The Breast DX Italy prospective study was designed to evaluate the impact of the 21‐gene Oncotype DX recurrence score assay on adjuvant treatment decisions for patients with early breast cancer. Results of the study are reported here.
Keywords: Early breast cancer, Adjuvant chemotherapy, Estrogen receptor positive, Recurrence score, Treatment change
Abstract
Background.
The Breast DX Italy prospective study evaluated the impact of the 21‐gene recurrence score (RS) result on adjuvant treatment decisions for patients with early breast cancer.
Materials and Methods.
Nine centers (two Hub and seven Spoke centers of the Veneto Oncology Network) participated. Consecutive patients with estrogen receptor positive, human epidermal growth receptor negative, T1–T3, N0–N1 early breast cancer were prospectively registered; only those meeting protocol‐defined clinicopathological “intermediate risk” criteria were eligible for the RS test. Pre‐RS and post‐RS physicians’ treatment recommendations and treatment actually received were collected.
Results.
A total of n = 124 N0 and n = 126 N1 patients underwent the RS assay. The majority had Grade 2 tumors (71%); median age was 55 years, median tumor size was 16 mm, and median Ki67 expression was 20%. Patients enrolled at Hub centers presented higher‐risk features. The distribution of RS results was <18 (60.8%), 18–30 (32.4%), and >30 (6.8%). The indication before RS was hormonal therapy (HT) alone in 52% of cases. An indication before RS of chemotherapy (CT)+HT was more frequent for patients with N1 versus N0 tumors (57% vs. 39%, p = .0035) and for patients enrolled at Hub versus Spoke centers (54% vs. 36%, p = .007).
The overall rate of change in treatment decision was 16% (n = 40), mostly from CT+HT to HT (n = 30). According to nodal status, rate of change in treatment decision was 12% for the N0 cohort and 20% for the N1 cohort. The proportion of patients recommended to CT+HT was significantly reduced from before to after RS (48% to 40%, p < .0016), especially in the N1 cohort (57% to 45%, p = .0027) and at Hub centers (54% to 44%, p = .001).
Conclusion.
Despite frequent indication of HT before RS, the use of the RS assay further contributed to sparing CT, especially for patients with N1 tumors and at Hub centers.
Implications for Practice.
This study shows that, although a high proportion of patients were recommended to receive endocrine treatment alone before knowing the recurrence score (RS) assay, the RS test further contributed in sparing chemotherapy for some of these patients, especially in case of the N1 stage or for patients enrolled at referral centers. These data highlight the need for further work in collaboration with health authorities and companies in order to define strategies for the implementation of the use of RS testing in clinical practice in the Italian setting.
摘要
背景.乳腺 DX 意大利前瞻性研究评估了21基因复发评分(RS)结果对早期乳腺癌患者的辅助治疗决策的影响
材料与方法.九个研究中心(威尼托肿瘤网络的两个Hub中心和七个Spoke中心)参与了研究。前瞻性地连续登记雌激素受体阳性、人表皮生长因子受体阴性、T1‐T3、N0‐N1早期乳腺癌患者;只有符合方案规定的临床病理学“中等风险”标准才有资格参加复发评分测试。收集复发评分前和复发评分后医生的治疗建议和实际接受的治疗
结果.总共124名N0患者和126名N1患者接受了复发评分法。大多数有2级肿瘤(71%);平均年龄为55岁, 中位肿瘤大小为16 mm, 中位Ki67表达为20%。在Hub中心入组的患者表现出更高风险特征。复发评分结果的分布为:<18(60.8%)、18‐30(32.4%)及>30(6.8%)。复发评分前的使用情况是在52%的病例中仅使用激素治疗(HT)。对于在化疗(CT)+ HT的复发评分前的使用情况, N1肿瘤患者较N0肿瘤患者相比更频繁(57% vs. 39%, p=0.0035), Hub中心入组的患者较Spoke中心入组的患者更频繁(54% vs. 36%, p=0.007)
治疗决策的总体变化率为16%(n=40), 主要来自CT+HT至HT(n=30)。根据淋巴结状态, N0队列的治疗决策变化率为12%, N1队列的治疗决策变化率为20%。推荐CT+ HT的患者比例从复发评分前到复发评分后显著降低(48%至40%, p<0.0016), 特别是在N1队列(57%至45%, p=0.0027)和Hub中心(54%至44%, p=0.001)
结论.尽管在复发评分之前频繁使用激素治疗, 但复发评分法的使用进一步减少了使用化疗, 尤其是对N1肿瘤患者和Hub中心的患者。
对临床实践的启示:这项研究表明, 尽管在知道复发评分(RS)法之前推荐较高比例的患者仅进行内分泌治疗, 但复发评分测试进一步促进了对这些患者中其中一些减少使用化疗, 特别是对N1期肿瘤患者或对在转诊中心入组的患者。这些数据证明, 有必要进一步与卫生主管部门和企业合作, 以确定在意大利临床实践中实施复发评分测试应用的策略
Introduction
Hormonal therapy (HT) is the mainstay of adjuvant treatment for patients with estrogen receptor (ER) positive/human epidermal growth receptor 2 (HER2) negative breast cancer (BC). Only a proportion of these patients benefit from the addition of chemotherapy (CT) [1]. No predictive biomarker for the efficacy of cytotoxic treatment is currently available, and the decision on whether to propose adjuvant chemotherapy is based on an estimation of the individual risk of relapse. According to the Early Breast Cancer Trialists’ Collaborative Group meta‐analysis, adjuvant CT for BC proportionally reduces the risk of recurrence by at least 30% in all subgroups [1]. A more precise estimation of the individual absolute risk of recurrence with endocrine treatment alone may allow evaluating whether the addition of CT might produce a meaningful benefit, taking into account the risk of early and late toxicities [2].
In clinical practice, classical clinicopathological factors are used to estimate the individual prognosis. Multigene prognostic tests have been developed to assist clinicians in the adjuvant decision‐making process. The 21‐gene Oncotype DX recurrence score (RS) assay (Genomic Health, Inc., Redwood City, CA) is a genomic prognosticator that has been shown to provide additional independent prognostic information beyond classical factors for patients with ER positive and HER2 negative BC [3], [4], [5]. Recently, the results from large prospective studies have further validated the ability of RS to predict prognosis. The Trial Assigning Individualized Options for Treatment (TAILORx) has demonstrated that patients with ER positive/HER2 negative, node‐negative BC with a RS <11 (n = 1,626) have a very low risk of distant recurrence at 5 years (0.7%) if treated with HT alone [6]. The PlanB trial reported similar results, showing a high rate of 3 years’ disease‐free survival (98.4%) for 348 patients with hormone receptor positive/HER2 negative, N0–N1 BC and RS ≤11 receiving HT alone. [7] A considerable amount of consistent outcome data is also coming from large real‐world cohorts, with RS results incorporated as part of clinical practice [8].
Moreover, retrospective studies conducted on samples from patients randomized in prospective phase III trials have reported that RS results may also predict the benefit from cytotoxic treatment [5], [9].
The RS test has been mentioned in most international guidelines as a useful tool in the adjuvant decision‐making process [10], [11] and was recently incorporated in the American Joint Committee on Cancer's eighth edition cancer staging manual [12]. However, in many countries, these tests are not reimbursed by the national health systems, limiting access to the tests. It is therefore important to provide additional evidence on the impact that the introduction of this test would have in clinical practice. Treatment patterns as well as health system regulations vary substantially across different countries, highlighting the need to conduct impact studies in a local perspective.
The Breast DX Italy prospective study was designed to evaluate for the first time in an Italian setting the impact of the 21‐gene RS assay on adjuvant treatment decisions for patients with early BC.
Materials and Methods
The Breast DX Italy is a multicentric, prospective decision‐impact study conducted within oncologic centers of the Veneto Region. Nine centers participated in the study: two major academic centers recognized as Hubs of the Veneto Oncology Network and 7 oncology units in community hospitals (Spoke centers). This distribution was planned to allow for the evaluation of the impact of test in different contexts. The protocol was approved by the Ethical Committees of all centers. All patients provided written informed consent.
The primary objective was to assess the impact of the RS test on adjuvant treatment decisions when used for patients with ER positive, HER2 negative, N0–N1 cancer presenting “intermediate risk” classical clinicopathological features. Secondary endpoints included (a) the frequency at which clinicians would consider the RS assay for all consecutive patients with ER positive, HER2 negative, N0–N1 early BC and (b) physician's confidence in treatment recommendation after RS and perception of assay utility.
Study Design
Study design is reported in Figure 1. After written informed consent, consecutive patients with ER positive, HER2 negative, T1–T3 early BC and with no more than three positive axillary nodes were prospectively registered in a dedicated database. Patients were not eligible in case of contraindication to systemic adjuvant therapy; multifocal, multicentric, or bilateral tumors; <2 mm invasive tumor; or prior history of BC. At baseline, physicians were asked whether they would deem the RS test useful in support of the clinical decision.
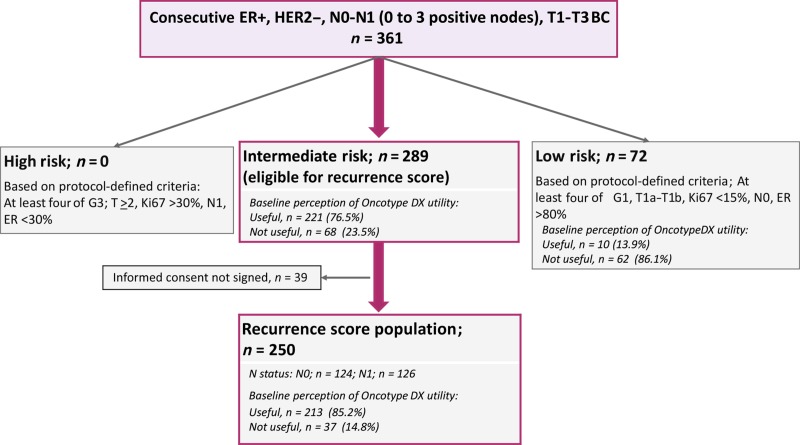
Figure 1.
Breast DX Italy study design and CONSORT diagram.
Abbreviations: ER+, estrogen receptor positive; G1, Grade 1; G3, Grade 3; HER2−, human epidermal growth receptor 2 negative.
Registered patients were classified in risk categories based on protocol‐defined classic criteria, which were derived and adapted from the St. Gallen Consensus Guidelines [13], [14], [15]: “low risk” (presence of at least four of the following features: Grade [G] 1, pT1a–pT1b, Ki67 <15%, N0, ER >80%), “high risk” (presence of at least four of the following features: G3, pT2 or higher, Ki67 >30%, N1, ER <30%), and “intermediate risk” (not classified as low or high risk).
Only “intermediate risk” patients were considered eligible for the RS test, irrespective of the presumed test utility reported by the physician at baseline. Patients were asked to sign a specific consent form for undergoing the RS assay. For each patient undergoing the RS test, the following data were collected: physician's recommendation of adjuvant treatment before RS, RS results, physician's recommendation after RS, and the type of adjuvant treatment finally received by the patient.
A final questionnaire asked physicians about confidence in their treatment recommendation after RS and their final perception of test utility.
Sample Size
The sample size was calculated assuming a rate of change in treatment recommendation from before to after RS of 25%, based on available data [16]. A sample size of 125 patients with node‐negative cancer and 125 patients with node‐positive cancer would allow determination of a 25% change rate in each group with a 95% confidence interval of 17.7%–33.4%. The population defined as “intermediate risk” according to the protocol was assumed to constitute 70% of all consecutive registered patients. Therefore, it was estimated to prospectively register in the study around n = 360 patients, to allow the sample size of 250 patients undergoing the RS. Enrollment in the study started in December 2014. In February 2016, enrollment of the N0 cohort of patients receiving the RS test was completed; thereafter no further registration of any patient with N0 cancer was allowed. Enrollment of the N1 cohort was completed in August 2016.
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Associations between variables were studied with the chi‐square test or the Kruskal‐Wallis test. The proportion of patients for whom treatment recommendation changed from before to after RS was calculated by nodal status (N0 and N1), RS category, and center type (Hub and Spoke). McNemar's test was used to investigate whether the proportion of patients recommended CT changed from before to after RS. Exploratory analyses comparing clinicopathological characteristics of patients with change versus patients without change in treatment recommendations were performed. All hypothesis tests were conducted at a two‐sided alpha level of 0.05.
Results
Patients’ Dispositions and Clinicopathological Characteristics
The CONSORT diagram is reported in Figure 1. A total of 361 consecutive patients with ER positive/HER2 negative BC were prospectively registered; their main clinicopathological characteristics are reported in Table 1. Physicians reported that they would need the RS assay in support of the adjuvant treatment decision for 64% (n = 231) of the registered patients. Factors associated with baseline perception of RS‐assay utility were N1 status, G2–3 rather than G1, lobular histology, larger tumor size, lower ER expression, lower progesterone receptor (PgR) expression, and higher Ki67 (Table 1).
Table 1. Clinical and pathological characteristics of the prospectively registered population of the Breast DX Italy study and distribution according to baseline physician's perception of RS test utility.
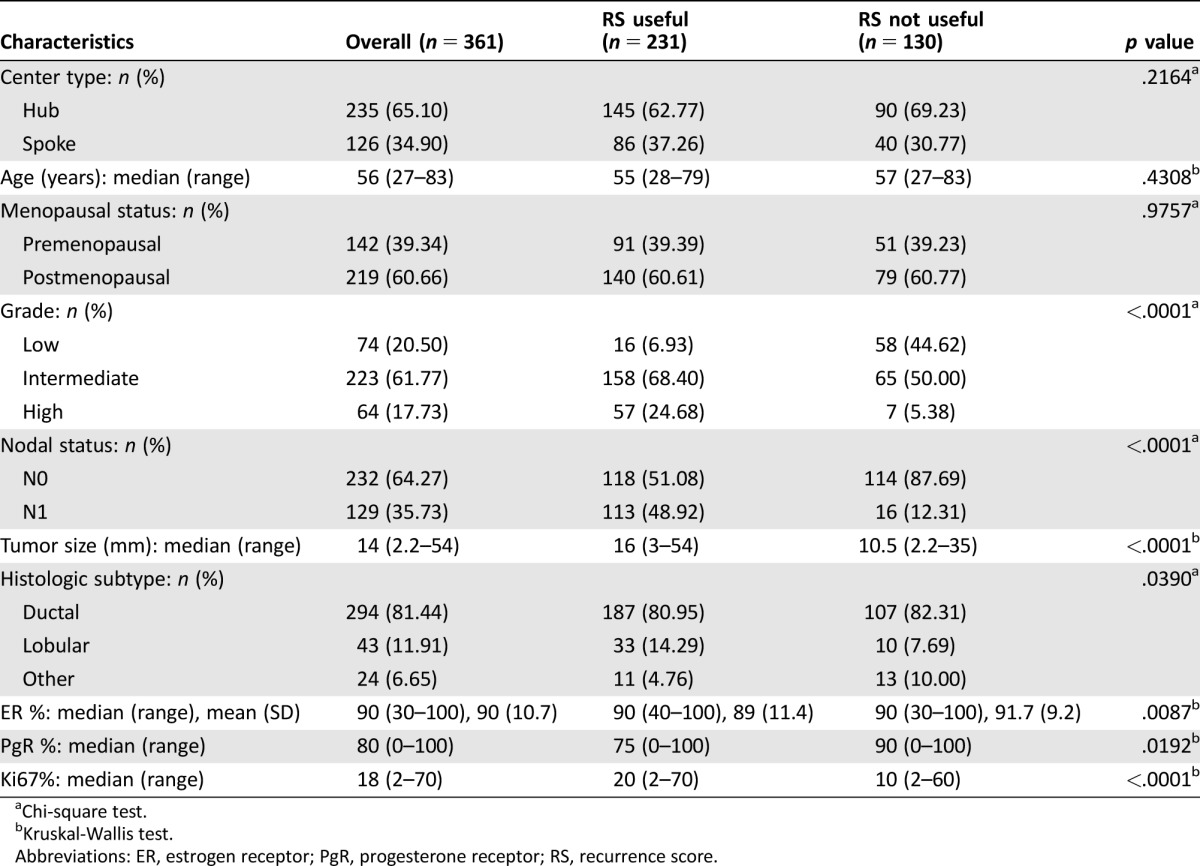
Chi‐square test.
Kruskal‐Wallis test.
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor; RS, recurrence score.
Based on protocol‐defined criteria, 289 of the 361 registered patients were classified as “intermediate risk” and therefore, irrespective of the physician's baseline perception of utility, were eligible for the RS assay. Among them, n = 250 patients signed the informed consent and underwent the RS test (RS population). For 85.2% of the patients included in the RS population, the physician would have deemed the RS assay useful in support of treatment decision at baseline (Fig. 1).
The main clinicopathological characteristics of the RS population are reported in Table 2. The median age was 55 years. Patients presented G2 tumors in the majority of cases (71%); however, G3 tumors were also represented (25%). The median tumor size was 16 mm, and the median Ki67 was 20% (range 2%–70%). Patients recruited at Hub centers presented higher risk features than patients referred to Spoke centers; they were younger and more frequently premenopausal and had tumors of higher grade and Ki67 levels and more frequently with ductal histology. Patients with N0 cancer presented smaller tumors than those with N1. Counterintuitively, patients with N1 cancer presented with tumors of lower grade and lower Ki67 levels than those with N0. This is probably due to the predefined “intermediate risk” study criteria that resulted in an enrichment of the RS population with patients with N0 cancer and high‐grade and/or rapidly proliferating tumors and with patients with N1 cancer with low and intermediate grade and/or slowly proliferating tumors.
Table 2. Characteristics of the recurrence score population of the Breast DX Italy study and distribution according to center type and nodal status.
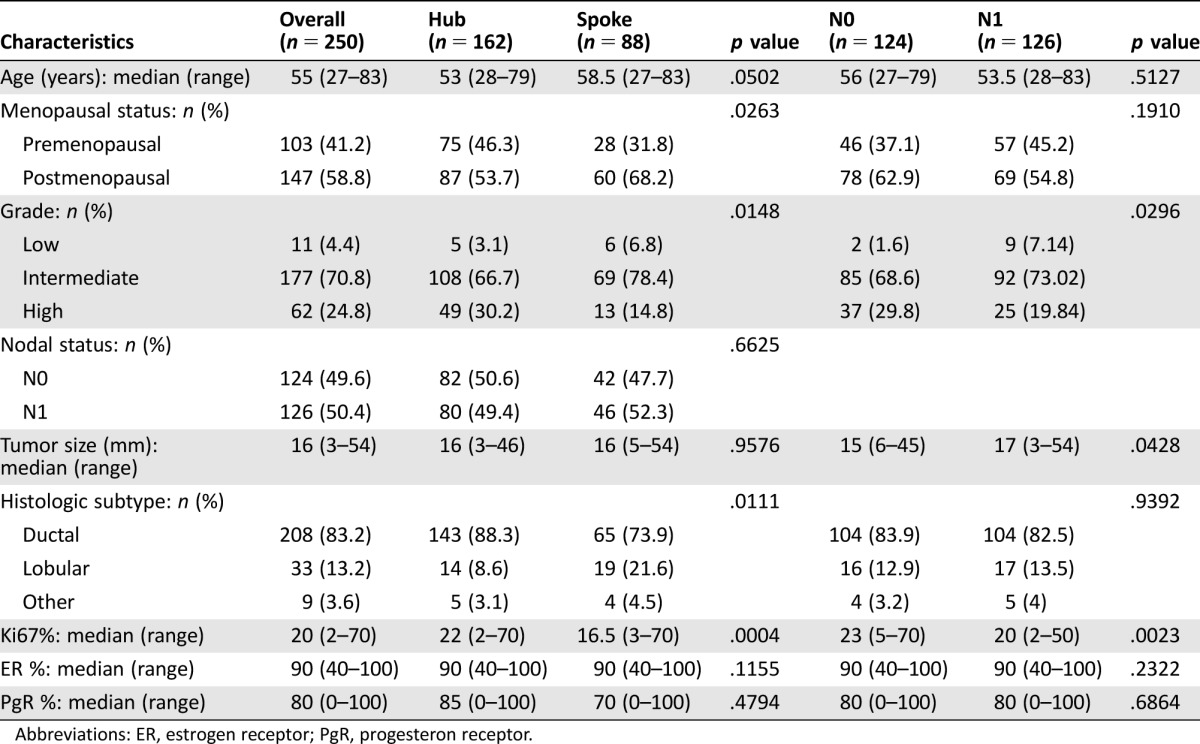
Abbreviations: ER, estrogen receptor; PgR, progesteron receptor.
RS Results
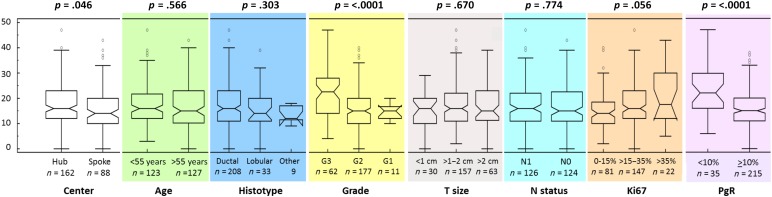
Overall, the median RS result was 16 (range 0–47), with 152 (60.8%), 81 (32.4%), and 17 (6.8%) patients classified as low (<18), intermediate (18–30), or high (>30) RS. According to the TAILORx categorization, distribution was as follows: low (<11; n = 53, 21.2%), midrange (11–25; n = 155, 62%), and high (>25; n = 42, 16.8%). As shown in Figure 2, significantly different distribution of RS values was observed according to histologic grade, PgR status, and Ki67. However, wide ranges of RS values were found across all subgroups. No significant association was observed between RS and other factors.
Figure 2.
Box plots showing recurrence score results according to main clinicopathological variables.
Abbreviations: G1, Grade 1; G2, Grade 2; G3, Grade 3; T size, tumor size; N status, nodal status; PgR, progesterone receptor.
The concordance between ER, PgR, and HER2 as assessed at local pathology departments with the single‐gene reporting provided by Oncotype DX was assessed. Positive correlations between ER and PgR levels with single gene reporting were found (Spearman's coefficient 0.424, p < .0001 for ER and Spearman's coefficient 0.821, p < .0001 for PgR; supplemental online Fig. 1). Concordance between positive and negative categories was 100% for ER (all cases were positive by local pathology and by single‐gene reporting) and 89% for PgR (16 cases were positive by local assessment and negative by Oncotype DX; 12 cases were negative by local assessment and positive by Oncotype DX). Concordance for HER2 status was high, with only 4 cases showing discordant results (2 cases scored as 0 by immunohistochemistry [IHC] at local assessment were positive by Oncotype DX, and 2 cases scored as 2+ by IHC and negative by fluorescent in situ hybridization at local assessment were equivocal by Oncotype DX).
Treatment Recommendation Before RS
In the entire RS population, physician's treatment recommendation before RS was HT alone for n = 130 (52%) cases and CT+HT for n = 120 (48%). More patients were initially recommended CT+HT at Hub centers (n = 88 of 162, 54%) compared with Spoke centers (n = 32 out of 88, 36%; p = .007), which is concordant with the higher risk profile based on classical features of patients recruited by Hub centers. Recommendations of CT before RS were more frequent for patients with N1 cancer than for those with N0 (57% vs. 39%, respectively; p = .0035). Other factors significantly associated with indication of CT+HT before RS were younger age, premenopausal status, higher histologic grade, larger tumor size, and higher Ki67 (Table 3).
Table 3. Association of clinicopathological characteristics of RS population with treatment recommendation before RS.
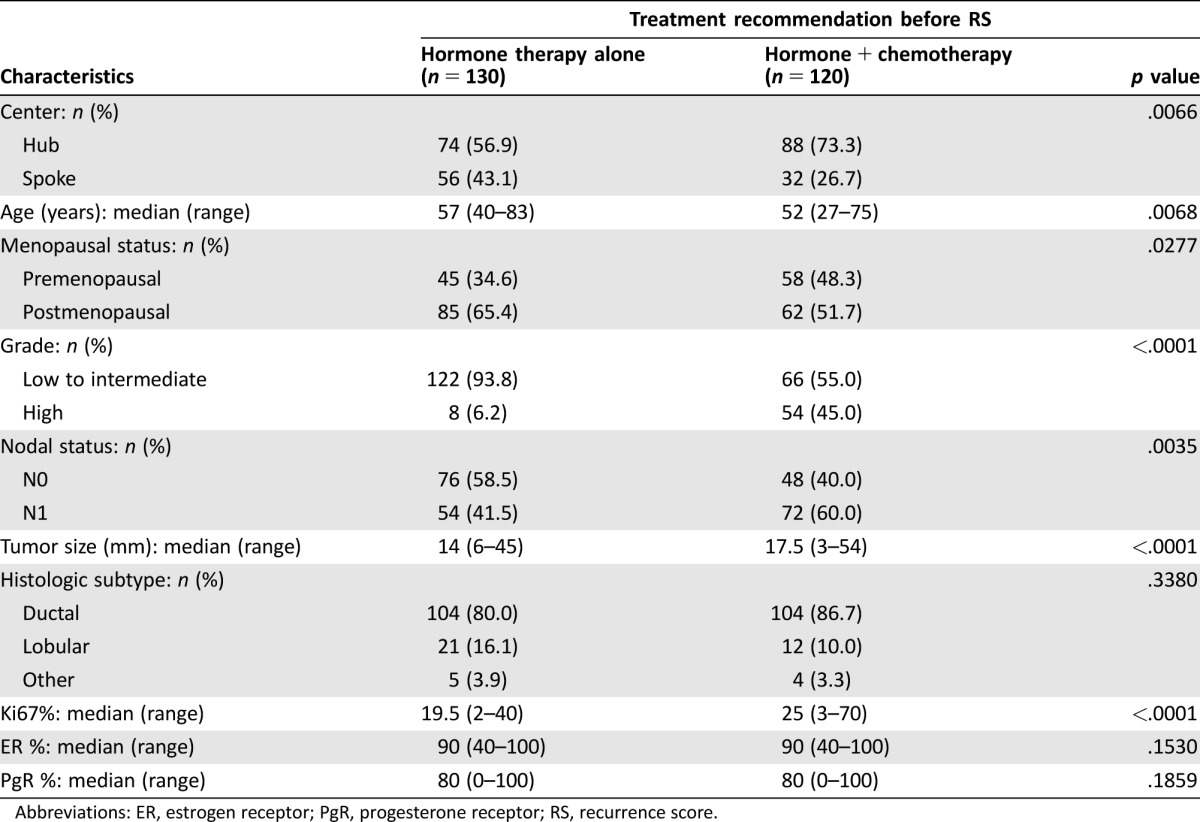
Abbreviations: ER, estrogen receptor; PgR, progesterone receptor; RS, recurrence score.
Change in Treatment Recommendation
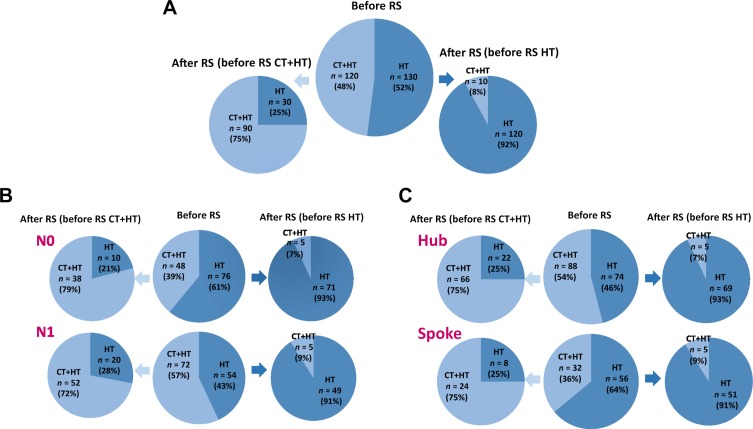
Overall, after knowing the RS results, physician's treatment recommendation changed for 16% of the patients (n = 40). Of these 40 patients, n = 30 (75%) changed from CT+HT to HT alone (Table 4). The direction of switch for those with RS <18 (n = 25) and those with RS >30 (n = 2) was consistent with RS results; for the 13 patients with RS between 18 and 30, change was from HT to CT+HT for 8 cases and from CT+HT to HT for 5 cases. According to the TAILORx categorization, among the 40 patients with recommendation change, test results in n = 10 were classified as low (RS <11, all switching from CT+HT to HT), n = 26 as midrange (RS 11–25, 77% switching to HT, 23% switching to CT+HT) and n = 4 as high (RS >25, all switching to CT+HT). As shown in Figure 3A, of the 120 patients initially recommended CT+HT, 25% had an indication of HT alone after RS. The distribution of recommendations of HT alone after RS according to RS category was 77.6%, 38.3%, and 5.9% of patients with RS <18, 18–30, and >30, respectively. We also explored the distribution of indications after RS according to different cutoffs. The rate of indication of CT after RS for patients with RS <18 was 22.4%; this included 8% of patients with RS <11 and 30% of patients with RS 11–17 (supplemental online Fig. 2). As RS approached 25 (RS 18–25), the recommendation of CT+HT after RS was 52%. Overall, following the RS result, the proportion of patients recommended to CT+HT was reduced from 48% (120/250) to 40% (100/250), McNemar's p < .0016.
Table 4. Change in treatment recommendation from before to after RS overall, by RS, center, and nodal status.
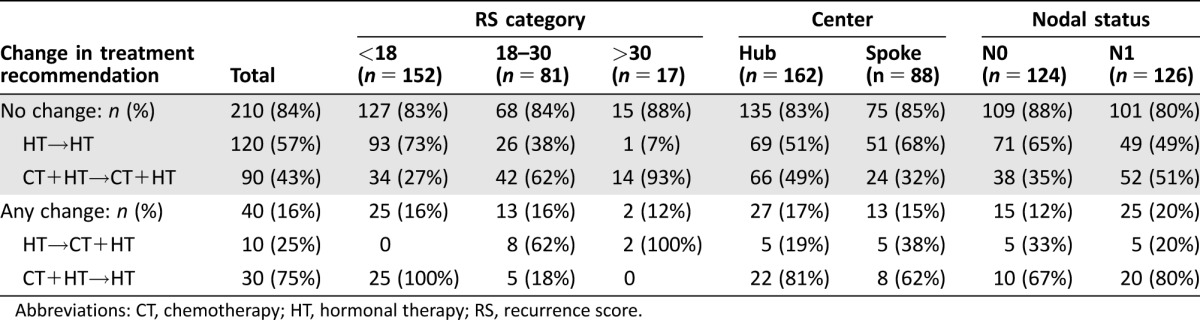
Abbreviations: CT, chemotherapy; HT, hormonal therapy; RS, recurrence score.
Figure 3.
Change in treatment recommendation (before → after RS) by indication before RS: in the whole RS population (A), separately for patients with N0 and N1 cancer (B) and separately for Hub and Spoke centers (C).
Abbreviations: CT, chemotherapy; HT, hormone therapy; RS, recurrence score.
For the 100 patients for whom the recommendation after RS was CT, 87 (87%) actually received it. One patient who was recommended HT alone after RS actually received CT (0.7%). When the actual number of CT given was compared with the recommendations before RS, the net reduction in the use of CT was 13% (from 48% to 35%, p < .0001).
Change in treatment recommendation was then evaluated according to nodal status and center type (Fig. 3B, 3C; Table 4). Change in treatment recommendation was more frequent for patients with N1 versus N0 (20% vs. 12%, respectively, p = .095). In each group, the most frequent change was from CT+HT to HT. This observation, coupled with significant difference in recommendations before RS (Table 3), resulted in a significant reduction in the proportion of patients recommended to CT+HT after RS testing in the N1 group only (from n = 72, 57% to n = 57, 45%; p = .0027 for N1; from n = 48, 39% to n = 43; 35%, p = .1967 for N0). The reduction in the use of CT from recommendation before RS to treatment actually received was 10% for N0 and 14% for N1 (p = .003 and p < .001, respectively). Indications of HT alone after RS according to RS result were as follows: 80%, 48.8%, and 12.5% of patients with RS <18, RS 18–30, and RS >30, respectively, in the N0 cohort; 75.3%, 27.5%, and none of patients with RS <18, RS 18–30, and RS >30, respectively, in the N1 cohort.
Despite significantly different treatment recommendations before RS (Table 3), the rate of change before and after RS was comparable in Hub and Spoke centers (17% and 15%, respectively). This resulted in a significant reduction from before to after RS in the proportion of patients with an indication to CT+HT at Hub centers (from n = 88, 54% to n = 71, 44%; p = .001) but not at Spoke centers (from n = 32, 36% to n = 29, 33%; p = .405). Considering the actual treatment received, the net reduction from recommendations of CT before RS to the proportion of patients who received CT was 14.5% at Hub (from 54% to 39.5%, p < .001) and 8% at Spoke centers (p = .052).
Further analyses were conducted to explore the association between clinicopathological characteristics and change in treatment recommendation. As reported in supplemental online Table 1, the only factor significantly associated with treatment recommendation change in the whole RS population was tumor size (median 18 mm vs. 15 mm in patients with and without change, respectively; p = .0153). In the group of patients initially recommended to HT (n = 130), only 10 patients had a switch to CT+HT after RS, associated with negative PgR status, lower ER expression, and larger tumor size. Among patients with indication of CT+HT before RS, those for whom the recommendation switched to HT alone after RS (n = 30) presented more frequently G1–G2 tumors and cancer of lobular histology and tended to show lower Ki67 levels and lower PgR expression.
Physicians' Perception of RS Utility After RS
Physicians agreed or strongly agreed that they were confident in treatment recommendation after the RS test in 87% of cases. Nevertheless, they agreed or strongly agreed that the RS test provided additional information in 63% of cases and that the RS result influenced their treatment recommendation in a lower proportion (36%).
Discussion
This is the first prospective study conducted in Italy evaluating the impact on adjuvant treatment decision of the use of the RS test for patients with early BC.
First, this study shows that physicians, at baseline, would have deemed the RS assay useful in support to the treatment decision process for two thirds of consecutive patients with ER positive/HER2 negative disease, T1–T3 and N0–N1 BC. For the remaining patients, physicians would have been confident in treatment decision based on the evaluation of classical clinicopathological factors. This attitude was indeed anticipated in the study design, which restricted the eligibility for the RS assay only to those patients meeting protocol predefined criteria of “intermediate risk,” presuming these could identify the subgroup of patients for whom the degree of physician's indecision was higher.
Distribution of RS results, in both the N0 and N1 cohort, is consistent with most of the European decision impact studies and postmarketing data in large population datasets [8], [14], with more than half of the patients presenting with low RS (<18), less than 10% presenting with high RS (>30), and the remainder being classified as intermediate RS (18–30). This distribution differs from original validation studies, in which populations were enriched with patients with higher RS [3], [5], probably due to different patient selection criteria.
In the full RS population, the rate of change in treatment recommendation was 16%, more frequently occurring for patients with N1 (20%) than for those with N0 cancer (12%). According to available literature and study assumptions, these rates are lower than expected. A recent pooled analysis including European studies confirmed that the use of RS assay led to a 31.9% change in treatment recommendation for patients with N0 cancer [17], which is considerably higher than the 12% observed in the N0 cohort of Breast DX Italy, although, as already mentioned, RS results in the two reports were similar. However, some differences in patients’ characteristics, recommendations before RS, and variables considered for decisions may in part explain these results. The N0 cohort of the Breast DX study included patients selected for presenting intermediate‐risk clinicopathological features. As a result, patients with G3 tumors were more represented in our study than in the pooled analysis (30% vs. 13.3%) [17]. However, recommendations of HT alone before RS were even more frequent in our study than in the pooled analysis: 61% compared with 54.6%, respectively, with levels reaching below 50% in individual studies [17]. The Breast DX study was conducted in more recent years as compared with the studies included in the pooled European analyses. New data showing a better outcome for patients with ER positive BC treated with contemporary endocrine therapy [6], [18], [19] may have contributed to the decreased physicians’ perception of the efficacy of chemotherapy in these patients. Interstudy heterogeneity in recommendations before RS was also noticed in the pooled analysis [18]. In part, this may also reflect geographical differences in the use of Ki67 in support of treatment decision. Ki67 is a biomarker with technical reproducibility issues [2], [20]; however, it has been shown to provide prognostic information and is widely available at low cost [21]. In Italy, the use of Ki67 is diffuse; based on our data, it represents one of the main factors significantly associated with recommendations before RS.
Despite a high proportion of recommendations of HT alone before RS in a population of patients with N0 cancer presenting with “intermediate risk,” the use of RS testing further helped in sparing CT, reducing the indication to CT+HT from 39% before RS to 35% after RS (statistically not significant). Of note, the proportion of indication to CT alone after RS was similar to the results of the pooled analysis (33.6%), as well as the distribution of indication after RS according to RS results [17]. In large, recent prospective or population‐based non‐European studies, indication of CT for patients with N0 cancer tested with RS was as low as 23%–24% [8], [22]. Distribution of RS scores was similar across these studies, further stressing geographical differences in treatment attitudes that mandate evaluating the impact of genomic tests on a local perspective.
Regarding patients with N1 cancer, there is a lack of data from European studies. In the N1 cohort of our study, indications before RS were CT+HT for 57% of the patients, and the use of RS significantly reduced it to 45% after RS. The German decision impact study included, overall, 366 patients with ER positive cancer, of whom 122 had N1 cancer. Only 8% of patients with N1 cancer had G3 tumors in that study, lower than in the Breast DX (20%). Treatment recommendation before to after RS changed for 39% of patients with N1 cancer (n = 47), more frequently from CT+HT to HT than the opposite (34/47, 72% and 13/47, 28%, respectively) [23]. In the Breast DX study, the rate of change was lower (20%). However, recommendations of CT+HT before RS were 75% in the German study and 57% in the Breast DX, with recommendations after RS of CT+HT similar in the two studies (46% in the German study and 45% in Breast DX). Another UK study assessing the impact of RS testing in clinical practice reported a rate of CT recommendations after RS for postmenopausal patients with ER positive, N1 cancer of 40% [24]. Lower rates (24.5%) of CT use for patients with N1 cancer after the RS results were reported in another work conducted in Israel; however, it should be pointed out that almost half of these patients presented with N1mi disease (n = 135 of 282, 48%) [25].
Therefore, the lower rate of change in treatment recommendation observed in the Breast DX study for patients with N1 cancer, similar to what was discussed for patients with N0 cancer, does not seem to be a consequence of physicians being less prone to revise their initial recommendation following RS testing. The main point is that recommendation of CT+HT before RS is less frequent than in other studies, concealing the impact of RS testing. In this context, however, the RS assay further contributed to significantly reduce indication to CT+HT (from 57% before RS to 45% after RS).
Another reason for low rate of change in treatment recommendation may be uncertainty about the optimal RS cutoffs to be applied for adjuvant treatment decisions. Indeed, large prospective validation studies have adopted more conservative cutoffs than the original ones [6], [7]. In Breast DX, we observed a progressive increase in indications of CT+HT after RS for higher RS values in the RS <18 category: 8% of patients with RS <11 and 30% of patients with RS 11–17 were recommended CT+HT after RS. In a recent US registry study, the proportion of patients with RS <17 receiving CT+HT was 8%, whereas rates of CT use were more similar to indications after RS in Breast DX in the case of RS >25 (42%, Breast DX, and 52%, US registry). The US study included a minority of patients with node‐positive cancer (16%), which in part may account for different results [26]. However, these data suggest that physicians in the Breast DX study were probably reluctant to avoid CT for patients with RS values falling in the “gray area” of 11–17. Other studies reported lower rates of CT+HT use in case of RS 18–25, but these cohorts included almost exclusively patients with node‐negative or N1mi cancer [27].
We observed a trend for higher recommendation change rate in Hub versus Spoke centers. Possible reasons include the following: clinicopathological characteristics of patients enrolled at Hub centers were more unfavorable, leading physicians to be more cautious in indications before RS; physicians at Hub centers might be more prone to revise their initial indication based on the result of genomic assay, because they are more used to being involved in clinical trials with novel approaches; and physicians at Hub centers may have accumulated more experience and confidence with the RS test during the study, because of the high number of patients enrolled.
In order to fully capture the impact on clinical practice of RS testing, treatment that was finally received by each patient has been collected. We observed a 5% (n = 13) degree of discordance between the indication after RS formulated by the physician and the treatment that was actually received by the patient, which is concordant with other studies reporting rates up to 12% [22], [23]. The reason why treatment actually received differed from recommendation after RS for these 13 patients was not collected in the Breast DX; however, for 12 of them, the treatment received was HT following a recommendation after RS of CT+HT. The use of the RS test might have reduced patients’ uncertainty in the discussion on treatment decision [22].
From a health economic perspective, it is relevant to identify patient subgroups with the highest rate of change in treatment recommendation. Although some hints could be derived from exploratory analyses in this paper, low sample size limits the possibility of drawing any conclusion. Moreover, from a clinical perspective, it is hard to decide to restrict the RS test use to limited subgroups, when the RS result may influence treatment decision for other patients too. Finally, a combination of features rather than one or a few clinicopathological characteristics dominates the treatment decision process, further jeopardizing the possibility of defining a precise algorithm on which to base strategies for test access.
Conclusion
The Breast DX study showed that, even in a context in which indication before RS is HT for the majority of patients and the rate of treatment recommendation change from before to after RS is lower than expected, the use of RS assay further helped in reduce overtreatment for patients with ER positive/HER2, N0–N1 BC. These data highlight the need for further work in collaboration with health authorities and companies in order to define strategies for the implementation of the use of RS testing in clinical practice in the Italian setting.
In the Breast DX study, for some patients meeting protocol‐defined criteria for intermediate risk, physicians still would not have deemed RS useful. Building on this experience, the ROXANE prospective study is ongoing in centers of the Veneto Region. In this study, access to the test is not restricted to a predefined intermediate risk population, but physicians have the opportunity to request the RS assay for any consecutive patient with ER positive/HER2 negative, N0–N1 BC for whom they feel unsure regarding treatment recommendation. The aim is to assess the impact of the RS assay in a setting more closely reflecting real‐world clinical practice.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The study was supported by a grant from Regione Veneto and Genomic Health‐PRIHTA 2013 call.
Author Contributions
Conception/design: Maria Vittoria Dieci, Valentina Guarneri, Pierfranco Conte, Gian Luca De Salvo
Provision of study material or patients: Maria Vittoria Dieci, Valentina Guarneri, Tommaso Giarratano, Marta Mion, Giampaolo Tortora, Costanza De Rossi, Stefania Gori, Cristina Oliani, Laura merlini, felice Pasini, Giorgio Bonciarelli, Gaia Griguolo, Enrico Orvieto, Silvia Michieletto, Tania Saibene, Pierfranco Conte
Collection and/or assembly of data: Maria Vittoria Dieci, Valentina Guarneri, Tommaso Giarratano, Marta Mion, Giampaolo Tortora, Costanza De Rossi, Stefania Gori, Cristina Oliani, Laura merlini, Felice Pasini, Giorgio Bonciarelli, Gaia Griguolo, Paola Del Bianco, Gian Luca De Salvo, Pierfranco Conte
Data analysis and interpretation: Maria Vittoria Dieci, Valentina Guarneri, Paola Del Bianco, Gian Luca De Salvo, Pierfranco Conte
Manuscript writing: Maria Vittoria Dieci, Paola del Bianco, Pierfranco Conte
Final approval of manuscript: Maria Vittoria Dieci, Valentina Guarneri, Tommaso Giarratano, Marta Mion, Giampaolo Tortora, Costanza De Rossi, Stefania Gori, Cristina Oliani, Laura Merlini, Felice Pasini, Giorgio Bonciarelli, Gaia Griguolo, Enrico Orvieto, Silvia Michieletto, Tania Saibene, Paola Del Bianco, Gian Luca De Salvo, Pierfranco Conte
Disclosures
Maria Vittoria Dieci: Genomic Health (RF); Marta Mion: Novartis, Roche (RF); PierFranco Conte: Genomic Health (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: An overview of the randomised trials. Lancet 2005;365:1687–1717. [DOI] [PubMed] [Google Scholar]
- 2. Harris LN, Ismaila N, McShane LM et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2016;34:1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paik S, Shak S, Tang G et al. A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N Engl J Med 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 4. Dowsett M, Cuzick J, Wale C et al. Prediction of risk of distant recurrence using the 21‐gene recurrence score in node‐negative and node‐positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol 2010;28:1829–1834. [DOI] [PubMed] [Google Scholar]
- 5. Albain KS, Barlow WE, Shak S et al. Prognostic and predictive value of the 21‐gene recurrence score assay in postmenopausal women with node‐positive, oestrogen‐receptor‐positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparano JA, Gray RJ, Makower DF et al. Prospective validation of a 21‐gene expression assay in breast cancer. N Engl J Med 2015;373:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gluz O, Nitz UA, Christgen M et al. West German Study Group phase III PlanB trial: First prospective outcome data for the 21‐gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 2016;34:2341–2349. [DOI] [PubMed] [Google Scholar]
- 8. Petkov VI, Miller DP, Howlader N et al. Breast‐cancer specific mortality in patients treated based on the 21‐gene assay: A SEER population‐based study. NPJ Breast Cancer 2016;2:16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paik S, Tang G, Shak S et al. Gene expression and benefit of chemotherapy in women with node‐negative, estrogen receptor‐positive breast cancer. J Clin Oncol 2006;24:3726–3734. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2.2017. April 6, 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June, 2017.
- 11. Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up Ann Oncol 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 12. Giuliano AE, Connolly JL, Edge SB et al. Breast Cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290–303. [DOI] [PubMed] [Google Scholar]
- 13. Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curigliano G, Burstein HJ, Winer EP et al. De‐escalating and escalating treatments for early‐stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2017;28:1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lo SS, Mumby PB, Norton J et al. Prospective multicenter study of the impact of the 21‐gene Recurrence Score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 2010;28:1671–1676. [DOI] [PubMed] [Google Scholar]
- 17. Albanell J, Svedman C, Gligorov J et al. Pooled analysis of prospective European studies assessing the impact of using the 21‐gene Recurrence Score assay on clinical decision making in women with oestrogen receptor‐positive, human epidermal growth factor receptor 2‐negative early‐stage breast cancer. Eur J Cancer 2016;66:104–113. [DOI] [PubMed] [Google Scholar]
- 18. Pagani O, Regan MM, Walley BA et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies C, Pan H, Godwin J et al. Long‐term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor‐positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andre F, Arnedos M, Goubar A et al. Ki67–No evidence for its use in node‐positive breast cancer. Nat Rev Clin Oncol 2015;12:296–301. [DOI] [PubMed] [Google Scholar]
- 21. Duffy MJ, Harbeck N, Nap M et al. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer 2017;75:284–298. [DOI] [PubMed] [Google Scholar]
- 22. Levine MN, Julian JA, Bedard PL et al. Prospective evaluation of the 21‐gene recurrence score assay for breast cancer decision‐making in Ontario. J Clin Oncol 2016;34:1065–1071. [DOI] [PubMed] [Google Scholar]
- 23. Eiermann W, Rezai M, Kümmel S et al. The 21‐gene recurrence score assay impacts adjuvant therapy recommendations for ER‐positive, node‐negative and node‐positive early breast cancer resulting in a risk‐adapted change in chemotherapy use. Ann Oncol 2013;24:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loncaster J, Armstrong A, Howell S et al. Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: Real world experience in Greater Manchester, UK. Eur J Surg Oncol 2017;43:931–937. [DOI] [PubMed] [Google Scholar]
- 25. Stemmer SM, Klang SH, Ben‐Baruch N et al. The impact of the 21‐gene Recurrence Score assay on clinical decision‐making in node‐positive (up to 3 positive nodes) estrogen receptor‐positive breast cancer patients. Breast Cancer Res Treat 2013;140:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vacirca J, Tsai M, Brufsky A et al. Initial results from the 21‐gene breast cancer assay registry: A prospective observational study in patients (pts) with ER+, early‐stage invasive breast cancer (EBC). J Clin Oncol 2013;31(suppl 15):565. 23295789 [Google Scholar]
- 27. Stemmer SM, Stelner M, Rizel S et al. Real‐life analysis evaluating 1594 N0/Nmic breast cancer patients for whom treatment decisions incorporated the 21‐gene Recurrence Score result: 5‐year KM estimate for breast cancer‐specific survival with recurrence score results ≤30 is >98%. Abstract presented at: San Antonio Breast Cancer Symposium; December 8–12, 2015; San Antonio, TX, P5‐08‐02.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.