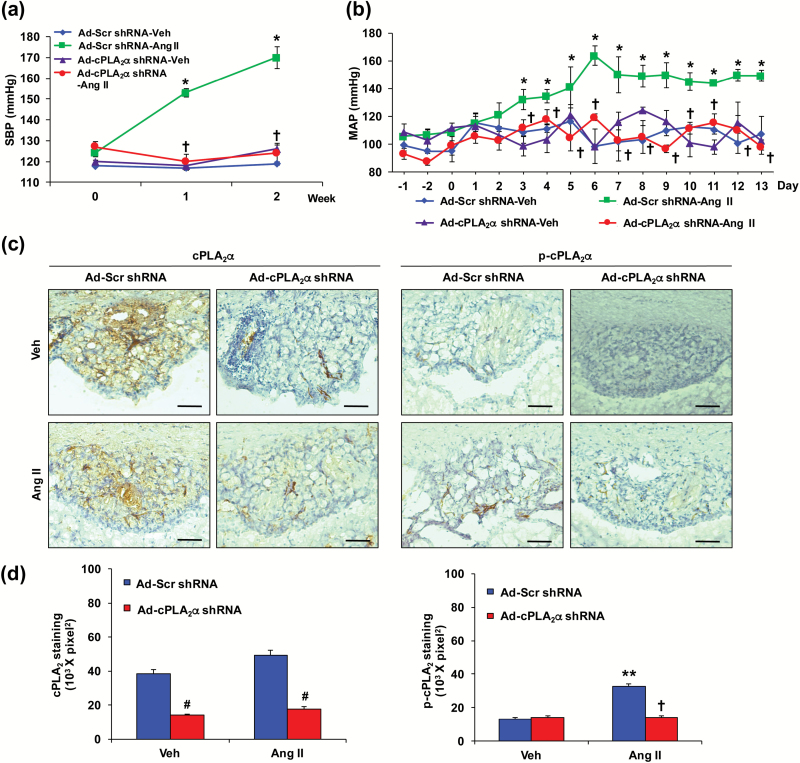
To determine the contribution of cPLA2α in the SFO to Ang II-induced hypertension, the SFO was transduced with Ad-cPLA2α shRNA. Infusion of Ang II by micro-osmotic pumps implanted subcutaneously increased SBP, measured by tail-cuff, in male cPLA2α+/+ BALB/c and cPLA2α+/+ C57BL/6 mice (Supplementary Figure S1A and B). Transduction of the SFO with Ad-cPLA2α shRNA but not its Ad-Scr shRNA prevented Ang II-induced increase in SBP in male cPLA2α+/+ BALB/c mice (Figure 1a). SBP was not altered by the adenoviruses during vehicle infusion (Figure 1a). Ad-cPLA2α shRNA but not its Ad-Scr shRNA also prevented Ang II-induced increase in mean arterial BP (MAP) measured by radio telemetry in cPLA2α+/+ C57BL/6 mice (Figure 1b). Transduction of the SFO with Ad probes was confirmed by expression of GFP in the SFO (Supplementary Figure S2A). cPLA2α expression in the SFO was abolished by Ad-cPLA2α shRNA but not Ad-Scr shRNA as determined by cPLA2α immunoreactivity using mouse anti-cPLA2 antibody in BALB/c mice (Figure 1c and d), and by RT-PCR in BALB/c and C57BL/6 mice (Supplementary Figures S2B and S3A, respectively). Ang II also increased cPLA2 activity measured by increased phospho-cPLA2 immunoreactivity in the SFO transduced with Ad-Scr shRNA, but not with Ad-cPLA2α shRNA in BALB/c (Figure 1c and d), and C57BL/6 mice (Supplementary Figure S3B and C). Transduction of the SFO with Ad-Scr shRNA or Ad-cPLA2α shRNA did not alter expression of cPLA2α in the PVN, heart, and kidney examined in BALB/c mice (Supplementary Figure S2C–E).
Figure 1.
cPLA2α gene disruption in subfornical organ (SFO) of cPLA2α+/+ mice with adenovirus (Ad)-green fluorescence protein (GFP)-cPLA2α shirt hairpin (sh) RNA (Ad-cPLA2α shRNA) abrogates Ang II-induced increase in blood pressure (BP) and cPLA2 phosphoimmunoreactivity. Ad-GFP scramble (Scr) shRNA (Ad-Scr shRNA) or Ad-cPLA2α shRNA was transduced into SFO. (a) systolic blood pressure (SBP) was measured by tail-cuff in BALB/c mice. (b) Mean arterial blood pressure (MAP) was measured by radio telemetry in C57BL/6 mice. (c) Expression of cPLA2, and its activity measured by its phosphorylation in SFO of BALB/c mice by immunohistochemical method. Scale bars: 50 µm. (d) Quantified data. Data are expressed as mean ± SEM. n = 5 per group. *, **P <0.05, Ad-Scr shRNA-Ang II vs. Ad-Scr shRNA-Veh (Vehicle); †P < 0.05, Ad-cPLA2α shRNA-Ang II vs. Ad-Scr shRNA-Ang II in cPLA2α+/+ BALB/c mice (a) and cPLA2α+/+ C57BL/6 mice (b). #P < 0.05, Ad-cPLA2α shRNA vs. Ad-Scr shRNA.
Ang II is known to cause cardiac and renal fibrosis.29,30 To determine if the alteration in cPLA2α expression in the SFO also affects the action of Ang II on cardiac and renal fibrosis, we examined the accumulation of collagen in these tissues in BALB/c mice. cPLA2α gene disruption in the SFO of cPLA2α+/+ mice by transduction with Ad-cPLA2α shRNA but not its Ad-Scr shRNA control infused with Ang II minimized accumulation of collagen in the heart and kidney (Supplementary Figure S4A and B).
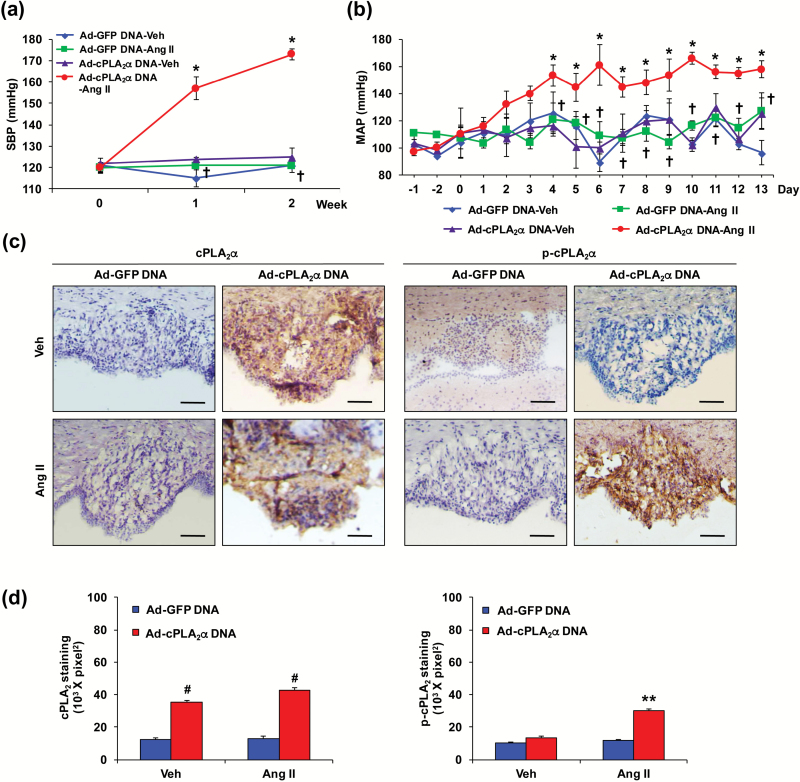
Transduction with Ad-ECFP-cPLA2α DNA, but not Ad-GFP DNA in the SFO of cPLA2α−/− mice restored the effect of Ang II to increase BP
Ang II failed to increase BP in cPLA2α−/− BALB/c mice (Supplementary Figure S1). Transduction with Ad-cPLA2α DNA but not Ad-GFP DNA in the SFO of cPLA2α−/− BALB/c mice restored the effect of Ang II to increase SBP measured by tail-cuff (Figure 2a). Ang II also increased MAP measured by radio telemetry in cPLA2α−/− C57BL/6 mice transduced with Ad-cPLA2α DNA, but not Ad-GFP DNA in the SFO (Figure 2b). The localization of ECFP-cPLA2α and GFP in the SFO transduced with Ad-cPLA2α DNA and Ad-GFP DNA, respectively, was confirmed by their fluorescence (Supplementary Figure S5A), and by RT-PCR in BALB/c (Supplementary Figure S5B) and C57BL/6 mice (Supplementary Figures S5A and S6A), and by immunohistochemistry using anti-cPLA2 antibody in BALB/c (Figure 2c and d) and C57BL/6 (Supplementary Figure S6B and C) mice. Ang II did not alter expression of cPLA2α, but it increased the cPLA2 activity, measured by phospho-cPLA2 immunoreactivity in the SFO transduced with Ad-cPLA2α DNA, but not Ad-GFP DNA in BALB/c mice (Figure 2c and d). cPLA2α mRNA expression in the PVN, heart, and kidney that was absent in cPLA2α−/− BALB/c, was not altered by transduction of the SFO with Ad-GFP DNA or Ad-cPLA2α DNA during infusion of Ang II (Supplementary Figure S5C–E).
Figure 2.
cPLA2α gene transduction in subfornical organ (SFO) of cPLA2α−/− mice with adenovirus (Ad)-enhanced cyan fluorescence protein (ECFP)-cPLA2α DNA (Ad-cPLA2α DNA) restores Ang II-induced increase in blood pressure (BP) and cPLA2 activity. Ad-green fluorescence protein (GFP) (Ad-GFP) DNA or Ad-cPLA2α DNA was transduced into SFO. (a) Systolic blood pressure (SBP) was measured by tail-cuff in cPLA2α−/− BALB/c mice. (b) Mean arterial blood pressure (MAP) was measured by radio telemetry in cPLA2α−/− C57BL/6 mice. (c) Expression, and activation of cPLA2 in SFO of BALB/c mice were measured by immunohistochemical method. Scale bars: 50 µm. (d) Quantified data. Data are expressed as mean ± SEM. n = 5 per group. *, **P < 0.05, Ad-cPLA2α DNA-Ang II vs. Ad-cPLA2α DNA-Veh (vehicle); †P < 0.05, Ad-cPLA2α DNA-Ang II vs. Ad-GFP DNA-Ang II in cPLA2α−/− BALB/c and C57BL/6 mice. #P < 0.05, Ad-cPLA2α DNA vs. Ad-GFP DNA.
We also determined the effect of Ang II on collagen accumulation in the heart and kidney of BALB/c cPLA2α−/− mice transduced with Ad-cPLA2α DNA and Ad-GFP DNA in the SFO, and found collagen accumulation in the former but not the latter group of mice (Supplementary Figure S7A and B).
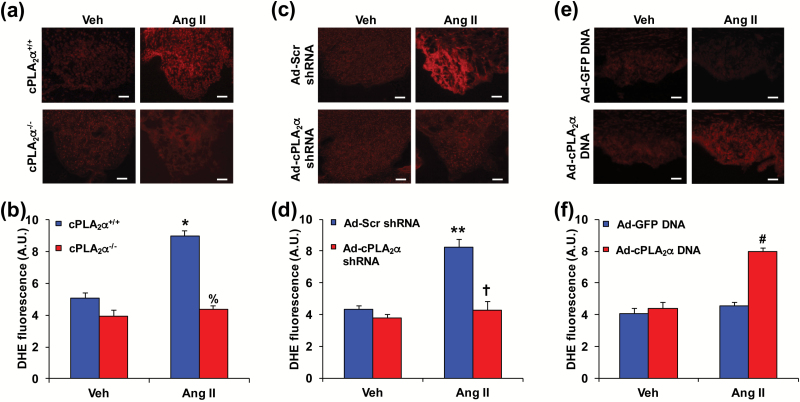
Transduction of SFO with cPLA2α shRNA in cPLA2α+/+ mice attenuated, and Ad-cPLA2α DNA in cPLA2α−/− mice restored Ang II-induced increase in ROS production
These studies were conducted in BALB/c mice that were infused with Ang II or its vehicle for the measurement of BP as described above. Infusion of Ang II also stimulated the production of ROS as indicated by enhanced 2-hydroxyethidium fluorescence in the SFO generated after staining with dihydroethidium as described,4 in cPLA2α+/+ mice but not in cPLA2α−/− mice (Figure 3a and b). Transduction of the SFO with Ad-cPLA2α shRNA but not its Ad-Scr shRNA inhibited dihydroethidium staining (Figure 3c and d) in cPLA2α+/+ mice. Infusion of Ang II in cPLA2α−/− mice failed to increase dihydroethidium staining, whereas transduction with Ad-cPLA2α DNA, but not Ad-GFP DNA in the SFO of these mice restored the effect of Ang II to increase dihydroethidium staining (Figure 3e and f).
Figure 3.
Transduction of subfornical organ (SFO) with Ad-cPLA2α shRNA attenuates, and Ad-cPLA2α DNA restores Ang II-induced increase in reactive oxygen species (ROS) production in cPLA2α+/+ BALB/c mice. ROS production was determined using dihydroethidium (DHE). (a) and (b) Ang II-induced increase in ROS production in SFO in cPLA2α+/+ but not cPLA2α−/− BALB/c mice. (c) and (d) Transduction of Ad-cPLA2α shRNA in SFO abrogated Ang II-induced increase in ROS production in cPLA2α+/+ BALB/c mice. (e) and (f) Transduction of Ad-cPLA2α DNA in SFO restored Ang II-induced increase in ROS production in cPLA2α−/− BALB/c mice. Panels a, c, and e, scale bars: 50 µm. Panels b, d, and f, Quantified data (A. U., arbitrary units). Data are expressed as mean ± SEM. n = 5 per group. *P < 0.05, Ang II vs. Veh (vehicle); %P < 0.05, cPLA2α−/−-Ang II vs. cPLA2α+/+-Ang II; **P < 0.05, Ad-Scr shRNA-Ang II vs. Ad-Scr shRNA-Veh; †P < 0.05, Ad-cPLA2α shRNA-Ang II vs. Ad-Scr shRNA-Ang II; #P < 0.05, Ad-cPLA2α DNA-Ang II vs. Ad-GFP DNA-Ang II.
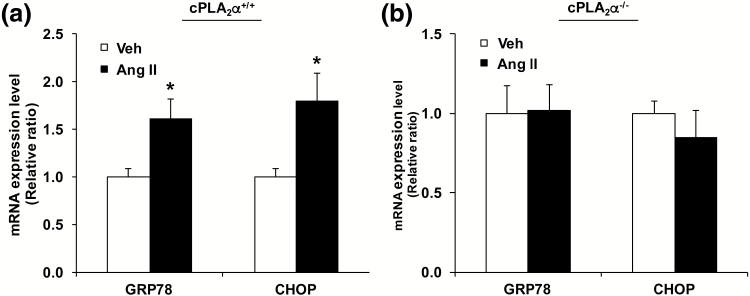
Ang II increased ER stress marker expression in SFO Of cPLA2α+/+ but not cPLA2α−/− mice
Ang II also increased ER stress as indicated by increased mRNA levels of markers of ER stress glucose-related protein 78 (GRP78), and C/EBP homologous protein (CHOP) in cPLA2α+/+ BALB/c mice (Figure 4a). Infusion of Ang II in cPLA2α−/− mice did not induce mRNA levels of GRP78 and CHOP (Figure 4b).
Figure 4.
Ang II increases endoplasmic reticulum (ER) stress marker expression in the subfornical organ (SFO) of BALB/c cPLA2α+/+ but not cPLA2α−/− mice. RNA was isolated from SFO, and real-time PCR (RT-PCR) was performed for glucose-related protein 78 (GRP78), and C/EBP homologous protein (CHOP). (a) mRNA expression of GRP78 and CHOP in SFO of cPLA2α+/+ mice. (b) mRNA expression of GRP78 and CHOP in SFO of cPLA2α−/− mice. *P < 0.05, Ang II vs. Veh (vehicle) (n = 4). Data are expressed as mean ± SEM.
Partial cPLA2α gene disruption (cPLA2α+/−) also prevented Ang-II-induced increase in BP in mice
Ang II did not increase BP in cPLA2α−/− BALB/c and C57BL/6 male mice (Supplementary Figure S1A and B). To determine if partial cPLA2α gene disruption reduces Ang II-induced increase in BP, we examined its effect in heterozygous cPLA2α (cPLA2α+/−) C57BL/6 male mice. Ang II (700 ng/kg/min) increased SBP measured by tail-cuff in cPLA2α+/+ but not cPLA2α+/− C57BL/6 male mice (Supplementary Figure S8). cPLA2α mRNA expression in the SFO, heart, and kidney of C57BL/6 cPLA2α+/− mice was lower (60–80%) than in C57BL/6 cPLA2α+/+ mice (Supplementary Figure S9).
DISCUSSION
The major findings of this sudy are that SFO is the principal site of action of cPLA2α in mediating the action of Ang II: (i) to increase BP; (ii) to stimulate ROS production and ER stress in the SFO, and (iii) to cause cardiac and renal fibrosis. These findings are based on our demonstration that cPLA2α selectively releases AA from tissue phospholipids,27,28 is expressed in the SFO, and that Ang II increased cPLA2 activity, as determined by its phosphoimmunoreactivity without altering its expression. However, Ang II has been shown to increase expression of phospholipase A2 in the organum vasculosum of the lamina terminalis, PVN, nucleus of the solitary tract, and middle cerebral artery of the rat.32 Whether this increase in phospholipase A2 expression by Ang II in these tissues represents primarily increased expression of cPLA2α, or other isoforms of phospholipase A2 is not known. We have previously reported that Ang II increases BP, and sympathetic outflow as determined from heart rate variability by power spectral analysis in cPLA2α+/+ but not cPLA2α−/− BALB/c mice.29 Our demonstration that cPLA2α gene disruption in the SFO by Ad-cPLA2α shRNA, but not its Ad-Scr shRNA, reduced cPLA2α expression and phospho-cPLA2 immunoreactivity and prevented Ang II-induced increase in BP in cPLA2α+/+ BALB/c and C57BL/6 mice, suggests that cPLA2α in the SFO is critical for Ang II-induced hypertension. Although Ang II 700 ng/kg/min used in this study would be expected to cause the increase in BP by its direct vascular action but it appears that cPLA2α in the SFO is primarily responsible for this effect of Ang II. Further supporting this conclusion was our finding that in cPLA2α−/− BALB/c and C57BL/6 mice, reconstitution of cPLA2α in the SFO by transduction with Ad-cPLA2α DNA but not Ad-GFP DNA increased cPLA2α expression and phospho-cPLA2 immunoreactivity, and restored the effect of Ang II to increase BP. That cPLA2α protein formed by transduction with Ad-cPLA2α DNA, but not Ad-GFP DNA is capable of releasing AA has been confirmed in vascular smooth muscle cells.34 The decrease in the expression of cPLA2α in the SFO transduced with Ad-Scr shRNA in cPLA2α+/+ mice, and the increase with Ad-cPLA2α DNA in cPLA2α−/− mice was selective because its expression in the PVN, heart and kidney were not altered in these mice. Ang II stimulates ROS production and ER stress in the SFO that leads to an increase in BP,3,4 most likely by increasing sympathetic activity.2 The increase in BP produced by Ang II 600 ng/kg/min, which is comparable to that obtained in the present study by 700 ng/kg/min of this peptide, is prevented by intracerebral ventricle administration of superoxide scavenger Ad-CuZn superoxide dismutase.4 Since (i) depletion of cPLA2α by Ad-cPLA2α shRNA in the SFO of cPLA2α+/+ mice reduced, and (ii) expression of cPLA2α by transduction with Ad-cPLA2α DNA in cPLA2α−/− BALB/c mice restored Ang II-induced ROS production and ER stress, this suggests that cPLA2α expression and activity mediates the effect of Ang II on ROS production and ER stress. Whether alteration in cPLA2α activity by Ang II in SFO also affects the ROS production and ER stress in PVN and rostral ventrolateral medulla remains to be determined. Ang II is known to produce cardiac and renal fibrosis, which is dependent on prohypertensive eicosanoids generated by activation of cPLA2α.29,30 Our demonstration that Ang II-induced cardiac and renal fibrosis, as indicated by collagen accumulation, was minimized by depletion of cPLA2α in the SFO by transduction with Ad-cPLA2α shRNA in cPLA2α+/+ BALB/c mice suggests that cPLA2α activation in the SFO contributes to this action of Ang II. Supporting this view was our observation that reconstitution of cPLA2α by Ad-cPLA2α DNA in cPLA2α−/− BALB/c mice caused Ang II to produce cardiac and renal fibrosis. Whether attenuation of Ang II-induced cardiac and renal fibrosis caused by decreased expression of cPLA2α by Ad-cPLA2α shRNA in the SFO of cPLA2α+/+ mice and restoration of fibrosis in these tissues by expression of cPLA2α by Ad-cPLA2α DNA in cPLA2α−/− mice, which could be due to changes in BP and/or sympathetic activity, remains to be determined.
cPLA2α activation by Ang II releases AA that is metabolized by COX, lipoxygenase, and cytochrome P450A into eicosanoids with prohypertensive and antihypertensive effects.11–22 Previously, we reported that prohypertensive eicosanoids generated by cPLA2α activation contributed to Ang II-induced hypertension and associated cardiac and renal pathogenesis.29,30 COX-1 inhibitor SC560 minimized Ang II-salt-induced hypertension which is associated with the increased sympathetic activity.32 Decrease in COX-2 expression by IL-10 in PVN is related to reduced neuronal sympathetic excitation in heart failure in rats after myocardial infarction.35 On the other hand, proinflammatory cytokines stimulate COX-2 expression in perivascular macrophages,36 and when injected in the SFO increase BP, heart rate, and renal sympathetic activity.37 Therefore, Ang II via production of proinflammatory cytokines could increase COX activity and PGE2 synthesis. Reduction in COX-1 and COX-2 expression by their respective siRNA in PVN also reduces deoxycorticosterone-induced hypertension.38 COX-generated AA metabolite PGE2 injected into the cerebroventricular system24,25 or rostral ventrolateral medulla26 increases BP and sympathetic activity via EP3 receptors, respectively.25,26 Ang II-induced increase in BP is inhibited in both EP1 and EP3 receptor knockout mice or by EP1 and EP3 receptor antagonists.19,21 PGE2 generated by COX-1 in the SFO via the EP1 receptor is required for ROS generation and hypertension caused by Ang II.33 AA-metabolizing enzymes are constitutively active, and the rate-limiting step in the production of eicosanoids is the availability of AA. Therefore, cPLA2α activation by Ang II in the SFO appears to be critical for AA release resulting in the production of PGE2, and generation of ROS and ER stress that increases BP and results in cardiac and renal fibrosis. The contribution in Ang II-induced hypertension of cPLA2α in the PVN and rostral ventrolateral medulla where PGE2 via EP3 receptors increases BP25,26 remains to be investigated and is one of the limitations of the present study. Like in our study in cPLA2α−/− mice, the COX1 or EP1 receptor gene disruption or the central administration of their pharmacological inhibitors attenuated the increase in BP produced byAng II (600 ng/kg/min)33 that was comparable to that obtained in the present study. At present, we have no explanation how the central cPLA2α/COX1/EP1 receptor in SFO masks the direct vasconstrictor effect of Ang II. Further studies are required to determine if alteration in cPLA2α/COX/EP receptors in SFO and other brain areas also prevent the effect of bolus injections or short-term infusion of Ang II.
An important finding in our study was that Ang II also failed to increase BP in the partially cPLA2α gene-disrupted mice (cPLA2α+/−) expressing reduced cPLA2α mRNA in the heart, kidney, and SFO in C57BL/6 mice. These observations further support the critical role of cPLA2α in Ang II-induced hypertension. Further studies on different levels of cPLA2α expression or its copy number in the SFO and other tissues should allow the determination of its relationship to BP in various models of hypertension and associated pathogenesis. cPLA2α gene disruption also prevented hypertension produced by the inhibitor of nitric oxide synthesis, L-NG-nitroarginine methyl ester,39 that is dependent on Ang II.40 Our preliminary data obtained in C57BL/6 mice showed that cPLA2α gene disruption abolished deoxycorticosterone-acetate-salt-induced hypertension and associated cardiac and renal fibrosis (C Y Song and K U Malik, unpublished results).
In conclusion, this study demonstrates that cPLA2α in the SFO is crucial in mediating the effect of systemic Ang II to cause ROS production and ER stress and hypertension, most likely by releasing AA and metabolizing it via COX producing PGE2. Our finding that the partial cPLA2α gene disruption (cPLA2α+/− mice) also prevented Ang II-induced hypertension supports the notion that cPLA2α activation is pivotal for the development of Ang II-induced hypertension. Therefore, development of selective orally active inhibitors of cPLA2α could be useful in the treatment of hypertension and its pathogenesis.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We thank Dr Richard Redfearn of the Office Scientific Writing in the Office of Research at UTHSC for editorial assistance. This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute grants R01HL-19134–42 (K.U.M.) and R21-NINDS-NS091593 (J.V.B.). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
REFERENCES
- 1. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59:251–287. [DOI] [PubMed] [Google Scholar]
- 2. Campese VM, Shaohua Y, Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 2005; 46:533–539. [DOI] [PubMed] [Google Scholar]
- 3. Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 2012; 122:3960–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 2004; 95:210–216. [DOI] [PubMed] [Google Scholar]
- 5. Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997; 95:588–593. [DOI] [PubMed] [Google Scholar]
- 6. Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance?Hypertension 2004; 44:248–252. [DOI] [PubMed] [Google Scholar]
- 7. Loperena R, Harrison DG. Oxidative stress and hypertensive diseases. Med Clin North Am 2017; 101:169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014; 126:267–274. [DOI] [PubMed] [Google Scholar]
- 9. Rao GN, Lassègue B, Alexander RW, Griendling KK. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem J 1994; 299 (Pt 1):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther 1998; 284:388–398. [PubMed] [Google Scholar]
- 11. McGiff JC. Prostaglandins, prostacyclin, and thromboxanes. Annu Rev Pharmacol Toxicol. 1981; 21:479–509. [DOI] [PubMed] [Google Scholar]
- 12. Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic Acid metabolites in the cardiovascular system: the role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol 2007; 50:609–620. [DOI] [PubMed] [Google Scholar]
- 13. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 2002; 82:131–185. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension 2000; 35:1129–1134. [DOI] [PubMed] [Google Scholar]
- 15. Nasjletti A. Arthur C. Corcoran Memorial Lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension 1998; 31:194–200. [DOI] [PubMed] [Google Scholar]
- 16. Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev 2014; 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, McNeish JD, Koller BH, Coffman TM. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol 1999; 277:H924–H930. [DOI] [PubMed] [Google Scholar]
- 18. Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 2009; 8:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Miao Y, Zhang Y, Dou D, Liu L, Tian X, Yang G, Pu D, Zhang X, Kang J, Gao Y, Wang S, Breyer MD, Wang N, Zhu Y, Huang Y, Breyer RM, Guan Y. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol 2012; 32:3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 2004; 43:364–369. [DOI] [PubMed] [Google Scholar]
- 21. Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest 2007; 117:2496–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 2014; 64:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asirvatham-Jeyaraj N, King AJ, Northcott CA, Madan S, Fink GD. Cyclooxygenase-1 inhibition attenuates angiotensin II-salt hypertension and neurogenic pressor activity in the rat. Am J Physiol Heart Circ Physiol 2013; 305:H1462–H1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okuno T, Lindheimer MD, Oparil S. Central effects of prostaglandin E2 on blood pressure and plasma renin activity in rats. Role of the sympathoadrenal system and vasopressin. Hypertension 1982; 4:809–816. [DOI] [PubMed] [Google Scholar]
- 25. Zhang ZH, Yu Y, Wei SG, Nakamura Y, Nakamura K, Felder RB. EP3 receptors mediate PGE2-induced hypothalamic paraventricular nucleus excitation and sympathetic activation. Am J Physiol Heart Circ Physiol 2011; 301:H1559–H1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rezq S, Abdel-Rahman AA. Rostral ventrolateral medulla EP3 receptor mediates the sympathoexcitatory and pressor effects of prostaglandin E2 in conscious rats. J Pharmacol Exp Ther 2016; 359:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 2009; 50(Suppl):S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leslie CC. Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res 2015; 56:1386–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan NS, Song CY, Jennings BL, Estes AM, Fang XR, Bonventre JV, Malik KU. Cytosolic phospholipase A2α is critical for angiotensin II-induced hypertension and associated cardiovascular pathophysiology. Hypertension 2015; 65:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan NS, Song CY, Thirunavukkarasu S, Fang XR, Bonventre JV, Malik KU. Cytosolic phospholipase A2α is essential for renal dysfunction and end-organ damage associated with angiotensin II-induced hypertension. Am J Hypertens 2016; 29:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balboa MA, Varela-Nieto I, Killermann Lucas K, Dennis EA. Expression and function of phospholipase A(2) in brain. FEBS Lett 2002; 531:12–17. [DOI] [PubMed] [Google Scholar]
- 32. Asirvatham-Jeyaraj N, Fink GD. Possible role for brain prostanoid pathways in the development of angiotensin II-salt hypertension in rats. Am J Physiol Regul Integr Comp Physiol 2016; 311:R232–R242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 2012; 59:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res 2008; 49:724–737. [DOI] [PubMed] [Google Scholar]
- 35. Yu Y, Zhang ZH, Wei SG, Chu Y, Weiss RM, Heistad DD, Felder RB. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res 2007; 101:304–312. [DOI] [PubMed] [Google Scholar]
- 36. Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 2010; 55:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 2015; 65:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 2015; 65:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka K, Yamamoto Y, Ogino K, Tsujimoto S, Saito M, Uozumi N, Shimizu T, Hisatome I. Cytosolic phospholipase A2alpha contributes to blood pressure increases and endothelial dysfunction under chronic NO inhibition. Arterioscler Thromb Vasc Biol 2011; 31:1133–1138. [DOI] [PubMed] [Google Scholar]
- 40. Pollock DM, Polakowski JS, Divish BJ, Opgenorth TJ. Angiotensin blockade reverses hypertension during long-term nitric oxide synthase inhibition. Hypertension 1993; 21:660–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.