Abstract
Glycogen storage disease type Ia (GSD-Ia) is caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC), a key enzyme in endogenous glucose production. This autosomal recessive disorder is characterized by impaired glucose homeostasis and long-term complications of hepatocellular adenoma/carcinoma (HCA/HCC). We have shown that hepatic G6Pase-α deficiency-mediated steatosis leads to defective autophagy that is frequently associated with carcinogenesis. We now show that hepatic G6Pase-α deficiency also leads to enhancement of hepatic glycolysis and hexose monophosphate shunt (HMS) that can contribute to hepatocarcinogenesis. The enhanced hepatic glycolysis is reflected by increased lactate accumulation, increased expression of many glycolytic enzymes, and elevated expression of c-Myc that stimulates glycolysis. The increased HMS is reflected by increased glucose-6-phosphate dehydrogenase activity and elevated production of NADPH and the reduced glutathione. We have previously shown that restoration of hepatic G6Pase-α expression in G6Pase-α-deficient liver corrects metabolic abnormalities, normalizes autophagy, and prevents HCA/HCC development in GSD-Ia. We now show that restoration of hepatic G6Pase-α expression normalizes both glycolysis and HMS in GSD-Ia. Moreover, the HCA/HCC lesions in L-G6pc−/− mice exhibit elevated levels of hexokinase 2 (HK2) and the M2 isoform of pyruvate kinase (PKM2) which play an important role in aerobic glycolysis and cancer cell proliferation. Taken together, hepatic G6Pase-α deficiency causes metabolic reprogramming, leading to enhanced glycolysis and elevated HMS that along with impaired autophagy can contribute to HCA/HCC development in GSD-Ia.
Keywords: glycolysis, hexose monophosphate shunt, hepatocellular adenoma/carcinoma, gene therapy
1. Introduction
Glycogen storage disease type Ia (GSD-Ia, MIM232200) is an autosomal recessive metabolic disorder caused by a deficiency in glucose-6-phosphatase-α (G6Pase-α or G6PC), an enzyme expressed primarily in the liver, kidney, and intestine [1, 2]. G6Pase-α catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose in the terminal step of glycogenolysis and gluconeogenesis and is a key enzyme for endogenous glucose production [1, 2]. The incidence of GSD-Ia is approximately 1 in 125,000 and GSD-Ia patients manifest impaired glucose homeostasis and long-term risks of hepatocellular adenoma (HCA) and carcinoma (HCC). The etiology of HCA/HCC in GSD-Ia is unknown. In gluconeogenic organs, there are multiple competing pathways utilizing intracellular G6P, including: G6Pase-α-mediated glucose production; glycolysis; hexose monophosphate shunt (HMS); and glycogen synthesis. GSD-Ia patients lacking a functional hepatic G6Pase-α cannot maintain blood glucose homeostasis. The inability to hydrolyze G6P to glucose in the liver leads to increased activities of the other G6P metabolic pathways. These are reflected in the clinical manifestations seen in GSD-Ia, including fasting hypoglycemia, nephromegaly, hepatomegaly, hyperlipidemia, hyperuricemia, and lactic acidemia [1, 2]. Hepatomegaly and nephromegaly are caused by excessive glycogen and/or neutral fat accumulation.
We hypothesized that hepatic G6Pase-α deficiency mediates a reprogramming of G6P metabolism which can contribute to HCA/HCC development in GSD-Ia. Aerobic glycolysis, a preferred way of glucose metabolism in cancer cells, is frequently linked to tumorigenesis [3]. The end product of glycolysis is pyruvate. Pyruvate can be converted either to lactate by lactate dehydrogenase (LDH) or to acetyl-CoA, by pyruvate dehydrogenase (PDH), for ATP production via mitochondrial oxidative phosphorylation [3]. Enhanced glycolysis can generate high levels of ATP that is required for cancer cell metabolism. Moreover, elevated glycolysis increases the supply of essential metabolic intermediates for the biosynthesis of nucleic acids, proteins, and phospholipids required for rapid cancer cell proliferation [3]. Similarly, the enhanced HMS supports cancer cells by generating increased NADPH [4], a cofactor for glutathione reductase that results in increased production of the reduced glutathione (GSH) [5], an antioxidant playing a pivotal role in maintaining the redox status and survival of cancer cells [6]. The enhanced HMS also results in increased pentose phosphates to support the high rate of macromolecular synthesis and proliferation of cancer cells [4]. While increased glycogen storage and elevated lactate accumulation are well-documented clinical manifestations of GSD-Ia [1, 2], perturbations in many components of the glycolytic and HMS pathways have not been carefully investigated. Recently, using the liver-specific G6pc-knockout (L-G6pc−/−) mice, we showed that hepatic steatosis mediated by G6Pase-α deficiency leads to defective autophagy, a process frequently associated with tumorigenesis [7].
In this study, we examine the metabolic reprogramming in glycolysis and HMS mediated by hepatic G6Pase-α deficiency. We show that hepatic G6Pase-α-deficiency leads to persistent enhancement of glycolysis and increased HMS, both of which can contribute to hepatocarcinogenesis. Moreover, the HCA/HCC lesions developed in L-G6pc−/− mice exhibit elevated levels of hexokinase 2 (HK2) and the M2 isoform of pyruvate kinase (PKM2) which favour aerobic glycolysis and cancer cell proliferation. Taken together, our results suggest that hepatic G6Pase-α plays a key role in liver homeostasis associated with the G6P metabolic pathways including glycolysis and HMS that can contribute to HCA/HCC development in GSD-Ia.
2. Materials and methods
2.1. Animals
All animal studies were conducted under an animal protocol approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee. The liver-specific G6pc-deficient (L-G6pc−/−) and L-G6pc+/− mice were generated by tamoxifen-mediated excision of the G6pc exon 3 in 6-week-old G6pcfx/fx.SAcreERT2/w and G6pcfx/w.SAcreERT2/w mice, respectively, as previously described [7]. To reconstitute hepatic G6Pase-α activity, L-G6pc−/− mice at 4 WP (weeks post G6pc gene deletion) were infused via retro-orbital sinus with rAAV-G6PC [8, 9], a G6Pase-α expressing, recombinant adeno-associated virus (rAAV) vector at 1 × 1012 viral particles (vp)/kg. Mouse phenotype was examined at 12 WP and liver samples were collected from mice following a 6-hour fast.
2.2. Primary hepatocyte isolation
Hepatocytes were isolated from control and L-G6pc−/− mice at 12 WP with the use of a two-step collagenase perfusion method. Liver was perfused via the portal vein with liver perfusion medium (Gibco, Waltham, MA, USA) prewarmed at 37°C for 5 min, and followed by liver digest medium (Gibco) prewarmed at 37°C for 5 min. The excised livers were further incubated in liver digest medium for 30 min at 37°C, and then passed through a 100 µm cell strainer (Falcon, Franklin Lakes, NJ, USA). The recovered hepatocytes were pelleted by centrifugation at 4°C, washed twice with hepatocyte wash medium (Gibco), and purified via 20% Percoll gradients (GE Healthcare, Waukesha, WI, USA). The resulting hepatocytes were resuspended in Willams E medium (Gibco). The viability of the isolated hepatocytes was analyzed by flow cytometry using the Guava ViaCount reagent (Millipore, St Charles, MO, USA).
2.3. Determination of metabolites and enzyme assays
Liver lysates were deproteinized using 14% perchloric acid, and then neutralized with 2 M KOH/0.2 M MOPS. The levels of lactate and GSH in deproteinized lysates were determined using the respective assay kits obtained from BioVision (Mountain View, CA, USA). Hepatic levels of NADPH were determined using the EnzyChrom NADP+/NADPH assay kit (BioAssay Systems, Hayward, CA, USA). The activities of LDH, PFK, and G6PD were determined using the respective BioVision assay kits.
2.4. Quantitative real-time RT-PCR and Western-blot analysis
The mRNA expression was quantified by real-time RT-PCR using the TaqMan probes (Life Technologies) in an Applied Biosystems QuantStudio 3 Real-Time PCR System (Foster City, CA, USA). Data were normalized to Rpl19 RNA. The antibodies used were c-Myc (#5605) and hexokinase 2 (HK2) (#2867) from Cell Signaling Technology (Danvers, MA, USA); the M2 isoform of pyruvate kinase (PKM2) (ab137791) from Abcam (Cambridge, MA, USA); β-actin (sc-47778), aldolase B (sc-393278), and glucose phosphate isomerase (GPI) (sc-33777) from Santa Cruz Biotechnolog (Dallas, TX, USA); and hypoxia-inducible factor-1α (HIF-1α) (NB100-449) from Novus Biologicals (Littleton, CO, USA). The monoclonal antibody against human G6Pase-α has been described [7].
2.5. Statistical analysis
The unpaired t test was performed using the GraphPad Prism Program, version 4 (San Diego, CA, USA). Values were considered statistically significant at p < 0.05.
3. Results
3.1. The L-G6pc−/− mice exhibit enhanced hepatic glycolysis and HMS
We have generated a G6pc−/− mouse strain that mimics the phenotype of human GSD-Ia [10]. However, even under intensive glucose therapy, the G6pc−/− mice rarely survive to weaning, making the study of long-term metabolic aberrations of the disorder difficult. We therefore generated a conditional liver-specific knockout strain, L-G6pc−/− mice, that are more amenable to longer term studies, and showed these mice are a good model to study long-term hepatic complications in GSD-Ia [7]. The liver-specific excision of G6pc exon 3 was induced at age 6 weeks [7]. In agreement with previous report [11], none of the L-G6pc−/− mice developed tumors at the pre-tumor stage of 12 WP (weeks post G6pc deletion), 30% developed HCA/HCC at the tumor-developing stage of 53 WP, and 100% developed HCA/HCC at the tumor-bearing stage of 78 WP.
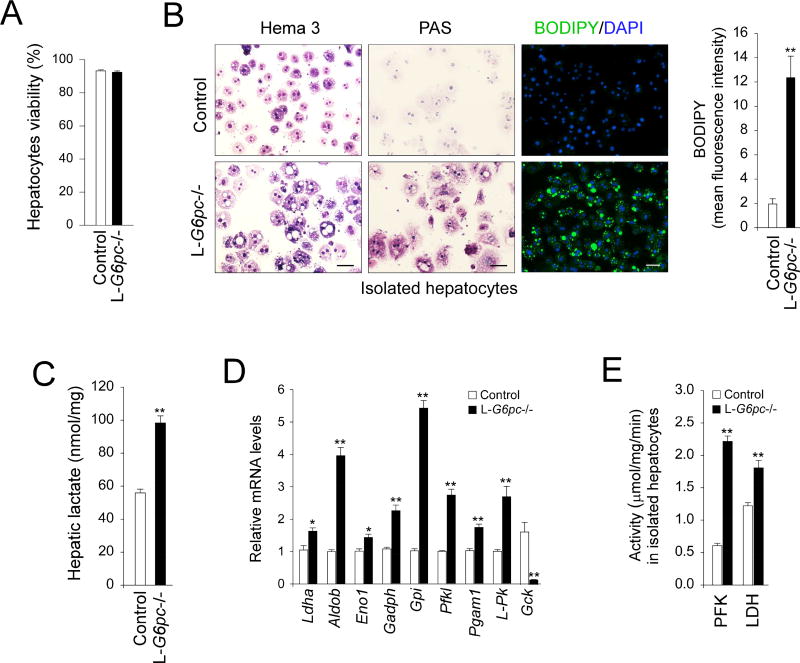
To accurately examine enzymatic activities in hepatocytes without interference by extracellular components and non-parenchymal cells, we isolated hepatocytes with similar viability (Fig. 1A) from the livers of control and L-G6pc−/− mice at 12 WP. Compared to hepatocytes from control mice, the G6Pase-α-deficient hepatocytes, resembling GSD-Ia hepatocytes, displayed an enlarged cytoplasm, and elevated accumulation of glycogen and neutral fat detected by increased periodic acid-Schiff (PAS) and BODIPY staining, respectively (Fig. 1B). One clinical manifestation of GSD-Ia is lactic acidemia [1, 2]. Consistent with this, lactate concentrations were markedly elevated in G6Pase-α-deficient livers (Fig. 1C). LDH, that converts pyruvate to lactate, also showed elevated mRNA expression (Fig. 1D) and enzymatic activity (Fig. 1E). There was also a marked increase in hepatic levels of mRNA for many glycolytic enzymes, including aldolase B (Aldob), enolase 1 (Eno1), glyceraldehyde-3-phosphate dehydrogenase (Gapdh), GPI (Gpi), PFK liver type (Pfkl), phosphoglycerate mutase 1 (Pgam1), and liver-type pyruvate kinase (L-Pk), although levels of glucokinase (Gck) mRNA were decreased (Fig. 1D). Hepatic activity of PFK, the rate-limiting enzyme in glycolysis was also increased in L-G6pc−/− mice (Fig. 1E). These findings were consistent with an enhanced hepatic glycolytic pathway in the L-G6pc−/− versus controls.
Fig. 1.
Enhanced hepatic glycolysis in L-G6pc−/− mice. Control and L-G6pc−/− mice at 12 WP were used. (A) The viability of hepatocytes isolated from control and L-G6pc−/− mice at 12 WP analyzed by flow cytometry. (B) Hema 3- and PAS-stained cytospins of hepatocytes, and immunofluorescence analysis of BODIPY-stained neutral fat (green) and DAPI-stained nuclei (blue) in hepatocytes, and quantitative flow cytometric analysis of BODIPY-positive hepatocytes (n = 4). Scale bar, 50 µm. (C) Hepatic lactate levels (n = 4). (D) Quantification of hepatic mRNA for glycolytic enzymes by real-time RT-PCR (n = 5–8). (D) PFK and LDH activities (n = 5). Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
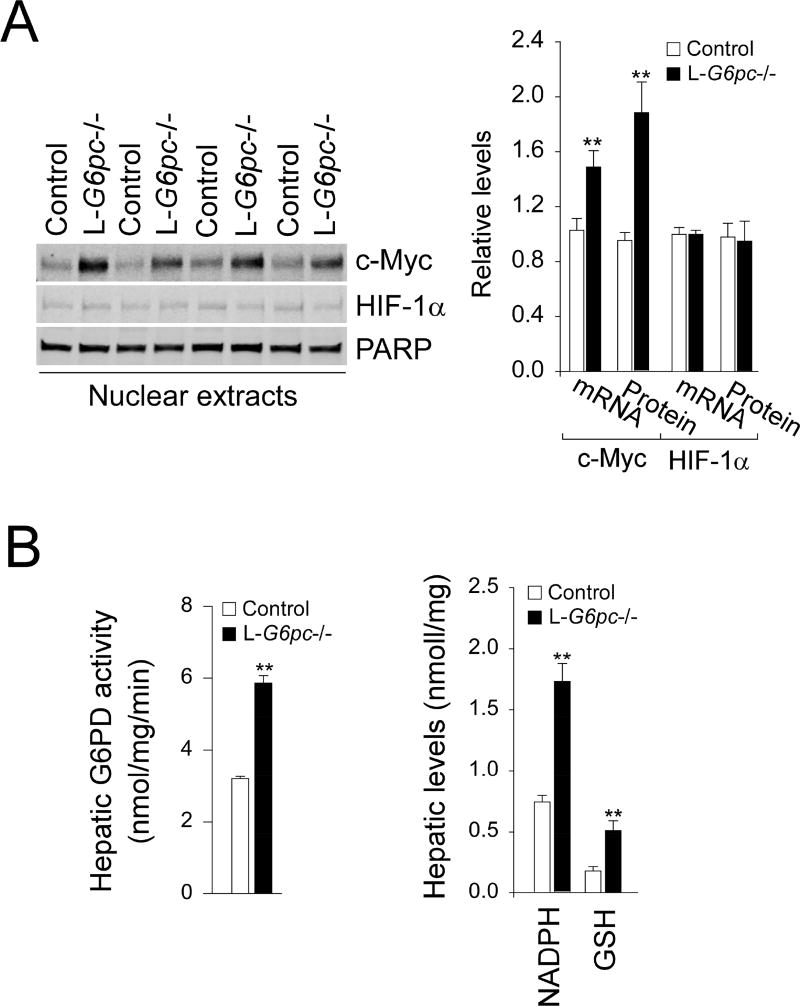
Furthermore, hepatic levels of c-Myc mRNA and nuclear c-Myc protein were significantly elevated in L-G6pc−/− mice, while those of HIF-1α mRNA and nuclear HIF-1α protein remained unchanged (Fig. 2A). Since c-Myc and HIF-1α are known to induce the expression of genes encoding glycolytic enzymes [12], this was further evidence of the increased glycolytic pathway activity. Hepatic G6Pase-α deficiency also promotes HMS that generates NADPH for the production of GSH, an antioxidant [5, 6]. The first committed step in HMS for NADPH production is catalyzed by glucose-6-phosphate dehydrogenase (G6PD) [4]. Compared to controls, hepatic G6PD activity was markedly increased in L-G6pc−/− mice at 12 WP (Fig. 2B). Likewise, hepatic levels of NADPH and GSH were both markedly increased in L-G6pc−/− mice at 12 WP (Fig. 2B), indicating enhanced HMS pathway.
Fig. 2.
Analysis of c-Myc, HIF-1α, NADPH and GSH in L-G6pc−/− mice. Control and L-G6pc−/− mice at 12 WP were used. (A) Western-blot analysis of c-Myc, HIF-1α and Poly (ADP-ribose) polymerase (PARP) in liver nuclear extracts, and quantification by densitometry (n = 4). (B) Hepatic G6PD activity (n = 5), and hepatic levels of NADPH (n = 6) and GSH (n = 6). Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
3.2. Hepatic G6Pase-α restoration normalizes glycolysis and HMS
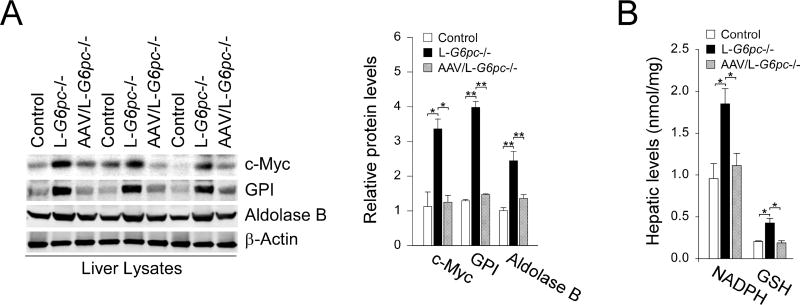
We have shown that rAAV-G6PC-mediated restoration of hepatic G6Pase-α expression in murine GSD-Ia corrects metabolic abnormalities, rectifies autophagy impairment, and prevent HCA/HCC development [7–9]. We therefore treated L-G6pc−/− mice at 4 WP with rAAV-G6PC and examined metabolic activities of the treated mice at 12 WP. Compared to untreated L-G6pc−/− mice, restoration of hepatic G6Pase-α expression in L-G6pc−/− mice normalized hepatic levels of c-Myc and c-Myc-regulated glycolytic enzymes, including GPI and aldolase B (Fig. 3A). Moreover, hepatic G6Pase-α restoration also normalized levels of NADPH and GSH in G6Pase-α-deficient livers (Fig. 3B). Taken together, restoration of hepatic G6Pase-α expression normalizes both glycolysis and HMS in L-G6pc−/− mice, demonstrating that hepatic G6Pase-α plays an essential role in the G6P metabolic pathways including glycolysis and HMS.
Fig. 3.
Hepatic G6Pase-α restoration normalizes glycolysis and HMS. L-G6pc−/− mice were treated with 1×1012 vp/kg of rAAV-G6PC at 4 WP and analyzed at 12 WP. (A) Western-blot analysis of c-Myc, GPI, aldolase B, and β-actin in liver lysates and quantification by densitometry (n = 4). (B) Hepatic levels of NADPH (n = 4) and GSH (n = 4). Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
3.3. Sustained hepatic elevation of glycolysis and HMS in all stages of tumor development
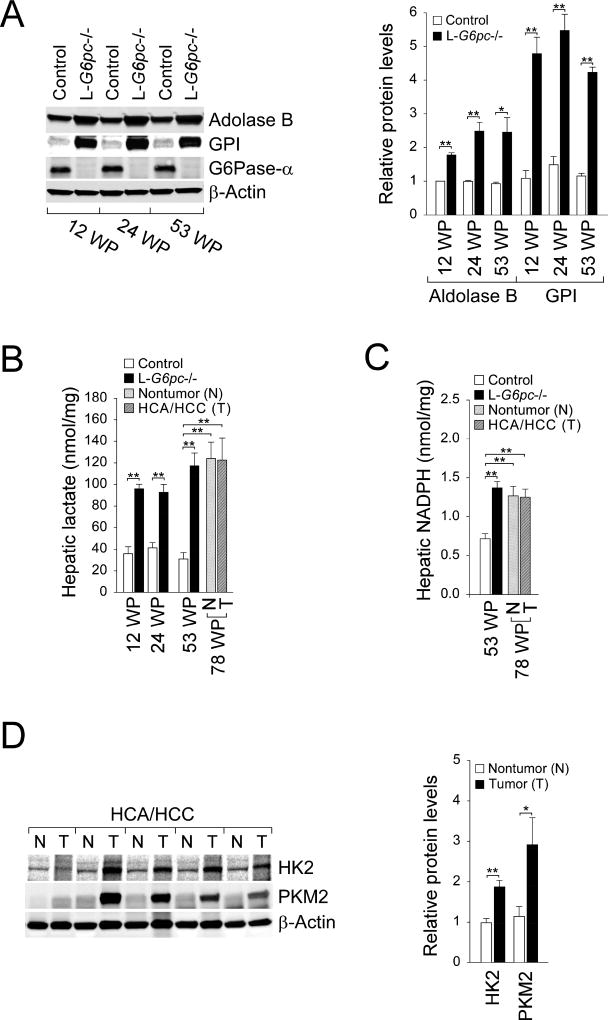
Next, we investigated chronic, long-term alterations in glycolysis and HMS which can contribute to HCA/HCC development in GSD-Ia. We first examined the status of G6Pase-α deletion and showed that hepatic G6Pase-α expression was absent in all three stages of tumor development (Fig. 4A). We then examined hepatic glycolysis and HMS during tumor development. Compared to control mice, hepatic levels of aldolase B and GPI (Fig. 4A) as wells as lactate (Fig. 4B), markers of glycolysis were increased in L-G6pc−/− mice at both pre-tumor and tumor-developing stages. Hepatic lactate levels remained elevated in the tumor-bearing stage and the HCA/HCC lesions (Fig. 4B). Likewise, compared to the controls, hepatic levels of NADPH, an indicator of HMS were increased in L-G6pc−/− mice at the pre-tumor stage (Fig. 2B), the tumor-developing stage (Fig. 4C), the tumor-bearing stage (Fig. 4C), and the HCA/HCC lesions (Fig. 4C).
Fig. 4.
The sustained enhancement in glycolysis and HMS. (A) Western-blot analysis of aldolase B, GPI, G6Pase-α, and β-actin in control and L-G6pc−/− mice at 12, 24 and 53 WP, and quantification by densitometry (n = 3). (B) Hepatic levels of lactate in control and L-G6pc−/− mice at 12, 24, and 53 WP (n = 4). The non-tumor liver tissues and HCA/HCC lesions were isolated from the L-G6pc−/− mice at 78 WP (n = 10). (C) Hepatic levels of NADPH in control and L-G6pc−/− mice at 53 WP (n = 5) and in the non-tumor liver tissues and HCA/HCC lesions of the L-G6pc−/− mice at 78 WP (n = 6). (D) Western-blot analysis of hepatic HK2, PKM2 and β-actin in the non-tumor (N) liver tissues and tumor (T) lesions of the L-G6pc−/− mice, and quantification by densitometry (n = 5). Data represent the mean ± SEM. *P < 0.05, **P < 0.005.
Hexokinases (HK) catalyze the first irreversible step in glycolysis, while pyruvate kinases (PK) catalyze the final rate-limiting step in glycolysis [13–15]. In particular, HK2 and the M2 isoform of PK (PKM2) play a critical role in aerobic glycolysis and tumor proliferation [13–15]. Thus, both proteins are frequently overexpressed in many cancers [13–15]. Notably, the HCA/HCC lesions in L-G6pc−/− mice displayed elevated levels of HK2 and PKM2 compared to the corresponding non-tumor regions (Fig. 4D). Collectively, the results show that the sustained enhancement in glycolysis and HMS, which are indispensable for the proliferation and survival of cancer cells, occurred in the livers of L-G6pc−/− mice.
4. Discussion
Patients affected by GSD-Ia are unable to maintain glucose homeostasis and present with fasting hypoglycemia, hepatomegaly, nephromegaly, hyperlipidemia, hyperuricemia, lactic acidemia, and growth retardation [1, 2]. One severe long-term complication in GSD-Ia is HCA that develops in 75% of patients over 25 years-old [1, 2, 16, 17]. In 10% of cases, HCA undergoes malignant transformation to HCC. It remains unclear why hepatic G6Pase-α deficiency leads to HCA/HCC development in GSD-Ia. To study the mechanism underlying tumor development in GSD-Ia, we generated a L-G6pc−/− mouse strain and showed that 30% of L-G6pc−/− mice developed hepatic tumors at 53 WP and 100% developed HCA/HCC at 78 WP. Using the L-G6pc−/− mice, we showed that hepatic G6Pase-α-deficiency leads to autophagy impairment [7] that has been linked to many metabolic disorders, including hepatocarcinogenesis. In the liver, there are four competing G6P metabolic pathways, the G6Pase-α-mediated glucose production, glycogen synthesis, glycolysis and HMS. The lack of glucose production by the liver to maintain blood glucose homeostasis and the increased glycogen synthesis are hallmarks of GSD-Ia. The glycogen shunt, a condition when glucose is shunted to glycogen and subsequently consumed through glycolysis, was recently proposed to be critical for cancer cell survival [18]. In GSD-Ia, the excess G6P accumulated in the liver increases glycogen storage and its subsequent consumption in glycolysis, a process mimicking glycogen shunt, suggesting increased glycogen storage can contribute to hepatocarcinogenesis. However, the other G6P metabolic pathways that is required for tumorigenesis in GSD-Ia have not been extensively characterized to date. Here, we provide evidence that hepatic G6Pase-α deficiency leads to persistent enhancement of glycolysis and HMS which can contribute to HCA/HCC development in GSD-Ia.
Studies have shown that cancer cells produce cellular ATP predominantly via aerobic glycolysis, a phenomenon known as the Warburg effect [19]. The metabolic and non-metabolic changes mediated by high rates of glycolysis offer several advantages essential for cancer cell proliferation and invasion. Firstly, when sufficiently elevated, glycolysis can generate higher rate of ATP production than oxidative phosphorylation for cancer cell proliferation [3]. Secondly, glycolysis can provide essential metabolic intermediates for the biosynthesis of nucleic acids, proteins, and phospholipids essential for rapid cancer cell proliferation [3]. Thirdly, lactic acidosis caused by enhanced glycolysis can suppress immune surveillance and promotes cancer growth and invasion [20]. Fourthly, the non-metabolic function of the glycolytic enzymes can promote tumorigenesis [21]. Using L-G6pc−/− mice, we show that G6Pase-α deficiency leads to enhanced hepatic glycolysis characterized by markedly increased lactate accumulation, increased expression of many glycolytic enzymes, along with elevated levels of mRNA and nuclear c-Myc which is known to induce the expression of glycolytic genes. Accordingly, hepatic G6Pase-α deficiency leads to sustained upregulation of glycolysis that can play multiple roles in tumor development in GSD-Ia.
The HCA/HCC lesions developed in L-G6pc−/− mice exhibited elevated levels of HK2 and PKM2 which play an important role in aerobic glycolysis and are frequently overexpressed in many cancers [13–15]. HK2 has a high affinity for substrate glucose and can bind to the outer mitochondrial membrane to facilitate access for mitochondrially generated ATP for phosphorylation of glucose [13]. On the other hands, in presence of mitogenic signal, PKM2 can switch from an active tetrameric form to a less active dimeric form, allowing to the accumulation of glycolytic intermediates that is essential for cancer cell proliferation [14]. Collectively, HK2 and PKM2 can contribute to HCA/HCC development in GSD-Ia via aerobic glycolysis.
The HMS utilizes G6P to synthesis ribonucleotides and fatty acids that can support the high rate of cancer cell proliferation [4]. Cancer cells have elevated levels of reactive oxygen species (ROS) due to their accelerated metabolism [22]. Although ROS can induce signaling pathways to promote cancer cell proliferation and survival, the ROS-mediated oxidative insults can also promote the death of cancer cells [22]. The HMS is a major source of NADPH for the production of the ROS scavenger GSH that helps tumor cells to escape oxidative insults and apoptosis [4]. We now show that hepatic G6Pase-α deficiency leads to enhanced HMS, characterized by elevated G6PD activity and increased hepatic production of NADPH and GSH. Accordingly, hepatic G6Pase-α deficiency also leads to upregulation of HMS that is required for tumor development in GSD-Ia.
In summary, hepatic G6Pase-α deficiency results in metabolic reprogramming of G6P metabolism, leading to enhanced glycolysis, increased HMS, and elevated glycogen storage, which along with the previously reported autophagy impairment can contribute to HCA/HCC development in GSD-Ia. Importantly, restoration of hepatic G6Pase-α expression normalizes all metabolic alterations associated with GSD-Ia and impaired autophagy, supporting the value of G6Pase-α gene therapy that corrects metabolic abnormalities and prevents HCA/HCC development.
Supplementary Material
Highlights.
Hepatic G6Pase-α deficiency leads to enhancement of hepatic glycolysis and HMS.
The enhanced hepatic glycolysis is characterized by increased expression of many glycolytic enzymes and increased lactate accumulation.
The increased HMS is reflected by increased G6PD activity and elevated production of NADPH and the reduced GSH.
Restoration of hepatic G6Pase-α expression normalizes both glycolysis and HMS.
The HCA/HCC lesions in L-G6pc−/− mice display elevated levels of HK2 and PKM2.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (HD000912-38), and The Children's Fund for Glycogen Storage Disease Research. We thank Dr. Pierre Chambon for the gist of the AlbCreERT2 mice.
Abbreviations
- rAAV
recombinant adeno-associated virus
- G6P
glucose-6-phosphate
- G6Pase-α
glucose-6-phosphatase-α
- G6PD
glucose-6-phosphate dehydrogenase
- GSD-Ia
glycogen storage disease type Ia
- GSH
reduced glutathione
- HCA
hepatocellular adenoma
- HCC
hepatocellular carcinoma
- HIF-1α
hypoxia-inducible factor-1α
- HMS
hexose monophosphate shunt
- LDH
lactate dehydrogenase
- PAS
periodic acid-Schiff
- PDH
pyruvate dehydrogenase
- PFK
phosphofructokinase
- PKL
liver-type pyruvate kinase
- HK
hexokinase
- PKM
muscle-type pyruvate kinase
- WP
weeks post G6pc gene deletion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Chou JY, Matern D, Mansfield BC, Chen YT. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr. Mol. Med. 2002;2:121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 2.Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat. Rev. Endocrinol. 2010;6:676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism. 2010;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 6.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato PA, Marinari UM, Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med.cell. longev. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JH, Kim GY, Pan CJ, Anduaga J, Choi EJ, Mansfield BC, Chou JY. Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLoS Genet. 2017;13:e1006819. doi: 10.1371/journal.pgen.1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yiu WH, Lee YM, Peng WT, Pan CJ, Mead PA, Mansfield BC, Chou JY. Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. Mol. Ther. 2010;18:1076–1084. doi: 10.1038/mt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YM, Jun HS, Pan CJ, Lin SR, Wilson LH, Mansfield BC, Chou JY. Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology. 2012;56:1719–1729. doi: 10.1002/hep.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei KJ, Chen H, Pan CJ, Ward JM, Mosinger B, Lee EJ, Westphal H, Mansfield BC, Chou JY. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type 1a mouse. Nat. Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- 11.Mutel E, Abdul-Wahed A, Ramamonjisoa N, Stefanutti A, Houberdon I, Cavassila S, Pilleul F, Beuf O, Gautier-Stein A, Penhoat A, Mithieux G, Rajas F. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J. Hepatol. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 13.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F. PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol. Lett. 2016;11:1980–1986. doi: 10.3892/ol.2016.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 16.Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I) Eur. J. Pediatr. 2002;161(Suppl 1):S20–34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 17.Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, Kishnani PS. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J. Inherit. Metab. Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 18.Shulman RG, Rothman DL. The glycogen shunt maintains glycolytic homeostasis and the Warburg effect in cancer. Trends Cancer. 2017;11:761–767. doi: 10.1016/j.trecan.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Li S. Non-metabolic functions of glycolytic enzymes in tumorigenesis. Oncogene. 2017;36:2629–2636. doi: 10.1038/onc.2016.410. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.