ABSTRACT
The Ca2+ entry mechanism that sustains the Ca2+ oscillations in fertilized pig oocytes was investigated. Stromal interaction molecule 1 (STIM1) and ORAI1 proteins tagged with various fluorophores were expressed in the oocytes. In some cells, the Ca2+ stores were depleted using cyclopiazonic acid (CPA); others were inseminated. Changes in the oocytes’ cytosolic free Ca2+ concentration were monitored, while interaction between the expressed fusion proteins was investigated using fluorescence resonance energy transfer (FRET). Store depletion led to an increase of the FRET signal in oocytes co-expressing mVenus-STIM1 and mTurquoise2-ORAI1, indicating that Ca2+ release was followed by an interaction between these proteins. A similar FRET increase in response to CPA was also detected in oocytes co-expressing mVenus-STIM1 and mTurquoise2-STIM1, which is consistent with STIM1 forming punctae after store depletion. ML-9, an inhibitor that can interfere with STIM1 puncta formation, blocked store-operated Ca2+ entry (SOCE) induced by Ca2+ add-back after a CPA treatment; it also disrupted the Ca2+ oscillations in fertilized oocytes. In addition, oocytes overexpressing mVenus-STIM1 showed high-frequency Ca2+ oscillations when fertilized, arguing for an active role of the protein. High-frequency Ca2+ oscillations were also detected in fertilized oocytes co-expressing mVenus-STIM1 and mTurquoise2-ORAI1, and both of these high-frequency Ca2+ oscillations could be stopped by inhibitors of SOCE. Importantly, in oocytes co-expressing mVenus-STIM1 and mTurquoise2-ORAI1, we were also able to detect cyclic increases of the FRET signal indicating repetitive interactions between STIM1 and ORAI1. The results confirm the notion that in pig oocytes, SOCE is involved in the maintenance of the repetitive Ca2+ transients at fertilization.
Keywords: oocyte, fertilization, pig, STIM1, ORAI1, calcium
Summary Sentence
The sperm-induced Ca2+ oscillations in pig oocytes are sustained by store-operated Ca2+ entry, which is mediated by repetitive interactions between STIM1 and ORAI1 proteins
Introduction
Ionized calcium (Ca2+) is a universal second messenger that regulates a wide range of biological events. It also plays a critical role in mammalian fertilization as the events of oocyte activation, i.e. cortical granule exocytosis, resumption of meiosis, and extrusion of the second polar body, are all stimulated by repetitive increases in the oocytes’ cytoplasmic free Ca2+ concentration [1]. These so-called Ca2+ oscillations are triggered by the fertilizing sperm and they are the result of cyclic release and re-uptake of Ca2+ by the intracellular stores known as the endoplasmic reticulum (ER) [2]. The initial Ca2+ rise begins soon after gamete fusion and is followed by a great number of additional elevations that last for hours. To maintain the train of Ca2+ spikes, an influx of Ca2+ across the plasma membrane (PM) is essential as demonstrated by the fact that the oscillations stop in the absence of Ca2+ in the holding medium [3]. However, despite the importance of the Ca2+ influx, the identity of the channels that deliver the extracellular Ca2+ into the cytoplasm and their regulation have not been completely elucidated.
In somatic cells, a major type of Ca2+ entry is known as store-operated Ca2+ entry. Stromal interaction molecule 1 (STIM1), located predominantly in the membrane of the ER, was identified as a single-pass transmembrane protein that senses the Ca2+ content in the ER with its luminal EF hand motif [4, 5]. In resting cells, STIM1 is distributed diffusely in the ER membrane. Following store depletion, STIM1 molecules oligomerize, which is followed by their translocation and accumulation into aggregates known as punctae at ER regions adjacent to the PM. The channel component of store-operated Ca2+ entry was identified as the ORAI protein [6–8]. ORAI1 is located in the PM and contains four transmembrane domains with cytosolic N- and C-termini. ORAI1 was demonstrated to be the pore-forming subunit of the channel that mediates Ca2+ entry as a result of store depletion [9–11]. Other experiments also confirmed the significance of ORAI1 in store-operated Ca2+ entry: its co-overexpression with STIM1 led to a significant increase in Ca2+ entry in response to store depletion [12]. At rest, ORAI1 is also diffusely distributed in the PM, and following Ca2+ release it co-localizes with STIM1 [13, 14]. It is now believed that store-operated Ca2+ entry is stimulated when Ca2+ is mobilized from the intracellular stores. When STIM1 senses that the Ca2+ concentration inside the ER lumen decreases, it forms aggregates, moves closer to the PM, and interacts with PM-resident ORAI1 to trigger Ca2+ entry that aids in refilling the stores.
Previous studies provided evidences that store-operated Ca2+ entry may play important roles in Ca2+ signaling in oocytes as well. Depletion of the ER stores leads to the generation of a Ca2+ influx across the PM in immature Xenopus oocytes [15–17] and mature oocytes of mice, pigs, and humans [18–20]. The STIM1 protein has been identified in oocytes and its involvement in store-operated Ca2+ entry has also been demonstrated [21–23]. Its role in store-operated Ca2+ entry was further confirmed when it was reported that Ca2+ influx induced by the mobilization of luminal Ca2+ was inhibited if STIM1 expression was suppressed [24]. In addition, fertilization of mouse oocytes revealed a significant and rapid re-localization of STIM1, which was similar to that found after pharmacological Ca2+ store depletion [22]. In porcine oocytes, downregulation of STIM1 expression using siRNAs completely abolished the Ca2+ oscillations at fertilization and had a negative effect on subsequent embryo development [24]. Meanwhile, mutations in the porcine STIM1 gene were identified and shown to significantly affect the number of piglets born, indicating that STIM1 might be tightly related to litter size in pigs [25]. The ORAI1 protein was also identified in porcine oocytes and was found to be essential to mediate Ca2+ entry after Ca2+ release from the ER, and also to maintain the long-lasting Ca2+ signal triggered by the sperm [26]. These findings clearly indicated the need for store-operated Ca2+ entry at fertilization.
The manner of communication between STIM1 and ORAI1 has been unclear. The involvement of a diffusible messenger was suggested earlier [27]. The calcium influx factor was proposed to form upon store depletion, move to the PM, and activate the ORAI1 channel. Other findings, however, pointed toward a direct physical association between STIM1 and ORAI1. This idea was supported by several lines of evidence, such as the close vicinity of the junctional ER to the PM [28]; the fact that STIM1 and ORAI1 co-immunoprecipitated after store depletion [10, 11]; and the finding that following the mobilization of luminal Ca2+, fluorescence resonance energy transfer (FRET) occurred between donor and acceptor fluorophores fused to STIM1 and ORAI1 proteins [29]. FRET is a physical phenomenon that occurs only over very short distances, and hence FRET technology is widely used to investigate molecular interactions. Here we applied FRET to study the role of STIM1 and ORAI1 in regulating the Ca2+ signal at fertilization in porcine oocytes.
Materials and methods
Chemicals
All chemicals used in this study were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO) unless otherwise indicated.
Oocyte maturation
Ovaries collected from prepubertal gilts were donated by a local slaughterhouse (Indiana Packers Corporation, Delphi, IN). Follicular liquid was aspirated from follicles (3–6 mm in diameter) using a syringe with a 20-G hypodermic needle. The cumulus–oocytes complexes (COCs) were rinsed twice in Hepes-buffered Tyrode's lactate (TL-Hepes) medium and then three times in maturation medium without hormones. They were then transferred into maturation medium (50 oocytes per 0.5 ml of medium) consisting of TCM-199 supplemented with 0.57 mM cysteine, 3.05 mM D-glucose, 0.91 mM sodium pyruvate, 10 ng/ml epidermal growth factor, 0.5 IU/ml ovine luteinizing hormone, 0.5 IU/ml porcine follicle stimulating hormone, 0.1% polyvinyl alcohol, 75 mg/ml penicillin, and 50 mg/ml streptomycin [30]. Maturation was conducted at 39°C and 5% CO2 in air with 100% humidity. After 44 h of maturation, the COCs were transferred into TL-HEPES containing 1 mg/ml hyaluronidase, and the cumulus cells were removed by vortexing for 1 min. Oocytes with intact PM, evenly dark cytoplasm, and an extruded first polar body were selected and used for the experiments.
Plasmids
To obtain constructs that express porcine ORAI1 and STIM1 tagged N-terminally with mTurquoise2 or mVenus, cDNA fragments of ORAI1 and STIM1 flanked with the 5ʹ-Xho I and 3ʹ-EcoR I sites were generated by PCR. Following a treatment with the restriction enzymes, the fragments were inserted into mVenus-C1 (Addgene, Plasmid #54651) or mTurquoise2-C1 (Addgene, Plasmid #54842) to generate the pmVenus-STIM1-C1, pmVenus-ORAI1-C1, pmTurquoise2-STIM1-C1, and pmTurquoise2-ORAI1-C1 plasmids. In order to facilitate in vitro transcription of the constructs, cDNA fragments with a T7 promoter sequence at their 5ʹ-end were amplified by PCR. To generate the T7-mVenus-ORAI1 and T7-mTurquoise2-ORAI1 cDNA, the following primers were used: forward T7 primer (5ʹ-AAGCGAATAATACGACTCACTATAGGGAAAACCACCATGGTGAGC-AAGGGCGAGGAG-3ʹ) and ORAI1 reverse primer (5ʹ-TTTTTTTTTTTTTTTTTTTTT-TTTTTTTTTCTAGGCATAGTGGCTGCCGG-3ʹ). To obtain the T7-mVenus-STIM1 and T7-mTurquoise2-STIM1 cDNA, the forward T7 primer was paired with the STIM1 reverse primer (5ʹ-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCTACTTCTTAAGAG-GCTTCC-3ʹ). The sequence of the expression vector constructs was confirmed by the DNA Sequencing Low Throughput Laboratory of Purdue University.
Complementary RNA synthesis
The PCR products were transcribed in vitro using the mMESSAGE mMachine kit, and the generated complementary RNA (cRNA) was then polyadenylated using the poly(A) tailing kit (both from Ambion, Austin, TX) according to the manufacturer's instructions. The cRNAs were diluted to a final concentration of 1 μM in DEPC-treated nuclease-free water (Invitrogen, Carlsbad, CA) and stored in 3 μl aliquots at –80°C until use.
Microinjection
In order to express the fluorophore-tagged proteins in the oocytes, cRNA of the tagged proteins was microinjected into the ooplasm. For microinjection, cumulus cells from the surface of the zona pellucida were removed by vortexing in the presence of 1 mg/ml hyaluronidase 30 h after the beginning of maturation. The denuded oocytes were then placed into nominally Ca2+-free TL-HEPES medium and microinjected with a small amount (approximately 40 pl) of cRNA by means of a FemtoJet microinjector (Eppendorf, Hamburg, Germany). The injected oocytes were transferred back to the maturation medium and incubated for about 14 h, until the end of the maturation period.
In vitro fertilization
Mature oocytes were rinsed in a modified Tris-buffered medium (mTBM) as fertilization medium consisting of 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2 × 2H2O, 20 mM Tris (crystallized free base), 11 mM glucose, 5 mM sodium pyruvate, 0.1% bovine serum albumin, and 1 mM caffeine. Groups of 20 to 30 oocytes were placed into 50 μl droplets of the medium covered with mineral oil. Fresh semen collected from a Large White boar was diluted in Modena extender and kept at 17°C until use. Right before in vitro fertilization, the semen was washed twice by centrifugation at 900 g for 4 min in Dulbecco's phosphate-buffered saline. The final sperm pellet was diluted with the fertilization medium and the sperm suspension, at a final concentration of 5 × 105 cells/ml, was added to the oocytes. The gametes were co-incubated for 2 h in a CO2 incubator at 39°C prior to the beginning of the fluorescence recordings.
Cytosolic free Ca2+ concentration measurements
The fertilized oocytes were loaded with the Ca2+ indicator dye fura-2 by incubation in the presence of 2 μM fura-2 LeakRes (AM) and 0.02% pluronic F-127 (Invitrogen) for 45 min. After incubation, they were transferred to a special chamber with a glass coverslip as the bottom (to allow fluorescent recordings) in a drop of TL-Hepes medium. Polyvinyl alcohol was omitted from this medium in order to attach the oocytes to the bottom of the chamber and prevent their movement during measurements. The chamber was placed on the heated stage of an inverted microscope (Nikon TE2000-U, Nikon Corporation, Tokyo, Japan). The changes in the intracellular free Ca2+ concentration of the oocytes were recorded using InCyt Im2, a dual-wavelength fluorescence imaging system (Intracellular Imaging, Inc., Cincinnati, OH). Fura-2 was excited alternately at 340 and 380 nm, and the emitted fluorescence was detected at 510 nm using a Pixelfly CCD camera. The emitted fluorescence intensities (F) were normalized by dividing them by the resting values (F0) and are presented as relative units. The measurements in each experiment were repeated several times (as indicated), and a representative measurement is presented in the figures.
Fluorescence resonance energy transfer measurements
The oocytes expressing the fluorescently tagged proteins were placed in the measuring chamber. They were imaged on a Nikon TE2000-U inverted microscope equipped with a ×10 objective (Nikon Superfluor, N/a 0.5), a 175W xenon arc lamp (Sutter Instrument, Novato, CA), and excitation and emission filter wheels (also from Sutter Instrument). The cells were illuminated with the mTurquoise2 (donor) excitation wavelength and the fluorescence emitted by mTurquoise2 and mVenus (acceptor) was recorded alternately. In order to minimize photobleaching, lamp output was reduced by means of a 0.6 neutral density filter. For the visualization of mTurquoise2, mVenus, and FRET fluorescence, the XF88-2 CFP/YFP standard fluorescence filter set (Omega Optical, Inc., Brattleboro, VT) was used that includes the following components: 455DRLP dichroic filter, 440BP20 excitation filter, 480AF30 emission filter, and 535AF26 emission filter. The time-lapse series of images were captured on a cooled CCD camera (Pixelfly, The Cooke Corporation, Auburn Hill, MI) at 6-s intervals at an exposure of 200 ms; image analysis was performed using the InCyt FRET software (Intracellular Imaging). First, the intensity from each filter set was corrected for background fluorescence detected by measuring noninjected oocytes. Next, because the FRET intensity contains spillover, i.e. contamination from directly excited fluorescence of the acceptor and from the long-wavelength tail of the donor emission spectrum, these components were removed to generate corrected FRET [31, 32]. Finally, normalized FRET was obtained by dividing the corrected FRET data by the acceptor intensity and displayed as time-dependent curves. For each experiment, a representative measurement of an oocyte is presented.
Statistical analysis
The results were compared by one-way analysis of variance using the IBM SPSS software (IBM Corporation, Armonk, NY). Differences were considered significant at P < 0.05. In the text, the “n” refers to the number of oocytes examined during the course of each experiment.
Results
Store depletion is followed by STIM1-ORAI1 interaction
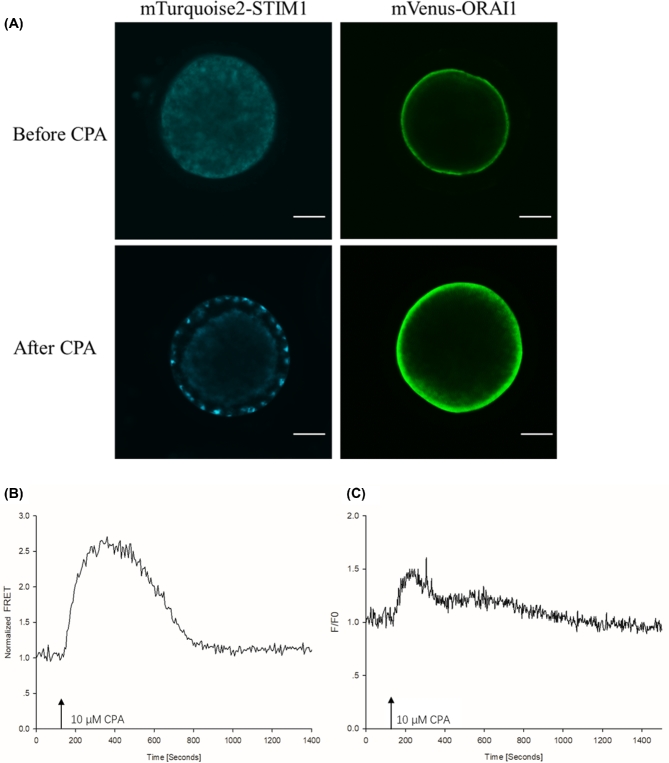
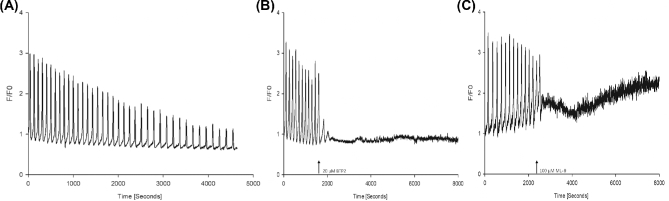
In the first experiment, we investigated whether the mobilization of intraluminal Ca2+ is associated with an interaction between STIM1 and ORAI1 proteins. For the visualization of the expressed proteins, the oocytes were injected with mTurquoise2-STIM1 and mVenus-ORAI1 cRNA (in preliminary experiments, we found that for confocal imaging this pairing of the fluorophores and proteins worked better). Imaging by laser-scanning confocal microscopy 14 h later revealed that the exogenously expressed STIM1 localized in the cortical region of the ooplasm while the distribution of ORAI1 was consistent with localization in the PM (n = 15 oocytes; Figure 1A). For FRET analysis, the oocytes were injected with mVenus-STIM1 and mTurquoise2-ORAI1 cRNA. Fourteen hours later, the injected oocytes were placed in Ca2+-free TL-Hepes medium in the measuring chamber, the chamber was placed on the stage of the inverted microscope, and the FRET signal was monitored. Cyclopiazonic acid (CPA) at a final concentration of 10 μM was then added to the oocytes. CPA is a known inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+-ATPases (SERCA pumps) and thus prevents reloading of the stores. Since Ca2+ is released continuously through the passive leak pathway, a CPA treatment causes the depletion of the intracellular Ca2+ stores [33]. In many cell types, CPA-induced store depletion leads to STIM1 translocation to the PM, opening of the ORAI1 channels, and an influx of extracellular Ca2+, a process known as store-operated Ca2+ entry (for a review, see [34]). We found that the addition of CPA triggered an increase of FRET between STIM1 and ORAI1 in 10 out of 10 oocytes (Figure 1B), followed by a gradual decrease of the elevated signal. On average, the Ca2+ level rose above the baseline 57.25 ± 2.58 s after the CPA treatment. Confocal microscopy revealed that the inhibitor caused the relocation of STIM1 and its arrangement into clusters below the PM (Figure 1A). When in a separate experiment, the oocytes were loaded with the Ca2+ indicator dye fura-2, the addition of CPA caused the discharge of Ca2+ after 68.25 ± 4.92 s (n = 12, Figure 1C). The fact that the two events, i.e. Ca2+ release and FRET increase overlap suggests that it is the depletion of the stores that causes the relocation of STIM1 and its interaction with ORAI1 in the PM.
Figure 1.
Interaction between fluorescently labeled STIM1 and ORAI1 in pig oocytes induced by Ca2+ store depletion. (A) Distribution of mTurquoise2-STIM1 and mVenus-ORAI1 before and after store depletion induced by the SERCA pump inhibitor CPA. Note the formation of STIM1 clusters after store depletion. The bar indicates 30 μm. (B) FRET increase between mVenus-STIM1 and Turquoise2-ORAI1 in response to the treatment with CPA. (C) A transient increase in the cytosolic free Ca2+ concentration stimulated by CPA. The arrow indicates the addition of the inhibitor to the oocytes.
Store depletion causes STIM1 oligomerization
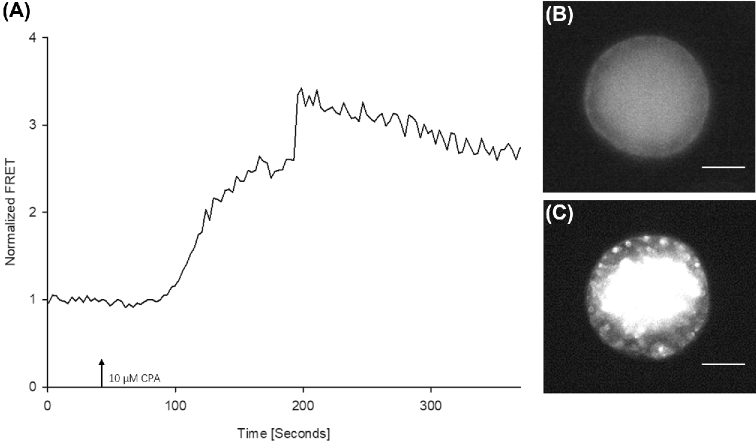
Next, we analyzed the effect of store depletion on the expressed STIM1 proteins. Complementary RNAs of mVenus- and mTurquoise2-tagged STIM1 were injected into the oocytes and following the expression of the labeled proteins the oocytes were used for FRET measurements. Again, after a short baseline recording in Ca2+-free medium, CPA was added to the holding medium. In 15 out of 15 oocytes, this led to an increase in FRET intensity indicating that depleting the Ca2+ stores caused the oligomerization and cluster formation of STIM1 (Figure 2A). Cluster formation could be verified directly under the fluorescence microscope of the imaging system (Figure 2B and C). These data indicate that STIM1 is activated and forms clusters in pig oocytes after the release of Ca2+ from the ER.
Figure 2.
FRET analysis of fluorescently tagged STIM1 after store depletion. Following mVenus-STIM1 and mTurquoise2-STIM1 expression, the oocytes were treated with CPA. (A) Treatment with the inhibitor led to an increase in the FRET signal indicating oligomerization of STIM1. (B) Image showing an oocyte with mVenus- and mTurquoise2- labeled STIM1 at the beginning of the measurement under the fluorescence microscope. (C) The same oocyte after the CPA treatment as seen during the FRET recording. Note the formation of STIM1 clusters in response to store depletion. The bar indicates 40 μm.
STIM1 sustains the fertilization Ca2+ signal via store-operated Ca2+ entry
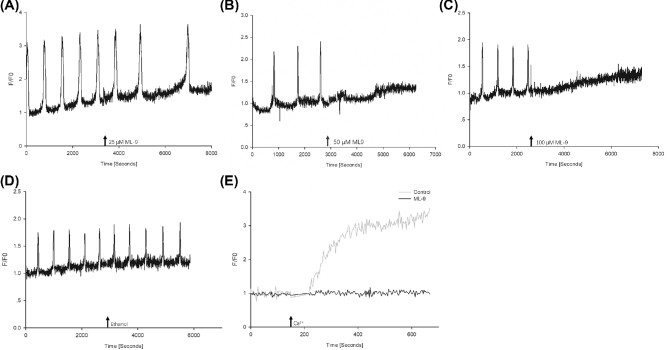
STIM1 is known to be a key player in the mediation of store-operated Ca2+ entry. This experiment was designed to better understand its significance in the maintenance of the Ca2+ oscillations at fertilization. Mature oocytes were placed in the measuring chamber, inseminated with capacitated boar spermatozoa, and kept in a CO2 incubator at 39°C for 2 h. In previous experiments, this time was sufficient for gamete fusion to occur and the initiation of the Ca2+ oscillations. After 2 h, the chamber was placed on the stage of an inverted microscope and changes in the intracellular Ca2+ levels were monitored. Once the oscillations were detected ML-9, an inhibitor that disrupts STIM1 puncta formation, was added to the gametes. We found that at 25 μM concentration, ML-9 markedly slowed down the oscillations, while at 50 and 100 μM the oscillations stopped completely (n = 34, Figure 3A–C). In control experiments, adding the carrier medium to the oocytes had no effect on the Ca2+ signal (n = 11, Figure 3D). This indicated that the sperm-induced Ca2+ signal was sustained by the action of STIM1. Proof that the mechanism STIM1 utilized to sustain the oscillations is store-operated Ca2+ entry came from the next experiment. When the oocytes were incubated in Ca2+-free TL-Hepes in the presence of CPA for 1 h, Ca2+ add-back (final conc. 2 mM) caused an immediate increase in the cytosolic Ca2+ levels, indicating the onset of store-operated Ca2+ entry (n = 9). Importantly, in the presence of 100 μM ML-9 no Ca2+ increase occurred (n = 10, Figure 3E). This suggests that ML-9 acts on STIM1 (i.e. blocks its function), which leads to the inhibition of store-operated Ca2+ entry, and in the absence of store-operated Ca2+ entry the sperm-induced Ca2+ signal is disrupted. Finally, STIM1 was overexpressed in oocytes by injecting mVenus-STIM1 cRNA into their cytoplasm. Fourteen hours later, the oocytes were inseminated and the cytosolic Ca2+ levels were monitored. We found that oocytes with elevated levels of STIM1 displayed high-frequency Ca2+ oscillations upon sperm penetration (n = 12, Figure 4A). In these oocytes, the average interval between transients was 1.57 ± 0.10 min, while that in the control oocytes was 8.22 ± 0.02 min (n = 15, data not shown). At the same time, ML-9 was able to stop these high-frequency Ca2+ oscillations (n = 13, Figure 4B). All these results imply that STIM1 is involved in the maintenance of the oscillations (as its level determines the frequency of the oscillations), and the results collected in these experiments indicate that the sperm-induced signal is mediated by STIM1.
Figure 3.
The involvement of STIM1 in the maintenance of the fertilization Ca2+ signal in pig oocytes. ML-9 is able to block STIM1 puncta formation. When added to the oocytes (arrow), at 25 μM concentration it slowed down the ongoing oscillations (A) while at 50 and 100 μM it completely disrupted the train of Ca2+ spikes (B and C). Addition of the carrier medium (ethanol) had no effect on the Ca2+ signal (D). ML-9 also blocked the Ca2+ influx caused by Ca2+ add-back in CPA-pretreated oocytes (E).
Figure 4.
Sperm-induced Ca2+ oscillations in oocytes overexpressing STIM1. Oocytes with high levels of STIM1 expression displayed high-frequency oscillations (A); the oscillations were stopped when ML-9 (arrow) was added to the oocytes (B). This indicates that STIM1 plays a critical role in sustaining the signal.
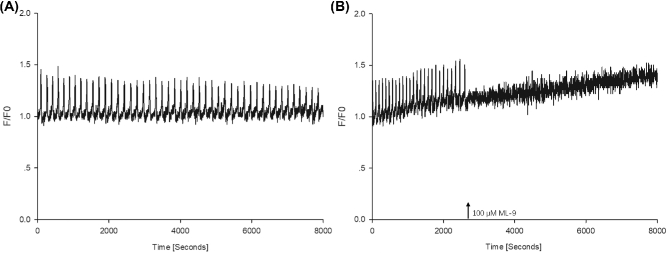
High-frequency Ca2+ oscillations in fertilized oocytes co-overexpressing STIM1 and ORAI1
To further investigate how STIM1 and ORAI 1 levels affect the fertilization Ca2+ signal, oocytes microinjected with cRNA of mVenus-STIM1 and mTurquoise2-ORAI1 were inseminated with capacitated boar spermatozoa and changes in the intracellular free Ca2+ concentrations were monitored. In these oocytes, the fertilizing sperm triggered Ca2+ oscillations with unusually high frequency (n = 14, Figure 5A). The average interval between two Ca2+ transients was 1.36 ± 0.07 min, while in the control noninjected oocytes it was 8.22 ± 0.02 min (n = 15, data not shown). Meanwhile, a treatment with ML-9 or BTP2 (the latter is a selective blocker of store-operated Ca2+ entry) can also stop these high-frequency Ca2+ oscillations (n = 14, Figure 5B and C). These observations indicate that elevated levels of STIM1 and ORAI1 in the oocytes lead to high-frequency Ca2+ oscillations at fertilization.
Figure 5.
Fertilization Ca2+ signal in oocytes co-overexpressing STIM1 and ORAI1. Such oocytes showed Ca2+ oscillations with very high frequency (A). The oscillations stopped when BTP2 (B) or ML-9 (C) was added to the oocytes, indicating that store-operated Ca2+ entry is necessary in the maintenance of the oscillations.
Repetitive STIM1/ORAI1 interaction in fertilized oocytes
To assess the possible interaction between STIM1 and ORAI1 proteins at fertilization, oocytes co-overexpressing mVenus-STIM1 and mTurquoise2-ORAI1 were inseminated. They were placed in a CO2 incubator for 2 h and subsequently, FRET was monitored in the fertilized gametes. Fertilization triggered an oscillating FRET signal in these oocytes. By the time the measurements began, the FRET signal has already been initiated. We found that each FRET signal started with a rapid increase that lasted for 26 ± 3.21 s (n = 3; Figure 6). Once it reached its maximum, the signal intensity dropped but remained elevated for several minutes (1.97 ± 0.29), during which time it was superimposed by small spikes. The signal then returned to baseline and another sharp rise soon followed. The average time between two subsequent rises was 2.18 ± 0.16 min; this frequency was very similar to that of the fertilization Ca2+ signal detected in such mVenus-STIM1/mTurquoise2-ORAI1-overexpressing oocytes (as described in the previous experiment), where each Ca2+ increase lasted for 34.2 ± 3.6 s and the average time between two subsequent rises was 2.34 ± 0.1 min. These results indicate that the two proteins repeatedly interact with each other during the sperm-induced Ca2+ oscillations.
Figure 6.
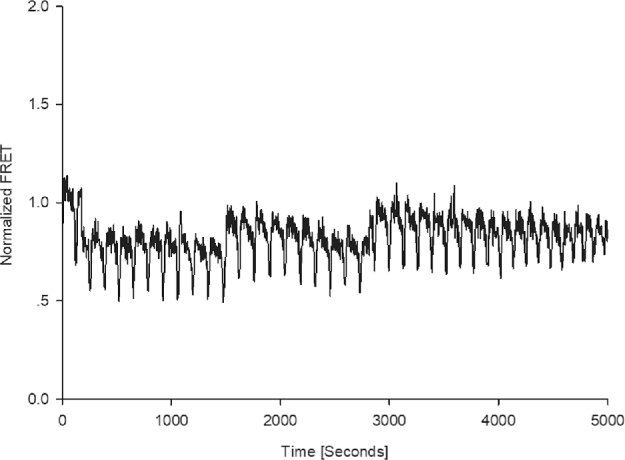
Repetitive FRET increases in fertilized pig oocytes expressing mVenus-STIM1 and mTurquoise2-ORAI1. The pattern of the signal implies that the expressed proteins repeatedly interact during the course of the signal, which is consistent with store-operated Ca2+ entry sustaining the oscillations.
Discussion
In all animal species studied, embryo development is stimulated by a transient increase in the intracellular free Ca2+ concentration at the time of fertilization [35]. In most animals, a single rise in the cytosolic Ca2+ level is responsible for the oocyte-to-embryo transition, whereas is mammals (and a number of marine invertebrates) the fertilizing sperm utilizes a series of low-frequency Ca2+ oscillations to induce development. The transients generally take the form of propagating Ca2+ waves that originate at the sperm entry point and sweep rapidly across the oocyte [36]. The waves are generated by inositol 1,4,5-trisphosphate-mediated Ca2+ release from the intracellular stores [37], and the frequency, amplitude, and duration of the oscillations are believed to code information important for normal development [38–40]. An influx of extracellular Ca2+ is essential to sustain the train of Ca2+ spikes but it is not completely clear what mechanism mediates the flow of Ca2+ across the PM. We have demonstrated previously that in pig oocytes, store-operated Ca2+ entry sustains the sperm-induced Ca2+ transients. This was primarily based on findings that downregulation of either STIM1 or ORAI1 levels in the oocytes abolished the oscillations, whereas overexpression of these proteins altered the pattern of the repetitive signal drastically. However, similar studies performed in mouse oocytes led to different conclusions. In this species, although both STIM1 and ORAI1 are expressed in oocytes, they seem to be involved in signaling during maturation rather than fertilization [22, 41–45]. Therefore, experiments of the current study were designed to gather further evidence regarding the involvement of store-operated Ca2+ entry in porcine fertilization, and to demonstrate the potential interactions between the key molecules in the regulation of the sperm-induced Ca2+ signal.
We utilized FRET to explore the interaction between the major proteins implicated in store-operated Ca2+ entry. The assay is based on the process of nonradiative energy transfer that occurs between the two fluorophores if they are sufficiently close (typically less than 10 nm) to each other. By exciting the donor and then monitoring the relative donor and acceptor emissions over time, it is possible to establish when energy transfer has occurred [46]. The most common FRET-based reporter pair consists of cyan and yellow fluorescent proteins (CFP and YFP) as fluorophores; in our experiments, we used their brighter variants mTurquoise2 as donor and mVenus as acceptor [47]. We found that store depletion by pharmacological means led to an increase in FRET in oocytes co-expressing mVenus-STIM1 and mTurquoise2-ORAI1. This indicates that store-operated Ca2+ entry is operational in pig oocytes, i.e. upon the release of Ca2+ from the ER, STIM1 relocates and interacts with ORAI1 channels in the PM. The phenomenon is similar to what has been reported in other cell types earlier. When Ca2+ release from the ER was stimulated in human embryonic kidney cells, it was followed by the redistribution of STIM1, which occurred in parallel with a pronounced increase in FRET between fluorophore-tagged STIM1 and ORAI1 [29, 48]. STIM1 is an ER-resident protein with a luminal EF-hand motif on its N-terminus that serves as a Ca2+ sensor and the STIM-ORAI activating region (SOAR) on its cytoplasmic C-terminus [49]. At rest, SOAR is obscured within the large C-terminus of the protein [50]. However, a decrease in the luminal Ca2+ level sensed by the EF hand motifs causes a conformational rearrangement that involves an unfolding and extension of the cytoplasmic domains [51]. The extended protein gets trapped in ER-PM junctions, contact sites between the ER and the PM. At the same time, the SOAR domain becomes exposed and therefore able to bind to ORAI1 channels in the PM, causing their activation to let extracellular Ca2+ enter the cell [50, 52]. The other key component of this signaling cascade, the ORAI1 channel, is a hexamer formed by six ORAI molecules [53]. The precise mechanism of the coupling between STIM1 and ORAI1 is still a matter of debate, but recent research indicates that the channel is opened when SOAR of the unfolded STIM1 molecules interacts with the STIM1-binding site on the cytoplasmic terminus of ORAI1 [54, 55]. The increased FRET signal we detected after the CPA treatment of the oocytes is indicative of this interaction. The conformational change caused by STIM1 binding is then propagated to the pore-forming domains of ORAI1, ultimately leading to the rearrangement of the pore and opening of the channel. The FRET signal eventually dropped spontaneously, which is somewhat puzzling. It implies that the two molecules move away from each other even without Ca2+ being added back to the medium. One possible explanation is that the inhibitory action of CPA is not a very strong one, and the SERCA pumps started to reload the released Ca2+ back to the ER, leading to the separation of STIM1 and ORAI1. This possibility could be tested by using thapsigargin, another inhibitor of SERCA pumps in a similar experiment. An alternative interpretation of the phenomenon is that store depletion triggers store-operated Ca2+ entry but then an additional type of Ca2+ influx is also stimulated that helps to refill the stores even after STIM1 and ORAI1 separation.
In resting cells, the EF hands on the luminal N-terminus of STIM1 bind Ca2+ through their negatively charged aspartate and glutamate residues [56]. Store depletion leads to the dissociation of Ca2+ from the EF hands, which causes STIM1 oligomerization. The SOAR region seems to be crucial in the process, indicating that SOAR is required not only for the binding and activation of ORAI1, but also for STIM1 oligomerization, the initial event to trigger store-operated Ca2+ entry [34]. Oligomerization involves conformational changes, giving rise to the above-mentioned extension of the cytoplasmic strand and the protein's relocation to ER-PM junctions [57]. The FRET increase we detected in mVenus-STIM1- and mTurquoise2-STIM1-expressing oocytes after store depletion indicates this oligomerization. A similar phenomenon has been reported before in other cell types: FRET between YFP-STIM1 and CFP-STIM1 increased once Ca2+ was released from the intracellular stores [29, 58, 59]. It occurred prior to FRET between STIM1 and ORAI1 (showing that STIM1 oligomerization precedes binding to ORAI1) and decreased once the stores were full again.
We have demonstrated previously that in fertilized pig oocytes STIM1 plays a critical role in the maintenance of the Ca2+ oscillations [60]. The inhibitor ML-9 further suggests participation of STIM1 in the process. ML-9 at lower doses slowed down the sperm-induced Ca2+ oscillations, whereas at higher concentrations it completely disrupted the Ca2+ signal. ML-9 is an inhibitor of myosin-light-chain kinase (MLCK) that also blocks the rearrangement of STIM1 into punctae; this was shown in human embryonic kidney cells where ML-9 reportedly inhibited store-operated Ca2+ entry and blocked the Ca2+ release-activated Ca2+ current [61]. ML-9 also prevented Ca2+ entry induced by Ca2+ add-back after a CPA-induced store depletion indicating that it caused disruption of the fertilization Ca2+ signal through inhibition of store-operated Ca2+ entry. Further indication that STIM1 is involved in the maintenance of the sperm-induced signal is the fact that manipulating STIM1 levels affected the Ca2+ signal. In oocytes overexpressing STIM1, the fertilizing sperm generated Ca2+ oscillations whose frequency was markedly higher than in control oocytes, and the oscillations were disrupted by ML-9. In human embryonic kidney cells, Ca2+ oscillations triggered by muscarinic-cholinergic receptor stimulation are also supported by store-operated Ca2+ entry mediated by STIM1 and ORAI1 proteins [62]. It is therefore not surprising that overexpression of STIM1, or co-overexpression of STIM1 and ORAI1, influences the signal pattern and leads to high-frequency oscillations. Interestingly, in our previous work, oocytes pre-injected with STIM1 and ORAI1 cRNA were not able to mount long-lasting Ca2+ oscillations following fertilization [60]. This apparent contradiction can be resolved if one considers the expression levels of external proteins and the stoichiometry of STIM1-ORAI1 coupling. Recent research using dimeric SOAR concatemers demonstrated that only one of the two sites in the SOAR dimer is needed for ORAI1 activation [55]. This coupling model predicts that six STIM1 dimers will interact with an ORAI1 channel, each binding to one of the six subunits of the hexameric multimer. The model justifies earlier findings that the greatest ORAI1 channel activation occurs when the STIM1: ORAI1 ratio is 2:1 [63, 64]. In addition, it also explains why the expression levels of STIM1 and ORAI1 determine the properties of the store-operated current [63, 65]. Overexpression of STIM1 or ORAI1 alone may have limited effect on the store-operated current (in fact, ORAI1 overexpression suppresses store-operated Ca2+ entry by reducing the STIM1: ORAI1 binding stoichiometry), but co-overexpression of the two proteins leads to dramatically increased store-operated Ca2+ entry [12, 26, 63]. Expression levels of the two proteins in the current experiments were such that they resulted in increased store-operated Ca2+ entry and elevated frequency of the sperm-induced Ca2+ signal. Most importantly, we were able to detect repetitive FRET signals in these oocytes at fertilization, and the frequency of the signal was identical to that of the Ca2+ oscillations. This indicates that during fertilization, STIM1 and ORAI1 repeatedly interact to generate a Ca2+ influx to sustain the oscillations (to our best knowledge, this is the first time repetitive STIM1-ORAI1 interaction is demonstrated in oocytes), and the similar pattern suggests that the two processes are interrelated. Each Ca2+ rise is associated with STIM1 translocating to ER-PM junctions and becoming juxtaposed with ORAI1. This leads to an increase in FRET and eventually, the opening of the Ca2+ influx channel in the PM.
Taken together, the findings reported here provide further evidence that in the pig, the Ca2+ oscillations that activate the oocyte and stimulate embryo development are supported by store-operated Ca2+ entry. This cascade seems to be a logical choice for the job but apparently, not all mammalian species opted for it. In mouse oocytes, inhibiting store-operated Ca2+ entry by specific blockers [42] or by the expression of exogenous protein fragments that prevented the interaction between STIM1 and ORAI1 molecules [43] did not stop the oscillations. Subsequently, in search of the mechanism that would deliver the extracellular Ca2+ in support of the oscillating signal, a number of candidate proteins have been tested. A transient receptor potential (TRPV3) channel was described that conducted a Ca2+ current when stimulated with an agonist [66]. The current was able to induce oocyte activation, but the gametes collected from transgenic females that lacked TRPV3 channels showed normal Ca2+ oscillations at fertilization. This clearly indicated that TRPV3 channels were not required for the maintenance of the sperm-induced Ca2+ signal. Later, a T-type voltage-gated channel CaV3.2 was proposed to be a component of the Ca2+ influx mechanism [67]. In the absence of a critical subunit of the CaV3.2 channel, only low levels of Ca2+ accumulated in the ER of during oocyte maturation and such cells were unable to mount normal Ca2+ oscillations upon fertilization. However, mice having only the modified, nonfunctional CaV3.2 channels were still fertile, which pointed toward the involvement of other Ca2+ influx mechanisms. Most recently, the transient receptor potential melastanin 7 (TRPM7)-like channel was identified in mouse oocytes [45, 68]. Activation of the channel with specific agonists generated a Ca2+ influx, whereas its inhibition disrupted the sperm-induced Ca2+ oscillations. The same researchers also investigated the potential function of store-operated Ca2+ entry by generating oocyte-specific conditional knockout mice for STIM1/STIM2, and also animals that globally lacked ORAI1 proteins [45]. As it turned out, such animals were fertile, their oocytes displayed Ca2+ entry upon store depletion, and they showed normal Ca2+ oscillations at fertilization. This implies that store-operated Ca2+ entry is not required for fertilization in mouse oocytes and it may be the TRPM7 channel that mediates the Ca2+ influx following sperm–oocyte fusion. It will be interesting to learn what cascade(s) other species utilize to maintain the fertilization Ca2+ signal, and eventually understand why certain mechanisms were chosen over the others during the course of evolution.
Notes
Edited by Dr. Monika A. Ward, PhD, University of Hawaii John A. Burns School of Medicine.
Footnotes
Grant support: This work was supported by Agriculture and Food Research Initiative Competitive Grant no. 2011-67015-30006 from the USDA National Institute of Food and Agriculture.
References
- 1. Whitaker M. Calcium at fertilization and in early development. Physiol Rev 2006; 86:25–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C, Machaty Z. Calcium influx in mammalian eggs. Reproduction 2013; 145:R97–R105. [DOI] [PubMed] [Google Scholar]
- 3. Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol 1983; 340:611–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JE, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005; 15:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 2005; 437:902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J-P. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 2006; 312:1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006; 441:179–185. [DOI] [PubMed] [Google Scholar]
- 8. Zhang SL, Yeromin AV, Zhang XH-F, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA 2006; 103:9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006; 443:230–233. [DOI] [PubMed] [Google Scholar]
- 10. Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006; 443:226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol 2006; 16:2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mercer JC, DeHaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem 2006; 281:24979–24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER–plasma membrane junctions. J Cell Biol 2006; 174:815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun 2006; 350:969–976. [DOI] [PubMed] [Google Scholar]
- 15. Lupu-Meiri M, Beit-Or A, Christensen S, Oron Y. Calcium entry in Xenopus oocytes: effects of inositol trisphosphate, thapsigargin and DMSO. Cell Calcium 1993; 14:101–110. [DOI] [PubMed] [Google Scholar]
- 16. Parekh A, Foguet M, Lübbert H, Stühmer W. Ca2+ oscillations and Ca2+ influx in Xenopus oocytes expressing a novel 5-hydroxytryptamine receptor. J Physiol 1993; 469:653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao Y, Parker I. Inositol trisphosphate-mediated Ca2+ influx into Xenopus oocytes triggers Ca2+ liberation from intracellular stores. J Physiol 1993; 468:275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem 1992; 267:17624–17630. [PubMed] [Google Scholar]
- 19. Macháty Z, Ramsoondar JJ, Bonk AJ, Bondioli KR, Prather RS. Capacitative calcium entry mechanism in porcine oocytes. Biol Reprod 2002; 66:667–674. [DOI] [PubMed] [Google Scholar]
- 20. Martín-Romero FJ, Ortíz-de-Galisteo JR, Lara-Laranjeira J, Domínguez-Arroyo JA, González-Carrera E, Álvarez IS. Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod 2008; 78:307–315. [DOI] [PubMed] [Google Scholar]
- 21. Koh S, Lee K, Wang C, Machaty Z. Characterization of STIM1 gene expression in porcine oocytes. Biol Reprod 2007; 77(Suppl_1):139. [Google Scholar]
- 22. Gómez-Fernández C, Pozo-Guisado E, Gañán-Parra M, Perianes MJ, Álvarez IS, Martín-Romero FJ. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction 2009; 138:211–221. [DOI] [PubMed] [Google Scholar]
- 23. Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci 2009; 106:17401–17406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee K, Wang C, Machaty Z. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol 2012; 367:154–162. [DOI] [PubMed] [Google Scholar]
- 25. Wu J, Peng X, Li F, Qiao M, Wu H, Mei S. Association of porcine STIM1 gene with litter size traits. Anim Sci Pap Rep 2016; 34:165–172. [Google Scholar]
- 26. Wang C, Lee K, Gajdócsi E, Papp ÁB, Machaty Z. Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Dev Biol 2012; 365:414–423. [DOI] [PubMed] [Google Scholar]
- 27. Csutora P, Peter K, Kilic H, Park KM, Zarayskiy V, Gwozdz T, Bolotina VM. Novel role for STIM1 as a trigger for calcium influx factor production. J Biol Chem 2008; 283:14524–14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 2006; 174:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem 2008; 283:8014–8022. [DOI] [PubMed] [Google Scholar]
- 30. Abeydeera LR, Wang W-h, Prather RS, Day BN. Maturation in vitro of pig oocytes in protein-free culture media: fertilization and subsequent embryo development in vitro. Biol Reprod 1998; 58:1316–1320. [DOI] [PubMed] [Google Scholar]
- 31. Hoc A, Phillips G. Calibration of fluorescence resonance energy transfer in microscopy using genetically engineered GFP derivatives on nickel chelating beads. Biotechnol Alia 1997; 3:1–18. [Google Scholar]
- 32. Kardash E, Bandemer J, Raz E. Imaging protein activity in live embryos using fluorescence resonance energy transfer biosensors. Nat Protoc 2011; 6:1835–1846. [DOI] [PubMed] [Google Scholar]
- 33. Plenge-Tellechea F, Soler F, Fernandez-Belda F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+ -atpases by cyclopiazonic acid. J Biol Chem 1997; 272:2794–2800. [DOI] [PubMed] [Google Scholar]
- 34. Prakriya M, Lewis RS. Store-operated calcium channels. Physiol Rev 2015; 95:1383–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kashir J, Deguchi R, Jones C, Coward K, Stricker SA. Comparative biology of sperm factors and fertilization-induced calcium signals across the animal kingdom. Mol Reprod Dev 2013; 80:787–815. [DOI] [PubMed] [Google Scholar]
- 36. Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S. Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal–vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev Biol 2000; 218:299–313. [DOI] [PubMed] [Google Scholar]
- 37. Miyazaki S-i, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 1992; 257:251–255. [DOI] [PubMed] [Google Scholar]
- 38. Swann K, Ozil J-P. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 1994; 152:183–222. [DOI] [PubMed] [Google Scholar]
- 39. Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil J-P. Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 2002; 250:280–291. [PubMed] [Google Scholar]
- 40. Ducibella T, Schultz RM, Ozil J-P. Role of calcium signals in early development. Semin Cell Dev Biol 2006; 17:324–332. [DOI] [PubMed] [Google Scholar]
- 41. Gómez-Fernández C, López-Guerrero AM, Pozo-Guisado E, Álvarez IS, Martín-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Hum Reprod 2012; 18:194–203. [DOI] [PubMed] [Google Scholar]
- 42. Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA 2012; 109:4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun 2013; 430:60–65. [DOI] [PubMed] [Google Scholar]
- 44. Cheon B, Lee HC, Wakai T, Fissore RA. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol Biol Cell 2013; 24:1396–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bernhardt ML, Padilla-Banks E, Stein P, Zhang Y, Williams CJ. Store-operated Ca2+ entry is not required for fertilization-induced Ca2+ signaling in mouse eggs. Cell Calcium 2017; 65:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Piston DW, Kremers G-J. Fluorescent protein FRET: the good, the bad and the ugly. Trends Biochem Sci 2007; 32:407–414. [DOI] [PubMed] [Google Scholar]
- 47. Goedhart J, Von Stetten D, Noirclerc-Savoye M, Lelimousin M, Joosen L, Hink MA, Van Weeren L, Gadella TW Jr, Royant A. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat Commun 2012; 3:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J Physiol 2008; 586:5383–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yeung PS-W, Yamashita M, Prakriya M. Pore opening mechanism of CRAC channels. Cell Calcium 2017; 63:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 2012; 109:5657–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P. Inside-out Ca2+ signalling prompted by STIM1 conformational switch. Nat Commun 2015; 6:7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG. Initial activation of STIM1, the regulator of store-operated calcium entry. Nat Struct Mol Biol 2013; 20:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012; 338:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McNally BA, Somasundaram A, Jairaman A, Yamashita M, Prakriya M. The C- and N-terminal STIM1 binding sites on Orai1 are required for both trapping and gating CRAC channels. J Physiol 2013; 591:2833–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou Y, Cai X, Nwokonko RM, Loktionova NA, Wang Y, Gill DL. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium 2017; 63:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frischauf I, Fahrner M, Jardín I, Romanin C. The STIM1: Orai interaction. In: Rosado JA. (ed). Calcium Entry Pathways In Non-Excitable Cells (Advances in Experimental Medicine Biology ), vol. 898 Switzerland: Springer International Publishing; 2016: 25–46. [DOI] [PubMed] [Google Scholar]
- 57. Stathopulos PB, Li G-Y, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of STIM1 via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem 2006; 281:35855–35862. [DOI] [PubMed] [Google Scholar]
- 58. Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA 2007; 104:9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell 2010; 21:1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang C, Zhang L, Jaeger LA, Machaty Z. Store-operated Ca2+ entry sustains the fertilization Ca2+ signal in pig eggs. Biol Reprod 2015; 93:25. [DOI] [PubMed] [Google Scholar]
- 61. Smyth JT, DeHaven WI, Bird GS, Putney JW. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci 2008; 121:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Putney JW, Bird GS. Cytoplasmic calcium oscillations and store-operated calcium influx. J Physiol 2008; 586:3055–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoover PJ, Lewis RS. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 2011; 108:13299–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res 2011; 21:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scrimgeour N, Litjens T, Ma L, Barritt G, Rychkov G. Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins. J Physiol 2009; 587:2903–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carvacho I, Lee HC, Fissore RA, Clapham DE. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep 2013; 5:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bernhardt ML, Zhang Y, Erxleben CF, Padilla-Banks E, McDonough CE, Miao YL, Armstrong DL, Williams CJ. CaV3.2 T-type channels mediate Ca2+ entry during oocyte maturation and following fertilization. J Cell Sci 2015; 128:4442–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carvacho I, Ardestani G, Lee HC, McGarvey K, Fissore RA, Lykke-Hartmann K. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci Rep 2016; 6:34236. [DOI] [PMC free article] [PubMed] [Google Scholar]