Abstract
The Retinoblastoma 1 (RB1) tumor suppressor, a member of the Retinoblastoma gene family, functions as a pocket protein for the functional binding of E2F transcription factors. About 1/3 of retinoblastoma patients harbor a germline RB1 mutation or deletion, leading to the development of retinoblastoma. Here, we demonstrate generation of a heterozygous deletion of the RB1 gene in the H1 human embryonic stem cell line using CRISPR/Cas9 nickase genome editing. The RB1 heterozygous knockout H1 cell line shows a normal karyotype, maintains a pluripotent state, and is capable of differentiation to the three germline layers.
Resource table
| Unique stem cell line identifier | CDMLe001-A |
| Alternative name(s) of stem cell line | H1-RB1(E16)−/+ |
| Institution | The University of Texas Health Science Center at Houston |
| Contact information of distributor | dung-fang.lee@uth.tmc.edu |
| Type of cell line | Human embryonic stem cell line |
| Origin | hESC line H1 (WA01) from WiCell Institute |
| Additional origin info | Sex: Male (46, XY) |
| Cell Source | Human blastocyst |
| Clonality | Clonal |
| Method of reprogramming | N/A |
| Genetic Modification | Yes |
| Type of Modification | Deletion of 50 bp in RB1 exon 16. |
| Associated disease | Hereditary retinoblastoma |
| Gene/locus | 13q14.1-14.2; RB1 exon 16 |
| Method of modification | CRISPR/Cas9 nickase |
| Name of transgene or resistance | None |
| Inducible/constitutive system | None |
| Date archived/stock date | 11/7/2017 |
| Cell line repository/bank | None |
| Ethical approval | Cell lines were used according to institutional guidelines. UTHealth approval number: SCRO-16-01 |
Resource utility
We generate a human embryonic stem cell (hESC) line with heterozygous RB1 deletion to facilitate the modeling of the cancer etiology of hereditary retinoblastoma.
Resource details
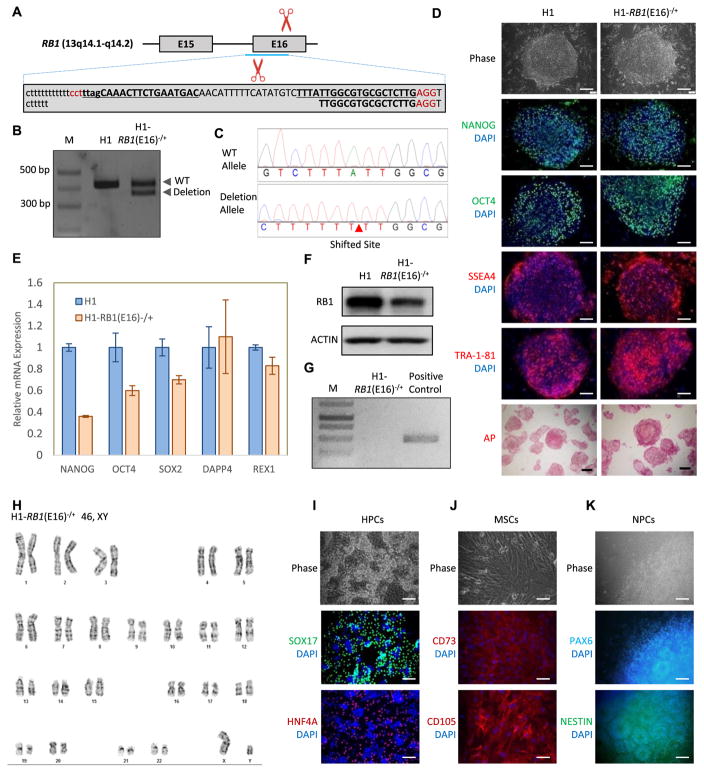
Hereditary retinoblastoma patients commonly carry a heterozygous RB1 mutation or deletion (Lin et al., 2017). Random sporadic damage to the remaining normal copy of RB1 in retinal cells initiates development of retinoblastoma. To provide a useful lab resource for scientists investigating hereditary retinoblastoma, we generated a heterozygous RB1 deletion hESC line by targeting exon 16 of the RB1 gene using the CRISPR/Cas9 nickase gene editing system (Fig. 1A, the Cas9/sgRNA target sites are underlined and RB1 introns and exons are presented in lowercase and uppercase, respectively).
Fig. 1.
Generation and characterization of the RB1 heterozygous knockout hESC line H1-RB1(E16)−/+. (A) Schematic overview of the gene targeting strategy to knockout RB1 exon 16 using CRISPR/Cas9 nickase. RB1 introns and exons are shown in lowercase and uppercase, respectively. The sgRNA target sites are underlined. (B) PCR confirms the deletion of the RB1 exon 16 in the H1-RB1(E16)−/+ line. (C) Sanger sequencing reveals the heterozygous deletion of the RB1 exon16. (D) The H1-RB1(E16)−/+ line expresses hESC pluripotency factors (NANOG and OCT4) and hESC surface markers (SSEA4 and TRA-1-81), and exhibits positive AP activity. Scale bar = 100 μm. (E) qRT-PCR reveals the expression of endogenous human NANOG, SOX2, OCT4, DPPA4, and REX1in H1-RB1(E16)−/+ line. PCR reactions are normalized to GAPDH and plotted relative to expression levels in human H1 ESCs. Error bars indicate ± SEM of triplicates. (F) The H1-RB1(E16)−/+ line presents the lower expression of RB1 protein. (G) The H1-RB1(E16)−/+ line is mycoplasma-free. (H) The H1-RB1(E16)−/+ line shows normal karyotype. (I–K) The H1-RB1(E16)−/+ line maintains pluripotency. Immunofluorescence staining reveals that H1-RB1(E16)−/+ line is capable of differentiating to endodermal (HPCs), mesodermal (MSCs) and ectodermal (NPCs) lineages. Scar bar = 100 μm.
hESC H1 cells were transfected with pX335-U6-Chimeric_BB-CBh-hSpCas9n(D10A) (PuroR) plasmids carrying sgRNAs targeting exon 16 of the RB1 gene. Electroporated cells were selected by puromycin. The puromycin-resistant clones were picked up, isolated, and expanded. The RB1 targeted regions were analyzed by PCR to demonstrate that only one allele was deleted (Fig. 1B). Primers surrounding exon 16 (amplifying both the wild-type and deleted alleles) were designed and the genetic region amplified by PCR and examined by Sanger sequencing. The mono-allelic 50-nucleotide-deletion was confirmed in the deleted allele, while the other allele retained its wild-type sequence (Fig. 1C). A premature stop codon was also noted in RB1 exon 17 in the allele with a 50-nucleotide-deletion in one of two heterozygous RB1 deletion hESC clones obtained and submitted for further characterization.
The H1-RB1(E16)−/+ line maintains a classical tightly packaged dome-shape hESC morphology and expresses high levels of pluripotency transcription factors (NANOG and OCT4), hESC surface markers (SSEA4 and TRA-1-81) as well as alkaline phosphatase (AP) (Fig. 1D, scale bar 100 μm). The expression of pluripotency genes (NANOG, OCT4, SOX2, DPPA4, and REX1) in the H1-RB1(E16)−/+ line was compared to the levels in parental H1 cells by quantitative real-time PCR. PCR reactions are normalized to GAPDH. The expression levels of all five pluripotency genes in the H1-RB1(E16)−/+ line were comparable to those in parental H1 cells (Fig. 1E, error bars indicate ± SEM of triplicates). Immunoblotting results suggested that RB1 expression is lower in the H1-RB1(E16)−/+ line than in parental H1 cells (Fig. 1F). PCR-based mycoplasma detection assay confirmed the cell line is mycoplasma-free (Fig. 1G). Karyotype analysis suggested that the H1-RB1(E16)−/+ line does not contain any chromosomal abnormality (Fig. 1H). Additionally, the short tandem repeat (STR) profile of H1-RB1(E16)−/+ cells was identical to that of their parental H1 cells (Supplementary Table S1). Furthermore, H1-RB1 (E16)−/+ cells maintained the potential to differentiate into all three germ layers as determined by their ability to differentiate to SOX17 and HNF4A-positive hepatic progenitor cells (HPCs, endodermal lineage) (Fig. 1I, scale bar 100 μm), CD73 and CD105-positive mesenchymal stem cells (MSCs, mesodermal lineage) (Fig. 1J, scale bar 100 μm) as well as PAX6 and NESTIN-positive neural progenitor cells (NPCs, ectodermal lineage) (Fig. 1K, scale bar 100 μm). In summary, the H1-RB1(E16)−/+ line is pluripotent and demonstrates a normal karyotype. The H1-RB1(E16)−/+ line provides a valuable cell resource to study the cancer etiology of hereditary retinoblastoma.
Materials and methods
Maintenance of hESCs
hESCs were maintained on Matrigel (Corning)-coated plates with StemMACS™iPS-Brew XF (Miltenyi Biotec). StemMACS™ Passaging Solution XF (Miltenyi Biotec) was used to passage hESCs and Rock inhibitor (Calbiochem) was utilized to improve cell survival rate during replating.
Generation of RB1 heterozygous deletion hESC line by CRIPSR/Cas9 nickase gene editing methodology
A CRISPR guide targeting exon 16 of RB1 was designed using CRISPR Design website (http://crispr.mit.edu). Two sgRNAs (TTAGCAAACTTCTGAGTGAC and TTTATTGGCGTGCGCTCTTG) targeting exon 16 of RB1 gene were selected and cloned into pX335-U6-Chimeric_BB-CBh-hSpCas9n(D10A)(PuroR), respectively to generate the guide plasmids. For electroporation, 107 cells were re-suspended with 0.6 ml Embryo Max Electroporation Buffer (Millipore), mixed with CRISPR/Cas9 nickase plasmids (25 μg each) and electroporated at 300 V/500 μF in a BIO-RAD Gene Pulser Xcell System. Following electroporation, cells were immediately dispensed into 10 cm MEF-coated plates in hESC medium (DMEM/F12 (Corning) with 20% KnockOut Serum replacement (Life Technologies), 1% Gibco GlutaMax (Life Technologies), 1% NEAA (Corning), 0.0007% β-mercaptoethanol (Sigma) and 10 ng/ml FGF2 (EMD Millipore)) supplemented with 10 μM ROCK inhibitor. After 48 h recovery, cells were treated with 1 μM puromycin (Sigma) for 48 h and then maintained in regular hESC medium for colony growth. The isolated genomic DNA from individual colonies was used for clonal identification by PCR using a RB1 exon 16 specific primer set (forward: 5′-TCTGTTTCAGGAAGAAGAACGAT-3′; reverse: 5′-ACCATGGAGGTTACAGCAGTG-3′). The PCR fragments of wild-type and deletion of RB1 exon 16 were examined by Sanger sequencing.
Quantitative real-time PCR
Total mRNA was isolated using TRIzol (Invitrogen) following the manufacturer’s instructions. 1 μg of RNA was used for reverse transcription. Real-time PCR analysis was performed on a CFX96 machine (Bio-Rad) using the SYBR Green PCR Master Mix (Bio-Rad). The PCR reaction consisted of 10 μl SYBR Green PCR Master Mix, 1 μl of 10 μM forward and reverse primers, and 1 μl of 3-times diluted template cDNA in a total volume of 20 μl. Samples were analyzed in triplicate and normalized to GAPDH expression. The primer sequences are shown in Table 1.
Table 1.
Characterization and validation.
| Classification | Test | Result | Data |
|---|---|---|---|
| Morphology | Photography | Normal | Fig. 1 panel D |
| Phenotype | Immunocytochemistry RT-qPCR |
NANOG, OCT4, SSEA4, TRA-1-81 and AP-positive. Comparable of NANOG, OCT4, SOX2, DPPA4, and REX1 expression with H1. |
Fig. 1 panel D Fig. 1 panel E |
| Genotype | Karyotype (G-banding) and resolution | 46 XY Resolution: 450–500 |
Fig. 1 panel H |
| Identity | Microsatellite PCR (mPCR) OR STR analysis |
N/A 14/14 sites matched |
N/A Supplementary Table S1 |
| Mutation analysis (IF APPLICABLE) | Sequencing Southern Blot OR WGS |
Heterozygous deletion of 50 bp in RB1 exon 16. N/A |
Fig. 1 panel B N/A |
| Microbiology and virology | Mycoplasma | Mycoplasma test shows negative. | Fig. 1 panel G |
| Differentiation potential | Directed in vitro differentiation | Expression of SOX17 and HNF4A in HPCs, CD105 and CD166 in MSCs, and PAX6 and NESTIN in NPCs. | Fig. 1 panel I, J, and K |
| Donor screening (OPTIONAL) | HIV 1 + 2 Hepatitis B, Hepatitis C | N/A | N/A |
| Genotype additional info (OPTIONAL) | Blood group genotyping HLA tissue typing |
N/A N/A |
N/A N/A |
In vitro differentiation
In vitro differentiation of H1-RB1(E16)−/+ cells to HPCs, MSCs, and NPCs was performed by well-defined differentiation protocols described previously (Chambers et al., 2009; Qin et al., 2016; Zhao et al., 2015). For cell characterization, SOX17 (R&D Systems) and HNF4A (Cell Signaling Technology) were used for HPCs, CD105 (Thermo Fisher Scientific) and CD73 (BD Biosciences) were used for MSCs, and PAX6 (BioLegend) and NESTIN (BioLegend) were used for NPCs.
Immunofluorescent staining and immunoblotting
H1-RB1(E16)−/+cells or differentiated cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, blocked with 10% serum in 0.1% PBST for 1 h and incubated with the indicated primary antibodies (Table 2) overnight. Cells were then washed with PBST, incubated with corresponding secondary antibodies for 1 h at room temperature, and detected by Leica DMi8. Immunoblotting was performed as described (Lee et al., 2007).
Table 2.
Reagents details.
| Antibodies used for immunocytochemistry
| |||
|---|---|---|---|
| Antibody | Dilution | Company Cat# and RRID | |
| Pluripotency markers | Goat anti-NANOG | 1:400 | R and D Systems Cat# AF1997 RRID:AB_355097 |
| Pluripotency markers | Rabbit anti-OCT4 | 1:250 | Santa Cruz Biotechnology Cat# sc-9081 RRID:AB_2167703 |
| Pluripotency markers | Mouse anti-SSEA4 | 1:200 | R and D Systems FA1435P-025 |
| Pluripotency markers | Mouse anti-TRA-1-85 | 1:400 | R and D Systems Cat# FAB3195A RRID:AB_663789 |
| Differentiation markers | Anti-CD105 | 1:400 | Thermo Fisher Scientific Cat# 12-1057-42 RRID:AB_1311123 |
| Differentiation markers | Anti-CD73 | 1:400 | BD Biosciences Cat# 550257 RRID:AB_393561 |
| Differentiation markers | Rabbit anti-HNF4a |
1:1000 | Cell Signaling Technology Cat# 3113S RRID:AB_2295208 |
| Differentiation markers | Goat anti-SOX17 | 1:500 | R and D Systems Cat# AF1924 RRID:AB_355060 |
| Differentiation markers | Rabbit anti-PAX6 | 1:200 | BioLegend Cat# 901301 RRID:AB_2565003 |
| Differentiation markers | Mouse anti-NESTIN | 1:200 | BioLegend Cat# 655102 RRID:AB_2562023 |
| RB1 (Western Blot) | Mouse anti-RB1 | 1:250 | BD Biosciences Cat# 554136 RRID:AB_395259 |
| Secondary antibodies | Goat anti-rabbit IgG(Alexa Fluor 488 conjugate) | 1:1000 | Jackson ImmunoResearch Labs Cat# 111-545-144 RRID:AB_2338052 |
| Secondary antibodies | Donkey Anti-Goat IgG (Alexa Fluor488 conjugate) | 1:1000 | Jackson ImmunoResearch Labs Cat# 705-545-003 RRID:AB_2340428 |
| Secondary antibodies | Donkey Anti-Mouse IgG (Alexa Fluor488 conjugate) | 1:1000 | Jackson ImmunoResearch Labs Cat# 715-545-150 RRID:AB_2340846 |
| Secondary antibodies | Donkey Anti-Rabbit IgG (Cy3 conjugate) | 1:1000 | Jackson ImmunoResearch Labs Cat# 711-165-152 RRID:AB_2307443 |
| Secondary antibodies | Donkey Anti-Rabbit IgG (Cy5 conjugate) | 1:1000 | Jackson ImmunoResearch Labs Cat# 711-175-152 RRID:AB_2340607 |
| Primers | |||
|
| |||
| Target | Forward/reverse primer (5′–3′) | ||
|
| |||
| Pluripotency markers (qPCR) | OCT4 | AACCTGGAGTTTGTGCCAGGGTTT/TGAACTTCACCTTCCCTCCAACCA | |
| Pluripotency markers (qPCR) | SOX2 | AGAAGAGGAGAGAGAAAGAAAGGGAGAGA/GAGAGAGGCAAACTGGAATCAGGATCAAA | |
| Pluripotency markers (qPCR) | NANOG | TTTGTGGGCCTGAAGAAAACT/AGGGCTGTCCTGAATAAGCAG | |
| Pluripotency markers (qPCR) | DPPA4 | GACCTCCACAGAGAAGTCGAG/TGCCTTTTTCTTAGGGCAGAG | |
| Pluripotency markers (qPCR) | REX1 | GCCTTATGTGATGGCTATGTGT/ACCCCTTATGACGCATTCTATGT | |
| House-keeping genes (qPCR) | GAPDH | CCACTCCTCCACCTTTGAC/ACCCTGTTGCTGTAGCCA | |
| Targeted mutation analysis/sequencing | RB1 exon16 | TTCTTTTTATAGAAGTAAGTATTTTATAATC/CTCAAAGGTCTTCGGAGGGA | |
Mycoplasma test
PCR mycoplasma test kit was used according to the manufacturer’s instructions (abm G238).
Karyotype analysis and STR analysis
The G-banding karyotype was performed by The T. C. Hsu Molecular Cytogenetics Facility in The University of Texas M.D. Anderson Cancer Center. Twenty metaphase chromosome spreads were analyzed with G-band resolution of 450–500. STR analysis for parent cell authentication was performed by the Characterized Cell Line Core Facility in The University of Texas M.D. Anderson Cancer Center. 14 STR loci were compared using the Promega Powerplex 16 HS kit. Fragments were amplified by PCR for further analysis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scr.2018.01.021.
Supplementary Material
Acknowledgments
We thank Dr. A.S. Multani for performing karyotype analysis and Dr. F. Zhang for CRISPR/Cas9 nickase plasmid. J.T. and Z.H. are supported by the Ke Lin Program of the First Affiliated Hospital of Sun Yat-sen University. D.H. is supported by State-sponsored Joint Ph.D. Program from China Scholarship Council (201606380093). R.Z. is supported by UTHealth Innovation for Cancer Prevention Research Training Program Pre-doctoral Fellowship (Cancer Prevention and Research Institute of Texas grant RP160015) and Wei Yu Family Endowed Scholarship (The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences). D.-F.L. is the CPRIT scholar in Cancer Research and supported by NIH Pathway to Independence Award R00 CA181496 and CPRIT Award RR160019.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. https://doi.org/10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK β Suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. https://doi.org/10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lin YH, Jewell BE, Gingold J, Lu L, Zhao R, Wang LL, Lee DF. Osteosarcoma: molecular pathogenesis and iPSC modeling. Trends Mol Med. 2017 doi: 10.1016/j.molmed.2017.06.004. https://doi.org/10.1016/j.molmed.2017.06.004. [DOI] [PMC free article] [PubMed]
- Qin J, Chang M, Wang S, Liu Z, Zhu W, Wang Y, Yan F, Li J, Zhang B, Dou G, Liu J, Pei X, Wang Y. Connexin 32-mediated cell-cell communication is essential for hepatic differentiation from human embryonic stem cells. Sci Rep. 2016:6. doi: 10.1038/srep37388. https://doi.org/10.1038/srep37388. [DOI] [PMC free article] [PubMed]
- Zhao Q, Gregory CA, Lee RH, Reger RL, Qin L, Hai B, Park MS, Yoon N, Clough B, McNeill E, Prockop DJ, Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci. 2015;112:530–535. doi: 10.1073/pnas.1423008112. https://doi.org/10.1073/pnas.1423008112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.