Abstract
The physis, or growth plate, is a cartilaginous region at the end of children's long bones that serves as the primary center for longitudinal growth and characterizes the immature skeleton. Musculoskeletal injury, including fracture, infection, malignancy, or iatrogenic damage, has risk of physeal damage. Physeal injuries account for 30% of pediatric fractures and may result in impaired bone growth. Once damaged, cartilage tissue within the physis is often replaced by unwanted bony tissue, forming a “bony bar” that can lead to complications such as complete growth arrest, angular or rotational deformities, and altered joint mechanics. Children with a bony bar occupying <50% of the physis usually undergo bony bar resection and insertion of an interpositional material, such as a fat graft, to prevent recurrence and allow the surrounding uninjured physeal tissue to restore longitudinal bone growth. Clinical success for this procedure is <35% and often the bony bar and associated growth impairments return. Children who are not candidates for bony bar resection due to a physeal bar occupying >50% of their physis undergo corrective osteotomy or bone lengthening procedures. These approaches are complex and have variable success rates. As such, there is a critical need for regenerative approaches to not only prevent initial bony bar formation but also regenerate healthy physeal cartilage following injury. This review describes physeal anatomy, mechanisms of physeal injury, and current treatment options with associated limitations. Furthermore, we provide an overview of the current research using cell-based therapies, growth factors, and biomaterials in the different animal models of injury along with strategic directions for modulating intrinsic injury pathways to inhibit bony bar formation and/or promote physeal tissue formation. Pediatric physeal injuries constitute a unique niche within regenerative medicine for which there is a critical need for research to decrease child morbidity related to this injurious process.
Keywords: : physis, growth plate, stem cells, biomaterials, bony bar, bone growth
Introduction
Injuries incurred by skeletally immature patients are unique both in their causes and gravity of their consequences. Physes, or growth plates, are cartilaginous regions at the ends of children's long bones that function as primary sites of bone elongation. Physeal injury may result from trauma, infection, metabolic abnormalities, or malignancy.
The major concern with physeal injury is that damaged cartilage within the physis can be replaced by bony repair tissue, forming a “bony bar” or “physeal bar”. Depending on the size and location of the injury within the physis, the bony bar may cause asymmetric growth arrest with subsequent angular deformity or complete cessation of longitudinal growth. The latter is a devastating outcome for children that have not yet reached their full height. Current treatment involves surgical resection of the bar and replacement with an interpositional material to preserve normal growth in the remaining physis. Bar reformation and additional growth effects, however, remain major complications of bar excision.
A critical need exists for developing effective treatments for children with physeal injuries, which not only prevent bony bar formation but also regenerate physiologic physeal cartilage and restore normal bone elongation. This review describes physeal anatomy, mechanisms of physeal injury, and current surgical therapies to treat complications resulting from physeal injuries. Furthermore, it discusses ongoing research efforts for physeal injury repair, including stem cell- and biomaterial-based tissue engineering strategies, as well as potential new avenues for physeal cartilage regeneration.
Anatomy, Physiology, and Injury of the Physis
The physis is a complex cartilaginous structure composed of peaks and valleys that lies between the epiphysis and metaphysis at both proximal and distal ends of long bones (Fig. 1). Longitudinal growth occurs in the physis through endochondral ossification, beginning in utero and continuing until the end of puberty.1 Chondrocyte proliferation then slows until the entire physis has undergone ossification, defined as skeletal maturity.2
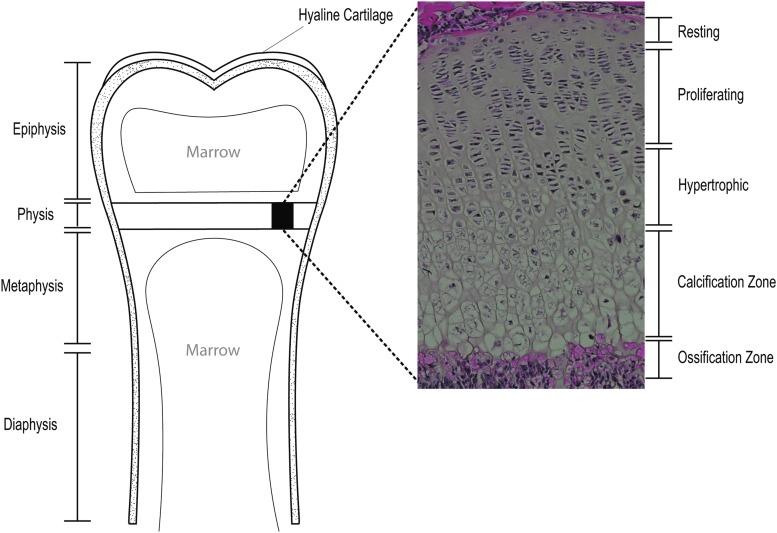
FIG. 1.
Anatomic location of the physis (or growth plate) in long bones. The physis is a cartilaginous region located between the epiphysis and the metaphysis at each end of long bones. Marrow compartments serve as a source of nutrition with vessels feeding the growth plate cartilage. The juxtaposition of hard cortical bone against relatively softer cartilage forms a weak point in the pediatric skeleton. (Expanded section): Five cartilaginous zones of the physis. Resting zone chondrocytes give way into rapidly proliferating chondrocytes that form vertical stacks in the proliferating zone. Cells in the hypertrophic zone enlarge by producing glycogen. In the calcification zone, chondrocytes undergo apoptosis and the extracellular matrix calcifies forming a network for osteoblasts to invade and form new bone in the ossification zone.
Chondrocytes exist within three distinct zones in the physis—the resting zone, the proliferating zone, and the hypertrophic zone (Fig. 1). Closest to the epiphysis, resting zone chondrocytes are hyaline cartilage cells, believed to be the progenitor cell population for the growth plate. Proliferative zone chondrocytes undergo rapid mitosis, forming vertical stacks of chondrocytes which form the basis for longitudinal growth.3 Hypertrophic zone chondrocytes exit the cell cycle, swell in size, and overproduce glycogen to increase extracellular matrix (ECM) volume. The hypertrophic state has long been thought to be the endpoint of chondrocyte differentiation.4
Following hypertrophy, chondrocytes undergo apoptosis leaving a network of calcified matrix for osteoblasts to invade and begin forming bone. Recent lineage-tracing experiments provide evidence that transdifferentiation of hypertrophic chondrocytes to osteoblasts also occurs in addition to apoptosis.5–7 The ECM is then mineralized to form mature bone, a process called ossification, which contributes to longitudinal expansion of the pediatric skeleton.
In addition to unique zonal cellular morphology, the composition of the ECM and mechanical properties change across the physis. For example, the resting zone is predominately made of horizontally aligned collagen II fibers and a low cell:ECM ratio, the proliferative zone has vertically aligned collagen II fibers and a moderate cell:ECM ratio, and the hypertrophic zone is composed predominately of collagen X and a high cell:ECM ratio. These structural properties, as well as others, lead to varying mechanical properties across the physis and have been reviewed elsewhere.8 Briefly, the resting zone is stiffer and more impermeable than the other zones,9,10 and mechanical properties are further influenced by loading, zonal height, and age.11
While both physeal and articular cartilages are variants of hyaline cartilage, they differ in structure and function. These differences are due, in part, to their differing developmental origins: physeal cartilage arises from limb mesenchyme condensations, while articular cartilage comes from the interzone, a thin mesenchyme lining on the ends of future limbs.12 Articular cartilage also has unique zonal organization similar in nature to the physis but with noticeable differences; for example, superficial zone chondrocytes are flattened and produce lubricin while deep zone chondrocytes form columns with vertically aligned collagen fibers that withstand compressive loading. The important difference between these two cartilages is function: the physis is a transient tissue that undergoes endochondral ossification to elongate long bones, while articular cartilage is a permanent tissue designed to protect joint surfaces. Articular cartilage does not calcify, except under pathological conditions.
The physis is vulnerable to injury in that the juxtaposition of relatively soft cartilage against hard bone serves as a weak point in the pediatric skeleton. Complications of physeal damage can range from inconsequential to the generation of a bony bar. The latter occurs when layers of physeal chondrocytes are damaged such that bony repair tissue forms and connects metaphyseal to epiphyseal bone. Lateral or medial physeal bony bar formation may result in asymmetric growth arrest, generating angular limb deformities.13 In severe cases, the bony bar results in complete growth arrest. Classically, injuries resulting in bony bar formation must undergo surgical correction to remove the bony bar and minimize further effects on the limb's growth potential.
Physeal Fractures and the Salter–Harris Classification System
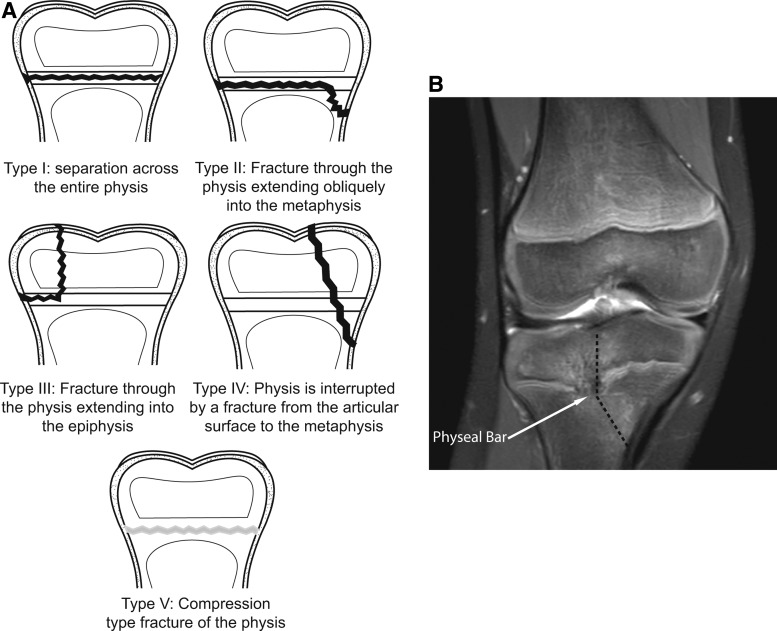
Fractures are one of the most common pediatric traumas, occurring in one in two males and one in three-to-four females.14,15 Of those, between 18% and 30% will involve the physis.16,17 The Salter–Harris (SH) Classification System classifies physeal fractures into five distinct patterns (types I-V) of physeal involvement (Fig. 2A).18 Fracture prognosis and predicting bony bar formation are somewhat dependent on this classification. Compression type fractures (SH type V) are the most likely fracture pattern to result in growth arrest. In the upper extremity, these are followed by fractures that cross the epiphyseal plate (SH types III, IV), which are more likely to demonstrate physeal bar formation (Fig. 2B).19 In the lower extremity, specifically distal femoral physeal fractures, SH types II, III, and IV are the next most common to result in growth disturbances, especially when the fracture is displaced.20
FIG. 2.
The Salter–Harris classification of growth plate fractures. (A) Approximately 5% of physeal fractures are Type I injuries and least likely to cause growth arrest. Type II injuries are the most common, 75% of physeal fractures, and have a moderate potential for arrest. Type III and Type IV each occur in ∼10% of fractures and are more likely to lead to growth arrest. Type V injuries are the least common, but the most likely to cause bony bar formation or growth arrest. (B) Anteroposterior MRI of a 9-year-old female who suffered a SHIV fracture (dashed line) of her right proximal tibial physis 10-months prior. The bony bar (arrow) begins central and extends lateral in location, causing significant misalignment of the knee with leg shortening.
The three most common fracture sites resulting in physeal injury are the wrist, the ankle, and the distal femur. Wrist fractures, specifically distal radius fractures, are one of the most common pediatric fracture types, resulting from high-energy sports trauma.21 Physeal arrest in distal radial fractures have an estimated incidence of 1–7%.22 Complications of physeal arrest in the distal radius include limb length discrepancy between the injured and uninjured arms, angular deformities of the radius leading to compromised biomechanics, and the potential to develop subsequent wrist arthritis.
Lower extremity physeal injuries occur primarily at the distal femoral physis (knee) or the distal tibial physis (ankle).23 Distal femoral fractures for the preadolescent child can be particularly devastating, as growth disturbance occurs in 52–90% of these injuries.20,24 The distal femoral physis accounts for up to 70% of longitudinal growth of the femur and up to 40% of total longitudinal growth of the lower extremity. Consequences of these injuries are dramatic, including significant leg length discrepancy and angular deformities leading to significant gait disturbances, low back pain, cosmetic deformity, and early-onset arthritis. Complete or partial premature closure of the distal tibial physis is the most common complication of SH types III and IV distal tibial fractures. Resulting growth discrepancies are less significant than those seen in distal femur fractures; however, rotational disturbances and altered ankle joint mechanics can also have long-term consequences for the individual.25,26
Rare Etiologies of Physeal Damage
Although less common than fracture, the physis may be damaged by infection, malignancy, or by iatrogenic damage, an unintended surgical complication. Infectious etiologies of physeal injury include hematogenous osteomyelitis of metaphyseal bone with extension into the growth plate. As blood flows through the narrow capillaries within the marrow compartment of long bones, low fluid flow rates can promote bacterial stasis and facilitate infection.27 In severe cases, resulting chondronecrosis and abscess formation resulting from infection lead to bony bar formation and its complications as described above.28
Pediatric bone tumors, including osteosarcoma and Ewing's sarcoma, may also lead to physeal damage.29 Damage may be secondary to the degree of tumor physeal involvement or from tumor resection surgery.30–32 Radiation therapy and chemotherapy regimens also may potentially cause physeal damage by interfering with normal chondrocyte physiology.33–37
During the treatment of pediatric musculoskeletal conditions, unintended physeal damage may occur. Premature physeal closure has occurred secondary to limb lengthening procedures.38,39 In addition, anterior cruciate ligament reconstruction may result in unintended physeal damage to either the distal femoral or proximal tibial physis, potentially leading to growth arrest.40,41 To prevent this, surgical techniques to avoid transphyseal instrumentation have been developed to preserve the physis.42–45
Current Therapeutic Techniques Following Physeal Arrest
Growth arrest, angular or rotational deformities, and subsequent altered joint mechanics are feared consequences of physeal injury in the immature skeleton and may develop up to 2 years postinjury. As such, patients with physeal injuries are followed for a longer duration than other musculoskeletal injuries. Depending on injury characteristics, physicians may choose nonoperative therapy, including casting, to ensure anatomic alignment of the limb with close radiologic follow-up for observation of bony bar formation.46 Severe injuries often require surgical intervention.
When bony bar formation occurs in patients with significant potential growth disturbance, the current gold standard therapy is bony bar resection.47–49 Typically, patients are younger and have a significant (50–70%) portion of healthy uninvolved physis.50 Following bony bar resection, the injury site is filled with an interpositional material such as fat, muscle, or silicone rubber to prevent reformation of bony tissue and allow the uninjured physeal cartilage to restore normal growth. Unfortunately, clinical success for resection ranges from 18% to 35%.51 Avascular fat grafts do not integrate into host tissue. Rather, they break down over time and fail to provide structural stability, leading to collapse of the injured growth plate area and either physeal closure or bony bar recurrence. Other graft materials, such as silicone rubber, are not ideal biomaterials because they do not incorporate within host tissues and may migrate from the surgical site causing subsequent problems. Current interpositional materials offer imperfect solutions and ultimately the bony bar may return and affect growth.52
If pronounced angular limb deformities following bony bar formation exist, corrective osteotomy to the affected limb may be performed to improve limb length and joint biomechanics.53 Osteotomy involves creating a wedge-shaped bone defect, then opening the wedge, lengthening, and correcting the angular deformity.54 Complications include infection, neurovascular injury, additional physeal damage, or recurrence of the angular deformity.55,56
In severe cases, ipsilateral epiphysiodesis, artificial closure of the physis, may be performed following bony bar formation to prevent further limb angulation. Generally, this is performed in cases with minimal residual growth potential, or cases where the bony bar occupies more than 50% of the physeal volume.57 Closing the injured physis limits the degree of subsequent angular deformity that can occur. In cases where several centimeters of limb growth is anticipated, but the bony bar occupies more than 50% of physeal volume, bilateral epiphysiodesis is performed, tethering both physes to minimize limb length discrepancy.58 Complications include unpredictable growth arrest leading to continued limb length discrepancies or worsened angular deformities.59,60
Successful restoration of growth following bony bar formation is limited with current therapeutic options. Existing interpositional materials are insufficient in restoring longitudinal growth in that they do not integrate into host tissues and they rely on the uninjured physeal cartilage to preserve growth. Surgical techniques are often limited by the extent of physeal injury. Reformation of the bony bar following resection occurs in up to 15–38% of cases, leading to additional growth disturbance or additional surgeries.51 The unpredictable nature of surgery coupled with imperfect graft materials results in high rates of bar reformation speaking to the critical need for novel, regenerative treatment methods.
Regenerative Approaches to Treat Physeal Injury
The morbidity and unpredictable nature of physeal injuries coupled with current therapeutic limitations establish a critical need to develop effective treatments for affected children. Successful treatments should prevent bony bar formation and simultaneously regenerate native physeal cartilage, restoring normal bone elongation. Regenerative approaches utilizing stem cells, growth factors, and biomaterials have the potential to overcome the shortcomings of current approaches by restoring physeal cartilage and, thus, may play an important role in the treatment of physeal injuries. An overview of the current animal models of physeal injury and research using cell-based therapies, growth factors, and biomaterials in the different animal models of injury along with strategic directions for modulating intrinsic injury pathways is presented below.
Animal models of physeal injury
To investigate the different regenerative medicine approaches, animal models of physeal injury have been developed where injury to the physis results in bony repair tissue, mimicking the bony bar formation seen in pediatric patients. In addition to bony bar formation, it has also been shown that tethers can form in the surrounding uninjured physis after injury and are another mechanism of growth dysfunction that should be evaluated.61,62
In small animal models, such as mice and rats, therapeutics can be tested immediately after injury to determine whether they prevent bony bar formation and restore bone elongation. Thus, they are a good initial model to test novel therapeutics. However, due to their small size, it is difficult to resect the bony bar that forms and implant a therapeutic material, as would be performed clinically.
Larger animal models such as the rabbit, miniature pig, or sheep have been used for these types of interventions.63–69 In the larger models, an injury to the physis is created and the bony bar allowed to form. A second intervention is then performed to remove the bony bar and implant a therapeutic material. The desired outcomes are prevention of bony bar reformation, prevention of angular deformities, and restoration of longitudinal growth.
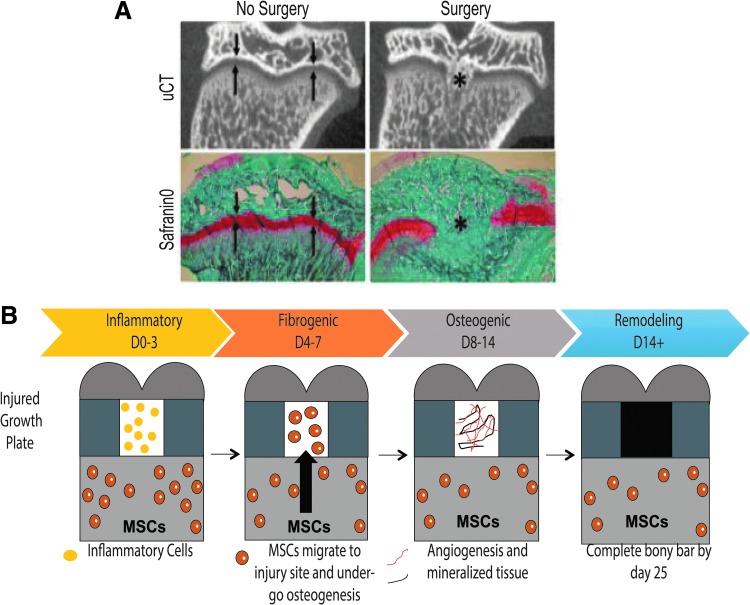
In addition to providing a means to test novel therapeutic strategies, animal models of physeal injury also offer the opportunity to study mechanisms of bony bar formation and identify potential targets for modulation.61,70–74 The rat model of physeal injury has been widely used to investigate pathophysiology.61,62,70,71,74–80 A drill-hole defect in the proximal tibial physis creates a bony bar in a predictable and reproducible manner (Fig. 3A). This well-established model has contributed to identifying four phases of injury repair: inflammatory, fibrogenic, osteogenic, and remodeling (Fig. 3B).70,71,74–81
FIG. 3.
(A) μCT and SafraninO/Fast Green images of rat physes 28 days after surgery. Both μCT and SafraninO staining for cartilage tissue (red) show an intact growth plate in the No Surgery rat, while the rat that underwent surgery displays bony tissue within the growth plate. Arrows show growth plate area. *Shows area of bony bar. (B) Schematic of repair phases after physeal injury in the rat model. MSCs, mesenchymal stem cells.
During the 3 days following injury, inflammatory cells infiltrate the injured area.75,78 From days 4 to 7, mesenchymal stem cells (MSCs) migrate from surrounding marrow compartments and go on to express osteogenic markers such as Runx2, alkaline phosphatase, and osteocalcin (Ocn).74,75,78 Evidence of angiogenesis and formation of mineralized tissue occurs between days 8 and 14.74 Bony remodeling occurs after 14 days, producing a bony bar by 28 days.74 Within each phase, specific cell signaling pathways play central roles, offering opportunities to inhibit osteogenesis or promote chondrogenesis. This injury model also suggests that endogenous MSCs play a central role in bony bar formation and are a potential target for physeal injury therapeutics.
Cellular based therapies
In early studies, implantation of articular cartilage or peripheral physeal cartilage as an interpositional material in a sheep model of growth plate injury inhibited bony bar formation.82 Despite correction of growth deformities, the transplanted cartilage demonstrated variable levels of apoptosis, and normal physeal cartilage was not regenerated.
To optimize integration of implanted chondrocytes into the injured physis, other groups have isolated and embedded chondrocytes within scaffolds. Rabbits treated with physeal implants composed of cultured chondrocytes embedded within agarose gels exhibited no growth arrest or angular deformity compared to untreated animals.83 After 2–4 weeks, implants formed columnar and prehypertrophic chondrocytes similar to native physeal cartilage.83 In pig models, cartilage-like discs generated from autologous articular chondrocytes prevented bony bar formation and growth arrest.84 Cultured epiphyseal cartilaginous disc implants containing epiphyseal chondrocytes also prevented bony bar formation in a sheep model.85 On further investigation, these discs integrated into host growth plate cartilage, forming columnar and prehypertrophic zones mimicking native physeal cartilage.86
These studies suggest that chondrocytes could be used to promote native-like cartilaginous repair tissue. However, using autologous chondrocytes clinically may be limited by the need to isolate cells from healthy pediatric tissues, thus creating secondary injury sites. This has led to the investigation of alternative cell sources such as MSCs.
MSCs are an attractive cell source for tissue engineering due to their availability, immune privilege, and multipotent differentiation capacity, especially toward the bone and cartilage lineages. MSCs originating from various tissue sources have been investigated for physeal injury treatment. MSCs derived from periosteum, bone marrow (BM-MSC), and adipose tissue (AT-MSC) were compared for their ability to treat partial growth arrest in a rabbit model.87 Periosteal and BM-MSC implants corrected angular deformities and growth arrest, while AT-MSCs did not. Furthermore, periosteal MSCs and BM-MSCs yielded native-like repair tissue with columnar chondrocyte arrangement and a prehypertrophic zone, while AT-MSCs resulted in irregular arrangement, suggesting that cell source may affect repair potential.87
Treatment of physeal injuries with MSCs has been successful in rabbit models,64,65,87–92 as well as in pigs.66 In addition, treatment of porcine physeal injuries with a co-MSC/chondrocyte graft yielded favorable repair tissue and prevented growth deformities.67 However, treatment of physeal injuries in a sheep model with BM-MSCs yielded dense, fibrous repair tissue.69 One possible explanation for this discrepancy is the chondrogenic predifferentiation of MSCs that occurred before implantation in the rabbit and porcine studies, but did not occur in the ovine study. A recent study further complicates these findings by reporting that treatment of rat physeal injuries with unstimulated BM-MSCs corrected growth arrest, while treatment with chondrogenically predifferentiated BM-MSCs did not.62 However, treatment with either cell type decreased tether formation in the adjacent, uninjured growth plate, which could reduce growth disturbance. Overall, these studies suggest that while MSCs are a potential cell source for the treatment of physeal injuries, further work is necessary to identify the optimal MSC source and differentiation state.
In addition to the implantation of exogenous MSCs for the treatment of physeal injuries, endogenous stem cells can also participate in tissue repair. As demonstrated in Figure 3, MSCs from adjacent marrow compartments migrate into the injured growth plate, undergo osteogenesis, and form bony repair tissue.74 However, MSCs are also capable of differentiating into a wide variety of connective tissue cells, including cartilage, and may be used to repair growth plate cartilage given the appropriate cues.93–96
MSCs express multiple chemokine receptors, including CXCR1, CXCR2, CCR2, and CXCR4,97–99 and can home to these chemokines, as well as stromal cell-derived factor 1 (SDF-1), interleukin-8, platelet derived growth factors, and transforming growth factor beta (TGF-β) isoforms.97,100–103 SDF-1 has been shown to recruit MSCs from the marrow and improve articular cartilage regeneration following injury, suggesting that SDF-1 may be a potential candidate for treatment of growth plate injuries.104–106
A strategic approach for physeal tissue engineering could include developing composite interpositional biomaterials that provide chemokine factors to recruit endogenous MSCs from nearby marrow compartments. Furthermore, they could provide factors that promote cartilage differentiation, encouraging endogenous MSCs to form cartilage rather than bone, leading to restoration of longitudinal growth and prevention of subsequent complications.
Enhancing chondrogenic potential with growth factors
Whether the cellular based approach for physeal cartilage regeneration relies on exogenous cells or endogenous cells, the cells will need to undergo chondrogenic differentiation to form cartilage tissue successfully. Three of the most extensively studied chondrogenic factors for MSCs and other progenitor cells are insulin like growth factor-1 (IGF-1), TGF-β1 and TGF-β3. Two separate studies demonstrated that treatment of physeal injuries with IGF-I encapsulated in poly(lactic-co-glycolic) acid (PLGA) scaffolds promoted cartilage regeneration and decreased bony repair tissue compared to empty scaffolds.63,107 Bone morphogenetic proteins (BMPs) also play a role in chondrogenesis. Differentiation of human BM-MSCs into proliferative zonal cartilage cells has been demonstrated using sequential exposure of MSCs to TGF-β3 followed by BMP-2.108 In a sheep model, treatment of physeal injuries with locally delivered BMP-7 resulted in an overall increase in growth plate height.109,110 These studies demonstrate the efficacy of growth factor induced reconstruction of growth plate cartilage when delivered in an appropriate manner.
Biomaterial-based approaches
For cells and chondrogenic molecules to have an effect at the site of physeal injury, they must be delivered locally by a material that can serve as a temporary scaffold while new tissue forms. Materials used in the treatment of physeal injuries have included collagen I and II,86 fibrin glue,87 hyaluronate-collagen-fibrin composites,65,92,93 collagen-chitin scaffolds,66,67,111 agarose,61,88 chitin,64 gelatin,69,90 and PLGA.63,107 The outcomes of the studies using these materials are outlined in Table 1.
Table 1.
Brief Overview of Biomaterials Used to Treat Growth Plate Injuries
| Reference | Model | Cell type | Biomaterial | Cell loading | Growth factor | Implantation method | Histology and overall results |
|---|---|---|---|---|---|---|---|
| Foster et al.86 | Sheep, prox. tib. | Lamb growth plate chondrocytes | — | — | — | Implanted, moldable | Growth plate-like repair tissue. No BB, AD, GA |
| Collagen 1 | Seeded | — | Implanted, moldable | BB formation. Immune reaction | |||
| Collagen 2 | Seeded | — | Implanted, moldable | Fibrous and collagenous repair tissue. Prevented BB, but immune reaction | |||
| Untreated | — | — | — | — | BB formation. | ||
| Hui et al.87 | Rabbit, prox. tib. | BM-MSCs | Fibrin | Mixed w cells | — | Injected | Growth plate-like repair tissue. No AD |
| Peri-MSCs | Fibrin | Mixed w cells | — | Injected | Growth plate-like repair tissue. No AD | ||
| AT-MSCs | Fibrin | Mixed w cells | — | Injected | Hyaline-like cartilage. Significant AD | ||
| — | Fibrin | — | — | Injected | BB formation. Significant AD. | ||
| Gál et al.91, Plánka et al.92 | Rabbit, prox. tib. | BM-MSCs | Hyaluronin/collagen/fibrin | Seeded | — | Implanted, rigid | Hyaline-like cartilage. Prevented AD, GA |
| — | Untreated | — | — | — | BB formation. Significant AD | ||
| Planka et al.66; Planka et al.67 | Pig, distal femur | BM-MSCs | Col1/chitosan | Seeded | — | Implanted, rigid | Hyaline-like cartilage. Prevented AD, GA |
| — | Col1/chitosan | — | — | Implanted, rigid | Fibrous repair tissue. Mild AD, GA | ||
| — | Untreated | — | — | — | BB formation. AD and GA | ||
| Chen et al.88 | Rabbit, prox. tib. | Peri-MSCs | Agarose | Seeded | — | Implanted, moldable | Growth plate-like repair tissue. No BB, AD, GA |
| — | Agarose | — | — | Implanted, moldable | BB formation. AD and GA | ||
| Li et al.64 | Rabbit, prox. tib. | Peri-MSCs | Chitin fiber | Seeded | — | Implanted, rigid | Growth plate-like repair tissue. No BB, AD, GA |
| — | Chitin fiber | — | — | Implanted, rigid | Fibrous and collagenous repair tissue. Prevented AD and GA | ||
| McCarty et al.69 | Sheep, prox. tib. | BM-MSCs | Gelfoam® | Seeded | TGFβ1 | Implanted, rigid | Dense fibrous repair tissue. Prevented progression of BB |
| — | Gelfoam | — | TGFβ1 | Implanted, rigid | Dense fibrous repair tissue. Prevented progression of BB | ||
| Ahn et al.90 | Rabbit, prox. tib. | BM-MSCs | Gelatin 5% | Seeded | — | Implanted, rigid | BB formation. Reduced AD |
| BM-MSCs | Gelatin 10% | Seeded | — | Implanted, rigid | BB formation. More reduced AD | ||
| BM-MSCs | Gelfoam | Seeded and cultured 1wk | TGFβ3 | Implanted, rigid | Growth plate-like repair tissue. No AD | ||
| — | Gelfoam | — | — | Implanted, rigid | BB formation. Significant AD | ||
| Sundararaj et al.107 | Rabbit, prox. tib. | — | PLGA | — | IGF-1 | Implanted, rigid | Hyaline-like cartilage |
| — | PLGA | — | — | Implanted, rigid | Delayed BB formation | ||
| — | Untreated | — | — | — | BB formation | ||
| Clark et al.63 | Rabbit, prox. tib. | — | PLGA | — | — | Implanted, rigid | BB formation. GA and AD |
| — | PLGA | — | IGF-1 | Implanted, rigid | Increased chondrogenic repair tissue. Mild AD | ||
| BM-MSCs | PLGA | Seeded | IGF-1 | Implanted, rigid | Increased chondrogenic repair tissue. Mild AD |
AD, angular deformity; AT-MSCs, adipose tissue mesenchymal stem cells; BB, bone bridge; BM-MSCs, bone marrow mesenchymal stem cells; Col1, collagen type I; GA, growth arrest; IGF-1, insulin-like growth factor 1; Peri-MSCs, periosteal mesenchymal stem cells; PLGA, poly(lactic-co-glycolic acid); prox. tib, proximal tibia; TGFβ1, transforming growth factor beta 1.
In all cases, treatment with MSC- or chondrocyte-laden biomaterials produced better results than biomaterials alone. Biomaterial constructs alone often delayed or prevented bony bar formation but ultimately resulted in fibrous repair tissue and mild growth abnormalities. This suggests that a variety of biomaterials may be used to introduce cells, but that cells are ultimately key to restoring physeal cartilage. Thus, it is important to use biomaterials that can effectively deliver biofactors and/or provide an environment that encourages cells to migrate into the material and that can perhaps also direct these cells toward the chondrogenic lineage and/or maintain their chondrogenic phenotype. Longer term studies are also necessary to fully evaluate the effect of biomaterials on skeletal growth.
Physiochemical cues from the microenvironment, such as cell-ECM interactions, cell–cell interactions, and dynamic mechanical forces, influence stem cell differentiation and tissue-synthesizing capabilities.112–117 Recent technology has allowed scientists to use biomaterials as building blocks to incorporate tissue mimetics such as ECM molecules and enzyme-sensitive cross-links for degradation. This can create a cartilage biomimetic environment to promote chondrogenic differentiation of stem cells coming into contact with the hydrogel and has led to promising in vivo results for cartilage repair.118–121
In addition to chemical cues, intrinsic biomaterial stiffness provides mechanical cues, further directing stem cell differentiation. This is especially important when the mechanical properties of the biomaterial direct cells away from the osteogenic lineage and toward the chondrogenic lineage.122 Such cartilage-biomimetic systems warrant further investigation as potential novel interpositional materials that could lead to improved physeal tissue engineering.
A common problem with current materials used clinically, such as fat grafts, is that they do not provide sufficient mechanical support to prevent collapse of the injury site. A scaffolding construct with a load-bearing structural component could minimize force differentials observed within the surrounding physeal cartilage. Recently, groups have investigated the use of three-dimensional (3D) printing to create scaffolding structures, as well as cell-laden hydrogels for use within scaffolds.123–125
Biomaterial constructs that offer a cartilage promoting environment through the presentation of physiochemical cues and a load-bearing structural component may be the future of interpositional materials used after bony bar resection as they would allow for improved physeal repair and restoration of longitudinal growth. These constructs may be ideal for children that are candidates for bony bar resection, as it may be sufficient to form a cartilage-like tissue that would prevent bony bar reformation and allow the uninjured physeal tissue to continue bone elongation. However, for children that are not candidates for resection because they have more than half their physis injured, and therefore insufficient uninjured physeal tissue to continue growth, the treatment strategy would need to be more robust. In these cases, it may be necessary to recapitulate the complex zonal microarchitecture and cellular organization of the physis to restore growth.
Three-dimensional printing technology offers the opportunity to design multiple layers and heterogenous structures within a biomaterial construct to mimic the different zones of the physis with increased accuracy, which would be difficult to achieve with conventional fabrication methods.126 Moreover, cells can be incorporated within the different layers and/or structures. Thus, combining cells, biomaterials, and 3D printing may ultimately be necessary to develop successful physeal tissue engineering approaches that can benefit all patients suffering from physeal injuries.
Modulating intrinsic injury pathways to prevent osteogenesis
Although promoting cartilage repair tissue after physeal injury is of utmost importance, preventing osteogenesis and bony bar formation is also a research avenue that should be pursued. Small animal models, including the rat model of physeal injury, have elucidated critical targets for modulation in physeal injury pathophysiology. In rat growth plate injuries, vascular endothelial growth factor (VEGF) and its receptors are detected during the first 28 days postinjury.80 Systemic delivery of bevacizumab, a humanized anti-VEGF antibody, demonstrated a reduction in osteogenic gene expression, fewer blood vessels, and decreased bony bar formation within the injury site.71 Systemic delivery also led to a reduction in bone growth in the contralateral limb, suggesting adverse effects of systemic delivery.71 Thus, VEGF inhibition warrants further investigation in preventing bony bar formation after physeal injury, specifically, localized antibody delivery to the injury may reduce adverse events. It is important to release the antibody during the repair process to prevent bony bar formation. However, since angiogenesis is necessary for normal bone elongation, the antibody should not remain in the area long term.127 Local, controlled, and timed delivery of antiangiogenic factors can be achieved with drug delivery systems and warrant further investigation in the treatment of growth plate injuries.
In addition to VEGF, other molecular pathways have been implicated in bony bar formation, including those related to Wnt/β-catenin signaling and BMP signaling.72 During late inflammatory and early fibrogenic phases, the Wnt/β-catenin pathway is a key regulator of the osteogenic differentiation of endogenous MSCs at the injury site.72,128 Inhibiting the Wnt/β-catenin pathway after physeal injury in rats by an orally administered inhibitor led to decreased expression of Wnt target genes, decreased osteogenesis and bony bar formation, and also led to increased cartilage tissue in the repair area.128 This suggests that the Wnt/β-catenin signaling pathway is another potential target for preventing bone formation after physeal injury, and this can also be achieved through localized biomaterial-based drug delivery systems.
Modulating pathways involved in ossification could also be achieved using short interfering RNA (siRNA), where key genes responsible for osteogenesis can be silenced, potentially inhibiting bony bar formation. Localized and sustained siRNA delivery has been shown to be possible through biomaterial-based delivery systems.129–131 siRNA molecules have been successfully encapsulated within degradable hydrogels demonstrating sustained release of siRNA molecules. Furthermore, successful integration of siRNA molecules into nanoparticle delivery systems and biodegradable solid polymers show promise for localized gene silencing.131,132 Localized, targeted, gene silencing through siRNA molecules may have a role in modulating intrinsic growth plate pathogenesis and warrant further investigation for correcting and preventing bony bar formation.
MicroRNA (miR) has recently become another target that can be modulated to affect tissue repair. Inhibiting miR-222 expression in vitro improved osteogenic differentiation of human MSCs as demonstrated by increased expression of Runx2, COL1A1, and BGLAP.133 These results translated into improved fracture healing in a rat fracture model when miR-222 inhibitor was administered.133 Since upregulation or inhibition of various miRNA expression patterns has the potential to promote or inhibit osteogenic and chondrogenic differentiation of MSCs,134–136 localized and controlled delivery of miRNA modulators to physeal injury sites may prove important to prohibiting the initial formation of a bony bar and promote physeal cartilage regeneration. Successes in identifying and manipulating key genes in fracture healing using miRNA offer insight into the possibilities of modulating pathways involved in physeal pathogenesis. These successes warrant additional investigation in elucidating and subsequently modulating patterns of miRNA expression in physeal injury.
Conclusion
Physeal injury remains a significant cause of morbidity among the pediatric population. The most significant complication of physeal injury is bony bar formation, either leading to angular limb deformities or complete growth arrest. Current management, surgical or otherwise, has significant limitations and may result in further morbidity in the form of additional surgeries, further development of growth arrest, or progression of angular limb deformities. As such, there is a critical need to develop new treatment strategies for physeal injury that not only prevent bony bar formation but also lead to regeneration of healthy growth plate cartilage, thus restoring normal bone elongation.
Methods under investigation include modulating intrinsic injury pathways to prevent osteogenesis, as well as recruiting or adding stem cells for regenerating damaged physeal cartilage. Additional methods include modulating cellular microenvironments of the injury site and creating interpositional materials with the structural support necessary to prevent collapse of the resection site. Development of an interpositional material with structural support has immediate translational potential as current materials used after bony bar resection, such as fat grafts, lead to poor outcomes. Further developing these interpositional materials using biomaterials that promote cartilage tissue will also be of great benefit to treat children undergoing bony bar resection.
In severe cases, where children are currently not candidates for bony bar resection due to a large physeal injury, it will likely be necessary to engineer a construct that mimics the complex zonal structural and cellular organization of the physis to ensure bone elongation. This will be more challenging, as proper signals will need to be available to the cells to progress from a resting state chondrocyte to a mineralizing cell. It may also be necessary to incorporate exogenous cells, which will increase the regulatory oversight needed to reach clinical trials. However, advances in cell-instructive biomaterials, delivery of cell signaling molecules, and 3D printing technology will allow progress to be made in the development of complex structures, ultimately allowing their clinical translation. Together, these will have important implications in preventing significant morbidity in the pediatric population affected by physeal injuries.
Acknowledgments
Funding support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) under Award Nos. 5R03AR068087, 1R01AR065441, and 1R01AR069060 and the National Institute of Child Health and Human Development of the NIH under Award No. 1R21HD090696. Also from National Science Foundation (NSF) Award No. 1342222. C.E. is supported by NIH/NCATS Colorado CTSA Grant No. UL1 TR001082. Additional funding provided by Children's Hospital Colorado. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NSF.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hosseinzadeh P., and Milbrandt T. The normal and fractured physis: an anatomic and physiologic overview. J Pediatr Orthop B 25, 385, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Ballock R.T., and O'Keefe R.J. The biology of the growth plate. J Bone Joint Surg Am 85-A, 715, 2003 [PubMed] [Google Scholar]
- 3.Wang W., Song B., Anbarchian T., Shirazyan A., Sadik J.E., and Lyons K.M. Smad2 and Smad3 regulate chondrocyte proliferation and differentiation in the growth plate. PLoS Genet 12, e1006352, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang K.Y., Chan D., and Cheah K.S. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev Growth Differ 57, 179, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Bahney C.S., Hu D.P., Taylor A.J., Ferro F., Britz H.M., Hallgrimsson B., Johnstone B., Miclau T., and Marcucio R.S. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res 29, 1269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X., von der Mark K., Henry S., Norton W., Adams H., and de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 10, e1004820, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L., Tsang K.Y., Tang H.C., Chan D., and Cheah K.S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A 111, 12097, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villemure I., and Stokes I.A.F. Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech 42, 1793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergerie K., Lacoursière M.-O., Lévesque M., and Villemure I. Mechanical properties of the porcine growth plate and its three zones from unconfined compression tests. J Biomech 42, 510, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Williams R.M., Zipfel W.R., Tinsley M.L., and Farnum C.E. Solute transport in growth plate cartilage: in vitro and in vivo. Biophys J 93, 1039, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wosu R., Sergerie K., Lévesque M., and Villemure I. Mechanical properties of the porcine growth plate vary with developmental stage. Biomech Model Mechanobiol 11, 303, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Koyama E. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol 316, 62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cass J.R., and Peterson H.A. Salter-Harris Type-IV injuries of the distal tibial epiphyseal growth plate, with emphasis on those involving the medial malleolus. J Bone Joint Surg Am 65, 1059, 1983 [PubMed] [Google Scholar]
- 14.Mäyränpää M.K., Mäkitie O., and Kallio P.E. Decreasing incidence and changing pattern of childhood fractures: a population-based study. J Bone Miner Res 25, 2752, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Naranje S.M., Erali R.A., Warner W.C., Sawyer J.R., and Kelly D.M. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop 36, e45, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Mann D.C., and Rajmaira S. Distribution of physeal and nonphyseal fractures in 2,650 long-bone fractures in children aged 0–16 years. J Pediatr Orthop 10, 713, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Mizuta T., Benson W.M., Foster B.K., Paterson D.C., and Morris L.L. Statistical analysis of the incidence of physeal injuries. J Pediatr Orthop 7, 518, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Salter R.B., and Harris W.R. Injuries involving the epiphyseal plate. J Bone Joint Surg Am 45, 587, 1963 [Google Scholar]
- 19.Sharrard W.J.W. Abnormalities of the epiphysis. Paediatric Orthopaedics and Fractures. 3rd ed. Cambridge, MA: Blackwell Scientific Publications, 1993, pp. 764 [Google Scholar]
- 20.Basener C.J., Mehlman C.T., and DiPasquale T.G. Growth disturbance after distal femoral growth plate fractures in children: a meta-analysis. J Orthop Trauma 23, 663, 2009 [DOI] [PubMed] [Google Scholar]
- 21.MacIntyre N.J., and Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther 29, 136, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Lee B.S., Esterhai J.L., and Das M. Fracture of the distal radial epiphysis. Characteristics and surgical treatment of premature, post-traumatic epiphyseal closure. Clin Orthop Relat Res 185, 90, 1984 [PubMed] [Google Scholar]
- 23.Mayer S., Albright J.C., and Stoneback J.W. Pediatric knee dislocations and physeal fractures about the knee. J Am Acad Orthop Surg 23, 571, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Graham J.M., and Gross R.H. Distal femoral physeal problem fractures. Clin Orthop Relat Res, 51, 1990 [PubMed] [Google Scholar]
- 25.Podeszwa D.A., and Mubarak S.J. Physeal fractures of the distal tibia and fibula (Salter-Harris Type I, II, III, and IV fractures). J Pediatr Orthop 32 Suppl 1, S62, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Kärrholm J., Hansson L.I., and Svensson K. Prediction of growth pattern after ankle fractures in children. J Pediatr Orthop 3, 319, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Browne L.P., Guillerman R.P., Orth R.C., Patel J., Mason E.O., and Kaplan S.L. Community-acquired staphylococcal musculoskeletal infection in infants and young children: necessity of contrast-enhanced MRI for the diagnosis of growth cartilage involvement. AJR Am J Roentgenol 198, 194, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson-Dahdal D., Wright J.E., Krupinski E., McCurdy W.E., and Taljanovic M.S. Transphyseal involvement of pyogenic osteomyelitis is considerably more common than classically taught. AJR Am J Roentgenol 203, 190, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Weitao Y., Qiqing C., Songtao G., and Jiaqiang W. Epiphysis preserving operations for the treatment of lower limb malignant bone tumors. Eur J Surg Oncol 38, 1165, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kumta S.M., Chow T.C., Griffith J., Li C.K., Kew J., and Leung P.C. Classifying the location of osteosarcoma with reference to the epiphyseal plate helps determine the optimal skeletal resection in limb salvage procedures. Arch Orthop Trauma Surg 119, 327, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Mercuri M., Capanna R., Manfrini M., Bacci G., Picci P., Ruggieri P., Ferruzzi A., Ferraro A., Donati D., and Biagini R. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res 264, 156, 1991 [PubMed] [Google Scholar]
- 32.Weisstein J.S., Goldsby R.E., and O'Donnell R.J. Oncologic approaches to pediatric limb preservation. J Am Acad Orthop Surg 13, 544, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Tamurian R.M., Damron T.A., and Spadaro J.A. Sparing radiation-induced damage to the physis by radioprotectant drugs: laboratory analysis in a rat model. J Orthop Res 17, 286, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Butler M.S., Robertson W.W., Rate W., D'Angio G.J., and Drummond D.S. Skeletal sequelae of radiation therapy for malignant childhood tumors. Clin Orthop Relat Res 251, 235, 1990 [PubMed] [Google Scholar]
- 35.Gawade P.L., Hudson M.M., Kaste S.C., Neglia J.P., Wasilewski-Masker K., Constine L.S., Robison L.L., and Ness K.K. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev 10, 249, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voss S.D., Glade-Bender J., Spunt S.L., DuBois S.G., Widemann B.C., Park J.R., Leary S.E., Nelson M.D., Adamson P.C., Blaney S.M., and Weigel B. Growth plate abnormalities in pediatric cancer patients undergoing phase 1 anti-angiogenic therapy: a report from the children's oncology group phase I consortium. Pediatr Blood Cancer 62, 45, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall A.P., Westwood F.R., and Wadsworth P.F. Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicol Pathol 34, 131, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sharma M., MacKenzie W.G., and Bowen J.R. Severe tibial growth retardation in total fibular hemimelia after limb lengthening. J Pediatr Orthop 16, 438, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Shapiro F. Longitudinal growth of the femur and tibia after diaphyseal lengthening. J Bone Joint Surg Am 69, 684, 1987 [PubMed] [Google Scholar]
- 40.Lemos S.E., Keating P.M., Scott T.P., and Siwiec R.M. Physeal-sparing technique for femoral tunnel drilling in pediatric anterior cruciate ligament reconstruction using a posteromedial portal. Arthrosc Tech 2, e483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauch C., Arnold M.P., Wirries A., Mayer R.R., Friederich N.F., and Hirschmann M.T. Anterior cruciate ligament reconstruction using quadriceps tendon autograft for adolescents with open physes—a technical note. Sports Med Arthrosc Rehabil Ther Technol 3, 7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo I.K., Bell D.M., and Fowler P.J. Anterior cruciate ligament injuries in the skeletally immature patient. Instr Course Lect 47, 351, 1998 [PubMed] [Google Scholar]
- 43.Guzzanti V., Falciglia F., and Stanitski C.L. Preoperative evaluation and anterior cruciate ligament reconstruction technique for skeletally immature patients in Tanner stages 2 and 3. Am J Sports Med 31, 941, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Chudik S., Beasley L., Potter H., Wickiewicz T., Warren R., and Rodeo S. The influence of femoral technique for graft placement on anterior cruciate ligament reconstruction using a skeletally immature canine model with a rapidly growing physis. Arthroscopy 23, 1309, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Seil R., Weitz F.K., and Pape D. Surgical-experimental principles of anterior cruciate ligament (ACL) reconstruction with open growth plates. J Exp Orthop 2, 11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen M.C., Bohm K.C., Rizkala A.R., and Ward C.M. Outcomes of Nonoperative Treatment of Salter-Harris II Distal Radius Fractures: a systematic review. Hand (N Y) 11, 29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badina A., Vialle R., Fitoussi F., and Damsin J.P. Case reports: treatment of traumatic triradiate cartilage epiphysiodesis: what is the role of bridge resection? Clin Orthop Relat Res 471, 3701, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eastwood D.M., deGheldere A., and Bijlsma P. Physeal injuries in children. Surgery 32, 1, 2014 [Google Scholar]
- 49.Mallick A., and Prem H. Physeal injuries in children. Surgery 35, 10, 2017 [Google Scholar]
- 50.Langenskiold A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop 1, 3, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Hasler C.C., and Foster B.K. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res 405, 242, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Marsh J.S., and Polzhofer G.K. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop 26, 255, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Monsell F.P., Barnes J.R., Kirubanandan R., and McBride A.M. Distal tibial physeal arrest after meningococcal septicaemia: management and outcome in seven ankles. J Bone Joint Surg Br 93, 839, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Bridgforth A.B., Burrus M.T., and Park J.S. Varus deformity of the distal tibia from physeal growth arrest treated using a titanium metal porous wedge. Foot Ankle Spec 9, 452, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Pinkowski J.L., and Weiner D.S. Complications in proximal tibial osteotomies in children with presentation of technique. J Pediatr Orthop 15, 307, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Abzug J.M., Ho C.A., Ritzman T.F., and Brighton B.K. Transphyseal fracture of the distal humerus. J Am Acad Orthop Surg 24, e39, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Epps C.H., and Bowen J.R. Complications in Pediatric Orthopaedic Surgery. Philadelphia: Lippincott, 1995 [Google Scholar]
- 58.Makarov M.R., Dunn S.H., Singer D.E., Rathjen K.E., Ramo B.A., Chukwunyerenwa C.K., and Birch J.G. Complications associated with epiphysiodesis for management of leg length discrepancy. J Pediatr Orthop 2016. [Epub ahead of print]; DOI: 10.1097/BPO.0000000000000835 [DOI] [PubMed] [Google Scholar]
- 59.Gaumétou E., Mallet C., Souchet P., Mazda K., and Ilharreborde B. Poor efficiency of eight-plates in the treatment of lower limb discrepancy. J Pediatr Orthop 36, 715, 2016 [DOI] [PubMed] [Google Scholar]
- 60.LaPorta G.A., and Susek M.M. Guided growth with temporary hemiepiphysiodesis to treat ankle valgus in a skeletally immature individual: a case report. J Foot Ankle Surg 55, 645, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Coleman R.M., Phillips J.E., Lin A., Schwartz Z., Boyan B.D., and Guldberg R.E. Characterization of a small animal growth plate injury model using microcomputed tomography. Bone 46, 1555, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Coleman R.M., Schwartz Z., Boyan B.D., and Guldberg R.E. The therapeutic effect of bone marrow-derived stem cell implantation after epiphyseal plate injury is abrogated by chondrogenic predifferentiation. Tissue Eng Part A 19, 475, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Clark A., Hilt J.Z., Milbrandt T.A., and Puleo D.A. Treating proximal tibial growth plate injuries using poly(lactic-co-glycolic acid) scaffolds. Biores Open Access 4, 65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L., Hui J.H., Goh J.C., Chen F., and Lee E.H. Chitin as a scaffold for mesenchymal stem cells transfers in the treatment of partial growth arrest. J Pediatr Orthop 24, 205, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Planka L., Gal P., Kecova H., Klima J., Hlucilova J., Filova E., Amler E., Krupa P., Kren L., Srnec R., Urbanova L., Lorenzova J., and Necas A. Allogeneic and autogenous transplantations of MSCs in treatment of the physeal bone bridge in rabbits. BMC Biotechnol 8, 70, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plánka L., Necas A., Srnec R., Rauser P., Starý D., Jancar J., Amler E., Filová E., Hlucilova J., and Kren L. Use of allogenic stem cells for the prevention of bone bridge formation in miniature pigs. Physiol Res 58, 885, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Planka L., Srnec R., Rauser P., Stary D., Filova E., Jancar J., Juhasova J., Kren L., Necas A., and Gal P. Nanotechnology and mesenchymal stem cells with chondrocytes in prevention of partial growth plate arrest in pigs. Biomed Pap Med Fac Univ Palacky Olomouc CzechRepub 156, 128, 2012 [DOI] [PubMed] [Google Scholar]
- 68.McCarty R.C., Gronthos S., Zannettino A.C., Foster B.K., and Xian C.J. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol 219, 324, 2009 [DOI] [PubMed] [Google Scholar]
- 69.McCarty R.C., Xian C.J., Gronthos S., Zannettino A.C., and Foster B.K. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J 4, 204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung R., Cool J.C., Scherer M.A., Foster B.K., and Xian C.J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol 80, 1272, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Chung R., Foster B.K., and Xian C.J. The potential role of VEGF-induced vascularisation in the bony repair of injured growth plate cartilage. J Endocrinol 221, 63, 2014 [DOI] [PubMed] [Google Scholar]
- 72.Macsai C.E., Georgiou K.R., Foster B.K., Zannettino A.C., and Xian C.J. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone 50, 1081, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Su Y.W., Chung R., Ruan C.S., Chim S.M., Kuek V., Dwivedi P.P., Hassanshahi M., Chen K.M., Xie Y., Chen L., Foster B.K., Rosen V., Zhou X.F., Xu J., and Xian C.J. Neurotrophin-3 induces BMP-2 and VEGF activities and promotes the bony repair of injured growth plate cartilage and bone in rats. J Bone Miner Res 31, 1258, 2016 [DOI] [PubMed] [Google Scholar]
- 74.Xian C.J., Zhou F.H., McCarty R.C., and Foster B.K. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res 22, 417, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Arasapam G., Scherer M., Cool J.C., Foster B.K., and Xian C.J. Roles of COX-2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem 99, 450, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Macsai C.E., Hopwood B., Chung R., Foster B.K., and Xian C.J. Structural and molecular analyses of bone bridge formation within the growth plate injury site and cartilage degeneration at the adjacent uninjured area. Bone 49, 904, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Ngo T.Q., Scherer M.A., Zhou F.H., Foster B.K., and Xian C.J. Expression of bone morphogenic proteins and receptors at the injured growth plate cartilage in young rats. J Histochem Cytochem 54, 945, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Zhou F.H., Foster B.K., Sander G., and Xian C.J. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone 35, 1307, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Garces G.L., Mugica-Garay I., Lopez-Gonzalez Coviella N., and Guerado E. Growth-plate modifications after drilling. J Pediatr Orthop 14, 225, 1994 [DOI] [PubMed] [Google Scholar]
- 80.Fischerauer E., Heidari N., Neumayer B., Deutsch A., and Weinberg A.M. The spatial and temporal expression of VEGF and its receptors 1 and 2 in post-traumatic bone bridge formation of the growth plate. J Mol Histol 42, 513, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Chung R., and Xian C.J. Recent research on the growth plate: mechanisms for growth plate injury repair and potential cell-based therapies for regeneration. J Mol Endocrinol 53, T45, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Wirth T., Byers S., Byard R., Hopwood J., and Foster B. The implantation of cartilaginous and periosteal tissue into growth plate defects. Int Orthop 18, 220, 1994 [DOI] [PubMed] [Google Scholar]
- 83.Lee E., Chen F., Chan J., and Bose K. Treatment of growth arrest by transfer of cultured chondrocytes into physeal defects. J Pediatr Orthop 18, 155, 1998 [PubMed] [Google Scholar]
- 84.Gál P., Nečas A., Adler J., Teyschl O., Fabian P., andBibrová Š. Transplantation of the autogenous chondrocyte graft to physeal defects: an experimental study in pigs. Acta Vet Brno 71, 327, 2002 [Google Scholar]
- 85.Hansen A.L., Foster B.K., Gibson G.J., Binns G.F., Wiebkin O.W., and Hopwood J.J. Growth-plate chondrocyte cultures for reimplantation into growth-plate defects in sheep. Characterization of cultures. Clin Orthop Relat Res 256, 286, 1990 [PubMed] [Google Scholar]
- 86.Foster B., Hansen A., Gibson G., Hopwood J., Binns G., and Wiebkin O. Reimplantation of growth plate chondrocytes into growth plate defects in sheep. J Orthop Res 8, 555, 1990 [DOI] [PubMed] [Google Scholar]
- 87.Hui J.H., Li L., Teo Y.H., Ouyang H.W., and Lee E.H. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng 11, 904, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Chen F., Hui J.H., Chan W.K., and Lee E.H. Cultured mesenchymal stem cell transfers in the treatment of partial growth arrest. J Pediatr Orthop 23, 425, 2003 [PubMed] [Google Scholar]
- 89.Yoshida K., Higuchi C., Nakura A., Nakamura N., and Yoshikawa H. Treatment of partial growth arrest using an in vitro-generated scaffold-free tissue-engineered construct derived from rabbit synovial mesenchymal stem cells. J Pediatr Orthop 32, 314, 2012 [DOI] [PubMed] [Google Scholar]
- 90.Ahn J.I., Canale S.T., Butler S.D., and Hasty K.A. Stem cell repair of physeal cartilage. J Orthop Res 22, 1215, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Gál P., Nečas A., Planka L., Kecova H., Křen L., Krupa P., Hlučilová J., and Usvald D. Chondrocytic potential of allogenic mesenchymal stem cells transplanted without immunosuppression to regenerate physeal defect in rabbits. Acta Vet Brno 76, 265, 2007 [Google Scholar]
- 92.Plánka L., Nečas A., Gál P., Kecova H., Filova E., Křen L., and Krupa P. Prevention of bone bridge formation using transplantation of the autogenous mesenchymal stem cells to physeal defects: an experimental study in rabbits. Acta Vet Brno 76, 253, 2007 [Google Scholar]
- 93.Huang Z., Godkin O., and Schulze-Tanzil G. The challenge in using mesenchymal stromal cells for recellularization of decellularized cartilage. Stem Cell Rev 13, 50, 2017 [DOI] [PubMed] [Google Scholar]
- 94.Mamidi M.K., Das A.K., Zakaria Z., and Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage 24, 1307, 2016 [DOI] [PubMed] [Google Scholar]
- 95.Tang X., Fan L., Pei M., Zeng L., and Ge Z. Evolving concepts of chondrogenic differentiation: history, state-of-the-art and future perspectives. Eur Cell Mater 30, 12, 2015 [DOI] [PubMed] [Google Scholar]
- 96.Pérez-Silos V., Camacho-Morales A., and Fuentes-Mera L. Mesenchymal stem cells subpopulations: application for orthopedic regenerative medicine. Stem Cells Int 2016, 3187491, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ringe J., Strassburg S., Neumann K., Endres M., Notter M., Burmester G.R., Kaps C., and Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem 101, 135, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Dar A., Goichberg P., Shinder V., Kalinkovich A., Kollet O., Netzer N., Margalit R., Zsak M., Nagler A., and Hardan I. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol 6, 1038, 2005 [DOI] [PubMed] [Google Scholar]
- 99.Wynn R.F., Hart C.A., Corradi-Perini C., O'Neill L., Evans C.A., Wraith J.E., Fairbairn L.J., and Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104, 2643, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Kitaori T., Ito H., Schwarz E.M., Tsutsumi R., Yoshitomi H., Oishi S., Nakano M., Fujii N., Nagasawa T., and Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 60, 813, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Chen Y., Xiang L.X., Shao J.Z., Pan R.L., Wang Y.X., Dong X.J., and Zhang G.R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med 14, 1494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee J.M., Kim B.-S., Lee H., and Im G.-I. In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther 20, 1434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendelson A., Frank E., Allred C., Jones E., Chen M., Zhao W., and Mao J.J. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J 25, 3496, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen P., Tao J., Zhu S., Cai Y., Mao Q., Yu D., Dai J., and Ouyang H. Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials 39, 114, 2015 [DOI] [PubMed] [Google Scholar]
- 105.Zhang W., Chen J., Tao J., Jiang Y., Hu C., Huang L., Ji J., and Ouyang H.W. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials 34, 713, 2013 [DOI] [PubMed] [Google Scholar]
- 106.Yu Y., Brouillette M.J., Seol D., Zheng H., Buckwalter J.A., and Martin J.A. Use of recombinant human stromal cell-derived factor 1alpha-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol 67, 1274, 2015 [DOI] [PubMed] [Google Scholar]
- 107.Sundararaj S.K., Cieply R.D., Gupta G., Milbrandt T.A., and Puleo D.A. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J Tissue Eng Regen Med 9, E202, 2015 [DOI] [PubMed] [Google Scholar]
- 108.Schmitt J.F., See K.H., Yang Z., Hui J.H., and Lee E.H. Sequential differentiation of mesenchymal stem cells in an agarose scaffold promotes a physis-like zonal alignment of chondrocytes. J Orthop Res 30, 1753, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Johnstone E.W., McArthur M., Solly P.B., Higginson K., Byers S., and Foster B.K. The effect of osteogenic protein-1 in an in vivo physeal injury model. Clin Orthop Relat Res 395, 234, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Thomas B.J., Byers S., Johnstone E., and Foster B. The effect of recombinant human osteogenic protein-1 on growth plate repair in a sheep model. J Orthop Res 23, 1336, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Plánka L., Starý D., Hlučilová J., Klíma J., Jančář J., Křen L., Lorenzová J., Urbanová L., Crha M., Srnec R., Dvořák M., and Gál P. Comparison of preventive and therapeutic transplantations of allogeneic mesenchymal stem cells in healing of the distal femoral growth plate cartilage defects in miniature pigs. Acta Vet Brno 78, 293, 2009 [Google Scholar]
- 112.Wang N., Butler J.P., and Ingber D.E. Mechanotransduction across the cell-surface and through the cytoskeleton. Science 260, 1124, 1993 [DOI] [PubMed] [Google Scholar]
- 113.Mullender M., El Haj A.J., Yang Y., van Duin M.A., Burger E.H., and Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput 42, 14, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Iqbal J., and Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun 328, 751, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Scadden D.T. The stem-cell niche as an entity of action. Nature 441, 1075, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Wang J.H.C., and Thampatty B.P. Mechanobiology of adult and stem cells. Int Rev Cell Mol Biol 271, 301, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Gieni R.S., and Hendzel M.J. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochemi 104, 1964, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Jin R., Teixeira L.S., Dijkstra P.J., van Blitterswijk C.A., Karperien M., and Feijen J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 31, 3103, 2010 [DOI] [PubMed] [Google Scholar]
- 119.Lai J.H., Kajiyama G., Smith R.L., Maloney W., and Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep 3, 3553, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen F., Yu S., Liu B., Ni Y., Yu C., Su Y., Zhu X., Yu X., Zhou Y., and Yan D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep 6, 20014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aisenbrey E.A., and Bryant S.J. Mechanical loading inhibits hypertrophy in chondrogenically differentiating hMSCs within a biomimetic hydrogel. J Mater Chem B Mater Biol Med 4, 3562, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang T., Lai J.H., and Yang F. Effects of hydrogel stiffness and extracellular compositions on modulating cartilage regeneration by mixed populations of stem cells and chondrocytes in vivo. Tissue Eng Part A 22, 1348, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Linnenberger A., Bodine M.I., Fiedler C., Roberts J.J., Skaalure S.C., Quinn J.P., Bryant S.J., Cole M., and McLeod R.R. Three dimensional live cell lithography. Opt Express 21, 10269, 2013 [DOI] [PubMed] [Google Scholar]
- 124.Xu T., Zhao W., Zhu J.M., Albanna M.Z., Yoo J.J., and Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34, 130, 2013 [DOI] [PubMed] [Google Scholar]
- 125.Fiedler C.I., Aisenbrey E.A., Wahlquist J.A., Heveran C.M., Ferguson V.L., Bryant S.J., and McLeod R.R. Enhanced mechanical properties of photo-clickable thiol-ene PEG hydrogels through repeated photopolymerization of in-swollen macromer. Soft Matter 12, 9095, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pedde R.D., Mirani B., Navaei A., Styan T., Wong S., Mehrali M., Thakur A., Mohtaram N.K., Bayati A., Dolatshahi-Pirouz A., Nikkhah M., Willerth S.M., and Akbari M. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater 29, 2017. DOI: 10.1002/adma.201606061 [DOI] [PubMed] [Google Scholar]
- 127.Petersen W., Tsokos M., and Pufe T. Expression of VEGF121 and VEGF165 in hypertrophic chondrocytes of the human growth plate and epiphyseal cartilage. J Anat 201, 153, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung R., Wong D., Macsai C., Piergentili A., Del Bello F., Quaglia W., and Xian C.J. Roles of Wnt/β-catenin signalling pathway in the bony repair of injured growth plate cartilage in young rats. Bone 52, 651, 2013 [DOI] [PubMed] [Google Scholar]
- 129.Krebs M.D., and Alsberg E. Localized, targeted, and sustained siRNA delivery. Chemistry 17, 3054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krebs M.D., Jeon O., and Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc 131, 9204, 2009 [DOI] [PubMed] [Google Scholar]
- 131.Hong C.A., and Nam Y.S. Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics 4, 1211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee S.H., Chung B.H., Park T.G., Nam Y.S., and Mok H. Small-interfering RNA (siRNA)-based functional micro- and nanostructures for efficient and selective gene silencing. Acc Chem Res 45, 1014, 2012 [DOI] [PubMed] [Google Scholar]
- 133.Yoshizuka M., Nakasa T., Kawanishi Y., Hachisuka S., Furuta T., Miyaki S., Adachi N., and Ochi M. Inhibition of microRNA-222 expression accelerates bone healing with enhancement of osteogenesis, chondrogenesis, and angiogenesis in a rat refractory fracture model. J Orthop Sci 2016 [DOI] [PubMed] [Google Scholar]
- 134.Fang T., Wu Q., Zhou L., Mu S., and Fu Q. miR-106b-5p and miR-17-5p suppress osteogenic differentiation by targeting Smad5 and inhibit bone formation. Exp Cell Res 347, 74, 2016 [DOI] [PubMed] [Google Scholar]
- 135.Li S., Hu C., Li J., Liu L., Jing W., Tang W., Tian W., and Long J. Effect of miR-26a-5p on the Wnt/Ca(2+) pathway and osteogenic differentiation of mouse adipose-derived mesenchymal stem cells. Calcif Tissue Int 99, 174, 2016 [DOI] [PubMed] [Google Scholar]
- 136.Li P., Wei X., Guan Y., Chen Q., Zhao T., Sun C., and Wei L. MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development. FASEB J 28, 3930, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]