Abstract
Carpal tunnel pressure is a key factor in the etiology of carpal tunnel syndrome. Numerous approaches have been conducted to measure carpal tunnel pressure. However, most techniques are invasive and take time and effort. We have developed an innovative approach to noninvasively assess the tunnel pressure by using the ultrasound surface wave elastography (USWE) technique. In a previous study it was shown that the shear wave speed in a tendon increased linearly with increasing tunnel pressure enclosed the tendon in a simple tendon model. This study aimed to examine the relationship between the carpal tunnel pressure and the shear wave speeds inside and outside the carpal tunnel in a human cadaveric model. The result showed that the shear wave speed inside the carpal tunnel increased linearly with created carpal tunnel pressure, while the shear wave speed outside the carpal tunnel remained constant. These findings suggest that noninvasive measurement of carpal tunnel pressure is possible by measuring the shear wave speed in the tendon. After fully establishing this technology and being applicable in clinic, it would be useful in the diagnosis of carpal tunnel syndrome. For that reason, further validation with this technique in both healthy controls and patients with carpal tunnel syndrome is required.
Keywords: Carpal tunnel syndrome, carpal tunnel pressure, ultrasound, elastography, human cadaver
Graphical abstract
For the purpose of deducing the carpal tunnel pressure noninvasively, this study was conducted to evaluate the relationship between the carpal tunnel pressure and the shear wave speeds propagating the tendon inside and outside the carpal tunnel in a human cadaveric model by using the ultrasound surface wave elastography technique. The result showed that the shear wave speed inside the carpal tunnel increased linearly with created carpal tunnel pressure, while the shear wave speed outside the carpal tunnel remained constant.
Introduction
Carpal tunnel syndrome (CTS) is a comparatively common disease. However, CTS etiology is still not well understood. Although there are various factors relating to the pathophysiology of CTS, elevated carpal tunnel pressure is the final common pathway leading to median nerve pathology and eventual sensory and motor changes 1–5 and ischemia 6–9. It is also well known that finger activities such as griping and pinching at a specific wrist angles induce higher carpal tunnel pressure compared with the wrist at rest10–12. Carpal tunnel pressure in patients with CTS is significantly higher than that in healthy people both at rest and during activities 13–16. An earlier identification of elevation in carpal tunnel pressure may allow for better treatment and prevention of CTS.
Electrophysiological examination and electromyography (EMG) is widely used to detect nerve conduction abnormality, which is a typical finding of CTS. This technique has been regarded as an objective examination and a test to validate the clinical diagnosis 17–19. EMG is invasive and uncomfortable for most patients. Moreover, in some cases positive findings may be absent in early stages of the disease, because abnormal EMG findings depend upon a substantial alteration in nerve function 20. Thus, it would be helpful if there was a less invasive and complicated means of assessing median nerve pathophysiology, not only to support a clinical diagnosis of CTS, but also as a way of noninvasively assessing the effect of various activities and postures on carpal tunnel pressure. Some clinicians have tried to measure carpal tunnel pressure directly with a percutaneous sensor 21–25. However, this is also invasive. In contrast, there are numerous techniques to examine the morphology of the median nerve non-invasively, to assist in the diagnosis of CTS 26–32. Among them, sonography has gained recognition as a convenient approach because of its noninvasiveness, ease of use in an office setting and requiring less time and effort 33. However, to this point these non-invasive methods have not been used to assess pathophysiology, such as nerve function, or intra-carpal tunnel pressure.
We have developed a novel surface wave elastography technique to measure skin and tissue viscoelasticity. In surface wave elastography, an electromechanical shaker is used to generate a local and short 0.1- second harmonic vibration on the skin. The resulting surface wave propagation on the skin or the shear wave propagation in the subcutaneous tissue is detected using an ultrasound probe. Surface wave elastography has widely been used for noninvasively evaluating the viscoelastic properties of soft tissues 34–37. A previous study showed that the shear wave speed (SWS) propagated in the Achilles tendon wrapped with canine skin increased linearly as pressure increased, suggesting usefulness in assessing compartmental pressures 38. The difference in SWS between tendon inside and outside the tunnel increased linearly with increased pressure. However, this study was done using an ideal model, i.e., a single tendon with a synthetic and homogeneous tunnel. The real carpal tunnel contains nine flexor tendons, median nerve and soft tissues such as subsynovial connective tissue (SSCT), in which the mechanical condition is much more complicated. The tunnel is oval and is surrounded partly by bone and partly by ligament. As the next step of aiming for clinical application, therefore, there is a need to verify surface wave elastography in a more practical condition which is using human cadaver hands to confirm the relationship between SWS and carpal tunnel pressure
This study is a continuation of our previous investigation on the biomechanical properties of tendons in a constricted space 38. We focused on the development of the relationship between carpal tunnel pressure and SWS propagation in the flexor digitorum superficialis of third finger (3rd FDS) inside and outside the carpal tunnel based on human cadaveric model.
Material and Method
This study was conducted with approval of our Institutional Review Board. A total of 10 fresh frozen human cadaveric forearm hands (six men and four women, and three right and seven left hands, from individuals aged 40–80 years) were obtained from the Anatomy Department at Mayo Clinic. Medical records were available for all donors. Exclusion criteria included a history of prior carpal tunnel release, volar wrist surgery, corticosteroid injection into the carpal tunnel or known hand/wrist tumor or deformity. Cadavers were also excluded if they had any of the following clinical diagnoses or conditions: cervical radiculopathy, inflammatory arthritis, osteoarthritis in the wrist, flexor tendinitis, hemodialysis, obesity (body mass index > 30 kg/m2), sarcoidosis, peripheral nerve disease, metabolic disorder, amyloidosis or major trauma to the ipsilateral wrist.
The cadaveric preparation included exposing all flexor tendons and the median nerve 6 cm proximal to the first wrist crease and maintaining an intact carpal tunnel region. A 2-cm incision was made on the palm of A1 pulley level of middle finger, and then A1 pulley was opened to expose flexor digitorum surperficialis (FDS) and flexor digitorum profundus (FDP) tendons. A customized guide pin with a small loop at the distal tip was inserted along the longitudinal axis of the middle finger between the space of FDP and phalanx from the distal insertion to the proximal interface between the interosseous membrane and tendons. Afterward, a string was looped to connect a 12 Fr Foley catheter (Foley Catheter, Silicone Elastomer Coated 30cc, Medline, Mundelein, IL) into which lumen a Kirschner wire with 1.2 mm diameter was inserted to stabilize the catheter balloon position in the carpal tunnel during the experiment. Then, the guide pin was pulled back to guide and secure the balloon part of the catheter at the area of the carpal tunnel beneath the FDP tendons. Next, the external fixator was installed between distal part of radius bone and metacarpal diaphysis of index finger to fix and keep wrist angle in neutral position. After that the specimen was mounted on a custom made apparatus by clamping the proximal ends of the radius and ulna bones, and all the fingers were kept in extension with a Velcro strip. The flexor tendons were exposed proximal to the edge of secured area and 50 gram weights were connected to median nerve and individual tendons except 3rd FDS to apply tension to maintain a physical load during all tests. Weights of 50, 200, 500, 1000 and 1500 gram were hung on the 3rd FDS to create different tendon tension conditions. The proximal balloon part of the Foley catheter was connected to a 10ml syringe containing 10 ml of saline and placed on syringe pump (Fusion100, Chemyx, Stafford, TX) to control saline infusion to inflate the distal end balloon steadily at 0.5 ml/min to create different levels of carpal tunnel pressure (baseline pressure, 10, 20, 30, 60, 90, 120 and 150 mmHg) at each 3rd FDS tendon tension condition. Because balloon pressure may not be equivalent to carpal tunnel pressure, a diagnostic pressure catheter system (Mikro-Cath, Millar Inc., Houston, TX) was used to measure real time carpal tunnel pressure. A 17 gauge surgical biopsy needle was used as a guide pin through which the pressure sensor was inserted into the carpal tunnel from 3 cm proximal to the first wrist crease. The sensor part of the catheter was secured to locate at the ulnar side of the 3rd FDS to detect the created carpal tunnel pressure, which simulated normal and CTS pressure conditions around the median nerve. The pressure sensor and balloon location were confirmed by ultrasound imaging (Fig. 1).
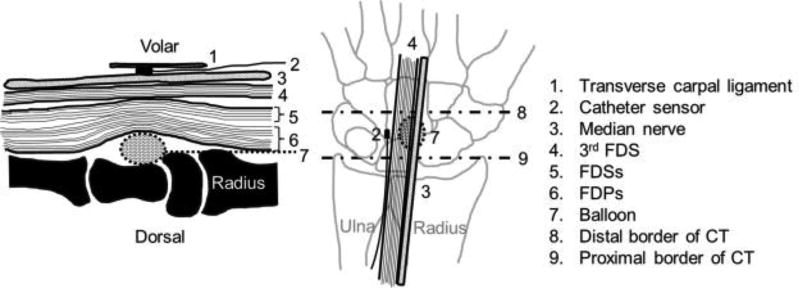
Fig. 1.
Schematic of positional relationship between devices and median nerve and 3rd FDS in sagittal plane (A) and coronal plane (B). The balloon located between carpal bones and FDPs and the sensor located between the transverse carpal ligament and FDSs. Both of them are secured within carpal tunnel.
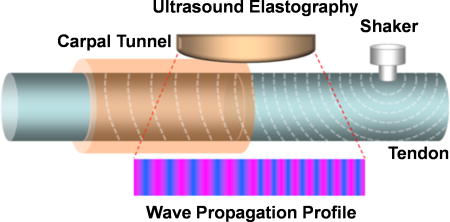
Overall setup of this experiment is illustrated in Fig. 2. The ultrasound probe was securely positioned over the carpal tunnel using a holder attached to the mounting frame and located at the volar aspect of the wrist along the longitudinal midline of the long finger and forearm with half of the contact surface on the intra carpal tunnel area (2 cm distal to the first wrist crease) and the other half on the outer carpal tunnel area (2 cm proximal to the first wrist crease) to detect the wave propagation applied onto 3rd FDS tendon through the skin (Fig. 3). A 0.1 second harmonic vibration at 100 Hz was generated by a function generator (Model: FG33120A, Hewlett Packard, Palo Alto, CA) amplified by an audio amplifier and applied to the skin using a ball-tipped (4 mm in diameter) indenter. The ball-tipped indenter was connected to an electromagnetic shaker which applied the 0.1 second vibration excitation onto the 3rd FDS proximal to the carpal tunnel. An ultrasound system (Verasonics, Inc, Kirkland, WA) with a linear array ultrasound probe L11-4 with central frequency of 6 MHz probe was used for detecting the generated shear wave propagations on the tendon. 8 ultrasound tracking lines inside the tunnel and 8 ultrasound tracking lines outside the tunnel were used to measure the wave motions at the selected 16 locations inside and outside the tendon at each pressure and at each tension level (Fig. 4). The phase of the propagating wave at each line is calculated with a cross-spectrum method. The cross-spectrum S(f) of two signals s1(t) and s2(t) is defined as 39,
where s1(f) and s2(f) are the Fourier transforms of s1(t) and s2(t), respectively; * denotes the complex conjugate and θ(f) is the phase delay between s1(t) and s2(t) at frequency f. Ideally, the phase of the shear wave θ at a particular frequency has a linear relationship with the distance, θ = α · l + β.
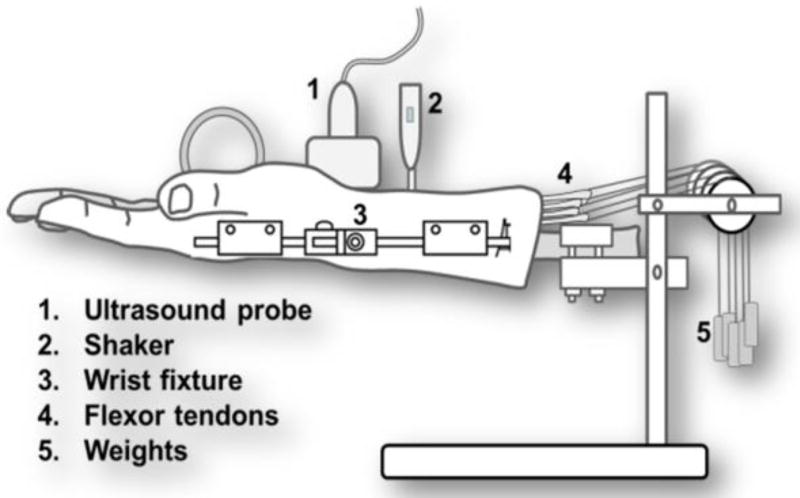
Fig. 2.
Schematic of the experimental setup. Multiple weights were applied to 3rd FDS and 50 gm weight was applied to the other tendons and median nerve.
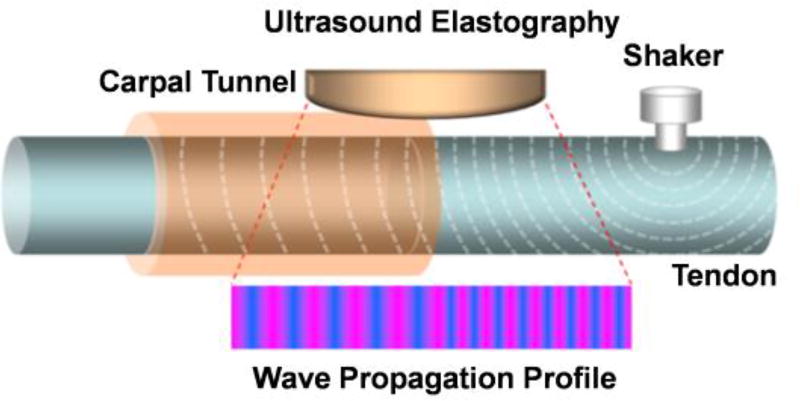
Fig. 3.
Schematic of the surface wave elastography setup. The probe was positioned longitudinally across the inlet of carpal tunnel just on the 3rd FDS through the skin. The shaker of wave generator was positioned a little proximal to the probe.
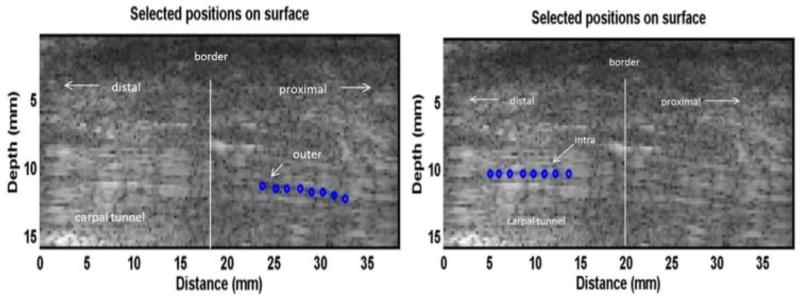
Fig. 4.
A B-mode image of the tendon inside and outside the carpal tunnel in the ex vivo human cadaveric hand testing. 8 locations over a length of 8 mm in the tendon inside the carpal tunnel and 8 locations over a length of 8 mm in the tendon outside the carpal tunnel were selected to measure the tissue motion using ultrasound tracking beams, respectively. The blue dots represent the relative locations of the selected points on the obtained ultrasound B-mode image.
So the shear wave speed can be calculated as,
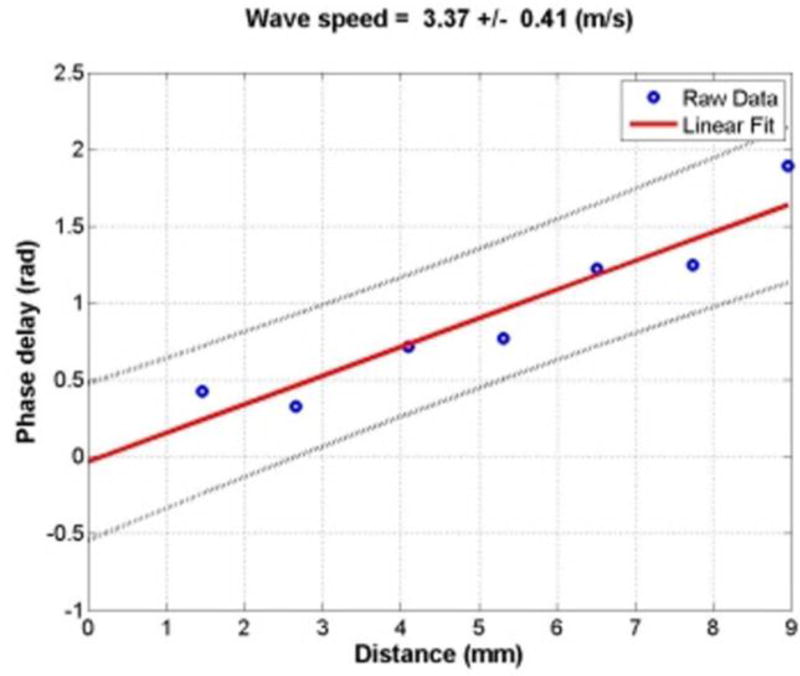
where ν is the wave speed to be determined, f is the excitation frequency in Hz and α is the slope of the linear regression between phase delay and distance 40–42(Fig. 5).
Fig. 5.
The wave phase delay of the remaining locations relative to the first one is used to measure the shear wave speed at 100 Hz. A linear robust regression is used to find the relationship between the two variables.
Measurements were performed and recorded three times at each condition. Mean values and standard deviation were calculated.
Statistical analysis
Mean values of intra carpal tunnel SWS (i-SWS), outer carpal tunnel SWS (o-SWS) and the difference of i-SWS and o-SWS (d-SWS) at multiple tensions and pressures were compared using a two-way ANOVA with critical p value = 0.05. Furthermore, a post hoc Bonferroni test with α = 0.05 was conducted to investigate the effect of tendon tension or carpal tunnel pressure on the SWS, respectively.
Results
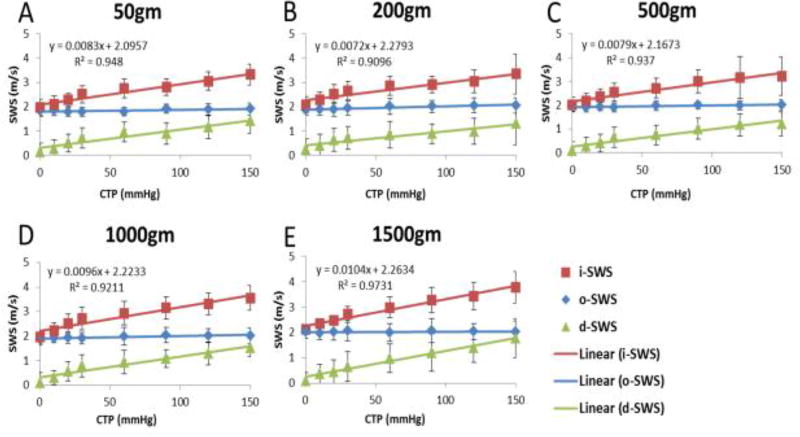
The results of i-SWS, o-SWS and d-SWS under different tendon tension are shown in Figure 6. Square dots indicate i-SWS, diamond dots indicate o-SWS and triangle dots are d-SWS, respectively. Each one dot and error bar indicate the mean value and standard deviation of the measurements of all ten specimens in each condition. From the statistical analysis, the o-SWS remained approximately constant with both tendon tension and carpal tunnel pressure. On the other hand, i-SWS significantly increased with carpal tunnel pressure. (p = .000). The pair-wise comparison for the main effect of carpal tunnel pressure using Bonferroni adjustment suggested significant differences between any two of the pressure conditions (p < .05) However, the i-SWS was not affected by tendon tension (p = .222) and there was no interaction between tendon tension and carpal tunnel pressure, either (p = .207). Linear regression model was applied on the results of i-SWS and the fitted parameters are shown along with the R2 values in Figure 6 which are all above 0.90. Linear relationships between i-SWS and carpal tunnel pressure and also between d-SWS and carpal tunnel pressure were obtained.
Fig. 6.
i-SWS, o-SWS and d-SWS under different carpal tunnel pressure and tendon tension. Linear curve fitting equation and R2 of intra carpal tunnel SWS are shown.
Discussion
The aim of this study was to investigate the relationship between carpal tunnel pressure and SWS of cadaveric hand model, thus developing a non-invasive technique that facilitates prediction of carpal tunnel pressure based on the surface wave elastography. Although electrophysiological examination is useful for the diagnosis of CTS from the viewpoint of documenting the abnormality in nerve physiology, false negative findings are not infrequent 20. Numerous approaches have been tried to measure carpal tunnel pressure in both a clinical and a laboratory setting 5; 13–16; 18; 21; 43–45. So far, all these methods have been invasive to some extent. A noninvasive assessment of carpal tunnel pressure would be ideal to reduce the burden and anxiety of patients, and to allow for both office based assessment and field evaluations. We believe that our innovative method is of importance in achieving this goal and hopefully can be useful in the diagnosis and assessment of risk factors for CTS.
Our results showed that i-SWS increased linearly (R2 > 0.90) with carpal tunnel pressure while o-SWS remained nearly constant with pressure changes. Moreover, the d-SWS linearly increased with carpal tunnel pressure (R2 > 0.88) as well. The above results were similarly observed under all tension loads. Furthermore, significant differences were found in i-SWS and d-SWS between any two of carpal tunnel pressure conditions (p < 0.05). In our previous study using the Achilles tendon, we confirmed that acoustic properties of the tendon are different between the portion of tendon outside the tunnel and the portion that is subjected to the pressure inside the tunnel. This difference in SWS stems from the pressure inside the tunnel. The tendon was compressed from the balloon below and its movement was restricted by FDPs on the dorsal and other soft tissues on medial and lateral sides. These results indicated that carpal tunnel pressure could be indirectly estimated by measuring i-SWS propagating 3rd FDS tendon. The reason why not median nerve itself but 3rd FDS tendon was used to measure SWS was that the tendon is quite uniform alone in the carpal tunnel region and rarely have the pathological changes that may affect wave propagation in addition to the pressure changes. Furthermore, the 3rd FDS tendon is just beneath the skin and easy to detect which provide the possibility to accurately locate a shaker to create the wave on it. On the other hand, median nerve in CTS patients is highly likely to have been denatured and degenerated locally, so the structural property of the flexor tendon is more uniform than median nerve as well as there is fewer possibility of degeneration, so to measure SWS is likely to be more accurate. Furthermore, it is the most superficial tendon and closest to median nerve in the carpal tunnel and it passes there through side by side. The 3rd FDS tendon could thus be served as a ‘pressure gauge’ for evaluation of carpal tunnel pressure by using surface wave elastography to quantify SWS. On the other hand, in this study we did not find an interaction between tendon tension and carpal tunnel pressure, so that we concluded the profile of SWS across different levels of carpal tunnel pressure was not affected by tendon tension ranging from 50g to 1500g. This might result from the anisotropic behavior of tendon, so that when we increased the tension, the load we applied influenced only slightly the mechanical property in the longitudinal direction, while the carpal tunnel pressure increase was more in a transverse direction. Therefore, SWS propagation speed was less sensitive to tendon tension changes. Consequently, in an overall view, the SWS we measured did not have a significant difference across all tendon tension conditions, but did increase with the carpal tunnel pressure.
It has been shown that acoustic properties of material are altered with deformation and pressurization, correspondingly changing the wave propagation speed. Moreover, local speed of wave propagation is directly related to material local stiffness. With tendon gradually being compressed with increasing carpal tunnel pressure, its local stiffness increases and so does the wave propagation speed. We agree the experimental setting is not the same as clinical condition. In this study, the pressure was increased quickly via balloon inflation. However, in the clinical condition, the hydrostatic pressure gradually builds up with a long period combining with complex soft tissue pathological alterations. Although there are different causes for the carpal tunnel pressure elevation, the effect of the pressure on the wave propagation in a tendon should be consistent. Our next step is to conduct an in vivo study for the further validation.
In this study, several measures were implemented in conducting the experiments. Firstly, measurements inside and outside the carpal tunnel were conducted simultaneously, enabling us to characterize the relationship of i-SWS and o-SWS with carpal tunnel pressure and thus deducing the relationship between d-SWS and carpal tunnel pressure. Comparing SWS at two points of single tendon at the same time and condition in different pressure environments made it more reliable and less affected by other factors. Secondly, during the insertion of balloon and thin catheter sensor in the carpal tunnel, unnecessary interference was maximally minimized by placing the balloon and sensor away from median nerve and 3rd FDS. Thirdly, carpal tunnel pressure was controlled by pressurization of the balloon with saline solution and continuously monitored. There may be a difference between using a balloon to increase pressure and in hydrostatic pressure in the carpal tunnel. However, this is an unavoidable limitation in the cadaveric study that cannot fully mimic clinical scenario. In vivo validation is definitely needed to further verify that this technology is accurate and useful for clinical application. Fourthly, a Kirschner wire was inserted through the lumen of the balloon in order to prevent it from sliding out of inlet or outlet of carpal canal when pressurized. Finally, the accurate locations of balloon and sensor as well as the balloon size were visually confirmed prior to and during the experiments via ultrasound imaging.
There were several limitations in this study. First, cadaveric specimens in this study were fresh frozen and were screened to have normal median nerve, flexor tendons and soft tissues including SSCT. However, soft tissues of CTS patients usually manifest pathological change in terms of edema, fibrosis, and hypertrophy 46–49. These pathologically modified soft tissues may alter their structural and mechanical properties and affect shear wave propagation. Under such circumstances, it is of importance to further conduct investigation on the SWS of CTS patients to validate our findings. However, since the SWS comparison is made within a single tendon at the same condition in different pressure environments, it may diminish the pathological effects. Second, the carpal tunnel pressure was manipulated with a balloon, which did not truly mimic the clinical scenario. In addition, the balloon inflation might slightly move the 3rd FDS tendon toward palmar side. However, we did not check if or how much the tendon moved during pressure was increased. Therefore, we don’t know if the tendon migration would affect shear wave propagation although we believe that the pressure change is the major driver for shear wave alteration. Third, we neglected the effect of initial pressure to the skin which might affect the results among different specimens. The ultrasound probe was placed onto the skin and held with a retort stand. An ultrasound gel was applied between the skin and ultrasound probe for better transmission quality. The ultrasound probe was gradually lowered to be in touch with the ultrasound gel to get ultrasound image and we tried our best to make sure the pressure applied on the skin was negligible so as not to affect our measurement of shear wave speed of tendon.
Finally, only one frequency of the tendon vibration (100 Hz) was studied. The SWS may be affected by different vibrating frequencies. However, based on a previous report, a range of 60–100 Hz was appropriate for soft tissues 50.
In this paper, we performed experiments on human cadaveric hands to investigate the relationship between carpal tunnel pressure and i-SWS propagated in a tendon that passes through the carpal tunnel using surface wave elastography. Our results showed a linear relationship between carpal tunnel pressure and i-SWS as well as between carpal tunnel pressure and d-SWS. This shows surface wave elastography is useful to indirectly estimate carpal tunnel pressure by measuring the SWS propagating 3rd FDS tendon and thus, potentially, for CTS early diagnosis and assessment. We verified the feasibility of an innovative and noninvasive method on cadaveric model and plan to validate this technique in both healthy controls and CTS patients in the future.
Acknowledgments
This study was supported by a grant from National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR067421).
Footnotes
[1] substantial contributions to research design, or the acquisition, analysis or interpretation of data: KK; BZ; YSC; BQ; THY; KNA; PCA; SLM; CZ; XZ
[2] drafting the paper or revising it critically: KK; BZ; YSC; KNA; PCA; SLM; CZ; XZ
[3] approval of the submitted and final versions: CZ; XZ
All authors were fully involved in the study and preparation of the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Gelberman RH, Hergenroeder PT, Hargens AR, et al. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63:380–383. [PubMed] [Google Scholar]
- 2.Werner R, Armstrong TJ, Bir C, et al. Intracarpal canal pressures: the role of finger, hand, wrist and forearm position. Clin Biomech (Bristol, Avon) 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 3.Luchetti R, Schoenhuber R, Nathan P. Correlation of segmental carpal tunnel pressures with changes in hand and wrist positions in patients with carpal tunnel syndrome and controls. J Hand Surg Br. 1998;23:598–602. doi: 10.1016/s0266-7681(98)80009-0. [DOI] [PubMed] [Google Scholar]
- 4.Szabo RM, Chidgey LK. Stress carpal tunnel pressures in patients with carpal tunnel syndrome and normal patients. J Hand Surg Am. 1989;14:624–627. doi: 10.1016/0363-5023(89)90178-0. [DOI] [PubMed] [Google Scholar]
- 5.Diao E, Shao F, Liebenberg E, et al. Carpal tunnel pressure alters median nerve function in a dose-dependent manner: a rabbit model for carpal tunnel syndrome. J Orthop Res. 2005;23:218–223. doi: 10.1016/j.orthres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Osamura N, Zhao C, Zobitz ME, et al. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech (Bristol, Avon) 2007;22:999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Ettema AM, Berglund LJ, et al. Gliding resistance of flexor tendon associated with carpal tunnel pressure: a biomechanical cadaver study. J Orthop Res. 2011;29:58–61. doi: 10.1002/jor.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osamura N, Zhao C, Zobitz ME, et al. Permeability of the subsynovial connective tissue in the human carpal tunnel: a cadaver study. Clin Biomech (Bristol, Avon) 2007;22:524–528. doi: 10.1016/j.clinbiomech.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Hargens AR, Romine JS, Sipe JC, et al. Peripheral nerve-conduction block by high muscle-compartment pressure. J Bone Joint Surg Am. 1979;61:192–200. [PubMed] [Google Scholar]
- 10.Zhao C, Ettema AM, Osamura N, et al. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: a biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 11.Rempel DM, Keir PJ, Bach JM. Effect of wrist posture on carpal tunnel pressure while typing. J Orthop Res. 2008;26:1269–1273. doi: 10.1002/jor.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rempel D, Bach JM, Gordon L, et al. Effects of forearm pronation/supination on carpal tunnel pressure. J Hand Surg Am. 1998;23:38–42. doi: 10.1016/S0363-5023(98)80086-5. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Osamura N, Tomita K. Segmental carpal canal pressure in patients with carpal tunnel syndrome. J Hand Surg Am. 2006;31:925–929. doi: 10.1016/j.jhsa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Kim IS, Sung JH, et al. Intraoperative dynamic pressure measurements in carpal tunnel syndrome: Correlations with clinical signs. Clin Neurol Neurosurg. 2016;140:33–37. doi: 10.1016/j.clineuro.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Hamanaka I, Okutsu I, Shimizu K, et al. Evaluation of carpal canal pressure in carpal tunnel syndrome. J Hand Surg Am. 1995;20:848–854. doi: 10.1016/S0363-5023(05)80442-3. [DOI] [PubMed] [Google Scholar]
- 16.Goss BC, Agee JM. Dynamics of intracarpal tunnel pressure in patients with carpal tunnel syndrome. J Hand Surg Am. 2010;35:197–206. doi: 10.1016/j.jhsa.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Rempel D, Evanoff B, Amadio PC, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. American journal of public health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SJ, Lin HS, Hsieh CH. Carpal tunnel pressure is correlated with electrophysiological parameters but not the 3 month surgical outcome. J Clin Neurosci. 2013;20:272–277. doi: 10.1016/j.jocn.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Rempel D, Evanoff B, Amadio PC, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundberg AB. Carpal tunnel decompression in spite of normal electromyography. J Hand Surg Am. 1983;8:348–349. doi: 10.1016/s0363-5023(83)80179-8. [DOI] [PubMed] [Google Scholar]
- 21.Coppieters MW, Schmid AB, Kubler PA, et al. Description, reliability and validity of a novel method to measure carpal tunnel pressure in patients with carpal tunnel syndrome. Man Ther. 2012;17:589–592. doi: 10.1016/j.math.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Marquardt TL, Gabra JN, et al. Pressure-morphology relationship of a released carpal tunnel. J Orthop Res. 2013;31:616–620. doi: 10.1002/jor.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundborg G, Gelberman RH, Minteer-Convery M, et al. Median nerve compression in the carpal tunnel--functional response to experimentally induced controlled pressure. J Hand Surg Am. 1982;7:252–259. doi: 10.1016/s0363-5023(82)80175-5. [DOI] [PubMed] [Google Scholar]
- 24.McGorry RW, Fallentin N, Andersen JH, et al. Effect of grip type, wrist motion, and resistance level on pressures within the carpal tunnel of normal wrists. Journal of Orthopaedic Research. 2014;32:524–530. doi: 10.1002/jor.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner CO, Elmqvist D, Ohlin P. Pressure and nerve lesion in the carpal tunnel. Acta Orthop Scand. 1983;54:312–316. doi: 10.3109/17453678308996576. [DOI] [PubMed] [Google Scholar]
- 26.Wong SM, Griffith JF, Hui AC, et al. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232:93–99. doi: 10.1148/radiol.2321030071. [DOI] [PubMed] [Google Scholar]
- 27.Paliwal PR, Therimadasamy AK, Chan YC, et al. Does measuring the median nerve at the carpal tunnel outlet improve ultrasound CTS diagnosis? J Neurol Sci. 2014;339:47–51. doi: 10.1016/j.jns.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Cartwright MS, Hobson-Webb LD, Boon AJ, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46:287–293. doi: 10.1002/mus.23389. [DOI] [PubMed] [Google Scholar]
- 29.Filius A, Scheltens M, Bosch HG, et al. Multidimensional ultrasound imaging of the wrist: Changes of shape and displacement of the median nerve and tendons in carpal tunnel syndrome. J Orthop Res. 2015;33:1332–1340. doi: 10.1002/jor.22909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altinok T, Baysal O, Karakas HM, et al. Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol. 2004;59:916–925. doi: 10.1016/j.crad.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Chen SF, Lu CH, Huang CR, et al. Ultrasonographic median nerve cross-section areas measured by 8-point "inching test" for idiopathic carpal tunnel syndrome: a correlation of nerve conduction study severity and duration of clinical symptoms. BMC Med Imaging. 2011;11:22. doi: 10.1186/1471-2342-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayrak IK, Bayrak AO, Tilki HE, et al. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007;35:344–348. doi: 10.1002/mus.20698. [DOI] [PubMed] [Google Scholar]
- 33.Pastare D, Therimadasamy AK, Lee E, et al. Sonography versus nerve conduction studies in patients referred with a clinical diagnosis of carpal tunnel syndrome. Journal of clinical ultrasound : JCU. 2009;37:389–393. doi: 10.1002/jcu.20601. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Kinnick R, Greenleaf JF. Viscoelasticity of lung tissue with surface wave method. 2008 IEEE Ultrasonics Symposium: IEEE. 2008:21–23. [Google Scholar]
- 35.Zhang X, Osborn T, Kalra S. A noninvasive ultrasound elastography technique for measuring surface waves on the lung. Ultrasonics. 2016;71:183–188. doi: 10.1016/j.ultras.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Qiang B, Hubmayr RD, et al. Preliminary study of human lung elasticity with the noninvasive surface wave technique. 2010 IEEE International Ultrasonics Symposium: IEEE. 2010:1153–1155. [Google Scholar]
- 37.Zhang X, Qiang B, Urban MW, et al. Quantitative surface wave method for measuring local viscoelasticity of lungs. 2009 IEEE International Ultrasonics Symposium: IEEE. 2009:479–482. [Google Scholar]
- 38.Wang Y, Qiang B, Zhang X, et al. A non-invasive technique for estimating carpal tunnel pressure by measuring shear wave speed in tendon: a feasibility study. Journal of biomechanics. 2012;45:2927–2930. doi: 10.1016/j.jbiomech.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Zheng Y, Mak AF. Estimating the effective Young's modulus of soft tissues from indentation tests—nonlinear finite element analysis of effects of friction and large deformation. Medical engineering & physics. 1997;19:512–517. doi: 10.1016/s1350-4533(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhou B, Sit AJ, Zhang X. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics. 2017 doi: 10.1016/j.ultras.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Osborn T, Zhou B, et al. Lung ultrasound surface wave elastography: a pilot clinical study. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2017 doi: 10.1109/TUFFC.2017.2707981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalra S, Osborn T, Bartholmai B, et al. LUNG ULTRASOUND SURFACE WAVE ELASTOGRAPHY-PRELIMINARY MEASUREMENTS IN PATIENTS WITH INTERSTITIAL LUNG DISEASES. RESPIROLOGY: WILEY 111 RIVER ST, HOBOKEN 07030-5774, NJ USA. 2017:90–90. [Google Scholar]
- 43.Okutsu I, Ninomiya S, Hamanaka I, et al. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg Am. 1989;71:679–683. [PubMed] [Google Scholar]
- 44.Hashizume H, Nanba Y, Shigeyama Y, et al. Endoscopic carpal tunnel pressure measurement: a reliable technique for complete release. Acta Med Okayama. 1997;51:105–110. doi: 10.18926/AMO/30783. [DOI] [PubMed] [Google Scholar]
- 45.Rempel D, Manojlovic R, Levinsohn DG, et al. The effect of wearing a flexible wrist splint on carpal tunnel pressure during repetitive hand activity. J Hand Surg Am. 1994;19:106–110. doi: 10.1016/0363-5023(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs PC, Nathan PA, Myers LD. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16:753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 47.Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998;23:1015–1024. doi: 10.1016/s0363-5023(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 48.Ettema AM, Amadio PC, Zhao C, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118:1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 49.Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg Br. 1987;12:229–232. doi: 10.1016/0266-7681_87_90020-9. [DOI] [PubMed] [Google Scholar]
- 50.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE transactions on ultrasonics, ferroelectrics, and frequency control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]