Abstract
Background: Bone and joint formation, maintenance, and regeneration are regulated by both chemical and physical signals. Among the physical signals there is an increasing realization of the role of pulsed electromagnetic fields (PEMF) in the treatment of nonunions of bone fractures. The discovery of the piezoelectric properties of bone by Fukada and Yasuda in 1953 in Japan established the foundation of this field. Pioneering research by Bassett and Brighton and their teams resulted in the approval by the Food and Drug Administration (FDA) of the use of PEMF in the treatment of fracture healing. Although PEMF has potential applications in joint regeneration in osteoarthritis (OA), this evolving field is still in its infancy and offers novel opportunities.
Methods: We have systematically reviewed the literature on the influence of PEMF in joints, including articular cartilage, tendons, and ligaments, of publications from 2000 to 2016.
Conclusions: PEMF stimulated chondrocyte proliferation, differentiation, and extracellular matrix synthesis by release of anabolic morphogens such as bone morphogenetic proteins and anti-inflammatory cytokines by adenosine receptors A2A and A3 in both in vitro and in vivo investigations. It is noteworthy that in clinical translational investigations a beneficial effect was observed on improving function in OA knees. However, additional systematic studies on the mechanisms of action of PEMF on joints and tissues therein, articular cartilage, tendons, and ligaments are required.
Keywords: : PEMF, articular cartilage, regeneration
Introduction
Osteoarthritis (OA) is a degenerative disorder that is prevalent in the aged population. More than 30 million Americans are currently affected and the number is increasing due to the aging of the population and due to the obesity epidemic. Articular cartilage is an anisotropic tissue and its properties are different depending on the depth from the surface.1 The characteristic of OA is progressive degeneration of articular cartilage in the joints; it is initiated from the surface and leads to the depth, causing functional joint failure and disability. However, articular cartilage in joints lacks the ability for self-repair, and, therefore, no treatment is available for complete cartilage repair and regeneration.
At present, pain relief by anti-inflammatory medications, physical therapy, and weight loss are the common treatments for amelioration of symptoms. Bone marrow stimulation, autologous chondrocyte implantation, novel biomaterial scaffolds, and stem-cell-based cartilage repair have been developed for OA treatment.2 However, as the outcomes are variable, arthroplasty is the most established treatment for advanced OA.3
One of the main goals of OA treatment is to regenerate a native articular cartilage, including a low friction coefficient. Lubricin/superficial zone protein (SZP) has been known to play an important role in the boundary lubrication in joints, and the genetic mutations of lubricin cause precocious OA.4,5 It is reported that recombinant lubricin prevents cartilage degeneration.6 Transforming growth factor β (TGF-β) and bone morphogenetic proteins (BMPs) are known to increase lubricin secretion from articular chondrocytes and synoviocytes.7,8 The pathways of stimulation by growth factors are one of the targets to regenerate the articular cartilage.
Tissue engineering is the emerging interdisciplinary field of the design and fabrication of spare parts for the human body. The goal is for functional restoration of lost parts due to trauma and diseases including osteoporosis and OA afflicting the musculoskeletal tissues such as bones and joints. Tissue engineering is the exiting final frontier of the wide-ranging field of bioengineering. It is based on principles of developmental biology and morphogenesis. Morphogenesis is the developmental cascade of pattern formation, the establishment of body plan incorporating the bilateral symmetry of musculoskeletal organs culminating in the adult human form. The three key ingredients of tissue engineering and regenerative medicine are signals for morphogenesis (TGFs and BMPs), cells (chondrocytes or stem cells) that respond to developmental and morphogenetic signals, and the scaffold of extracellular matrix.9
The ultimate goal of tissue engineering is to design functional tissues in vitro for implantation in vivo to repair, replace, restore, and regenerate new tissues with the utmost fidelity of function. Regeneration, in general, recapitulates embryonic development and morphogenesis. Among the musculoskeletal tissues, bone has high potential for regeneration as part of the repair process in response to injury, as well as during skeletal development.10 However, articular cartilage at the ends of bone lacks the ability to regenerate because of the limitation of blood supply in cartilage and is a formidable challenge to new investigations.
The tissue engineering triad consists of signals, stem cells, and scaffolds and is now well established.9, 11 However, considerable progress has been made in the chemical identification of morphogenetic signals such as BMPs. On the other hand, the progress in our understanding of the physical signals including, but not limited to, mechanical forces and pulsed electromagnetic fields (PEMF) has lagged behind.
Articular cartilage is an anisotropic structure with a zonal design and consists of three zones: superficial, middle, and deep zones. The superficial zone contains low proteoglycan (PG) content, and type II collagen is lined parallel to the surface.12 The superficial zone chondrocytes secrete lubricin, also known as SZP, which plays an important role in the lubrication of joints.4 The middle zone consists of higher PG content and randomly oriented type II collagen.12 This zone is critical for resistance to compressive forces.13 The deep zone has the highest concentration for PGs, and type II collagen is aligned perpendicular to the articular surface.12 In this region, extracellular matrix is mineralized and plays an integral role in connecting cartilage to bone. This region is responsible for resistance to the greatest amount of compressive forces.13
PEMF, a remedy for delayed union and nonunions of bone fractures, has also been suggested as an alternative treatment for OA.14 PEMF promotes bone and cartilage growth based on basic principles of physics: Wolff's law, the piezoelectric properties of collagens, and the concept of streaming potentials.15 The safety and efficacy of the PEMF is well established.16 PEMF has been known to increase morphogens to promote osteogenesis.17, 18 However, the therapeutic effects of PEMF on OA treatment are still debated and not settled.19 Therefore, the aim of this article is to review the potential benefits of PEMF for the regeneration of articular cartilage.
What Is PEMF?
Although there were known reports of success in bone healing using electrical stimulation as early as 1841, the use of this treatment did not progress until the 1950s.20 In 1953, Japanese scientist Yasuda reported the new bone formation by continuous current in rabbits.21 Since then, many studies about the effect of electricity on bone healing have been developed. In 1964, Bassett et al. revealed that the medullary cavity of caine femora was completely filled by new bone growth by direct electrical current.22 Brighton's group first applied this technology to nonunions in fracture healing.23 Becker et al. demonstrated the treatment of a variety of nonunions of fractures with a success rate of 77%.24 In 1979, the U.S. Food and Drug Administration (FDA) approved PEMF therapy for use in treating nonunion fractures. After Sisken reported the effect of specific frequencies in an electromagnetic field on soft tissue healing in 1995, clinical research of PEMF therapy increased dramatically.25 In 1997, Zhuang et al. demonstrated that PEMF increased the expression of TGF-β1 in osteoblasts to promote osteogenesis.17 In 1998, Bodamyali et al. demonstrated that PEMF has an osteogenic effect through upregulation of BMP-2 and BMP-4.18 Zhou et al. revealed that PEMF can prevent ovariectomy-induced bone loss through activation of Wnt signaling.26 However, the applied magnetic fields vary in their amplitude, frequency, waveform, or stimulation durations. Thus, there is still a need for more mechanistic investigations of the mechanisms underlying the actions of PEMF on skeletal structures (Fig. 1).
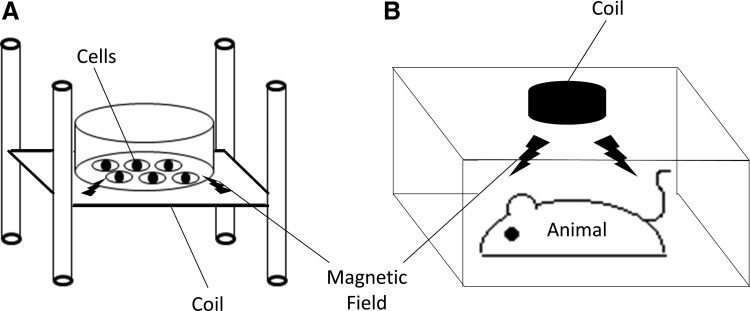
FIG. 1.
The schema of the applications of PEMF (A) In vitro system. Culture plates are placed on the stand surrounded by coils, which generates electromagnetic fields. (B) In vivo system. Small animals are placed in a cage, and PEMF is applied to the animal's whole body. PEMF, pulsed electromagnetic fields.
Electromagnetic fields are considered to play a role in bone healing through the same principles as the influence of mechanical stress on tissues.27 When mechanical stress is applied to a bone, strain gradients are created, resulting in changes in pressure gradients in the interstitial fluid. These drive fluid through the canalicular network in bone from regions of high to low pressure, exposing osteocyte membranes to flow-related shear stress as well as to electrical potentials in response to the streaming potentials.20 Thus, the mechanical stress is applied on bone transfers to the electrical potentials in the same manner as PEMF. These signals are possibly transduced to transmembrane receptors and accelerate bone repair.28 PEMF also has a beneficial effect in the tissue engineering of cartilage to promote matrix synthesis and, at the same time, to limit the inflammatory cytokines.29 However, the precise cellular mechanisms are still not well elucidated. In this article, we have reviewed the articles published on the influence of PEMF on joints, including articular cartilage, tendons, and ligaments, in the synovial joints published between the years between 2000 and 2016.
The Effects of PEMF on Articular Cartilage-Derived Chondrocytes and Stem Cells In Vitro
To regenerate intact articular cartilage surface is challenging due to the lack of the innate ability for self-repair. Chang et al. reported that 24 h exposure of PEMF had a possibility to increase chondrocyte proliferation and mRNA expressions of aggrecan, type I and X collagen in porcine (Table 1). However, glycosaminoglycan (GAG) production was decreased by PEMF treatment in their study.30 Others also analyzed the effect of PEMF on porcine chondrocytes and reported that 3 weeks of 2 h per day treatment of PEMF increased GAG and type II collagen.31 Bovine explant cultures revealed that PEMF increased matrix synthesis,32,33 and the effect was age dependent and not seen in damaged explants.32 However, Veronesi et al. reported that PEMF counteracted the progression of OA in bovine explants treated with interleukin-1β (IL-1β).34
Table 1.
The Effects of Pulsed Electromagnetic Fields on Chondrocytes and Stem Cells In Vitro
| Species | Culture | Treatment | Effect | Main results | Reference |
|---|---|---|---|---|---|
| Chondrocytes from pig knees | Cultured in collagen gel | After 24 h of PEMF exposure, cells were cultured for 3 weeks | Positive | PEMF increased proliferation but decreased GAG production. | Chang et al.30 |
| Frequency: 75 Hz Intensity: 1.8-3 mT |
PEMF increased mRNA expression of aggrecan, type I and X collagen. | ||||
| Articular chondrocytes of porcine | Cultured on chitosan films | PEMF: 2 h per day for 3 weeks | Positive | PEMF increased GAG and type II collagen. | Chang et al.31 |
| Frequency: 75 Hz | |||||
| Intensity: 1.8-3 mT | |||||
| Cartilage explants from metacarpophalangeal joints of calves and adult cows | Explants culture | PEMF: Continuous for 7 days with IL1β | Positive | PEMF promoted matrix synthesis in intact explants but had no effect on damaged explants. | Babacz et al.32 |
| Frequency: 200Hz | |||||
| Metacarpophalangeal joints of bovine | Full-thickness explants culture | PEMF: Different frequency and intensity for 1–24 h | Positive | PEMF increased PG synthesis with maximal effect at 1.5 mT. | De Mattei et al.33 |
| Frequency: 2–110 Hz Intensity: 0.5-2 mT |
No effect of pulse frequency was observed on PG synthesis. | ||||
| Metacarpophalangeal joints of bovine | High-density monolayer culture | Frequency: 75 Hz | Positive | PEMF upregulated A2A and A3 receptors. | Varani et al.40 |
| Intensity: 1.5 mT | |||||
| Bovine cartilage explants from metacarpophalangeal joints | Full-thickness explants culture | PEMF: continuous for 24 h, 7 or 21 days with high-dose IL1β | Positive | PEMF counteracted the progression of OA acting on both cartilage cellularity and ECM treated with IL1β. | Veronesi et al.34 |
| Frequency: 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Human healthy chondrocytes from hip | Low- and high-density monolayer culture | PEMF: 1–18 h or 3–6 days Frequency: 75 Hz Intensity: 2.3 mT |
Positive | PEMF increased proliferation at 9 and 18 h in both low- and high-density culture. | De Mattei et al.35 |
| PEMF increased proliferation during the first 3 days in low-density culture. | |||||
| Human OA chondrocytes | Cultured in alginate gel | PEMF: 3 h a day with IL1β | Positive | PEMF restored the PG concentration in the medium. | Fioravanti et al.38 |
| Frequency: less than 30Hz | |||||
| Human chondrocytes from femoral condyles | Monolayer culture | PEMF: 6 h | Positive | PEMF changed chondrocyte morphology from stellate to spherical. | Jahns et al.39 |
| Frequency: 100 Hz | |||||
| Human chondrocytes | Monolayer culture | PEMF: 30 min per day for 4 days | Positive | PEMF increased DNA content by 150% at 72-h stimulation. | Fitzsimmons et al.37 |
| Frequency: 4150Hz | PEMF increased nitric oxide in medium and cGMP in cells within the 30-min exposure. | ||||
| Human chondrocytes from OA knee | Cultured in type I collagen gel | PEMF: continuous for 14 days | None | No effects on gene expressions of type II and aggrecan were seen. | Schmidt-Rohlfing et al.44 |
| Frequency: 16.7 Hz | |||||
| Intensity: 2 mT | |||||
| Human cartilage explants from OA knee | Explants culture | PEMF: continuous for 7 days with IL1β or IGF-1 | Positive | PEMF and IGF-1 increased S-sulfate incorporation and counteracted the inhibitory effect of IL1β. | Ongaro et al.43 |
| Frequency: 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Human chondrocytes from OA knee | Explants culture | PEMF: 4 h per day for 4 days | None | PEMF did not influence DNA content and GAG content in the explants. | Sadoghi et al.45 |
| Frequency: 75 Hz | |||||
| Intensity: 1.6 mT | |||||
| Human immortalized chondrocytes (T/C-28a2) | Monolayer culture | PEMF: 24 h with IL1β | Positive | PEMF upregulated A2A and A3 receptor expression and decreased pro-inflammatory cytokine release by IL1β. | Vincenzi et al.41 |
| Frequency: 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Human chondrocytes from OA knee | Monolayer culture | PEMF: 1 h per day for 3 days | Positive | PEMF with 0.1HZ and 1.95 μT produced the most favorable response on cell viability, ECM production, and proliferation. | Anbarasan et al.36 |
| Frequency: 0.1–10 Hz | |||||
| Intensity: 0.65–1.95 μT | |||||
| Human umbilical cord-derived mesenchymal stem cells | Monolayer culture | PEMF: 8 h per day for 21 days | Positive | PEMF increased cell proliferation and accelerated chondrocyte differentiation. | Esposito et al.46 |
| Frequency: 75Hz | |||||
| Human ADSCs | 2D hydroxyapatite culture | PEMF: 2, 4, or 8 h per day Frequency: 15 Hz |
Positive | PEMF enhanced the chondrogenic differentiation of human ADSCs in both 2D and 3D cultures. | Chen et al.47 |
| 3D pellet culture | |||||
| Human bone marrow-derived stromal cells (BMSCs) | Pellet culture | PEMF: continuous for 3 weeks Frequency: 15 Hz Intensity: 0.4T |
Positive | PEMF has a synergistic effect with TGF-β3 on chondrogenic differentiation. PEMF alone caused TGF-β secretion in culture. |
Amin et al.48 |
2D, two-dimensional; 3D, three-dimensional; ADSCs, adipose-derived stem cells; ECM, extracellular matrix; GAG, glycosaminoglycan; IGF-I, insulin-like growth factor I; IL, interleukin; OA, osteoarthritis; PEMF, pulsed electromagnetic fields; PG, proteoglycan; TGF-β, transforming growth factor β.
PEMF also increased proliferation in human healthy chondrocytes as well as in human OA chondrocytes.35,36 Fitzsimmons et al. revealed that PEMF increased human chondrocyte proliferation through nitric oxide signaling.37 PEMF has positive effects on PG synthesis in human OA chondrocytes38 and preservation of chondrocyte morphology in monolayer culture.39 Further, PEMF has a protective effect on the catabolic environment. PEMF upregulated A2A and A3 adenosine receptor expression, resulting in the decrease in pro-inflammatory cytokine release.40,41 Insulin-like growth factor I (IGF-I) plays an anabolic role in chondrocyte metabolism,42 and PEMF enhances the effect of IGF-I.43 However, controversial effects of PEMF are still reported. Schmidt-Rohlfing et al. reported that PEMF had no effect on gene expression of type II collagen44 and aggrecan in human OA chondrocyte culture, and Sadoghi et al. reported that PEMF had no effect on proliferation and GAG synthesis in human OA explant culture.45 There is no established protocol for PEMF treatment, and many different waveforms of PEMF were used. Thus, this may affect the controversial results.
The beneficial effect of PEMF on chondrogenic differentiation from stem cells was also reported. Esposito et al. treated human umbilical cord-derived stem cells with PEMF and revealed that PEMF enhanced cell proliferation and chondrogenic differetiation.46 Chen et al. also reported that PEMF enhanced the chondrogenic differentiation from human adipose-derived stem cells in both two-dimensional and three-dimensional cultures.47 Amin et al. reported that PEMF upregulates TGF-β secretion and promotes chondrogenic differentiation through the TGF-β pathway.48 Thus, PEMF might have beneficial effects on chondrocyte proliferation, differentiation from stem/progenitor cells release of anti-inflammatory cytokines, and upregulation of extracellular matrix synthesis through adenosine receptors and the TGF-β pathway. However, the different cell sources as well as the different protocols of PEMF might affect the different outcomes.
The Effects of PEMF on Articular Cartilage In Vivo
Positive effects of PEMF on chondrocytes and cartilage were also demonstrated in in vivo studies (Table 2). Zhou et al. revealed that PEMF improved histological scores and reduced MMP-13 expression in the anterior cruciate ligament transection model of rats.49 A similar effect was seen in ovariectomized rats and PEMF also upregulated X-linked inhibitor of apoptosis (XIAP), which is considered the most potent caspase inhibitor.50 Fini et al. treated aged guinea pigs with PEMF up to 12 weeks and found that PEMF preserved morphology of articular cartilage as well as subchondral bone thickness.51,52 Ciombor et al. adopted the same model and treated it with PEMF for 6 months. They showed that PEMF preserved morphology of articular cartilage for a long time and also found that PEMF increased the numbers of cells immunopositive to TGF-β and decreased those immunopositive to IL-1.53 TGF-β plays a significant role in the anabolism of chondrocytes.54 Therefore, PEMF is believed to have both anabolic and anti-catabolic effects. Veronesi et al. investigated two different frequencies of PEMF (37 and 75 Hz) in aged guinea pigs and revealed that PEMF at 75 Hz had more beneficial effects on cartilage preservation.55 They applied this system to a cartilage defect model of rabbit and found that PEMF improved the cartilage regeneration and the combination with bone marrow concentrate further improved the regeneration.56 Boopalan et al. also used a cartilage defect model of rabbit and showed similar results.57 Benazzo et al. adapted the osteochondral autografts model of sheep and revealed that PEMF increased TGF-β1 and decreased IL1 and TNFα, and, as a result, PEMF improved the regeneration of the defect site.58 These studies demonstrated that PEMF has beneficial effects on cartilage regeneration.
Table 2.
The Effects of Pulsed Electromagnetic Fields on Articular Cartilage In Vivo
| Species | Model | Treatment | Effect | Results | Reference |
|---|---|---|---|---|---|
| Sprague-Dawley rats | Ovariectomized | PEMF: 40 m per day for 30 days | Positive | PEMF inhibited OA progression. In ovariectomized rats, PEMF upregulated XIAP expression and downregulated Bax expression. |
Li et al.50 |
| Frequency: 8 Hz | |||||
| Intensity: 3.8 mT | |||||
| Sprague-Dawley rats | ACLT | PEMF: 40 m per day for 12 weeks Frequency: 20 Hz |
Positive | Mankin scores in the PEMF group were significantly lower than in the ACLT group. | Zhou et al.49 |
| MMP-13 expression was significantly higher in the ACLT group than in the sham group; however, PEMF reduced the expression after ACLT induction. | |||||
| Dunkin Hartley guinea pig 12 months old | Natural OA | PEMF: 1 h per day for 6 months Frequency: 1.5Hz |
Positive | PEMF preserved the morphology of articular cartilage and retarded OA development. | Ciombor et al.53 |
| PEMF increased the numbers of cells immunopositive to TGF-β and decreased those immunopositive to IL1. | |||||
| Dunkin Hartley guinea pig 12 months old | Natural OA | PEMF: 6 h per day for 3 months | Positive | PEMF preserved the morphology of articular cartilage and slowed OA progression | Fini et al.51 |
| Frequency: 75 Hz | |||||
| Intensity: 1.6 mT | |||||
| Dunkin Hartley guinea pig 12 months old | Natural OA | PEMF: 6 h per day for 6 months | Positive | PEMF improved histochemical score, cartilage thickness, and subchondral bone thickness of OA lesion. | Fini et al.52 |
| Frequency: 75 Hz | |||||
| Intensity: 1.6 mT | |||||
| Dunkin Hartley guinea pig 24 months old | Natural OA | PEMF: 6 h per day for 3 months | Positive | At both frequencies, PEMF significantly improved histological score; however, PEMF at 75 Hz produced more beneficial effects. | Veronesi et al.55 |
| Frequency: 37 or 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Rabbit | Full-thickness cartilage defect filled with calcium phosphate scaffold | PEMF: 1 h per day for 6 weeks | Positive | Histological score was significantly better in the PEMF-treated group. | Boopalan et al.57 |
| Frequency: 1Hz | |||||
| Rabbit | Cartilage defect filled with bone marrow concentrate (BMC) and collagen scaffold | PEMF: 4 h per day for 40 days | Positive | PEMF improved both cell and matrix parameters compared with scaffold alone. The combination with BMC and PEMF further improved osteochondral regeneration. |
Veronesi et al.56 |
| Frequency: 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Sheep | Osteochondral autografts | PEMF: 6 h per day for 6 months Frequency: 75 Hz Intensity: 45 μT |
Positive | PEMF increased TGF-β1 and decreased IL1 and TNFα in synovial fluid. | Benazzo et al.58 |
| One month after intervention, larger bone formation was observed in PEMF-treated grafts. | |||||
| In the long term, PEMF limited the bone resorption in subchondral bone. |
ACLT, anterior cruciate ligament transaction; XIAP, X-linked inhibitor of apoptosis.
The Effects of PEMF on Ligaments and Tendons
Diarthrodial joints consist not only of bone and cartilage but also of ligaments and tendons (Table 3). We were unable to find any articles about the regeneration of ligaments and tendons in the human joints in vivo; however, there are several articles regarding PEMF and tendon repair in animal models and human tenocytes culture. PEMF increased in tensile strength of repaired Achilles tendon of rats59 and also improved early tendon healing in the rotator cuff repair model of rats.60 A similar effect was seen in human tenocytes culture.61 de Girolamo et al. treated human tenocytes with PEMF up to 12 h and revealed that PEMF increased tendon-specific markers, such as scleraxis and COL1A1, in a dose-dependent manner.62,63 PEMF has been also shown to enhance gene expression of growth factors in human tenocytes culture under inflammatory condition.64 Further, PEMF maintained stem cell properties of human tendon stem cells.65
Table 3.
The Effects of Pulsed Electromagnetic Fields on Ligaments and Tendons
| Species | Culture or model | Treatment | Effect | Results | Reference |
|---|---|---|---|---|---|
| Rat | Achilles' tendon repair model | PEMF: Twice daily for 30-m sessions for 3 weeks | Positive | PEMF increased in tensile strength of repair site up to 60%. | Strauch et al.59 |
| Frequency: 27.12 MHz | |||||
| Sprague-Dawley rats | Rotator cuff repair model | PEMF: 3 h per day for 16 weeks | Positive | PEMF improved early tendon healing. | Tucker et al.60 |
| Frequency: 3.85 kHz | |||||
| Intensity: 0.5 mT | |||||
| Human tendon cells from semitendinosus and gracilis tendon | Monolayer culture | PEMF; 4, 8, and 12 h | Positive | PEMF increased SCX and COL1A1 in a dose-dependent manner. PEMF for 8 and 12 h significantly increased anti-inflammatory cytokines. |
de Girolamo et al.62 |
| Frequency: 75 Hz | |||||
| Intensity: 1.5 mT | |||||
| Human tendon fibroblasts from patella tendon | Monolayer culture | PEMF at 33 Hz for 10 m per day or PEMF at 7.8 Hz for 20 m per day | Positive | The mean time for bridging the scraped gap was significantly shorter in the PEMF-treated group. | Seeliger et al.61 |
| Intensity: 0.25–3.16 μT | |||||
| Human tendon cells from semitendinosus and gracilis tendon | Monolayer culture | PEMF; 8 h or 12 h | Positive | Proliferation was enhanced by both intensities; however, 1.5 mT PEMF elicited the highest upregulation of SCX, VEGF-A, and COL1A1. | de Girolamo et al.63 |
| Intensity: 1.5 or 3 mT | |||||
| Human tendon stem cells from supraspinatus tendon | Monolayer culture | After PEMF treatment for 1 h, cells were cultured up to 48 h. | Positive | PEMF did not affect proliferation, viability, migration, and morphology. However, PEMF-treated cells maintained a higher expression of stem cell markers. |
Randelli et al.65 |
| Frequency: 10–30 Hz | |||||
| Intensity: 0.5–1.5 mT | |||||
| Human rotator cuff tenocytes | Monolayer culture | PEMF: 3 h per day for 2 weeks with IL1α | Positive | PEMF enhanced gene expression of growth factors under inflammatory conditions. | Liu et al.64 |
| Frequency: 3.85 kHz | |||||
| Intensity: 0.5 mT |
SCX, scleraxis.
The Clinical Effects of PEMF on Joints
PEMF has been used for more than 20 years for the treatment of OA joints (Table 4). However, there appears to be a lack of consensus, and recent studies demonstrated that PEMF has a moderate effect on OA treatment. Bagnato et al. revealed that 1-month treatment of PEMF significantly reduced pain and improved functional scores in knee OA.66 The effects of PEMF on knee OA were seen especially in early OA and in younger patients who are less than 65 years.67,68 In other studies, PEMF was combined with surgical treatments. Zorzi et al. combined arthroscopic cartilage abrasion and PEMF treatment. They showed that functional scores were significantly better in the PEMF-treated group 90 days after surgery, and the numbers of completely recovered patients were significantly higher in the PEMF-treated group at 3 years follow-up.69 Osti et al. combined microfracture and PEMF for the treatment of knee OA and reported that the clinical and functional outcomes were better in the PEMF-treated group after 5 years follow-up.70 However, Reilingh et al. treated osteochondral talar defects by microfracture and PEMF, but they did not find any beneficial effects of PEMF.71 The continuing progress in this area permits one to conclude that PEMF might have a beneficial effect on the treatment of knee OA. However, there are no established clinical protocols for PEMF treatment for the regeneration of articular cartilage in the diarthrodial joints.
Table 4.
The Clinical Effects of Pulsed Electromagnetic Fields on Joints
| Model | Treatment | Effect | Results | Reference |
|---|---|---|---|---|
| Knee OA | PEMF: 2 h per day for 6 weeks | Positive | PEMF significantly improved stiffness after 2 weeks in patients <65 years. | Thamsborg et al.67 |
| Frequency: 50Hz | ||||
| Arthroscopic treatment of knee cartilage | PEMF: 6 h per day for 90 days | Positive | Knee injury and OA outcome score at 90 days was significantly higher in the PEMF-treated group. At 3 years follow-up, the numbers of completely recovered patients were significantly higher in the PEMF-treated group. |
Zorzi et al.69 |
| Frequency: 75 Hz | ||||
| Intensity: 1.5 mT | ||||
| Early knee OA | PEMF: 4 h per day for 45 days | Positive | PEMF significantly improved symptoms, function, and activity at 1 year follow-up. | Gobbi et al.68 |
| Frequency: 75 Hz | ||||
| Intensity: 1.5 mT | ||||
| Microfracture for knee OA | PEMF: 6 h per day for 60 days | Positive | At 5 years follow-up, clinical and functional outcomes were better in the PEMF-treated group. | Osti et al.70 |
| Frequency: 75 Hz | ||||
| Intensity: 1.5 mT | ||||
| Knee OA | PEMF: 12 h per day for 1 month | Positive | PEMF induced a significant reduction in visual analog scale pain and The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. | Bagnato et al.66 |
| Frequency: 27.12MHz | ||||
| Microfracture of osteochondral talar defects | PEMF: 4 h per day for 60 s | None | There were no significant between-group differences in sport resumption and bone repair. | Reilingh et al.71 |
| Frequency: 75 Hz | ||||
| Intensity: 1.5 mT |
Conclusions
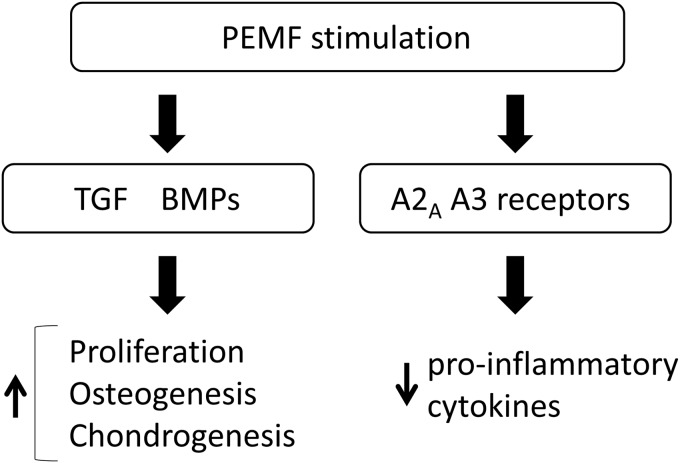
PEMF has a beneficial effect on chondrocyte proliferation, matrix synthesis, and chondrogenic differentiation by upregulation of TGF-β and BMPs, and it decreases anti-inflammmatory cytokines via A2A and A3 adenosine receptors in in vitro studies (Fig. 2). In in vivo studies, PEMF has beneficial effects on OA progression and cartilage defects. PEMF also has a positive effect on tendon repair. In clinical translational investigations, PEMF has a beneficial effect on pain and functions of OA knees. On the other hand, some of the studies showed no effect of PEMF on cell proliferation and matrix synthesis may be due to different protocols such as waveforms or stimulation duration. Although PEMF might have a beneficial effect on cartilages and tendons, it is important to establish an optimized protocol for PEMF treatments.
FIG. 2.
The mechanisms of PEMF for cartilage regeneration. PEMF upregulates the expression of TGF and BMPs to increase cell proliferation, osteogenesis, and chondrogenesis. PEMF inhibits pro-inflammatory cytokines through adenosine receptors A2A and A3. BMPs, bone morphogenetic proteins.
Acknowledgments
This investigation was supported by the Lawrence J. Ellison Endowed Chair in Musculoskeletal Molecular Biology at the University of California, Davis, and in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, AR 061496. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Schinagl R.M., Gurskis D., Chen A.C., and Sah R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res 15, 499, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Ivkovic A., Marijanovic I., Hudetz D., Porter R.M., Pecina M., and Evans C.H. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 3, 923, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Pavone V., Boettner F., Fickert S., and Sculco T.P. Total condylar knee arthroplasty: a long-term followup. Clin Orthop Relat Res 18, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Swann D.A., Slayter H.S., and Silver F.H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem 256, 5921, 1981 [PubMed] [Google Scholar]
- 5.Marcelino J., Carpten J.D., Suwairi W.M., et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23, 319, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Flannery C.R., Zollner R., Corcoran C., et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum 60, 840, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Niikura T., and Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum 56, 2312, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Lee S.Y., Niikura T., and Reddi A.H. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A 14, 1799, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Reddi A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol 16, 247, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Einhorn T.A. The cell and molecular biology of fracture healing. Clin Orthop Relat Res S7, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Rai V., Dilisio M.F., Dietz N.E., and Agrawal D.K. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A 105, 2343, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Mow V.C., Ratcliffe A., and Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 13, 67, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Abrikosova S., and Balabolkin M.I. [Hormonal function of the thyroid gland in chronic alcoholism]. Sov Med 112, 1987 [PubMed] [Google Scholar]
- 14.Sharma L. Nonpharmacologic management of osteoarthritis. Curr Opin Rheumatol 14, 603, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Shupak N.M., Hensel J.M., Cross-Mellor S.K., Kavaliers M., Prato F.S., and Thomas A.W. Analgesic and behavioral effects of a 100 microT specific pulsed extremely low frequency magnetic field on control and morphine treated CF-1 mice. Neurosci Lett 354, 30, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Trock D.H., Bollet A.J., Dyer R.H., Jr., Fielding L.P., Miner W.K., and Markoll R. A double-blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J Rheumatol 20, 456, 1993 [PubMed] [Google Scholar]
- 17.Zhuang H., Wang W., Seldes R.M., Tahernia A.D., Fan H., and Brighton C.T. Electrical stimulation induces the level of TGF-beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun 237, 225, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Bodamyali T., Bhatt B., Hughes F.J., et al. Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Commun 250, 458, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Bjordal J.M., Johnson M.I., Lopes-Martins R.A., Bogen B., Chow R., and Ljunggren A.E. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord 8, 51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannouche D., Petite H., and Sedel L. Current trends in the enhancement of fracture healing. J Bone Joint Surg Br 83, 157, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Yasuda I. The classic: fundamental aspects of fracture treatment by Iwao Yasuda, reprinted from J. Kyoto Med. Soc., 4:395–406, 1953. Clin Orthop Relat Res 5, 1977 [PubMed] [Google Scholar]
- 22.Bassett C.A., Pawluk R.J., and Becker R.O. Effects of electric currents on bone in vivo. Nature 204, 652, 1964 [DOI] [PubMed] [Google Scholar]
- 23.Friedenberg Z.B., Harlow M.C., and Brighton C.T. Healing of nonunion of the medial malleolus by means of direct current: a case report. J Trauma 11, 883, 1971 [DOI] [PubMed] [Google Scholar]
- 24.Becker R.O., Spadaro J.A., and Marino A.A. Clinical experiences with low intensity direct current stimulation of bone growth. Clin Orthop Relat Res 75, 1977 [PubMed] [Google Scholar]
- 25.Sisken B. Therapeutic aspects of electromagnetic fields for soft-tissue healing. Am Chem Soc 250, 277, 1995 [Google Scholar]
- 26.Zhou J., He H., Yang L., et al. Effects of pulsed electromagnetic fields on bone mass and Wnt/beta-catenin signaling pathway in ovariectomized rats. Arch Med Res 43, 274, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Victoria G., Petrisor B., Drew B., and Dick D. Bone stimulation for fracture healing: what's all the fuss? Indian J Orthop 43, 117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciombor D.M., and Aaron R.K. The role of electrical stimulation in bone repair. Foot Ankle Clin 10, 579, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Fini M., Pagani S., Giavaresi G., et al. Functional tissue engineering in articular cartilage repair: is there a role for electromagnetic biophysical stimulation? Tissue Eng Part B Rev 19, 353, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Chang C.H., Loo S.T., Liu H.L., Fang H.W., and Lin H.Y. Can low frequency electromagnetic field help cartilage tissue engineering? J Biomed Mater Res A 92, 843, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Chang S.H., Hsiao Y.W., and Lin H.Y. Low-frequency electromagnetic field exposure accelerates chondrocytic phenotype expression on chitosan substrate. Orthopedics 34, 20, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Bobacz K., Graninger W.B., Amoyo L., and Smolen J.S. Effect of pulsed electromagnetic fields on proteoglycan biosynthesis of articular cartilage is age dependent. Ann Rheum Dis 65, 949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Mattei M., Fini M., Setti S., et al. Proteoglycan synthesis in bovine articular cartilage explants exposed to different low-frequency low-energy pulsed electromagnetic fields. Osteoarthritis Cartilage 15, 163, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Veronesi F., Fini M., Giavaresi G., et al. Experimentally induced cartilage degeneration treated by pulsed electromagnetic field stimulation; an in vitro study on bovine cartilage. BMC Musculoskelet Disord 16, 308, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Mattei M., Caruso A., Pezzetti F., et al. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res 42, 269, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Anbarasan S., Baraneedharan U., Paul S.F., Kaur H., Rangaswami S., and Bhaskar E. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes: an experimental study. Indian J Orthop 50, 87, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzsimmons R.J., Gordon S.L., Kronberg J., Ganey T., and Pilla A.A. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J Orthop Res 26, 854, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Fioravanti A., Nerucci F., Collodel G., Markoll R., and Marcolongo R. Biochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapy. Ann Rheum Dis 61, 1032, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahns M.E., Lou E., Durdle N.G., et al. The effect of pulsed electromagnetic fields on chondrocyte morphology. Med Biol Eng Comput 45, 917, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Varani K., De Mattei M., Vincenzi F., et al. Characterization of adenosine receptors in bovine chondrocytes and fibroblast-like synoviocytes exposed to low frequency low energy pulsed electromagnetic fields. Osteoarthritis Cartilage 16, 292, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Vincenzi F., Targa M., Corciulo C., et al. Pulsed electromagnetic fields increased the anti-inflammatory effect of A(2)A and A(3) adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 8, e65561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonassar L.J., Grodzinsky A.J., Frank E.H., Davila S.G., Bhaktav N.R., and Trippel S.B. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res 19, 11, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Ongaro A., Pellati A., Masieri F.F., et al. Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics 32, 543, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Rohlfing B., Silny J., Woodruff S., and Gavenis K. Effects of pulsed and sinusoid electromagnetic fields on human chondrocytes cultivated in a collagen matrix. Rheumatol Int 28, 971, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Sadoghi P., Leithner A., Dorotka R., and Vavken P. Effect of pulsed electromagnetic fields on the bioactivity of human osteoarthritic chondrocytes. Orthopedics 36, e360, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Esposito M., Lucariello A., Costanzo C., et al. Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo 27, 495, 2013 [PubMed] [Google Scholar]
- 47.Chen C.H., Lin Y.S., Fu Y.C., et al. Electromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitro. J Appl Physiol (1985) 114, 647, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Amin H.D., Brady M.A., St-Pierre J.P., Stevens M.M., Overby D.R., and Ethier C.R. Stimulation of chondrogenic differentiation of adult human bone marrow-derived stromal cells by a moderate-strength static magnetic field. Tissue Eng Part A 20, 1612, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J., Liao Y., Xie H., et al. Pulsed electromagnetic field ameliorates cartilage degeneration by inhibiting mitogen-activated protein kinases in a rat model of osteoarthritis. Phys Ther Sport 24, 32, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Li S., Luo Q., Huang L., Hu Y., Xia Q., and He C. Effects of pulsed electromagnetic fields on cartilage apoptosis signalling pathways in ovariectomised rats. Int Orthop 35, 1875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fini M., Giavaresi G., Torricelli P., et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res 23, 899, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Fini M., Torricelli P., Giavaresi G., et al. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother 62, 709, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Ciombor D.M., Aaron R.K., Wang S., and Simon B. Modification of osteoarthritis by pulsed electromagnetic field—a morphological study. Osteoarthritis Cartilage 11, 455, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Grimaud E., Heymann D., and Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev 13, 241, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Veronesi F., Torricelli P., Giavaresi G., et al. In vivo effect of two different pulsed electromagnetic field frequencies on osteoarthritis. J Orthop Res 32, 677, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Veronesi F., Cadossi M., Giavaresi G., et al. Pulsed electromagnetic fields combined with a collagenous scaffold and bone marrow concentrate enhance osteochondral regeneration: an in vivo study. BMC Musculoskelet Disord 16, 233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boopalan P.R., Arumugam S., Livingston A., Mohanty M., and Chittaranjan S. Pulsed electromagnetic field therapy results in healing of full thickness articular cartilage defect. Int Orthop 35, 143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benazzo F., Cadossi M., Cavani F., et al. Cartilage repair with osteochondral autografts in sheep: effect of biophysical stimulation with pulsed electromagnetic fields. J Orthop Res 26, 631, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Strauch B., Patel M.K., Rosen D.J., Mahadevia S., Brindzei N., and Pilla A.A. Pulsed magnetic field therapy increases tensile strength in a rat Achilles' tendon repair model. J Hand Surg Am 31, 1131, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Tucker J.J., Cirone J.M., Morris T.R., et al. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model. J Orthop Res 35, 902, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeliger C., Falldorf K., Sachtleben J., and van Griensven M. Low-frequency pulsed electromagnetic fields significantly improve time of closure and proliferation of human tendon fibroblasts. Eur J Med Res 19, 37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Girolamo L., Stanco D., Galliera E., et al. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem Biophys 66, 697, 2013 [DOI] [PubMed] [Google Scholar]
- 63.de Girolamo L., Vigano M., Galliera E., et al. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field. Knee Surg Sports Traumatol Arthrosc 23, 3443, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Liu M., Lee C., Laron D., et al. Role of pulsed electromagnetic fields (PEMF) on tenocytes and myoblasts-potential application for treating rotator cuff tears. J Orthop Res 35, 956, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Randelli P., Menon A., Ragone V., et al. Effects of the pulsed electromagnetic field PST(R) on human tendon stem cells: a controlled laboratory study. BMC Complement Altern Med 16, 293, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagnato G.L., Miceli G., Marino N., Sciortino D., and Bagnato G.F. Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology (Oxford) 55, 755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thamsborg G., Florescu A., Oturai P., Fallentin E., Tritsaris K., and Dissing S. Treatment of knee osteoarthritis with pulsed electromagnetic fields: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 13, 575, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Gobbi A., Lad D., Petrera M., and Karnatzikos G. Symptomatic early osteoarthritis of the knee treated with pulsed electromagnetic fields: Two-year follow-up. Cartilage 5, 78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zorzi C., Dall'Oca C., Cadossi R., and Setti S. Effects of pulsed electromagnetic fields on patients' recovery after arthroscopic surgery: prospective, randomized and double-blind study. Knee Surg Sports Traumatol Arthrosc 15, 830, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Osti L., Del Buono A., and Maffulli N. Application of pulsed electromagnetic fields after microfractures to the knee: a mid-term study. Int Orthop 39, 1289, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Reilingh M.L., van Bergen C.J., Gerards R.M., et al. Effects of pulsed electromagnetic fields after debridement and microfracture of osteochondral talar defects: Response. Am J Sports Med 44, NP61, 2016 [DOI] [PubMed] [Google Scholar]