Abstract
Lessons Learned.
Panitumumab has no clinical activity in metastatic RAS wild‐type small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC), possibly due to the foregut and midgut derivation of small bowel and ampulla.
These results, along with findings from genomic characterization of SBA, suggest that SBA represents a unique intestinal malignancy and treatments should not be habitually extrapolated from colorectal cancer.
Further studies evaluating the benefit of targeted therapies exclusively in SBA and AAC are warranted.
Background.
Given the benefit of epidermal growth factor receptor (EGFR) monoclonal antibodies in colorectal cancer (CRC), we sought to evaluate the efficacy of panitumumab in metastatic RAS wild‐type small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC).
Methods.
We conducted a single‐center, open‐label, single‐arm, Bayesian phase II trial. The primary objective was response rate (RR). Panitumumab was administered at a dose of 6 mg/kg intravenously (IV) every 14 days.
Results.
Nine patients (male/female 7:2, median age: 61 years [range: 40–74], Eastern Cooperative Oncology Group [ECOG] performance status 0/1: 2/7) were enrolled from September 2013 to October 2015. One patient had AAC (pancreaticobiliary subtype) and eight patients had SBA (three duodenal, five jejunal/ileal). Acneiform rash was the most common toxicity. The study was stopped early due to futility with no responses, stable disease (SD) in two patients, and progression of disease (PD) in seven patients. Median progression‐free survival (PFS) and overall survival (OS) were 2.4 and 5.7 months, respectively. No patients had extended RAS mutations (exons 2/3/4), but two patients had BRAF G469A and one patient had PIK3CA H1074R mutations.
Conclusion.
Panitumumab had no clinically meaningful activity in patients with metastatic RAS wild‐type SBA and AAC. Our findings may relate to the primarily midgut and foregut derivation of the small bowel and ampulla.
Abstract
经验总结
• 帕尼单抗在转移性RAS野生型小肠腺癌(SBA)和壶腹腺癌(AAC)中不具有临床活性, 可能是由于小肠和壶腹部是前肠和中肠来源的原因
• 这些结果以及SBA基因组特征的研究结果表明, SBA代表了一种独特的肠道恶性肿瘤, 不应习惯性地从结直肠癌外推治疗
• 有必要进一步研究评估SBA和AAC专门针对性治疗的益处
摘要
背景. 鉴于表皮生长因子受体(EGFR)单克隆抗体在结直肠癌(CRC)中的益处, 我们试图评估帕尼单抗在转移性RAS野生型小肠腺癌(SBA)和壶腹腺癌(AAC)中的疗效
方法. 我们进行了单中心、开放标签的单组贝叶斯II期试验主要目的是了解反应率(RR)每14天以6 mg/kg的剂量静脉内(IV)给予帕尼单抗。
结果. 从2013年9月至2015年10月期间有9名患者[男性/女性7:2, 中位年龄:61岁(范围:40‐74), 东部肿瘤协作组(ECOG)体能状态0/1:2/7]入组。1例患者患AAC(胰胆亚型), 8例患者患SBA(3例十二指肠, 5例空肠/回肠)。痤疮样皮疹是最常见的毒性症状。由于无效, 没有反应, 提前终止了研究, 其中2名患者疾病稳定(SD), 7名患者疾病进展(PD)。中位无进展生存期(PFS)和总生存期(OS)分别为2.4和5.7个月。患者没有扩展的RAS突变(外显子2/3/4), 但两名患者有BRAF G469A, 一名患者有PIK3CA H1074R突变。
结论. 帕尼单抗在转移性RAS野生型SBA和AAC患者中没有具临床意义的活性。我们的发现可能与小肠和壶腹部主要是中肠和前肠起源有关。
Discussion
Panitumumab is a U.S. Food and Drug Administration‐approved anti‐EGFR monoclonal antibody with a demonstrated RR of 31% and improvement in mean PFS from 1.7 to 5.2 months when compared with best supportive care in RAS wild‐type refractory metastatic CRC. Given their rarity and proximity to the large bowel, SBA and AAC are often treated in a similar manner to CRC with treatment data extrapolated from studies in CRC.
We performed a single‐arm trial evaluating efficacy of panitumumab monotherapy in refractory metastatic RAS wild‐type SBA and AAC. The primary endpoint of this study was RR. A sample size of 17 was required to demonstrate an RR of 17% using a binomial one‐sample test with two‐sided alpha of 0.05 and power of 90%. Between September 2013 and October 2015, nine patients were enrolled. Per continuous Bayesian monitoring criteria, the study was stopped early when the probability of determining a 17% RR was <5%.
Median age of the study population was 61 (range 40–74) years. One patient had AAC (pancreaticobiliary subtype) and eight patients had SBA (duodenal in three, jejunal/ileal in five). Of nine patients, poorly differentiated histology was present in five (55.6%) and mucinous histology was present in three (33.3%). Inflammatory bowel disease was present in one (11.1%) patient, and two (22.2%) patients had a known history of Lynch syndrome.
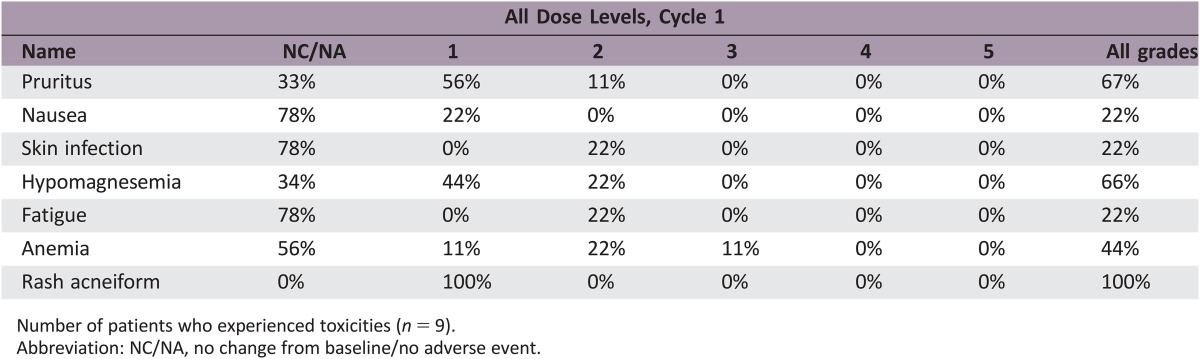
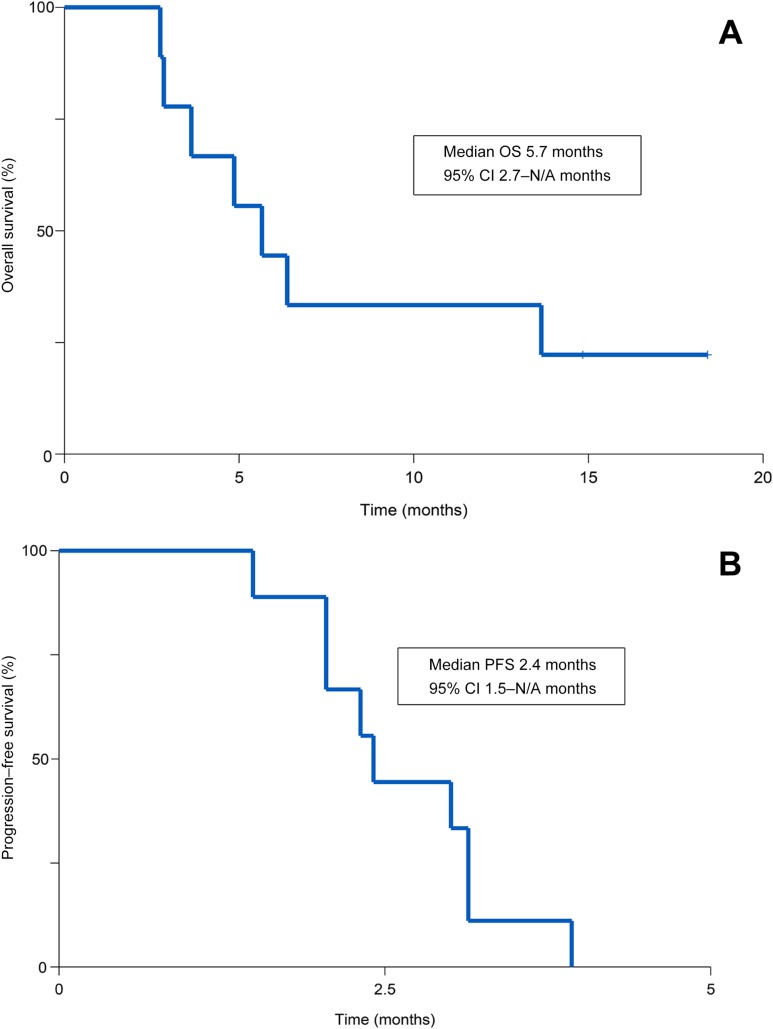
In nine patients, panitumumab demonstrated no responses, two SD, and seven PD (Fig. 1). Median PFS was 2.4 months and median OS was 5.7 months at median follow‐up time of 16.6 months. Treatment was otherwise well tolerated, with expected common toxicities of acneiform rash (100%), anemia (33%), fatigue (22%), hypomagnesemia (22%), and skin infection (22%).
Figure 1.
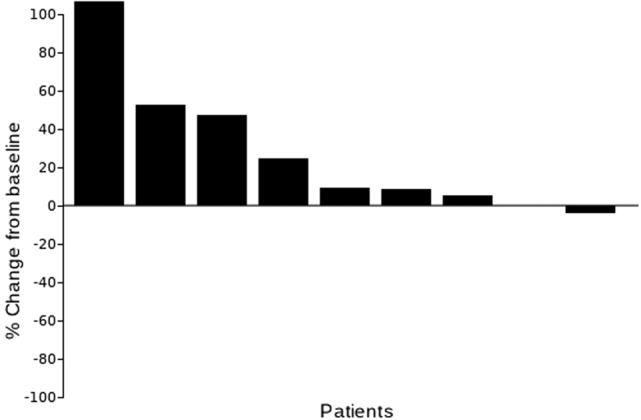
Waterfall plot with best tumor response as per Response Evaluation Criteria in Solid Tumors v1.1.
We evaluated several key mutational hotspots (BRAF, PIK3CA and ERBB2 genes) associated with resistance to EGFR blockade in RAS wild‐type metastatic CRC and identified two patients with BRAF G469A mutation, and one patient with PIK3CA H1047R mutation. Given recent findings suggesting that right‐sided colon cancers (midgut derivation) benefit less from anti‐EGFR therapy compared with left‐sided colon cancers (hindgut derivation), we propose that our findings may relate to the primarily midgut (distal duodenum to ileum) and foregut (proximal duodenum) derivation of the small bowel and ampulla.
To our knowledge, this is the first prospective clinical trial evaluating anti‐EGFR therapy in SBA and AAC. Taken together with recent findings from the first large‐scale genomic comparison of SBA with colorectal and gastric cancers, we propose that SBA is a molecularly unique intestinal malignancy and treatment paradigms should not be extrapolated from CRC to SBA and AAC without dedicated investigations. Further studies evaluating the benefit of targeted therapies in SBA and AAC are warranted.
Trial Information
- Disease
Small bowel and ampullary cancer
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
1 prior regimen
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Progression‐free survival
- Secondary Endpoint
Overall survival
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- Study Design The study was an open‐label, single‐arm, single‐institution, Bayesian phase II study conducted at University of Texas MD Anderson Cancer Center. This clinical trial was originally designed to evaluate the addition of panitumumab to capecitabine and oxaliplatin in patients with SBA and AAC. Oxaliplatin was dosed at 110 mg/m2 on day 1, panitumumab was dosed at 9 mg/kg on day 1, and capecitabine 750 mg/m2 p.o. b.i.d. on days 1–14 every 21 days (1 cycle). However, due to toxicity, the trial was modified to investigate single‐agent panitumumab administered at a dose of 6 mg/kg intravenously every 14 days (1 cycle). Imaging studies were conducted every 4 cycles. Treatment was continued until progression of disease, intercurrent illness preventing further administration of treatments, severe predefined treatment‐related toxicities, or treatment delay of more than 4 weeks due to toxicity.
- Statistical Analysis The primary endpoint of this study was RR to single‐agent panitumumab per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 criteria [1] in the evaluable population. Data from a previous study of single‐agent panitumumab in KRAS wild‐type CRC demonstrated a 17% response rate [2], [3]. Assuming a null hypothesis of ≤1% RR, a sample size of 17 patients would be able to demonstrate a RR of 17% using a binomial one‐sample test with two‐sided alpha of 0.05 and power of 90%. Continuous Bayesian monitoring for efficacy was conducted, requiring study termination if the probability of RR of 17% was <5% [4], [5]. Monitoring for response allowed up to 8 cycles from their first dose prior to nonresponder determination and study enrollment was continuous. Evaluable patients were defined as patients who had restaging imaging to enable response determination.
- Secondary endpoints included toxicity rate, PFS, and OS. Toxicities to be included in toxicity monitoring included definite or probably treatment‐related grade 3 or 4 nonhematological toxicities, excluding grade 3 rash and grade 3 hypomagnesemia, which are both expected and manageable toxicities. PFS was defined as the interval between start of treatment to the date of first documentation of progression or symptomatic deterioration or death due to any cause. OS was defined as the time from first study treatment to date of death or last follow‐up. Comparisons were conducted using Wilcoxon rank sum test, Fisher's exact test, or log‐rank test. Kaplan‐Meier curves were used to estimate unadjusted OS and PFS time distributions. All computations were carried out in SAS version 9.4 (SAS Institute Inc., Cary, NC) and TIBCO Spotfire S+ version 8.2 (TIBCO Software Inc., Palo Alto, CA).
- Investigator's Analysis
Inactive because results did not meet primary endpoint
Drug Information for Phase II Treatment
- Drug 1
- Generic/Working Name
Panitumumab
- Trade Name
Vectibix
- Company Name
Amgen
- Drug Type
Antibody
- Drug Class
EGFR
- Dose
6 milligrams (mg) per kilogram (kg)
- Route
IV
- Schedule of Administration
6 mg/kg intravenously every 14 days
Patient Characteristics for Phase II Treatment
- Number of Patients, Male
7
- Number of Patients, Female
2
- Stage
IV
- Age
Median (range): 61 (40–74)
- Number of Prior Systemic Therapies
Median (range): 1
- Performance Status: ECOG
-
0 — 2
1 — 7
2 — 0
3 — 0
Unknown — 0
Primary Assessment Method for Phase II Treatment
- Title
Total patient population
- Number of Patients Screened
9
- Number of Patients Enrolled
9
- Number of Patients Evaluable for Toxicity
9
- Number of Patients Evaluated for Efficacy
9
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 0 (0%)
- Response Assessment SD
n = 2 (22.2%)
- Response Assessment PD
n = 7 (77.8%)
- Response Assessment OTHER
n = 0 (0%)
- (Median) Duration Assessments PFS
16.6 months
- (Median) Duration Assessments OS
16.6 months
Phase II Treatment Adverse Events
Number of patients who experienced toxicities (n = 9).
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Inactive because results did not meet primary endpoint
Small bowel adenocarcinoma (SBA) and ampullary adenocarcinoma (AAC) are rare tumors with an estimated incidence of 10,190 in the U.S. in 2017, of which approximately 40% will be adenocarcinomas [6]. The vast majority of patients present with metastatic disease secondary to frequent delays in diagnosis [6]. Although there are no randomized clinical trials comparing the efficacy of various chemotherapy regimens in patients with advanced SBA, there have been five prospective studies, four of which used fluoropyrimidine and oxaliplatin as the backbone chemotherapy [7], [8], [9], [10], [11]. We recently published the first prospective clinical trial evaluating the use of targeted therapies in SBA and AAC, in which we found that capecitabine and oxaliplatin (CAPOX) with bevacizumab is a safe and effective regimen [11].
Epidermal growth factor receptor (EGFR) plays a key role in tumor‐associated proliferation, angiogenesis, invasion/metastasis, antiapoptosis, and chemotherapy resistance [12]. EGFR is expressed in approximately 67% cases of AAC [13] and 72% cases of SBA [14]. Panitumumab is a U.S. Food and Drug Administration‐approved anti‐EGFR monoclonal antibody that demonstrated response rate (RR) of 31% and improvement in mean progression‐free survival (PFS) from 1.7 to 5.2 months when compared with best supportive care in RAS wild‐type refractory metastatic colorectal cancer (CRC) patients [2], [3]. Given their rarity and proximity to the large bowel, SBA and AAC are treated in a similar manner to CRC [15]. Although several case reports have suggested benefit from anti‐EGFR therapies in SBA [16], [17], [18], the role of anti‐EGFR targeted agents has never been prospectively studied in the treatment of SBA and AAC.
This clinical trial was originally designed to evaluate the addition of panitumumab to CAPOX in patients with SBA and AAC. We initially enrolled three patients (two with SBA, one with AAC [pancreaticobiliary subtype]) to receive CAPOX (dosing as described previously [7]) along with panitumumab (9 mg/kg intravenously [IV] on day 1 of each 21‐day cycle). Two of three patients had partial response at the time of the first restaging scan, whereas one of three patients had progression of disease. However, all three patients developed grade 3 toxicities, which led to dose reduction and/or discontinuation of treatment on protocol. More specifically, the first patient had grade 3 nausea and grade 3 vomiting requiring dose reduction of oxaliplatin, the second patient had grade 2 nausea and grade 3 diarrhea requiring dose reduction of both oxaliplatin and capecitabine, and the third patient had grade 3 mucositis, grade 2 hand‐foot syndrome, and grade 3 paronychia requiring dose reduction of capecitabine and discontinuation of panitumumab. Based on the aforementioned toxicities and the subsequent publications of the COIN and REAL3 trials, which suggested an antagonistic interaction between oxaliplatin, capecitabine, and anti‐EGFR antibodies [19], [20], we modified the protocol to maximize patient safety and instead performed a single‐arm trial evaluating efficacy of panitumumab monotherapy dosed at 6 mg/kg IV on day 1 of each 14‐day cycle in refractory metastatic RAS wild‐type SBA and AAC.
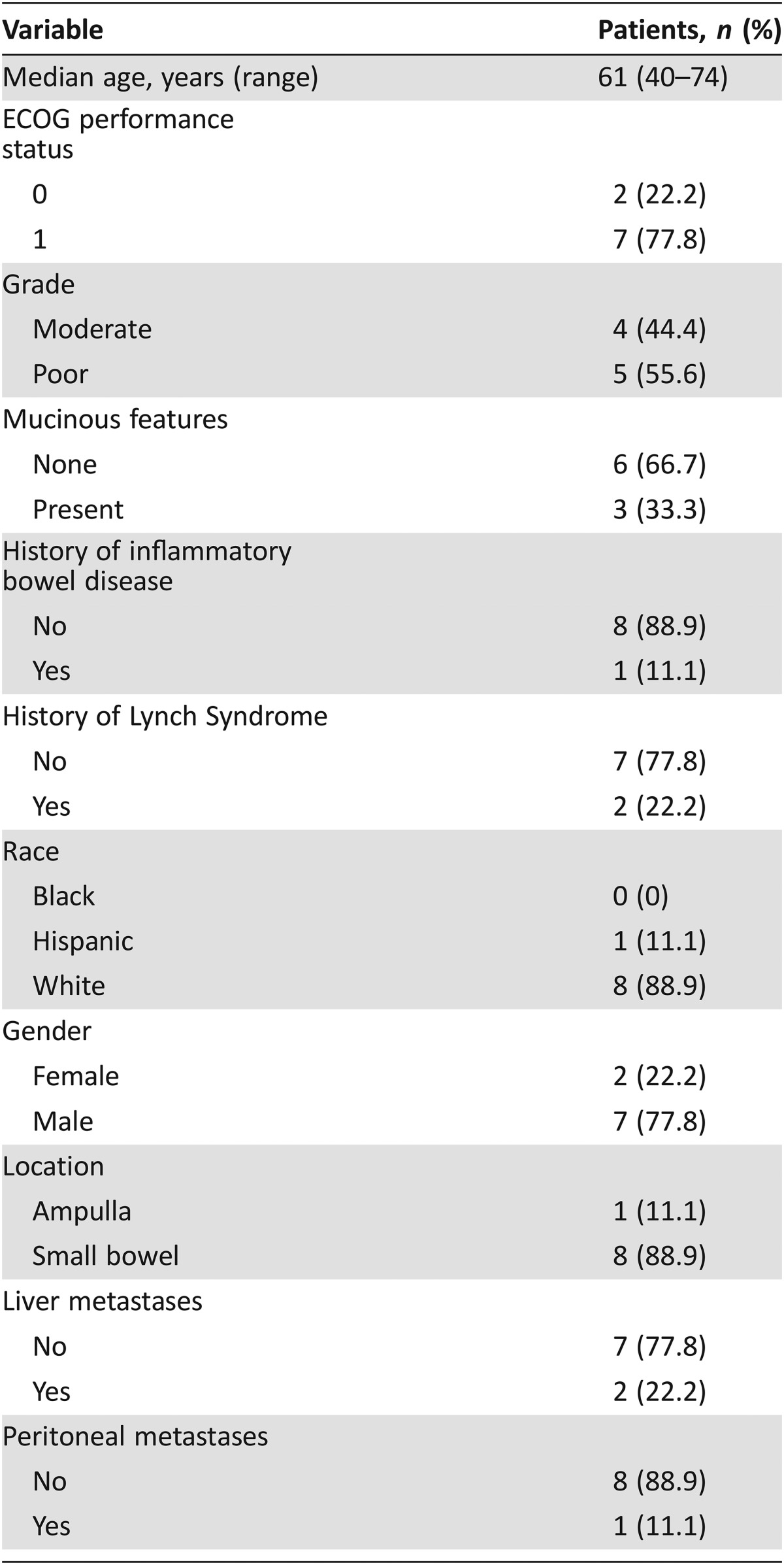
Between September 2013 and October 2015, nine patients with advanced SBA or AAC were enrolled. The baseline characteristics of the study population are listed in Table 1. The median age of the study population was 61 (range 40–74) years. Of nine patients, one (11.1%) had AAC (pancreaticobiliary subtype) and eight (88.9%) had SBA (duodenal in three, jejunal/ileal in five). Poorly differentiated histology was present in five (55.6%) patients and mucinous histology was present in three (33.3%) patients. Inflammatory bowel disease was present in one (11.1%) patient, and two (22.2%) patients had a known history of Lynch syndrome.
Table 1. Baseline patient characteristics (n = 9).
Outcomes related to the efficacy of this regimen are listed in Table 2. The primary endpoint for this study was RR. We found that panitumumab has limited clinical activity in this population with no responses noted; two patients had stable disease, whereas the remaining seven patients had progression of disease. Figure 1 depicts a waterfall plot of best tumor response per Response Evaluation Criteria In Solid Tumors criteria. Secondary endpoints included PFS, overall survival (OS), and toxicity. At a median follow‐up time of 16.6 months, median PFS was 2.4 months (95% confidence interval [CI]: 1.5 months – not applicable [N/A]) and median OS was 5.7 months (95% CI: 2.7 months – N/A; Fig. 2; Table 2). The most common treatment‐related grade 1–4 adverse events are listed in Table 3. Treatment was well tolerated, with the most common toxicity being grade 1 acneiform rash (100% of patients). The most common grade 2/3 toxicities were anemia (33%), fatigue (22%), hypomagnesemia (22%), and skin infection (22%). There were no treatment‐related deaths.
Table 2. Efficacy analysis (n = 9).
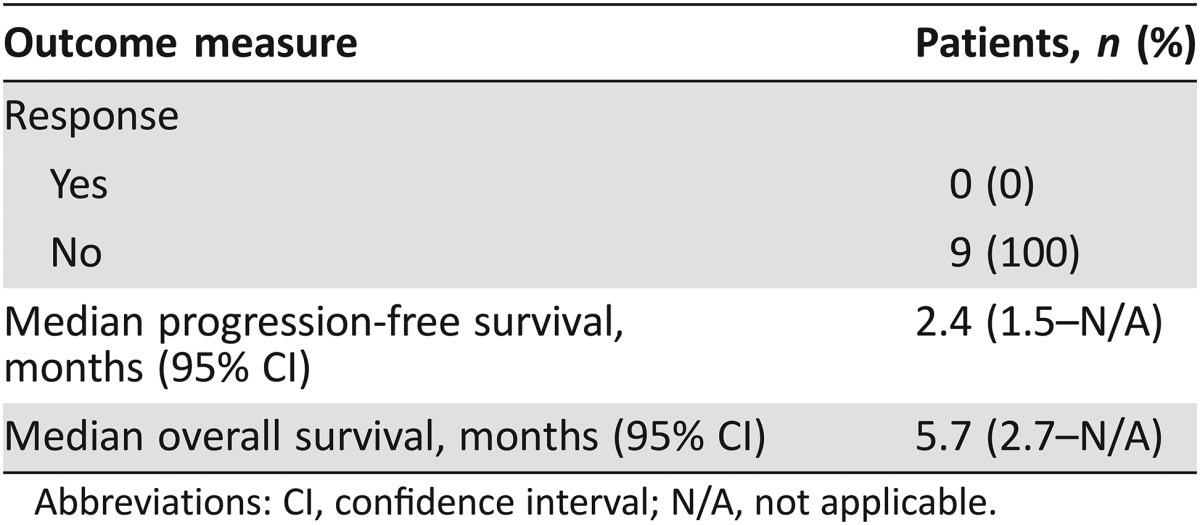
Abbreviations: CI, confidence interval; N/A, not applicable.
Figure 2.
Kaplan‐Meier estimates of overall survival (A) and progression‐free survival (B) for panitumumab in metastatic RAS wild‐type small bowel adenocarcinoma and ampullary adenocarcinoma.
Abbreviations: CI, confidence interval; N/A, not applicable; OS, overall survival; PFS, progression‐free survival.
Table 3. Number of patients who experienced toxicities (n = 9).
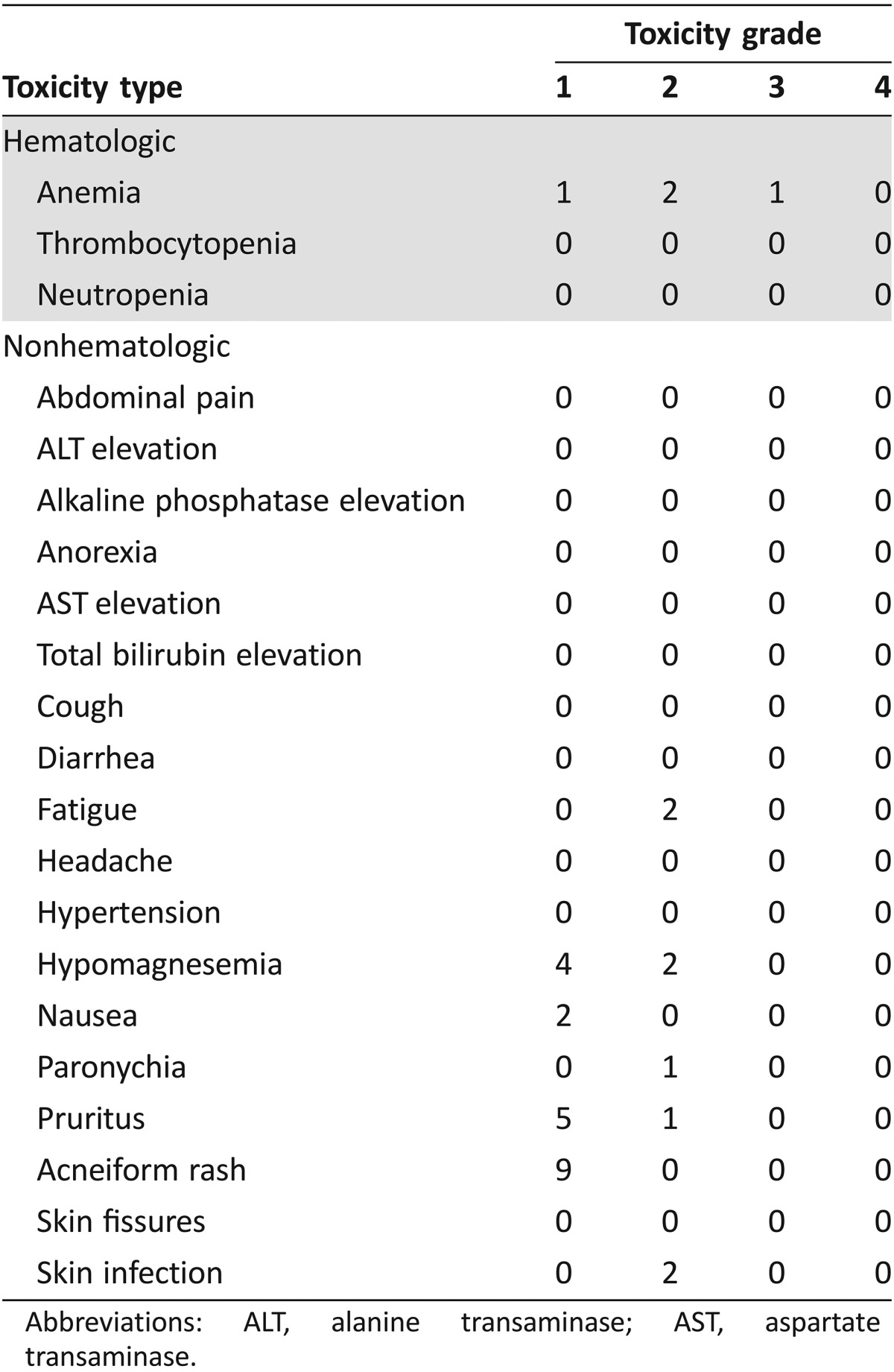
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase.
We evaluated several key mutational hotspots associated with resistance to EGFR blockade in metastatic CRC [21]. Extended RAS mutational testing identified no patients with mutations in KRAS or NRAS. Further genomic analysis of genes relevant to anti‐EGFR activity (BRAF, PIK3CA and ERBB2) identified two of nine patients with BRAF G469A mutation, one of nine patients with PIK3CA H1047R mutation, and no patients with ERRB2 mutations. We recently reported the largest genomic profiling of SBA along with comparison to neighboring intestinal tumors, which demonstrated that SBA represents a unique genomic entity with distinct alterations compared with CRC [22]. In that study, we reported mutation rates of 9.1% (29/317), 16.1% (51/317), and 9.5% (30/317) for BRAF, PIK3CA and ERRB2 in SBA, respectively [22]. Given the large number of targetable genomic alterations (91% of patients) noted in SBA, further studies evaluating the benefit of targeted therapies in SBA and AAC are warranted [22].
In conclusion, panitumumab is a well‐tolerated treatment with limited clinical activity in SBA and AAC. Toxicities were limited and there were no treatment‐related deaths. To our knowledge, this is the first prospective clinical trial evaluating the use of EGFR‐targeted antibodies in SBA and AAC. Given recent findings suggesting that right‐sided colon cancers (midgut derivation) benefit less from anti‐EGFR therapy compared with left‐sided colon cancers (hindgut derivation) [23], we propose that our findings may relate to the primarily midgut (distal duodenum to ileum) and foregut (proximal duodenum) derivation of the small bowel and ampulla.
Figures and Tables
See http://www.TheOncologist.com for supplemental material available online.
Footnotes
ClinicalTrials.gov Identifier: NCT01202409
Sponsor(s): Amgen
Principal Investigator: Michael J. Overman
IRB Approved: Yes
See the commentary on page 275.
Disclosures
Michael J. Overman: Amgen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guidelines (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Peeters M, Siena S et al. Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–1664. [DOI] [PubMed] [Google Scholar]
- 3. Kim TW, Elme A, Kusic Z et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild‐type KRAS or RAS metastatic colorectal cancer. Br J Cancer 2016;115:1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single‐arm clinical trials with multiple outcomes. Stat Med 1995;14:357–379. [DOI] [PubMed] [Google Scholar]
- 5. Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 1998:17:1563–1580. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 7. Overman MJ, Varadhachary GR, Kopetz S et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol 2009;27:2598–2603. [DOI] [PubMed] [Google Scholar]
- 8. Gibson MK, Holcroft CA, Kvols LK et al. Phase II study of 5‐fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. The Oncologist 2005;10:132–137. [DOI] [PubMed] [Google Scholar]
- 9. McWilliams RR, Mahoney MR, Marchello BT et al. Pharmacogenetic dosing by UGT1A1 genotype as first‐line therapy for advanced small‐bowel adenocarcinoma: A North Central Cancer Treatment Group (NCCTG) trial. J Clin Oncol 2012;30(suppl 4):314a. [Google Scholar]
- 10. Xiang XJ, Liu YW, Zhang L et al. A phase II study of modified FOLFOX as first‐line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 2012;23:561–566. [DOI] [PubMed] [Google Scholar]
- 11. Gulhati P, Raghav K, Shroff RT et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single‐center, open‐label, phase 2 study. Cancer 2017;123:1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saltz L, Easley C, Kirkpatrick P. Panitumumab. Nat Rev Drug Discov 2006;5:987–988. [DOI] [PubMed] [Google Scholar]
- 13. Lee CS, Pirdas A. Epidermal growth factor receptor immunoreactivity in gallbladder and extrahepatic biliary tract tumours. Pathol Res Pract 1995;191:1087–1091. [DOI] [PubMed] [Google Scholar]
- 14. Overman MJ, Pozadzides J, Kopetz S et al. Immunophenotype and molecular characterization of adenocarcinoma of the small intestine. Br J Cancer 2010;102:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raghav K, Overman MJ. Small bowel adenocarcinomas–Existing evidence and evolving paradigms. Nat Rev Clin Oncol 2013;10:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Overman MJ, Wolff RA, Wang H. Reply: Cetuximab in small bowel adenocarcinoma: A new friend? Br J Cancer 2010;103:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poddar N, Raza S, Sharma B et al. Small bowel adenocarcinoma presenting with refractory iron deficiency anemia – Case report and review of literature. Case Rep Oncol 2011; 4:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santini D, Fratto ME, Spoto C et al. Cetuximab in small bowel adenocarcinoma: A new friend? Br J Cancer 2010;103:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maughan TS, Adams RA, Smith CG et al. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomized phase 3 MRC COIN trial. Lancet 2011;377:2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waddell T, Chau I, Cunningham D et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol 2017;14:235–246. [DOI] [PubMed] [Google Scholar]
- 22. Schrock AB, Devoe CE, McWilliams R et al. Genomic profiling of small‐bowel adenocarcinoma. JAMA Oncol 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Venook AP. Right‐sided vs left‐sided colorectal cancer. Clin Adv Hematol Oncol 2017;15:22–24. [PubMed] [Google Scholar]