Abstract
The use of synthetic mesh in the abdominal compartment has recently become a topic of debate as high profile public cases have called into question their safety. Several case reports have demonstrated significant complications due to intra-abdominal mesh. Furthermore, some studies have suggested that the rates of these severe complications are underestimated. We present the case of a patient who developed an enteroenteric and enterocutaenous fistulae, an abdominal wall collection and an intraperitoneal inflammatory mass from intraluminal migration of a synthetic mesh inserted during laparoscopic incisional hernia repair. We discuss the considerations and complications of using synthetic mesh for ventral hernia repair and discuss the scientific evidence behind the increasingly apparent ‘mesh problem’.
Keywords: gastrointestinal surgery, general surgery
Background
Ventral hernias are commonly repaired laparoscopically using a mesh to bridge the defect and to stop visceral eventration. Recently, there has been much speculation about the safety of placing synthetic mesh into the abdominal compartment. In particular, the product Physiomesh has been withdrawn from the market due to its increased complication profile.1 In gynaecology, there have been recent enquiries into the validity of using synthetic mesh tape for the treatment of stress urinary incontinence2 and vaginal prolapse.3 Furthermore, recent media reports of high complication rates after laparoscopic ventral rectopexy have led to the suspension of surgeons from practice and calls for a national enquiry in the UK into the use of mesh for the treatment of pelvic organ prolapse and urinary incontinence. There are case reports demonstrating bowel erosion and mesh fistulation4 after laparoscopic ventral hernia repair, and it is thought that the frequency and severity of complications caused by synthetic mesh are underestimated.5 6
As this debate continues, the true complication rates of synthetic mesh remain unknown. It is therefore important that cases of mesh migration and fistulation are reported in the literature. In this paper, a case of synthetic mesh migration is presented; after a simple laparoscopic ventral hernia repair, this patient had devastating consequences from the placement of synthetic mesh into the abdominal domain.
Case presentation
An 80-year-old woman of Asian origin presented to clinic at our tertiary centre (specialised in abdominal wall reconstruction) with a history of a year’s worsening chronic abdominal pain and 2 months of discharge from the right iliac fossa.
The patient had a medical history of type 2 diabetes, hypercholesterolaemia, hypertension and a raised body mass index (BMI) of 39. She had a surgical history of a laparoscopic cholecystectomy in 2004, which subsequently led to a large umbilical port site incisional hernia. In 2010, this was repaired laparoscopically using an intraperitoneal onlay synthetic mesh (a single piece of polypropylene mesh) and non-absorbable titanium tacks. Subsequently, the repair failed leading to an umbilical hernia recurrence, intermittent abdominal pain and enterocutaneous fistula formation in the right iliac fossa. The patient’s CT scan showed multiple loops of small bowel adherent to the mesh resulting in an enterocutaneous fistula (ECF), ileal-ileal fistula, an anterior abdominal wall collection and an inflammatory mass in the right iliac fossa (figures 1 and 2). The patient was referred to our tertiary abdominal wall unit from her district general hospital for removal of the infected mesh, ECF repair, ventral hernia repair and abdominoplasty. Initially, a US-guided drainage of the anterior abdominal wall collection was performed. The aspirated fluid grew Escherichia coli and the patient’s infection was treated with intravenous coamoxiclav (amoxicillin/clavulanic acid). After treatment of her intra-abdominal sepsis, the patient was consented for abdominal wall reconstruction.
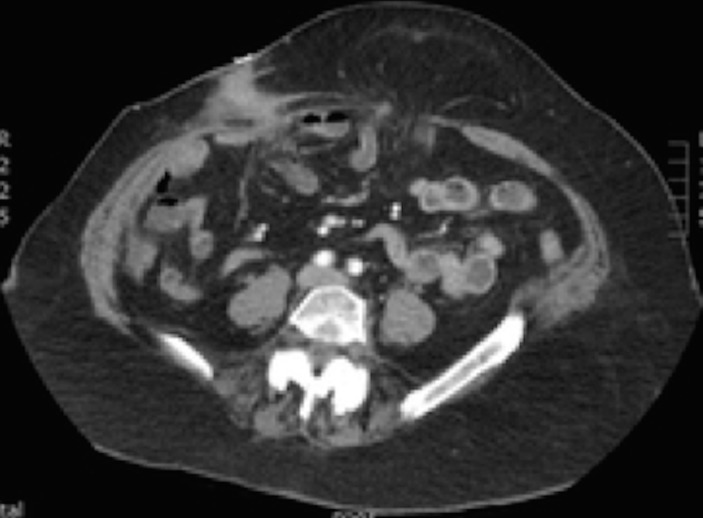
Figure 1.
Transverse CT image showing the enterocutaneous fistula in the right iliac fossa and the recurrent umbilical hernia in the midline.
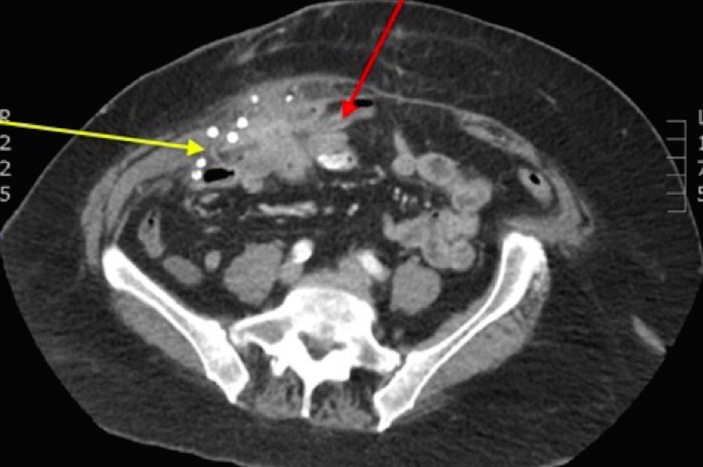
Figure 2.
Transverse CT image showing the inflammatory mass in the right iliac fossa. The non-absorbable titanium tacks are clearly seen. This inflammatory mass incorporates the synthetic mesh (which has migrated laterally, shrunk and become coiled, yellow arrow) and loops of bowel (red arrow).
During the patient’s operation, the mesh was found to have coiled into a cylindrical shape and was intraluminal at a confluence of three adjoining small bowel fistulae (figures 3–5). The infected mesh and fistulae were removed with a single 50 cm resection of small bowel and a subsequent side-to-side anastomosis was created using the proximate linear cutter 75 (Ethicon, Somerville, New Jersey, USA) stapler. No mesh was inserted, as in a contaminated setting, adding a mesh (either biological or synthetic) is known to significantly increase the likelihood of fistula recurrence.7 Consequently, the narrow ventral hernia defect (3.5 cm wide, figure 1) was easily repaired using primary fascial closure. Postoperatively, the patient spent 24 hours in the intensive care unit where her main issues were nausea and pain. Once deemed stable, she was stepped down to the ward, and the remainder of her inpatient stay was complicated by diarrhoea and a postoperative haemoglobin count of 79 g/L which required a blood transfusion of two units. Stool samples revealed no significant growth and no Clostridium difficile infection. No source of bleeding was identified.
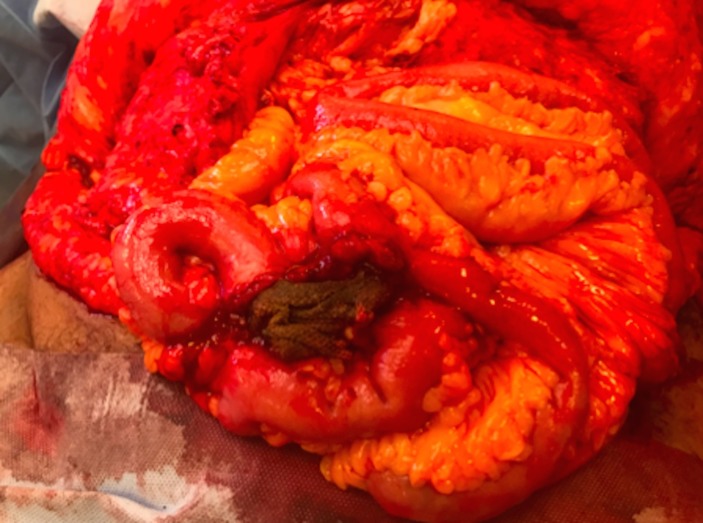
Figure 3.
After adhesiolysis and small bowel mobilisation the mesh appears to have formed a curved cylindrical shape and to be intraluminal.
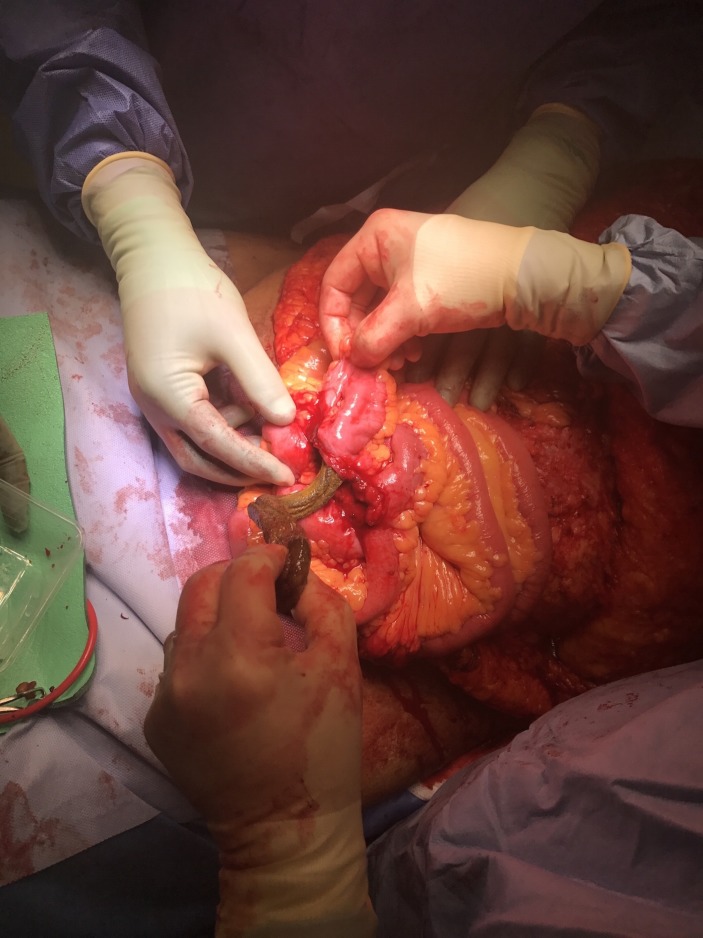
Figure 4.
Removing the cylindrical mesh from the small bowel lumen.
Figure 5.
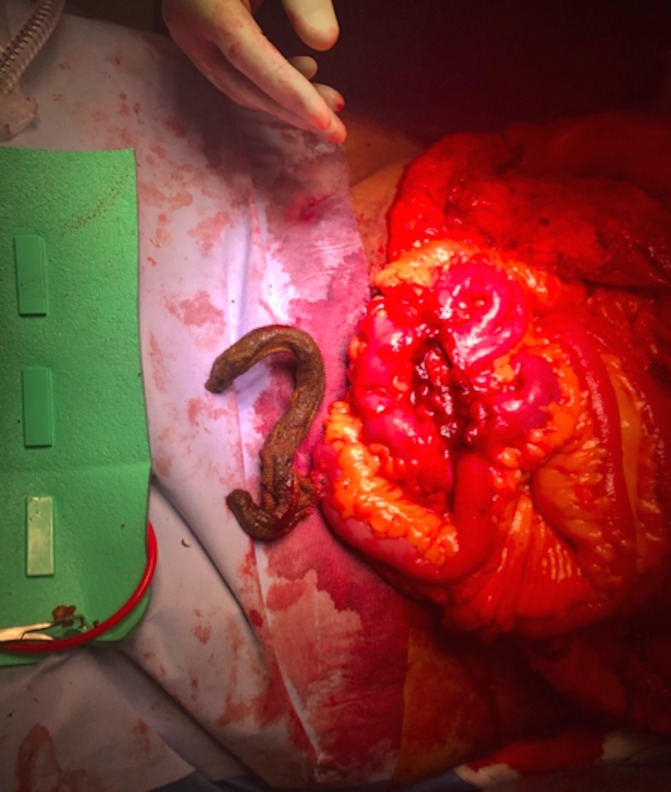
After removal from the small bowel the coiled cylindrical mesh can be seen.
Outcome and follow-up
Postoperatively, the patient was complaining of loose stool for a short period of time; however, she recovered well from her major abdominal surgery. She was followed up in clinic and no longer described symptoms of daily abdominal pain and on examination was found to have well-healed wounds.
Discussion
The use of mesh for ventral hernia repair is common practice and reinforces a tension-free repair. This reduces ventral hernia recurrence rates.8 The severe complications that can arise from the use of intra-abdominal synthetic material have previously been reported in the literature.9 10 In this case, we describe mesh shrinkage and detachment from the abdominal wall and intraluminal mesh migration, bowel erosion, enteroenteric fistula and enterocutaneous fistula formation. For this patient, the use of an intra-abdominal synthetic (polypropylene) mesh has been catastrophic and has resulted in chronic disability leading to major reconstructive surgery.
The literature reports that synthetic mesh acts as a foreign body creating a local inflammatory reaction.9 The surrounding inflammation is sometimes known as an inflammatory granuloma or capsule.11 When intra-abdominal synthetic mesh comes in contact with the bowel, this can lead to adhesions, bowel erosion and fistula formation.12 Mesh migration also seems to be more common when the mesh is in direct contact with the bowel compared with implanting the mesh into other planes.13 In our patient, an enterocutaneous fistula was the presenting complication; however, other cases have reported fistulae involving the bladder and rectum.4 14 Once a mesh has migrated into the bowel causing a fistula, a bowel resection is required to remove the mesh and restore bowel continuity. As closure in a contaminated abdomen has a higher risk of mesh infection, the abdominal wall defect can be repaired either by direct primary closure or with a biological mesh.8 If a mesh infection was to occur, salvage of the mesh using intravenous antibiotics is more successful after using a biological mesh as opposed to a synthetic mesh15; therefore, a biological mesh is more commonly used in contaminated cases. On occasion, the surgeon may feel that a mesh is not required as a strong primary fascial closure can easily be achieved, as in this case. Furthermore, even though a mesh would reduce the risk of hernia recurrence, using a mesh is known to significantly increase the risk of both wound morbidity (6) and fistula recurrence (7) and is not always indicated. Fistula recurrence is the main complication to avoid in cases involving fistula repair. In addition, by not using a mesh, the risk of wound morbidity is reduced resulting in better wound healing and a lower the risk of hernia recurrence. This patient also had an abdominoplasty, which reduces the abdominal wall adiposity, BMI and the risk of hernia recurrence.
For mesh fixation, the previous surgeons used titanium tacks and these were removed along with the mesh during the patient’s reconstructive surgery. The authors note that (perhaps counter intuitively) permanent titanium tacks have been reported to cause higher rates of mesh shrinkage and migration when compared with absorbable tacks16 and suture fixation.17
General surgeons carrying out ventral hernia repairs have to make many decisions about their surgical approach and repair technique. Level one evidence exists to support the use of a mesh,8 but with over 200 meshes on the market18 which mesh they should use remains a difficult question for practising surgeons to answer. To add further confusion, mesh companies do not test their implants in a standardised manner in both the in vitro and in vivo settings. Before implantation, mechanical testing can involve either uniaxial, biaxial or ball-burst tensile strength testing. Further tests can involve suture retention strength or tear resistance testing.19 In vivo biocompatibility is commonly assessed using H&E staining, but the time periods used to assess tissue ingrowth after mesh implantation are seldom the same and histological grading scales used to assess neovascularisation and periprosthetic inflammation vary.20 21 To add further confusion to the ‘mesh problem’, the orientation of the mesh implant also seems to affect mesh shrinkage and migration rates, as the anisotropy of the abdominal wall requires a mesh with similar anisotropic tensile strength and stiffness.22 This phenomenon is little known to most general surgeons and a comprehensive study analysing the orientations of every mesh and their optimal orientation for biocompatibility does not exist.
It is important for surgeons to be aware that there is a lack of data reporting the mesh-associated complication rates. At a recent consensus meeting at abdominal wall reconstruction (AWR) Europe,23 surgeons called for a national UK mesh register, so that accurate long-term follow-up data can be collected and the rates of mesh migration, bowel erosion and fistula formation can be discovered. Mesh infection and explantation are reported in the literature but are highly heterogeneous. As recent large systematic review, reported the mesh infection and explantation rates at 1.9% and 1.2%, respectively, after the repair of Ventral hernia working group (VHWG) grade I and II hernias.24 For contaminated hernias, mesh infection and explantation rates have been reported at 38% and 5%, respectively, after biological mesh implantation.25 However, some case series of contaminated abdominal wall defects report mesh infection and explantation rates of 0% after both synthetic mesh26 and biological mesh27 hernia repair, implying that these rates are highly operator dependent. Before carrying out AWR, surgeons must have an awareness of which patients are at increased risk of a mesh infection before consenting and preoperative risk evaluation. High BMI, smoking, American Society of Aneasthesiologists (ASA) grade ≥3, chronic obstructive pulmonary disease, emergency operation, prior AAA repair, prior wound infection, concomitant bowel procedure, longer operation time, enterotomy and ECF repair have all shown a significant association with the development of mesh infection.28–31
For academic hernia surgeons to discover the most biocompatible mesh with the lowest hernia recurrence and mesh complication rates, standardisation of laboratory mesh testing is required. Currently, the lack of standardisation makes the data in the literature heterogeneous and comparing the biocompatibility of different meshes impossible. Consensus is required to identify the main mechanical parameters that provide a comprehensive analysis of a mesh and its biocompatibility. For example, mesh stiffness (tension/change in width) may be one of the main parameters to measure as significant differences between the host tissue and the implant can cause pain and other complications.32 With standardisation, the postoperative outcomes of each mesh will correlate with their descriptive parameters. Achieving such standardisation will be a significant step in the search for the ideal mesh implant causing the lowest postoperative complication rates.
Learning points.
Although widely used, synthetic mesh use in the abdominal compartment can lead to significant complications for patients.
Intraluminal mesh migration may occur and prompt treatment is required to prevent morbidity.
There is an increasingly evident ‘mesh problem’ and a more standardised approach to testing meshes is clearly required.
Footnotes
Contributors: RP, THR and SGP have contributed towards a literature search on the complications of a mesh hernia repair in addition to the write- up of the case report and discussion. AW has performed the surgical procedure detailed within the case report and supervised the write-up of this case.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Köckerling F, Simon T, Hukauf M, et al. The importance of registries in the postmarketing surveillance of surgical meshes. Ann Surg 2017;1:1 10.1097/SLA.0000000000002326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keltie K, Elneil S, Monga A, et al. Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women. Sci Rep 2017;7:12015 10.1038/s41598-017-11821-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morling JR, McAllister DA, Agur W, et al. Adverse events after first, single, mesh and non-mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997-2016: a population-based cohort study. Lancet 2017;389:629–40. 10.1016/S0140-6736(16)32572-7 [DOI] [PubMed] [Google Scholar]
- 4. Adeyemo D. Case report: open access International Journal of Surgery case reports mesh fistulation into the rectum after laparoscopic ventral mesh rectopexy. Int J Surg Case Reports 2014;5:152–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hampel C, Naumann G, Thüroff JW, et al. [Management of complications after sling and mesh implantations]. Urologe A 2009;48:496–509. 10.1007/s00120-009-1978-4 [DOI] [PubMed] [Google Scholar]
- 6. Kokotovic D, Bisgaard T, Helgstrand F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA 2016;316:1575–82. 10.1001/jama.2016.15217 [DOI] [PubMed] [Google Scholar]
- 7. Hodgkinson JD, Maeda Y, Leo CA, et al. Complex abdominal wall reconstruction in the setting of active infection and contamination: a systematic review of hernia and fistula recurrence rates. Colorectal Dis 2017;19:319–30. 10.1111/codi.13609 [DOI] [PubMed] [Google Scholar]
- 8. Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 2000;343:392–8. 10.1056/NEJM200008103430603 [DOI] [PubMed] [Google Scholar]
- 9. Picchio M, Muggianu A, Mancini F, et al. Complete mesh migration into the small bowel after incisional hernia repair: a case report and literature review. Acta Chir Belg 2017;117:118–21. 10.1080/00015458.2016.1229399 [DOI] [PubMed] [Google Scholar]
- 10. Basoglu M, Yildirgan MI, Yilmaz I, et al. Late complications of incisional hernias following prosthetic mesh repair. Acta Chir Belg 2004;104:425–8. [PubMed] [Google Scholar]
- 11. Binnebösel M, Klink CD, Otto J, et al. Impact of mesh positioning on foreign body reaction and collagenous ingrowth in a rabbit model of open incisional hernia repair. Hernia 2010;14:71–7. 10.1007/s10029-009-0580-4 [DOI] [PubMed] [Google Scholar]
- 12. Norton C, Culver A, Mostafa G. Intraluminal mesh migration after ventral hernia repair. J Gastrointest Surg 2016;20:1920–2. 10.1007/s11605-016-3178-z [DOI] [PubMed] [Google Scholar]
- 13. Yang GPC. From intraperitoneal onlay mesh repair to preperitoneal onlay mesh repair. Asian J Endosc Surg 2017;10:119–27. 10.1111/ases.12388 [DOI] [PubMed] [Google Scholar]
- 14. Raghavendran M, Kumar KG, Prasad S, et al. Post incisional hernia meshplasty vesicocutaneous fistula: a rare complication. Urol Case Rep 2017;13:149–51. 10.1016/j.eucr.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slater NJ, van der Kolk M, Hendriks T, et al. Biologic grafts for ventral hernia repair: a systematic review. Am J Surg 2013;205:220–30. 10.1016/j.amjsurg.2012.05.028 [DOI] [PubMed] [Google Scholar]
- 16. Harsløf S, Zinther N, Harsløf T, et al. Mesh shrinkage depends on mesh properties and anchoring device: an experimental long-term study in sheep. Hernia 2017;21:107–13. 10.1007/s10029-016-1528-0 [DOI] [PubMed] [Google Scholar]
- 17. Beldi G, Wagner M, Bruegger LE, et al. Mesh shrinkage and pain in laparoscopic ventral hernia repair: a randomized clinical trial comparing suture versus tack mesh fixation. Surg Endosc 2011;25:749–55. 10.1007/s00464-010-1246-0 [DOI] [PubMed] [Google Scholar]
- 18. Le D, Deveney CW, Reaven NL, et al. Mesh choice in ventral hernia repair: so many choices, so little time. Am J Surg 2013;205:602–7. 10.1016/j.amjsurg.2013.01.026 [DOI] [PubMed] [Google Scholar]
- 19. Todros S, Pavan PG, Pachera P, et al. Synthetic surgical meshes used in abdominal wall surgery: Part II-Biomechanical aspects. J Biomed Mater Res B Appl Biomater 2017;105:892–903. 10.1002/jbm.b.33584 [DOI] [PubMed] [Google Scholar]
- 20. Melman L, Jenkins ED, Hamilton NA, et al. Histologic and biomechanical evaluation of a novel macroporous polytetrafluoroethylene knit mesh compared to lightweight and heavyweight polypropylene mesh in a porcine model of ventral incisional hernia repair. Hernia 2011;15:423–31. 10.1007/s10029-011-0787-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozog Y, Konstantinovic ML, Werbrouck E, et al. Shrinkage and biomechanical evaluation of lightweight synthetics in a rabbit model for primary fascial repair. Int Urogynecol J 2011;22:1099–108. 10.1007/s00192-011-1440-1 [DOI] [PubMed] [Google Scholar]
- 22. Anurov MV, Titkova SM, Oettinger AP. Biomechanical compatibility of surgical mesh and fascia being reinforced: dependence of experimental hernia defect repair results on anisotropic surgical mesh positioning. Hernia 2012;16:199–210. 10.1007/s10029-011-0877-y [DOI] [PubMed] [Google Scholar]
- 23. Sanders DL, Torkington J, Smart NJ, et al. A meeting of UK AWR surgeons, 2nd February, AWR Europe. London: Royal College of Physicians, 2018. [Google Scholar]
- 24. Bueno-Lledó J, Torregrosa-Gallud A, Sala-Hernandez A, et al. Predictors of mesh infection and explantation after abdominal wall hernia repair. Am J Surg 2017;213:50–7. 10.1016/j.amjsurg.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 25. Atema JJ, de Vries FE, Boermeester MA. Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 2016;212:982–95. 10.1016/j.amjsurg.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 26. Carbonell AM, Criss CN, Cobb WS, et al. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg 2013;217:991–8. 10.1016/j.jamcollsurg.2013.07.382 [DOI] [PubMed] [Google Scholar]
- 27. Fayezizadeh M, Majumder A, Belyansky I, et al. Outcomes of retromuscular porcine biologic mesh repairs using transversus abdominis release reconstruction. J Am Coll Surg 2016;223:461–8. 10.1016/j.jamcollsurg.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 28. Mavros MN, Athanasiou S, Alexiou VG, et al. Risk factors for mesh-related infections after hernia repair surgery: a meta-analysis of cohort studies. World J Surg 2011;35:2389–98. 10.1007/s00268-011-1266-5 [DOI] [PubMed] [Google Scholar]
- 29. Sanchez VM, Abi-Haidar YE, Itani KM. Mesh infection in ventral incisional hernia repair: incidence, contributing factors, and treatment. Surg Infect 2011;12:205–10. 10.1089/sur.2011.033 [DOI] [PubMed] [Google Scholar]
- 30. Petro CC, Posielski NM, Raigani S, et al. Risk factors for wound morbidity after open retromuscular (sublay) hernia repair. Surgery 2015;158:1658–68. 10.1016/j.surg.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 31. Cuomo R, Nisi G, Grimaldi L, et al. Immunosuppression and abdominal wall defects: use of autologous dermis. In Vivo 2015;29:753–5. [PubMed] [Google Scholar]
- 32. Awad ZT, Puri V, LeBlanc K, et al. Mechanisms of ventral hernia recurrence after mesh repair and a new proposed classification. J Am Coll Surg 2005;201:132–40. 10.1016/j.jamcollsurg.2005.02.035 [DOI] [PubMed] [Google Scholar]