Abstract
Background
Adolescent females have a higher prevalence of depression in comparison to their male peers - a disparity that has been increasing over the past decade. Depression is of concern as it is associated with chronic disease and to immune dysregulation, which may be one mechanism linking depression to future pathology. This study examined the extent to which sex moderated the association between depressive symptoms and immune dysregulation during adolescence using Epstein-Barr virus (EBV) reactivation, a biomarker of cellular immune response, as a model.
Methods
A representative community sample of 259 female and 279 male adolescents aged 11 to 17 years who were EBV IgG positive were examined. Trained interviewers collected the data during two home visits, one week apart. Depressive symptoms were measured at the first visit using the 9 item short-form of the Center for Epidemiologic Studies-Depression scale. EBV biomarkers were collected via saliva at the second visit and included a qualitative measure of EBV viral capsid antigen immunoglobulin G to assess prior EBV infection and a quantitative measure of EBV DNA to assess the number of viral copies shed in the saliva.
Results
In multivariable logistic regression analyses, increasing depressive symptoms were significantly associated with salivary shedding of EBV DNA for adolescent females only (logit=0.66, se=0.30, p<0.05), and the interaction between sex and depressive symptoms on salivary shedding of EBV DNA was statistically significant (logit= −1.19, se=0.42, p<0.01). Sensitivity analyses were conducted in which sex was examined as a moderator in the relationship between depressive symptoms and salivary EBV DNA quantitative copies via Tobit regression; results were consistent with the presented findings.
Conclusions
Depressive symptoms are associated with EBV reactivation among EBV positive female adolescents, but not males. Future research is needed to examine EBV reactivation in female adolescents as a mechanism linking depression to future chronic disease and the role of sex hormones in explaining sex differences in the relationship between depressive symptoms and EBV reactivation.
1.0 Introduction
The prevalence of depression among U.S. adolescents has increased from 8.7% to 11.3% over the past decade.(Mojtabai et al., 2016) Adolescent females have been disproportionately affected in both the overall prevalence and the increasing trend with 17.3% of adolescent females reporting a major depressive episode in the year prior to 2014 (up from 13.1% in 2005) in comparison to 5.7% of adolescent males (up from 4.5% in 2005).(Mojtabai et al., 2016) The increasing rate of depression is of significant concern as suicide rates among adolescents have also increased over time, particularly among female adolescents aged 10 to 14 years in which rates have increased 200% since 1999.(Curtin et al., 2016) In addition to suicide, adolescent depression is associated prospectively with obesity,(Mannan et al., 2016; Zhu et al., 2016) substance use,(Hussong et al., 2017) accelerated atherosclerosis, and early cardiovascular disease.(Goldstein et al., 2015)
A growing body of research has found that depression,(Haeri et al., 2011; Rector et al., 2014; Zhu et al., 2013) consistent with other forms of psychosocial distress (e.g. attachment anxiety,(Fagundes et al., 2014) psychosocial stress,(Glaser et al., 1985; Glaser et al., 1994) and exposure to adverse life events(McDade et al., 2000)), is also be associated with reactivation of latent herpes viruses, including the Epstein-Barr virus (EBV) – a latent virus linked to certain autoimmune disorders,(Niller et al., 2008) cancer(Li et al., 2016) and athersclerosis.(Espinola-Klein et al., 2002) In the U.S., primary infection with EBV is nearly ubiquitous by young adulthood, and although the virus is most well- known for causing mononucleosis during adolescence, the majority of the population experience primary infection earlier in childhood with few to no symptoms.(Christian et al., 2009; Ford and Stowe, 2013; Glaser et al., 1991) The DNA of the virus remains latent in the B-lymphocytes after the primary infection, kept in balance through the action of cytotoxic T-cells in individuals with competent immune systems. However, physical or psychosocial stress can decrease leukocyte production and functioning enabling reactivation of the herpes virus with increased replication of the viral DNA and antiviral antibodies.(Ford and Stowe, 2013; Stowe et al., 2010) Thus, EBV reactivation may be one pathway through which depression contributes to future health outcomes.
However, despite the higher prevalence of depression reported in females than males,(Mojtabai et al., 2016) research examining the potential for sex differences in the effect of depression on latent herpes virus reactivation has been limited as prior studies have focused primarily on pregnant women.(Christian et al., 2012; Haeri et al., 2011; Zhu et al., 2013) or they controlled for sex in analyses.(Rector et al., 2014) Evidence from animal and human models that sex differences in the stress response exist is strong,(Bale and Epperson, 2015; Bekhbat and Neigh, 2017) and that it may vary across developmental stage with females more susceptible to stress and stress-associated affective disorders during peripubertal and pubertal development than males.(Bale and Epperson, 2015) For example, in one pilot study, exposure to a greater number of adverse life events was significantly associated with higher EBV IgG antibody levels in EBV seropositive female youth aged 9 to 13 years, but not in male youth.(McDade et al., 2000) Similarly, in an investigation of youth aged 7 to 13 years, higher levels of depressive symptoms were associated with increased natural killer cell cytotoxicity, but only among the stratified sample of older female youth with no significant associations found for younger females or for male youth regardless of age.(Caserta et al., 2011) Thus, to advance our understanding of potential sex differences in the linkages between depression and EBV reactivation we build on the prior research through an examination of the relationships between depressive symptoms and salivary shedding of EBV DNA among a representative community sample of EBV IgG positive adolescent females and males.
2.0 Methods
2.1 Study Design
The current study examines data from two linked studies: (1) The Adolescent Health and Development in Context study – a two wave representative study on the impact of activity space exposures on the health and well-being of urban and suburban youth aged 11–17 years and (2) the Linking Biological and Social Pathways to Adolescent Health and Wellbeing study, which added the collection of immune function biomarkers (saliva for EBV VCA IgG and viral DNA) on a representative subsample of youth participating in the first wave of the AHDC study (N=674).
The study takes place in Franklin County, OH – a large, metropolitan area that is representative of the average U.S. metropolitan area in terms of social and economic characteristics. The study area incorporates Columbus and the suburban municipalities that border, or are contained within, the boundaries of Columbus for comparisons between youth residing in low income urban neighborhoods and those residing in the highest income suburban locations. The sampling procedures for the study have been previously described elsewhere.(Ford et al., 2016) Briefly, households were mailed a flyer describing the study with instructions to call if they were interested in participating. Trained interviewers also called to determine interest and eligibility (youth aged 11–17 years, 1 primary caregiver and English speaking). If the household had more than one youth eligible for the study, one focal youth was randomly selected for participation in the AHDC study. Youth were then eligible for the biomarker collection if they had not taken corticosteroids in the previous month. Both studies were approved by the university institutional review board and parental consent and youth assent were obtained prior to data collection.
As the main objective of this study was to examine the potential for EBV reactivation through salivary EBV DNA shedding, the sample for this analysis included only those youth who were EBV VCA IgG positive (had evidence of past or primary EBV infection) determined through an adapted ELISA method using saliva.(Stowe et al., 2014) To date, the measurement of EBV VCA IgG has been most commonly assessed using serum, but researchers have successfully employed salivary ELISA methods in a large sample of children.(Crowcroft et al., 1998) Furthermore, our pilot study (N=50 young adults, unpublished) found a 0.92 correlation coefficient between serum and salivary EBV IgG antibodies in which an antibody titer greater than 0.02 was suggestive of prior EBV infection. Thus, youth in this study who had a salivary EBV VCA IgG antibody level greater than 0.02 were considered EBV positive (N=538 or 81.0% of the youth who had saliva collected for EBV).
2.2 Data Collection
All data were collected by trained interviewers over a weeklong period. A face-to-face interview and self-administered survey with both the focal youth and his or her primary caregiver were conducted at the beginning of the week during which items specific to mental and physical health and health behaviors were queried via the self-administered portion of the survey. A seven day smartphone-based Global Positioning System tracking and Ecological Momentary Assessment collection followed the entrance survey in which real-time data were collected on the youth’s physical and social environmental exposures and health behaviors. At the end of the week, the interviewer returned to the home for a final face-to-face interview with the youth and an additional self-administered survey for the parent that asked about their perceptions of their physical and social environments. The interviewer also collected the saliva for immune function biomarkers at this second visit via passive drool. Specimens were then transported from the home to the survey research center where they were stored at −20C and then transferred on dry ice to the −80C freezers at the university laboratory until assay.
2.3 Measures
2.31 Dependent Variable
Salivary shedding of EBV DNA is a dichotomous measure created to compare youth who had 10 or more copies (the lower bound detectable level) of EBV DNA in their saliva (yes=1) to those youth who were below the detectable level. A quantitative measure of the logged EBV DNA copy number was also created and examined in sensitivity analyses. Assessment of the EBV DNA viral load was accomplished using PCR methodology as performed in numerous studies from Stowe’s laboratory.(Mehta et al., 2013; Stowe et al., 2007) DNA was isolated from saliva using the QiaAmp blood kit (Qiagen, Valencia, CA). EBV copy numbers were measured in samples using real-time PCR using PCR primers that amplify a portion of the BALF5 gene. Real-time fluorescence measurements were taken over 40 cycles using an Mx3005P real-time PCR instrument, and unknowns were compared to a standard curve (serially-diluted plasmids containing single copy viral genes). Copy numbers were then calculated automatically using the Strategene software.
2.32 Primary Independent Variables
Depressive symptoms is the primary independent variable of interest, which was measured with a short form of the original 20 item Center for Epidemiologic Studies-Depression scale (CES-D) (Cole et al., 2004) in an effort to reduce respondent burden. The 10 item CES-D short form scale was developed and validated by Cole and colleagues through Rasch modeling and confirmatory factor analysis. In the 10 item scale, youth were queried about depressive symptoms that occurred during the prior week (e.g. bothered by things, could not shake of the blues, my life has been a failure, hopeful for the future, trouble keeping mind on task, lonely, people unfriendly, afraid, everything an effort, and felt as good as others) with 4 response options ranging from “rarely/none of the time/1 day” to “most/all of the time/5–7 days”. The positive affect items (hopeful for the future and felt as good as others) were reverse coded and higher scores indicate increasing depressive symptoms. The 10 item scale does incorporate items from each of the 4 original CES-D subscales (negative affect, positive affect, somatic symptoms and interpersonal relations), however, Cole et al. found in their analysis that the total score (one factor) was a better model fit than the 4 separate subscales (4 factors) for their 10 item short form as well as the original 20 item CES-D. Thus, they concluded that the use of the total score is appropriate in studies investigating depressive symptoms.
We conducted exploratory factors analysis (EFA) with this study to examine the latent structure of the 10 item scale with our data, specifically to determine if the data from the parent study supported the use of a single scale measuring a total score of depressive symptoms or if the use of the 4 sub-scales was more appropriate. Our EFA results (based on scree plot, Eigen value and factor loadings) as well as the Cronbach alpha scores (total score α=0.71, negative affect subscale α=0.64, positive affect subscale α=0.50, somatic symptoms subscale α=0.36, interpersonal relations subscale α=0.51) supported the use of a total score only. In addition, the item, “I felt everything I did was an effort” was found to have a negative and low correlation with all other items in the EFA and correlation analyses, thus the item was removed for a 9 item total scale (α=0.74 for pooled total sample; α=0.76 for the stratified female sample; α=0.74 for the stratified male sample). The scores to the items were summed and averaged for the participants who had complete data on all 9 items; those missing a response to any item were set to missing on the scale.
Sex was based on youth self-identification as male or female and coded 1 for male and 0 for female
2.33 Covariate Measures
Additional measures based on prior research and theory that could influence immune function and potentially confound the relationships between depression and EBV were included in the analyses. The measures were based on youth or caregiver self-report and included the following: age (11–17 years); race/ethnicity (three categories were developed: (1) non-Hispanic black; (2) non-Hispanic white – reference; and, (3) “other” – due to small, but geographically representative sample sizes, youth who self-reported their race/ethnicity as “other”, Hispanic or Asian were collapsed into one group and categorized as ‘other’; caregiver married or cohabitating (married/cohabiting vs single/divorced/widowed – reference); caregiver level of education (less than high school or high school degree, some college, bachelor’s degree, and master’s degree or higher – reference); household size; in school during data collection (yes=1); exposure to household tobacco smoke (yes=1); anti-depressant medication use (yes=1); body mass index (BMI) z-score (the modified z-score measure of BMI adjusted for age and sex were calculated according to the Centers for Disease Control and Prevention child and adolescent BMI guidelines(Kuczmarski et al., 2002)); and puberty (youth self-reported sex-specific scales adapted from Petersen et al.(Petersen et al., 1988) in which youth were asked to rate their change in pubertal development. Response options included no development, development barely begun, development definitely underway or development complete with scores ranging from 1 to 4 and higher scores indicating more advanced pubertal development. Both males and females were asked to rate their development on the following: growth spurt in height, growth of pubic hair, and skin changes (pimples/acne). Males were asked to rate changes in voice and growth of facial hair whereas females were asked to rate breast growth and if they had ever menstruated, which included a yes or no response option with yes=4 and no=1. The scores to the items were summed and averaged for the participants who had complete data on all 5 items; those missing a response to any item were set to missing on the scale).
2.4 Analytic Strategy
Missing data of the measures were examined and the highest proportion of missing data was found for the depression and puberty scales (many of the “missing” puberty responses were due to unknown responses). Specifically, 80% of the total sample had complete data on all 9 items of the depression scale; the 20% who were missing data on 1 item or more was evenly distributed by sex. For the puberty measure, approximately 78% of the total sample had complete data on the 5 item scale with females having slightly more missing data (25%) than males (20%). Analysis of the missing data found that those missing were less likely to report depressive symptoms and more likely to report advanced pubertal development in comparison to those without any missing data. Thus to minimize bias, multiple imputation of the depression and puberty scales as well as the other covariates comprised of single items was conducted for the multivariable analyses using 20 sets of imputation. In addition, several statistical approaches to address the missing data were conducted as sensitivity analyses. First, we conducted a complete case analysis (N=334). Second, we created depression and puberty scales for participants who had responded to at least 50% of the items in each of the scales and imputed data only for those participants missing responses to more than 50% of the items. Third, because puberty has 2 sex specific items out of the 5 total, we examined stratified analyses by sex so that we could impute individual items versus the total scale. The findings of these 3 approaches yielded similar results to the multiple imputation approach presented in Table 2. (Results available upon request).
Table 2.
Multivariable logistic regression analysis on the associations between depressive symptoms and salivary shedding of EBV DNA and interaction by sex in EBV VCA IgG positive adolescents, N=538
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Logit | (se) | Logit | (se) | |
|
| ||||
| Intercept | 0.66 | (0.15)*** | 0.62 | (0.15)*** |
| Male | 0.02 | (0.22) | 0.03 | (0.23) |
| Age | −0.15 | (0.08) | −0.16 | (0.08) |
| Race/ethnicity | ||||
| Black/African American | 0.58 | (0.24)* | 0.59 | (0.24)* |
| White (reference) | ||||
| “other” | 0.33 | (0.35) | 0.31 | (0.36) |
| Primary caregiver level of education | ||||
| High school degree or less | −0.30 | (0.33) | −0.34 | (0.34) |
| Some college | −0.42 | (0.29) | −0.41 | (0.30) |
| Bachelor degree | −0.20 | (0.28) | −0.22 | (0.28) |
| Master degree or more (reference) | ||||
| Primary caregiver married or cohabitating | −0.07 | (0.23) | −0.11 | (0.24) |
| Household size | 0.22 | (0.07)** | 0.25 | (0.07)*** |
| In school during data collection | 0.06 | (0.21) | 0.09 | (0.22) |
| Exposed to household smoke | 0.27 | (0.25) | 0.19 | (0.26) |
| BMI z-score | −0.08 | (0.07) | −0.07 | (0.07) |
| Puberty score | 0.13 | (0.23) | 0.17 | (0.25) |
| Antidepressant medication use | 0.25 | (0.42) | 0.19 | (0.42) |
| Depressive symptoms | 0.07 | (0.21) | 0.66 | (0.30)* |
| Interaction: male* depressive symptoms | −1.19 | (0.42)** | ||
p<0.001;
p<0.01;
p<0.05
(se)=standard error
All variables are mean centered except for the binary dependent variable and moderator
In addition to the missing data analyses, descriptive analyses were conducted to understand the characteristics of the sample, including bivariate analyses to examine potential sex differences in the sample characteristics. Multivariable analyses were then conducted with multiple imputation as described previously using Proc MI in SAS 9.4 (Cary, NC). Two models were analyzed in which the dichotomous measure – salivary shedding of EBV DNA was regressed on the level of depressive symptoms in model 1 and then an interaction term was added to the equation to examine the extent to which the association between depressive symptoms and salivary shedding of EBV DNA was moderated by sex (Model 2). All variables except the binary dependent variable and the binary moderator were mean centered for the multivariable analyses. Sensitivity analyses were also conducted in which the relationship between depressive symptoms and the logged EBV DNA measure was examined via Tobit regression analyses. The findings were consistent with the logistic regression results (available upon request).
3.0 Results
Table 1 presents the findings of the descriptive statistics for the male and female sample. The prevalence of salivary shedding of EBV DNA was approximately 65% for both the female and male samples, along with a similar number of viral copies shed in the saliva (female logged mean=5.89 vs male logged mean=5.61; not shown in the table); no statistically significant differences in the EBV measures between the two samples were found. The mean depressive symptom score was relatively low for both samples (female mean=0.78, male mean=0.62, range 0–3), however, depressive symptoms were significantly higher for females in comparison to males (p=0.001). The findings for the covariates were consistent between the two samples with respect to race and ethnicity, age, caregiver marital and educational status, timing of data collection occurring during the school year, household size, exposure to household smoking, and BMI. However, the use of prescription antidepressants was statistically higher among females than males (8.3% vs 4.0%, p=0.04) as was self-reported pubertal development (mean 3.27 vs 2.75, p=<0.001).
Table 1.
Descriptive characteristics and bivariate sex differences between the female and male samples
| Female Sample (N=259) | Male Sample (N=279) | ||||||
|---|---|---|---|---|---|---|---|
| Dependent Variable | N | n | % | N | n | % | p |
| Salivary EBV DNA shedding | 259 | 279 | |||||
| Yes | 170 | 65.6 | 180 | 64.5 | NS | ||
| No | 89 | 34.4 | 99 | 35.5 | |||
| Primary Independent Variable | N | Mean | (sd) | N | Mean | (sd) | |
| Depressive symptoms (range 0–3) | 214 | 0.78 | (0.53) | 222 | 0.62 | (0.48) | 0.001 |
| Covariates | N | n | % | N | n | % | |
| Race/ethnicity | 259 | 279 | NS | ||||
| Black/African American | 108 | 41.7 | 117 | 41.9 | |||
| White | 133 | 51.3 | 136 | 48.8 | |||
| “other” | 18 | 7.0 | 26 | 9.3 | |||
| Primary caregiver level of education | 254 | 277 | NS | ||||
| High school degree or less | 46 | 18.1 | 57 | 20.6 | |||
| Some college | 90 | 35.4 | 92 | 33.2 | |||
| Bachelor degree | 67 | 26.4 | 70 | 25.3 | |||
| Master degree or more | 51 | 20.1 | 58 | 20.9 | |||
| Primary caregiver married or cohabitating | 252 | 273 | NS | ||||
| Yes | 174 | 69.1 | 179 | 65.6 | |||
| No | 78 | 30.9 | 94 | 34.4 | |||
| Exposure to household smoking | 253 | 276 | NS | ||||
| Yes | 59 | 23.3 | 69 | 25.0 | |||
| No | 194 | 76.7 | 207 | 75.0 | |||
| In school during data collection | 259 | 279 | NS | ||||
| Yes | 199 | 76.8 | 199 | 71.3 | |||
| No | 60 | 23.2 | 80 | 28.7 | |||
| Antidepressant medication | 254 | 273 | 0.03 | ||||
| Yes | 21 | 8.3 | 11 | 4.0 | |||
| No | 233 | 91.7 | 262 | 96.0 | |||
| N | Mean | (sd) | N | Mean | (sd) | ||
| Age (range 11–17) | 259 | 14.4 | (1.89) | 279 | 14.4 | (1.77) | NS |
| Household size (range 2–14) | 259 | 4.65 | (1.47) | 279 | 4.66 | (1.68) | NS |
| Puberty score (range 1–4) | 195 | 3.27 | (0.64) | 225 | 2.75 | (0.67) | <0.001 |
| Modified BMI Z-score (range −2.71–7.98) | 247 | 0.48 | (1.23) | 268 | 0.62 | (1.51) | NS |
Bivariate sex differences assessed with available sample size (no multiple imputation)
SD=standard deviation
NS=not significant (p>0.05)
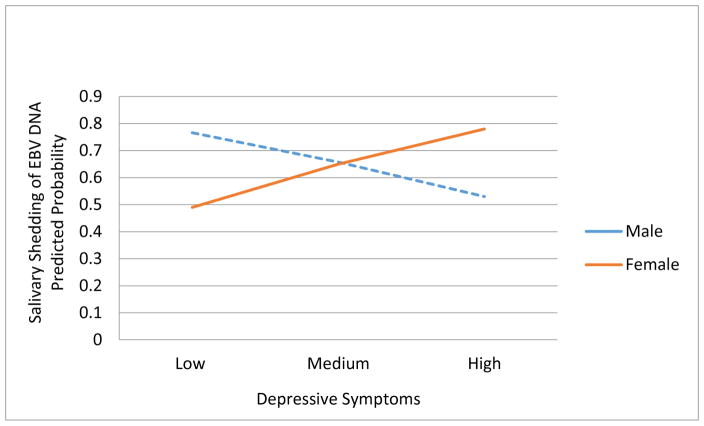
The findings of the multivariable logistic regression analyses, adjusted for the covariates, are depicted in Table 2. Specifically, in model 1, increasing depressive symptoms was not significantly associated with salivary shedding of EBV DNA (logit=0.07, p>0.05) in this sample of EBV positive adolescents. However, with the addition of the interaction term into the regression equation, increasing depressive symptoms was significantly associated with salivary shedding of EBV DNA for adolescent females only (logit=0.66, p<0.05), and the difference in the effect of depressive symptoms on salivary shedding of EBV DNA by sex was significant (logit=−1.19, p< 0.01); please see Figure 1 for the graphical representation of the findings. With respect to significant findings for covariates, racial disparities in salivary shedding of EBV DNA were found as non-Hispanic black/African American EBV positive youth were more likely to shed EBV DNA in their saliva than their non-Hispanic white counterparts (logit=0.59, p<0.05). In addition, EBV positive adolescents living in larger households had an increased odds of salivary shedding of EBV DNA than their peers from smaller households (logit=0.25, p<0.001).
Figure 1.
Moderating Effect of Sex on the Association between Depressive Symptoms and Salivary Shedding of EBV DNA
4.0 Discussion
Our study found significant sex differences in the association between depressive symptoms and salivary shedding of EBV DNA. Specifically, EBV positive adolescent females who reported higher levels of depressive symptoms in the week prior to the first visit in the data collection were significantly more likely to orally shed EBV DNA one week later in comparison to their female peers with lower levels of depressive symptoms. However, no significant association between depressive symptoms and salivary shedding of EBV DNA was found for the male adolescents and the difference in the effect of depressive symptoms on salivary shedding of EBV DNA by sex was significant. Our results are consistent with the aforementioned research in that significant associations between other forms of adversity (e.g. adverse life events) and EBV reactivation(McDade et al., 2000) or depressive symptoms and other immune function biomarkers (e.g. natural killer cell function)(Caserta et al., 2011) were found for female, but not male youth. Together, the findings highlight the need to investigate both the potential for, and the explanation underlying sex differences in immune response to depressive symptoms, particularly during peripubertal and pubertal development.
To date, explanations for the observed sex difference in physiologic stress responses during adolescence have centered on the areas of the brain (e.g. hippocampus, amygdala, prefrontal cortex) responsible for emotion regulation and stress responsivity. These areas of the brain undergo structural and functional re-organization during the pubertal period and are highly sensitive to the stress hormone, cortisol, as well as to the sex hormones – interacting systems that play a role in the development of the stress response system.(Bale and Epperson, 2015; Eiland and Romeo, 2013; Romeo, 2010; Romeo and McEwen, 2006) For example, the increasing level of testosterone during puberty is linked to blunting of the cortisol stress response among males while the variations in estrogen and progesterone over the monthly menstrual cycle can also influence the stress response in females differentially depending on the timing of the cycle.(Bale and Epperson, 2015) In addition, estrogen is known to trigger B-cell mediated diseases and may play a role in EBV reactivation as the virus infects and remains latent in B-lymphocytes unless reactivation occurs with physical or psychosocial stress.(Christian et al., 2009; Ford and Stowe, 2013; Glaser et al., 1991; Stowe et al., 2010) Because depression(Andersson et al., 2015; Euesden et al., 2017) and EBV(Niller et al., 2008) are both linked to certain autoimmune disorders (e.g. systemic lupus erythematosus) known to occur more frequently in females,(Brandt et al., 2015; Dunn et al., 2015; Ngo et al., 2014) further research testing causal pathways and the role of sex hormones in EBV reactivation and immune irregularities among adolescents is warranted.
Significant associations between several of the covariates and salivary shedding of EBV DNA were also found in our study. Specifically, black/African American adolescents were significantly more likely than their non-Hispanic white peers to shed EBV DNA in their saliva. These findings are in accordance with prior research, (Dowd et al., 2014; Ford and Stowe, 2013) but explanations for the disparity are poorly understood. For example, in the Dowd et al. (2014) study, neither perceived stress nor the number of adverse life events explained the black-white disparity in EBV IgG antibody levels among EBV seropositive young adults. Further examination of the mechanisms underlying the racial disparity in EBV reactivation is needed, including the potential effects of racism on immune function. In addition to the racial disparity, adolescents in our study who lived in larger households had an increased odds of salivary shedding of EBV DNA. Although these findings are in contrast with our previous research examining EBV reactivation in a national sample of adolescents,(Ford and Stowe, 2013) the range for the household size in the current study was much wider (2 to 14 persons) than the range in our earlier research (2 to 7). Others also using salivary IgG methodology found that sharing a room was associated with higher IgG antibody levels.(Crowcroft et al., 1998) Because household crowding can increase exposure to infectious pathogens as well as contribute to psychosocial(Regoeczi, 2008; Riva et al., 2014a) and physiologic stress (e.g. allostatic load, cardiovascular reactivity), (Johnston-Brooks et al., 1998; Riva et al., 2014b) further research on the effect and mechanisms of household crowding on the immune system is warranted.
Several limitations to this study warrant further discussion. First, prior research has found that immune dysregulation, including inflammation (Kim et al., 2014; Valkanova et al., 2013) and reactivation of the latent herpes virus – cytomegalovirus (Simanek et al., 2014) predicts depression. Our study was designed to collect the biomarkers at the end of the week long data collection period, thus longer-term and reciprocal effects could not be assessed. However, future research with repeated measures is needed to better understand the reciprocal relationships. Second, although EBV DNA levels are routinely assessed in saliva, EBV VCA IgG antibody levels have been primarily assayed in blood. The results of our pilot study indicate a high correlation between the serum and salivary qualitative EBV VCA IgG antibody measure (r=0.92), but further advances in salivary methodologies will be useful to enhance participation and cost efficiency particularly in community-based studies that include children and adolescents.
Despite these limitations, the study is among the first to examine sex differences in the relationship between depressive symptoms and EBV reactivation during adolescence. The findings highlight the need for researchers to examine differences in the immune response to psychosocial stress and depression between males and females and potential explanations for sex disparities. Furthermore, longitudinal research is needed as EBV reactivation may have utility as a predictive biomarker for females of worsening depressive symptoms, suicide or in the development of future chronic disease.
Highlights.
Increasing depressive symptoms was associated with an increased likelihood of salivary shedding of EBV DNA in EBV VCA IgG positive adolescent females, but not males.
Black/African American adolescents who were EBV VCA IgG positive were more likely than their non-Hispanic white counterparts to orally shed EBV DNA.
Household crowding was associated with an increased likelihood of salivary shedding of EBV DNA in EBV VCA IgG positive adolescent females and males.
Acknowledgments
This study was funded by National Institutes of Health, National Institute on Drug Abuse (Ford, 1R21DA034960 and Browning, 1R01DA032371) and National Institute of Child Health and Development (Casterline, 2P2CHD058484).
Footnotes
Conflict of Interest
Drs. Jodi Ford and Raymond Stowe have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jodi L Ford, The Ohio State University College of Nursing, 1585 Neil Ave., Columbus, OH 43210.
Raymond P. Stowe, Senior Scientist, Microgen Laboratories, 903 Texas Avenue, La Marque, TX 77568.
References
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jorgensen P, Goodwin RD. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med. 2015;45:3559–3569. doi: 10.1017/S0033291715001488. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nature neuroscience. 2015;18:1413–1420. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN. Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun. 2017 doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt JE, Priori R, Valesini G, Fairweather D. Sex differences in Sjogren’s syndrome: a comprehensive review of immune mechanisms. Biology of Sex Differences. 2015;6:19. doi: 10.1186/s13293-015-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Wyman PA, Wang H, Moynihan J, O’Connor TG. Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Dev Psychopathol. 2011;23:1139–1147. doi: 10.1017/S0954579411000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Deichert NT, Gouin JP, Graham JE, Kiecolt-Glaser JK. Psychological influences on neuroendocrine and immune outcomes. In: Cacioppo JT, Berntson GG, editors. Handbook of Neuroscience for the Behavioral Sciences. John Wiley and Sons; Hoboken, NJ: 2009. pp. 1260–1279. [Google Scholar]
- Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun. 2012;26:1280–1287. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Rabin AS, Smith TL, Kaufman AS. Development and validation of a Rasch- derived CES-D short form. Psychological Assessment. 2004;16:360–372. doi: 10.1037/1040-3590.16.4.360. [DOI] [PubMed] [Google Scholar]
- Crowcroft NS, Vyse A, Brown DW, Strachan DP. Epidemiology of Epstein-Barr virus infection in pre-adolescent children: application of a new salivary method in Edinburgh, Scotland. J Epidemiol Community Health. 1998;52:101–104. doi: 10.1136/jech.52.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin SC, Warner M, Hedegaard H. Statistics, N.C.f.H, editor. Increase in Suicide in the United States, 1999–2014. 2016. [PubMed] [Google Scholar]
- Dowd JB, Palermo T, Chyu L, Adam E, McDade TW. Race/ethnic and socioeconomic differences in stress and immune function in The National Longitudinal Study of Adolescent Health. Soc Sci Med. 2014;115:49–55. doi: 10.1016/j.socscimed.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Gunde E, Lee H. Sex-based differences in multiple cclerosis (MS): Part II: Rising incidence of multiple sclerosis in women and the vulnerability of men to progression of this disease. Current Topics in Behavioral Neurosciences. 2015;26:57–86. doi: 10.1007/7854_2015_370. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke; a journal of cerebral circulation. 2002;33:2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- Euesden J, Danese A, Lewis CM, Maughan B. A bidirectional relationship between depression and the autoimmune disorders - New perspectives from the National Child Development Study. PLoS One. 2017;12:e0173015. doi: 10.1371/journal.pone.0173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Jaremka LM, Glaser R, Alfano CM, Povoski SP, Lipari AM, Agnese DM, Yee LD, Carson WE, 3rd, Farrar WB, Malarkey WB, Chen M, Kiecolt-Glaser JK. Attachment anxiety is related to Epstein-Barr virus latency. Brain Behav Immun. 2014;41:232–238. doi: 10.1016/j.bbi.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JL, Boch SJ, McCarthy DO. Feasibility of hair collection for cortisol measurement in population research on adolescent health. Nursing Research. 2016;65:249–255. doi: 10.1097/NNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JL, Stowe RP. Racial-ethnic differences in Epstein-Barr virus antibody titers among U.S. children and adolescents. Ann Epidemiol. 2013;23:275–280. doi: 10.1016/j.annepidem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. Journal of behavioral medicine. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao HY, Kiecolt-Glaser JK. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991;5:219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- Haeri S, Johnson N, Baker AM, Stuebe AM, Raines C, Barrow DA, Boggess KA. Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstetrics and Gynecology. 2011;117:862–866. doi: 10.1097/AOG.0b013e31820f3a30. [DOI] [PubMed] [Google Scholar]
- Hussong AM, Ennett ST, Cox MJ, Haroon M. A systematic review of the unique prospective association of negative affect symptoms and adolescent substance use controlling for externalizing symptoms. Psychology of Addictive Behaviors. 2017;31:137–147. doi: 10.1037/adb0000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston-Brooks CH, Lewis MA, Evans GW, Whalen CK. Chronic stress and illness in children: the role of allostatic load. Psychosom Med. 1998;60:597–603. doi: 10.1097/00006842-199809000-00015. [DOI] [PubMed] [Google Scholar]
- Kim JW, Szigethy EM, Melhem NM, Saghafi EM, Brent DA. Inflammatory markers and the pathogenesis of pediatric depression and suicide: a systematic review of the literature. The Journal of Clinical Psychiatry. 2014;75:1242–1253. doi: 10.4088/JCP.13r08898. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics. Series. 2002;11:1–190. [PubMed] [Google Scholar]
- Li H, Liu S, Hu J, Luo X, Li N, AMB, Cao Y. Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis. International Journal of Biological Sciences. 2016;12:1309–1318. doi: 10.7150/ijbs.16564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan M, Mamun A, Doi S, Clavarino A. Prospective Associations between depression and obesity for adolescent males and females- A systematic review and meta-analysis of longitudinal studies. PLoS One. 2016;11:e0157240. doi: 10.1371/journal.pone.0157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Stallings JF, Angold A, Costello EJ, Burleson M, Cacioppo JT, Glaser R, Worthman CM. Epstein-Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62:560–567. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Crucian BE, Stowe RP, Simpson RJ, Ott CM, Sams CF, Pierson DL. Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine. 2013;61:205–209. doi: 10.1016/j.cyto.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016:138. doi: 10.1542/peds.2016-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Frontiers in Neuroendocrinology. 2014;35:347–369. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, Stalder T, Hoffman K, Fischer JE, Bosch JA. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun. 2014;38:133–141. doi: 10.1016/j.bbi.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Regoeczi WC. Crowding in context: an examination of the differential responses of men and women to high-density living environments. J Health Soc Behav. 2008;49:254–268. doi: 10.1177/002214650804900302. [DOI] [PubMed] [Google Scholar]
- Riva M, Larsen CV, Bjerregaard P. Household crowding and psychosocial health among Inuit in Greenland. International Journal of Public Health. 2014a;59:739–748. doi: 10.1007/s00038-014-0599-x. [DOI] [PubMed] [Google Scholar]
- Riva M, Plusquellec P, Juster RP, Laouan-Sidi EA, Abdous B, Lucas M, Dery S, Dewailly E. Household crowding is associated with higher allostatic load among the Inuit. J Epidemiol Community Health. 2014b;68:363–369. doi: 10.1136/jech-2013-203270. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology. 2014;50:139–148. doi: 10.1016/j.psyneuen.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Perez NA, Yetman DL, Cutchin MP, Goodwin JS. Herpesvirus reactivation and socioeconomic position: a community-based study. J Epidemiol Community Health. 2010;64:666–671. doi: 10.1136/jech.2008.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Ruiz RJ, Fagundes CP, Stowe RH, Chen M, Glaser R. An ELISA method to compute endpoint titers to Epstein-Barr virus and cytomegalovirus: application to population-based studies. J Immunol Methods. 2014;408:64–69. doi: 10.1016/j.jim.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. Journal of Affective disorders. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Zhu K, Allen K, Mountain J, Lye S, Pennell C, Walsh JP. Depressive symptoms, body composition and bone mass in young adults: a prospective cohort study. International Journal of Obesity. 2016;41:576–581. doi: 10.1038/ijo.2016.214. [DOI] [PubMed] [Google Scholar]
- Zhu P, Chen YJ, Hao JH, Ge JF, Huang K, Tao RX, Jiang XM, Tao FB. Maternal depressive symptoms related to Epstein-Barr virus reactivation in late pregnancy. Scientific Reports. 2013;3:3096. doi: 10.1038/srep03096. [DOI] [PMC free article] [PubMed] [Google Scholar]