Abstract
Campylobacter spp. is a major cause of foodborne diseases in humans, particularly when transmitted by the handling or consumption of undercooked poultry meat. Most Campylobacter infections are self-limiting, but antimicrobial treatment (e.g., fluoroquinolones and macrolides) is necessary in severe or prolonged cases. The indiscriminate use of these drugs, both in clinical medicine and animal production, has a major impact on public health. The aim of the present study was to identify Campylobacter strains, isolated from turkey and broilers, using both PCR and the matrix-assisted laser desorption–ionization time-of-flight (MALDI-TOF) methods to reveal the accuracy of identification, as well to evaluate the antimicrobial and genetic resistance of the investigated strains. MALDI-TOF and PCR methods were used to show differences, if any, in the specificity of that test. In this study, MALDI-TOF mass spectrometry gave the same results as multiplex PCR, in all cases. The highest rate of resistance (i.e., 100% of turkey and broiler strains) was detected against ciprofloxacin, whereas 58.1% of turkey and 78.6% of broiler strains were resistant to tetracycline. Multidrug-resistant isolates were not found in the study. All ciprofloxacin-resistant strains had a mutation in the gyrA gene, at the Thr-86 position. The presence of the tetO gene was found in 71% of turkey and in 100% of broiler strains. All resistant to tetracycline strains included tetO gene. Additionally, in five turkey and three broiler strains, susceptible to tetracycline, tetO gene was present. These results indicate the high prevalence of Campylobacter strains, which are phenotypically and genetically resistant to fluoroquinolones and tetracycline.
Keywords: : Campylobacter, antimicrobials, MALDI-TOF MS, PCR
Introduction
Campylobacter spp. is a major cause of foodborne illness worldwide.1 According to EFSA Reports presented in the last few years, the prevalence of Campylobacter spp. in humans has an increasing prominence.2 Among Campylobacter species, C. jejuni and C. coli are the most common bacterial cause of human gastroenteritis.2 Poultry and poultry products are considered to be the main reservoir and source of transmission of Campylobacter, to humans.2,3 Campylobacteriosis is usually a self-limiting infection that typically does not require any antimicrobial therapy; children, the elderly, and patients who are immunocompromised are at a higher risk for severe diarrhea that requires hospitalization. C. jejuni infections occasionally lead to complications, such as bacteremia and postinfection reactive arthritis or Guillain–Barré Syndrome.4,5
For many decades, Campylobacter spp. has been identified using serological and biochemical methods, which are time consuming and unreliable. The PCR method has more complementary value, but it is also time consuming. In more recent years, matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) has been developed. This method was used as a suitable technique for the specification of Campylobacter strains, to their genera, based on “species-identifying,” biomarker ions.6 Comparing with genetic approach of microbial identification, MALDI-TOF Biotyper is found to be less demanding in laboratory probe preparation, definitely cheaper, and time-saving method. Based on the obtained “protein fingerprint” results MALDI-TOF Biotyper software allows direct construction of dendrograms showing phylogenetic relation of strains. A further study on full-spectral MALDI-TOF MS analysis allowed the establishment of a reference database of selected Campylobacter strains, based on a list of up to 70 peaks, obtained from a set of mass spectra. This was a starting point, to the confirmation of a main spectrum, containing information about mean peak masses, mean peak intensities, and peak frequency.7
An increasing trend within the antibiotic resistance of Campylobacter spp. has been observed, in the last few years. Campylobacter spp., as a zoonotic bacteria, is exposed to antibiotics, used in both animal production and clinical medicine. The drug of choice for the treatment of human campylobacteriosis is erythromycin (i.e., a macrolide), but ciprofloxacin (i.e., a fluoroquinolone) is also frequently applied, because of its broad antibacterial spectrum. Alternatively, with a systemic infection of Campylobacter spp., tetracycline has also been applied.8 In poultry industry, these antimicrobials are used for the treatment of respiratory or gastrointestinal disorders. Furthermore, it is well known that ciprofloxacin-resistant C. jejuni may colonize poultry intestinal tracts better than the isogenic susceptible C. jejuni, even in the absence of fluoroquinolone selection pressure.9
Regarding molecular mechanism explaining antimicrobial resistance of Campylobacter spp. to different groups of antimicrobials, several genes were identified. The main resistance mechanism of Campylobacter spp. to fluoroquinolones is a point mutation in the gyrA gene (most commonly leading to Thr-86-Ile), which encodes part of the DNA gyrase, in the quinolone resistance-determining region.10 Resistance to tetracycline is primarily mediated by a plasmid-encoded tetO gene.11,12 High-level resistant Campylobacter spp. to erythromycin, is mediated by a mutation at position A2074C or at A2075G, in domain V of the 23S rRNA.13,14 Multidrug efflux pumps, such as CmeABC and CmeDEF, are also involved in the resistance of Campylobacter, to a broad spectrum of antimicrobials. It has been found that CmeABC functions synergistically, with target mutations, in conferring and maintaining high-level resistance to fluoroquinolones and macrolides.15,16
The aim of this study was to identify Campylobacter spp., isolated from turkeys and broilers, using two selected methods (PCR and MALDI-TOF) and to evaluate the antibiotic resistance of investigated strains. Regarding the development of microorganism identification methods, MALDI-TOF Biotyper is more recent than PCR method, so it was important to check its effectiveness for speciation of thermophilic Campylobacter isolated from turkeys and broilers in Poland. Moreover, the aim of our research was to analyze the prevalence of point mutation or the presence of genes, responsible for antimicrobial resistance to fluoroquinolones, erythromycin and tetracycline, and the prevalence of multidrug efflux pump genes in the population of Campylobacter spp. strains.
Materials and Methods
Sample preparation for Campylobacter spp. identification
Fresh stool samples were collected, in 2015, in Lower Silesia region, from five commercial turkey farms (MK, MS, Z, O, and Ko) and two broiler farms (1 and 3). The strains marked as MK1, 2, 4–6 (five isolates), and MK9-11 (three isolates) were collected from two separate turkey farms, which were closely located, less than 1 km. Therefore, they are marked the same name—MK. On each farm, few turkey or broiler houses were located (from 3 to 7). From each turkey or broiler house only one strain of Campylobacter spp. was isolated. In general, 31 of turkey and 14 of broiler houses (flocks) were sampled (Table 1). Material was taken with sterile swabs and placed into a Bolton Selective Enrichment Broth (Oxoid, Basingstoke, Hampshire, UK). Swabs were then transported to the laboratory, at a temperature of 4°C (±2°C), not exceeding a shipment time of 6 hours.
Table 1.
Species Identification of Campylobacter Isolates (n = 45) Using the Matrix-Assisted, Laser Desorption Ionization Biotyper and Multiplex PCR
| Identification | |||
|---|---|---|---|
| Source and isolate number | MALDI-TOF Biotyper | Score | Multiplex PCR |
| Reference strains | |||
| Campylobacter coli ATCC 33559 | C. coli | 2.41 | C. coli |
| Campylobacter jejuni ATCC 33560 | C. jejuni | 2.29 | C. jejuni |
| Turkey strains | |||
| MK1, MK2, MK4, MK5, MK6, MK9, MK10, MK11 MS1, MS2, MS3, MS4, MS8, MS10 | C. jejuni (n = 30) | 2.12–2.42 | All listed strains confirmed as C. jejuni |
| Z1, Z2, Z5, Z7 | |||
| O1, O2, O3, O4, O5, O6 | |||
| Ko4, Ko5, Ko6, Ko7, Ko8, Ko9 | |||
| Z3 | C. coli (n = 1) | 2.03 | Confirmed as C. coli |
| Broiler strains | |||
| 1.3, 1.4, 1.5, 1.6, 1.8, 1.9, 1.10 | C. jejuni (n = 11) | 2.10–2.43 | All listed strains confirmed as C. jejuni |
| 3.3, 3.4, 3.5, 3.6 | |||
| 3.2, 3.7, 3.8 | C. coli (n = 3) | 2.20–2.30 | All listed strains confirmed as C. coli |
MALDI-TOF, matrix-assisted laser desorption-ionization time-of-flight mass spectrometry.
The collected samples were swabbed onto Columbia Blood Agar plates (Oxoid) with a Campylobacter Selective Supplement, SR0069 (Oxoid). Agar plates were incubated at 42°C, for 48 hours, in microaerophilic conditions (85% N2, 10% CO2, and 5% O2). One typical Campylobacter colony (smooth, flat, and colorlessly translucent to gray) was selected for further biochemical investigation; positive motility, and curved shape, Gram-negative and an oxidase- and catalase-positive test. All positive isolates were stored frozen, in glycerol broth, at −70°C, for further molecular identification and antimicrobial resistance.
MALDI-TOF MS analysis for Campylobacter spp. identification
Two to five colonies of actively growing cultures, incubated for 24 hours on a blood agar at 42°C, were suspended in 300 μl of double-distilled water. Then, 900 μl of absolute ethanol was added. The sample was centrifuged twice (at 13,000 g, for 3 minutes) and the sediment was dried at room temperature. Lysates were prepared by adding 50 μl of 70% formic acid to the bacterial pellet, mixing thoroughly, adding 50 μl of acetonitrile, before mixing the sample again. Following further centrifugation (at 13,000 g, for 2 minutes) the supernatant was transferred to a fresh tube and 1 μl of the bacterial protein lysate was applied to a 384 ground steel MALDI target plate (Bruker Daltonics, Bremen, Germany) and air dried at room temperature. Next, the sample was overlaid with 1 μl of α-cyano-4-hydroxycinnamic acid matrix solution (Bruker Daltonics) and air dried, again. Measurements were performed using a Bruker Daltonics UltrafleXtreme spectrometer. Spectra were recorded in the positive linear mode, for a mass range of 2,000–20,000 Da (laser frequency 200 Hz; ion source voltage one, 25 kV; ion source voltage two, 23.5 kV; lens voltage, 6.0 kV). Each spectrum was obtained by averaging 1,500 laser shots, acquired from three spot positions, under the control of FlexControl software 3.1 (Bruker Daltonics). Spectra were externally calibrated using an Escherichia coli DH5-alpha standard (Bruker Daltonics). The calibrant consisted of six ribosomal proteins from E. coli, with added RNAse A and myoglobin, to cover a range of 3,637.8–16,952.3 Da. Biotyper 3.1 software (Bruker Daltonics) and a database containing 4,613 entries was used for identification. According to the manufacturer, the following score values were used; less than 1.7—identification not reliable, 1.7–2.0—probable genus identification, 2.0–2.3—secure genus identification and probable species identification, and more than 2.3—highly probable species identification.
For dendrogram preparation, principal component analysis (PCA) of sets of spectra was used. This statistical analysis provides information on the hetero/homogeneity of a dataset. PCA was managed by an external MATLAB software tool that was integrated into the MALDI Biotyper.
DNA extraction
Genetic material was isolated using Genomic Mini (A&A Biotechnology, Gdańsk, Poland) in accordance with the manufacturer's recommendations. DNA was quantified spectrophotometrically (BioPhotometer, Eppendorf, Hamburg, Germany) and stored at −20°C.
Multiplex PCR
A single PCR method was used to identify genus Campylobacter, whereas the species level of C. jejuni or C. coli was determined using multiplex PCR. Primer sequences, specific for the simultaneous amplification of the mapA gene (C. jejuni) and the ceuE gene (C. coli) are listed in Table 2.17–19 Protocol for single and multiplex PCR was described by Denis et al.20 The final volume of reaction mixture was 25 μl and contained 1 U of Dream Taq DNA Polymerase (5 U/μl) (Thermo Fisher Scientific, Tewksbury, MA). The PCR program for single and multiplex PCR was as follows: 10 minutes at 95°C, 35 cycles consisted of 30 seconds at 95°C, 1.5 minutes at 59°C, 1 minute at 72°C, and final extension step of 10 minutes at 72°C. Strains used as positive controls were C. jejuni ATCC 33560 and C. coli ATCC 33559. Products obtained by amplification were separated, through the electrophoresis method, in a 2% agarose gel. DNA bands were stained with a Midori Green DNA Stain (Nippon Genetics Europe GmbH, Dueren, Germany) and visualized with an UV transilluminator.
Table 2.
Primer Sequences Used for Campylobacter Species Identification
| Species | Gene | Sequence (5′→3′) | Amplicon (bp) | References |
|---|---|---|---|---|
| C. spp. | 16S rRNA | AGTCTTGGCAGTAATGCACCTAACG | 408 | Wangroongsarb et al.19 |
| ATATGCCATTGTAGCACGTGTGTCG | ||||
| C. jejuni | mapA | CTATTTTATTTTTGAGTGCTTGTG | 589 | Stucki et al.18 |
| GCTTTATTTGCCATTTGTTTTATTA | ||||
| C. coli | ceuE | AATTGAAAATTGCTCCAACTATG | 462 | Gonzalez et al.17 |
| TGATTTTATTATTATTTGTAGCAGCG |
Antimicrobial resistance
Nine antimicrobial agents (azithromycin, ciprofloxacin, erythromycin, gentamicin, tetracycline, florfenicol, nalidixic acid, telithromycin, and clindamycin) were tested to show the susceptibility of Campylobacter strains. Some of these antimicrobials, especially fluoroquinolones and macrolides, are the drugs of choice in the treatment of Campylobacter infection in humans. The minimal inhibitory concentration (MIC) of each drug was determined by broth microdilution, using a Sensititre™ Campylobacter MIC Plate (TREK Diagnostic System, West Sussex, UK) according to the manufacturer's instructions. Colonies were harvested on a Columbia Blood Agar Base (Oxoid) supplemented with 7% of sheep blood, incubated in microaerophilic conditions, for 48 hours, at 42°C, and then seeded in a Mueller-Hinton Broth Supplement with blood, and added to microtiter plates. Plates were incubated for 24 hours, in the same conditions and then screened. Assays were repeated twice, each in duplicate, to confirm the reproducibility of the MIC data. Interpretation of the obtained results was performed to the interpretative standard, as recommended by the Clinical and Laboratory Standards Institute.21,22 The reference strains of C. jejuni ATCC 33560 and C. coli ATCC 33559 were used as test controls.
Molecular methods for detection of antimicrobial resistance
Ciprofloxacin resistance
To detect a point mutation in the gyrA gene, which correlates to fluoroquinolone resistance, PCR-RFLP was used. The sequences of the primers used in this study are presented in Table 3. PCR amplification of the gyrA gene of C. jejuni and C. coli was performed, using primers and the reaction condition described by Alonso et al.23 and Wardak et al.24 A PCR product of size 179-bp was digested by restriction enzyme RsaI (Thermo Fisher Scientific, Waltham, MA) with an effect at 10 U/μl.
Table 3.
List of Primers Used for the Molecular Detection of Antimicrobial Resistance
| Gene | Primers | Sequence (5′→3′) | Amplicon (bp) | References |
|---|---|---|---|---|
| Ciprofloxacin | ||||
| gyrA | cjgyrM1 | AAATCAGCCCGTATAGTGGGTGCTGTTATAGGTCGTTATCACCCACACATGGAGGT | 179 | Wardak et al.24 |
| cjgyrA2 | TCAGTATAACGCATCGCAGC | |||
| Tetracycline | ||||
| tetO | DMT 1 | GGCGTTTTGTTTATGTGCG | 559 | Gibreel et al.12 |
| DMT 2 | ATGGACAACCCGACAGAAGC | |||
| Erythromycin | ||||
| 23S rRNA | F2-campy-23S | AATTGATGGGGTTAGCATTAGC | 316 | El-Adawy et al.25 |
| R2-campy-23S | CAACAATGGCTCATATACAACTGG | |||
| Efflux pumps | ||||
| cmeB | EP 1 | TCCTAGCAGCACAATATG | 241 | Obeng et al.26 |
| EP 2 | AGCTTCGATAGCTGCATC | |||
Tetracycline resistance
The conserved primers, DMT 1 and DMT 2, were used to amplify a 559-bp PCR product of the tetO gene that is associated with resistance to tetracycline, in Campylobacter isolates. PCR was done as previously described.12
Erythromycin resistance
All isolates were tested for the presence of mutation in the 23S rRNA gene, using PCR-RFLP, according to the protocols described by El-Adawy et al.25 The amplified PCR products of 316-bp were digested with a BsaI restriction enzyme (Thermo Fisher Scientific, Waltham, MA).
Efflux pumps
All Campylobacter spp. were tested for the presence of the cmeB gene. PCR was carried out, using the amplification protocols described by Obeng et al.26 The lengths of various amplicons, obtained in each PCR, were determined by comparing them with a GeneRuler 100 bp DNA Ladder (Thermo Fisher Scientific, Waltham, MA).
Results
Bacterial identification
In total, 45 Campylobacter strains were isolated from turkeys (31; 68.9%) and broilers (14; 31.1%). Among all investigated strains, 41 (91.1%) were identified as C. jejuni, whereas only 4 (8.9%) as C. coli. The number of C. jejuni and C. coli isolated from turkeys were as follows: 30 (96.8%) and only 1 (3.2%), whereas the number of C. jejuni and C. coli obtained from broilers were, 11 (78.6%) and 3 (21.4%), respectively (Table 1).
All isolates from turkeys and broilers were also identified, with the MALDI Biotyper method. The results presented in Table 3 confirm multiplex PCR data, recognizing 41 strains as C. jejuni and 4 strains as C. coli. Based on score values over 2.00, obtained for all 45 samples, species identification was deemed as complete.
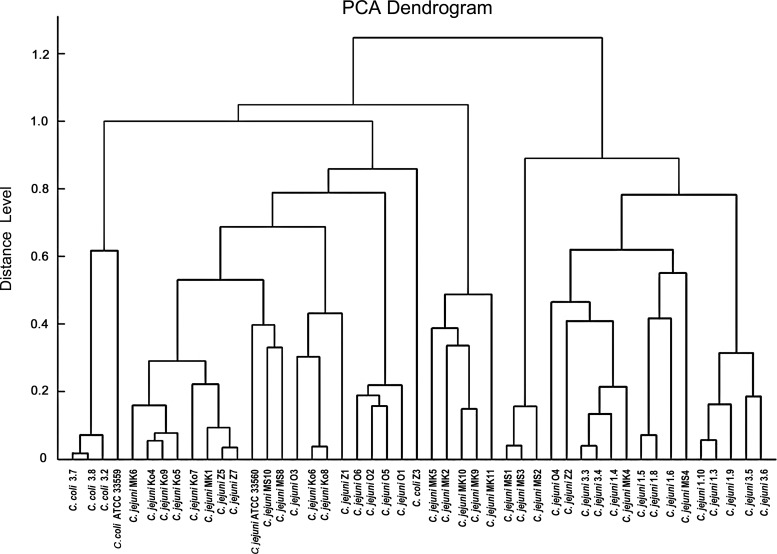
Furthermore, based on MALDI Biotyper spectra, a PCA Dendrogram of all the studied strains was obtained. As presented in Fig. 1, a cluster separation of C. coli strains, from the C. jejuni strains, with a high distance level, was observed. Interestingly, isolate 3.2, identified as C. coli, was positioned in a branch together with C. jejuni, as with other strains isolated from turkeys.
FIG. 1.
Principal component analysis dendrogram of Campylobacter isolates and reference strains.
Antimicrobial resistance
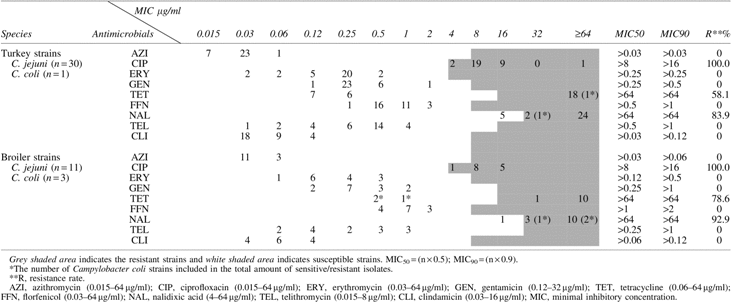
The results of the resistance of Campylobacter strains, to selected antimicrobials, and the rate of resistance to each antimicrobial agent are shown in Table 4. All investigated strains, isolated from turkeys and broilers, were susceptible to azithromycin, erythromycin, gentamicin, florfenicol, telithromycin, and clindamycin, whereas all strains were resistant to ciprofloxacin. Also, 92.9% and 78.6% of broiler strains were resistant to nalidixic acid and tetracycline, whereas 83.9% and 58.1% of turkey isolates were resistant to nalidixic acid and tetracycline, respectively. Similar resistance patterns were observed between C. jejuni and C. coli isolated from turkeys, whereas all C. coli isolated from broilers (n = 3) were susceptible to tetracycline, in comparison to C. jejuni, where all strains were resistant to this antibiotic. Multidrug resistance to three or more classes of antimicrobial agents was not found between the investigated strains.
Table 4.
Antimicrobial Resistance of C. jejuni and C. coli, Isolated from Turkeys and Broilers
 |
Molecular detection of antimicrobial resistance
All phenotypically, ciprofloxacin-resistant strains had a mutation in the gyrA gene, at the Thr-86 position (Table 5). The presence of the tetO gene was found in 21 (70%) of C. jejuni and in 1 (100%) of C. coli strains isolated from turkeys. Among the C. jejuni obtained from broilers, all tetracycline-resistant strains (11/100%) possessed the tetO gene. Notably, C. coli isolated from broilers, which were phenotypically tetracycline-susceptible, were found to encode the tetO and cmeB genes, as well. Comparing the presence of the cmeB gene between turkey and broiler isolates, 4 (13.3%) of the C. jejuni isolated from turkeys had a cmeB gene, whereas among broiler strains, 3 of the C. coli (100%) were found to encode the cmeB gene. All investigated strains possessing a cmeB gene, were obtained from one farm, in the case of turkey and broiler isolates, separately. The results of PCR-RFLP showed that no strain had a restriction site for BsaI, demonstrating the absence of mutation in all of the erythromycin-susceptible strains.
Table 5.
Presence of Resistant Genes/Mutations in C. jejuni and C. coli, Isolated from Turkeys and Broilers
| Turkey isolates (n = 31) | Broiler isolates (n = 14) | |||
|---|---|---|---|---|
| Resistant genes/mutations | C. jejuni (n = 30) | C. coli (n = 1) | C. jejuni (n = 11) | C. coli (n = 3) |
| Mutation in gyrA | 30 (100.0) | 1 (100.0) | 11 (100.0) | 3 (100.0) |
| tetO | 21 (70.0) | 1 (100.0) | 11 (100.0) | 3 (100.0) |
| Mutation in 23S rRNA | 0 | 0 | 0 | 0 |
| cmeB | 4 (13.3) | 0 | 0 | 3 (100.0) |
Discussion
The development of MALDI-TOF MS technology, for bacterial strain identification, is a simple, low-cost, and rapid method. In this study, MALDI-TOF MS produced correct identifications for all investigated Campylobacter strains, in comparison with a PCR method. The accuracy of MALDI-TOF MS identification has also been proved by other authors.7,27,28 Bessède et al.27 analyzed over one thousand strains and the results were compared with the gold standard of Campylobacter species identification, using real-time PCR and the sequencing of a 444-base-pair fragment of the gyrA gene. The accuracy of MALDI-TOF MS reached 100%, compared with the gold standard, for all of the Campylobacter species, except C. jejuni (99.4%). For the confirmation and comparison purposes, the classifications achieved through these techniques were compared with 16S rRNA sequence-based phylogenetic analysis.
A recent study focused on employing MALDI-TOF MS, together with Raman and FT-IR spectroscopies, combined with a multivariate statistical analysis, for the differentiation of Campylobacter, down to the subspecies level.29 The classifications achieved, through these techniques, were compared with a 16S rRNA sequence-based phylogenetic analysis, for confirmation and evaluation purposes. Results demonstrated that such metabolomic approaches, combined with molecular biology techniques, may provide critical information and knowledge, related to risk factors, virulence, and understanding of the distribution and transmission routes associated with different strains of foodborne Campylobacter spp.
Clustering analysis, using PCA dendrograms, generated by MALDI Biotyper mass spectra, for bacterial isolates, allows specification of the phylogenetic affiliation of the individual isolates, during population-based study.30
Thermophilic Campylobacter are zoonotic pathogens; therefore, the development of antimicrobial resistance, in these bacteria, is a matter of great concern. There is a hypothesis that unregulated use of antimicrobial agents, in food-animal production, has led to the emergence and spread of antibiotic resistance, among Campylobacter spp. The prevalence of resistant strains is very low, in countries where the use of antibiotics in poultry industry is uncommon.31 Norström et al. indicated a very low resistance rate to oxytetracycline (1.3%), whereas resistance to quinolones was not observed. Other authors, from Italy, revealed a high level of resistance to ciprofloxacin (62.8%), tetracycline (55.9%), and nalidixic acid (55.2%).32 High levels of the resistance of Campylobacter isolates to fluoroquinolones have also been found in Latvia, Lithuania, Spain, and Iran.33–35 Zhao et al.36 have shown 13 multidrug resistance profiles of C. jejuni and C. coli, isolated from humans, retail meats, and cecal samples of food production animals (e.g., chicken and turkey). Strains were resistant to tetracycline, gentamicin, azithromycin, clindamycin, telithromycin, erythromycin, and ciprofloxacin. The highest rate of resistance was observed for tetracycline, 94.7% of all isolates. A similar, high incidence of tetracycline resistance of C. jejuni and C. coli, isolated from broiler carcasses, was reported in Brazil and the United States, 75% and over 96%, respectively.37,38 The present study provides the highest rate of resistance to fluoroquinolones (i.e., ciprofloxacin and nalidixic acid), and to tetracycline. Both of these antimicrobial groups are often used in veterinary and human medicine, especially regarding enteric infections. All strains isolated from turkeys and broilers were resistant to ciprofloxacin, whereas 83.9% of turkey isolates and 92.9% of broiler isolates were resistant to nalidixic acid. The rate of resistance to fluoroquinolones and tetracycline in poultry has increased over the last dozen years, in Poland. Woźniak39,40 reported that the rate of resistance of Campylobacter, isolated from broilers, to tetracycline, rose from 0% in 1994–1996 to 18.7% in 2005–2008, and to ciprofloxacin, rose from 43.6% in 1994–1996 to 85.4% in 2005–2008.
In the current study, all of the Campylobacter isolates were susceptible to azithromycin, erythromycin, gentamicin, florfenicol, telithromycin, and clindamycin, probably because of the rare use of these antimicrobials in Polish poultry production. Other authors25,32,37 have revealed similar results, where all isolates were susceptible to gentamicin and less than 5% of strains were resistant to chloramphenicol. In general, the rate of resistance to erythromycin seems to be low,26,33,35,37,41 which is in agreement with the results of this study.
Our study revealed a high correlation between phenotypic resistance to a given drug tested and the presence of the gene/mutation expected to confer resistance to that drug, which is in agreement with previous research.24,25,34,42 With regard to fluoroquinolones, mutation Thr86Ile is the most prevalent in isolates, among other described mutations (i.e., Thr86Ala, Thr86Lys, Ala87Pro, Asp90His, and Asp90Tyr).43,44 In this study, in relation to ciprofloxacin and nalidixic acid, there was a 100% correlation to the presence of a Thr86Ile mutation and the observed quinolone resistance phenotype. Our findings are similar to El-Adawy et al.25 who revealed the same mutation in all ciprofloxacin-resistant Campylobacter strains.
Resistance to tetracycline is mostly associated with the presence of the tetO resistant gene. In this study, all 18 tetracycline-resistant strains isolated from turkeys and 11 tetracycline-resistant strains isolated from broilers carried the tetO gene, whereas 5 tetracycline-susceptible C. jejuni isolated from turkeys and 3 tetracycline-susceptible C. coli obtained from broilers were also carrying the tetO gene. These findings indicate that the tetO gene, in these Campylobacter isolates, may have been inactive or were not being expressed. Obeng et al.26 found 3.8% of tetracycline-susceptible C. jejuni, from chickens, with the presence of the tetO gene. Pratt and Korolik45 revealed that the tetO gene was found in all tetracycline-resistant Campylobacter, of human and poultry origin, in Australia, whereas PCR amplification was successful in detecting the tetO gene in 97.8% of investigated strains.
The main mechanisms conferring resistance against macrolides, in Campylobacter, is transition A2075G.46 This study found no strains with mutation A2075G, which is in agreement with their susceptibility to this antimicrobial and with other studies.25 Pérez-Boto et al.34 revealed only 1 C. coli strain resistant to erythromycin, where the mutation at A2075G (23S r DNA) was responsible for macrolide resistance. Wirz et al.47 detected the A2075G transition, only in 3.1% of C. coli isolates from broilers.
In this study, the cmeB gene was carried by a low number (13.3%) of C. jejuni strains from turkeys, whereas the same was found in all (100%) C. coli isolated from broilers. The occurrence of this gene was significantly higher in C. coli than in C. jejuni, which is consistent with other studies.26,48
Conclusions
Campylobacter identification, using the MALDI-TOF MS method, considering the speed with which reliable identification can be obtained, is well suited for large-scale research and diagnostic analyses. Additionally, this technology allows the analysis of the phylogenetic origin of investigated strains.
In this study, Campylobacter from poultry production, showed resistance to a relatively narrow range of antimicrobials. The significant usage of fluoroquinolones and tetracycline in poultry production, in our country, has led, as shown in our study, to the emergence of quinolone and tetracycline-resistant Campylobacter strains. Monitoring of antimicrobial resistance for Campylobacter and the appropriate use of antimicrobials in animal-food production are essential, for decreasing drug resistance in bacterial pathogens.
Acknowledgment
The Publication was supported by the Wroclaw Center of Biotechnology, program of the Leading National Research Center (KNOW), for years 2014–2018.
Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. 2012. The global view of campylobaceriosis: report of an expert consultation. 9–11 July, Utrecht, The Netherlands [Google Scholar]
- 2.EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 13:4329 [Google Scholar]
- 3.Hermans D., Pasmans F., Messens W., Martel A., Van Immerseel F., Rasschaert G., Heyndrickx M., Van Deun K., and Haesebrouck F. 2012. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonot. 12:89–98 [DOI] [PubMed] [Google Scholar]
- 4.Keo T., Collins J., Kunwar P., Blaser M.J., and Iovine N.M. 2011. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence 2:30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki N. 2010. Human gangliosides and bacterial lipo-oligosaccharides in the development of autoimmune neuropathies. Methods Mol. Biol. 600:51–65 [DOI] [PubMed] [Google Scholar]
- 6.Mandrell R.E., Harden L.A., Bates A., Miller W.G., Haddon W.F., and Fagerquist C.K. 2005. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 71:6292–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alispahic M., Hummel K., Jandreski-Cvetkovic D., Nöbauer K., Razzazi-Fazeli E., Hess M., and Hess C. 2010. Species-specific identification and differentiation of Arcobacter, Helicobacter and Campylobacter by full-spectral matrix-associated laser desorption/ionization time of flight mass spectrometry analysis. J. Med. Microbiol. 59:295–301 [DOI] [PubMed] [Google Scholar]
- 8.Luangtongkum T., Jeon B., Han J., Plummer P., Logue C.M., and Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 4:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo N., Pereira S., Sahin O., Lin J., Huang S., Michel L., and Zhang Q., Q. 2005. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc. Natl. Acad. Sci. U. S. A. 102:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakanen A., Jalava J., Kotilainen P., Jousimies-Somer H., Siitonen A., and Huovinen P. 2002. gyrA polymorphism in Campylobacter jejuni: detection of gyrA mutations in 162 C. jejuni isolates by single-strand conformation polymorphism and DNA sequencing. Antimicrob. Agents Chemother. 46:2644–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engberg J., Aarestrup F.M., Taylor D.E., Gerner-Smidt P., and Nachamkin I. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibreel A., Tracz D.M., Nonaka L., Ngo T.M., Connell S.R., and Taylor D.E. 2004. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 48:3442–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladely S.R., Meinersmann R.J., Englen M.D., Fedorka-Cray P.J., and Harrison M.A. 2009. 23S rRNA gene mutations contributing to macrolide resistance in Campylobacter jejuni and Campylobacter coli. Foodborne Pathog. Dis. 6:91–98 [DOI] [PubMed] [Google Scholar]
- 14.Payot S., Bolla J.M., Corcoran D., Fanning S., Mégraud F., and Zhang Q. 2006. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect. 8:1967–1971 [DOI] [PubMed] [Google Scholar]
- 15.Cagliero C., Mouline C., Payot S., and Cloeckaert A. 2005. Involvement of the CmeABC efflux pump in the macrolide resistance of Campylobacter coli. J. Antimicrob. Chemother. 56:948–950 [DOI] [PubMed] [Google Scholar]
- 16.Luo N., Sahin O., Lin J., Michel L.O., and Zhang Q. 2003. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 47:390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez I., Grant K.A., Richardson P.T., Park S.F., and Collins M.D. 1997. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J. Clin. Microbiol. 35:759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stucki U., Frey J., Nicolet J., and Burnens A.P. 1995. Identification of Campylobacter jejuni on the basis of a species-specific gene that encodes a membrane protein. J. Clin. Microbiol. 33:855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wangroongsarb P., Jittaprasatsin C., Suwannasing S., Suthivarakom K., and Khamthalang T. 2011. Identification of genus Campylobacter and four enteropathogenic Campylobacter species by PCR. J. Trop. Med. Parasitol. 34:17–29 [Google Scholar]
- 20.Denis M., Soumet C., Rivoal K., Ermel G., Blivet D., Salvant G., and Colin P. 1999. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 29:406–410 [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2010. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guidelines, second edition, Pennsylvania [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2014. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. Pennsylvania [Google Scholar]
- 23.Alonso R., Mateo E., Girbau C., Churruca E., Martinez I., and Fernández-Astorga A. 2004. PCR-restriction fragment length polymorphism assay for detection of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli. Antimicrob. Agents Chemother. 48:4886–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardak S., Szych J., and Cieślik A. 2005. PCR-restriction fragment length polymorphism assay (PCR-RFLP) as an useful tool for detection of mutation in gyrA gene at 86-THR position associated with fluoroquinolone resistance in Campylobacter jejuni. Med. Dosw. Mikrobiol. 57:295–301 [PubMed] [Google Scholar]
- 25.El-Adawy H., Hotzel H., Düpre S., Tomaso H., Neubauer H., and Hafez H.M. 2012. Determination of antimicrobial sensitivities of Campylobacter jejuni isolated from commercial turkey farms in Germany. Avian Dis. 56:685–692 [DOI] [PubMed] [Google Scholar]
- 26.Obeng A.S., Rickard H., Sexton M., Pang Y., Peng H., and Barton M. 2012. Antimicrobial susceptibilities and resistance genes in Campylobacter strains isolated from poultry and pigs in Australia. J. Appl. Microbiol. 113:294–307 [DOI] [PubMed] [Google Scholar]
- 27.Bessède E., Solecki O., Sifré E., Labadi L., and Mégraud F. 2011. Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin. Microbiol. Infect. 17:1735–1739 [DOI] [PubMed] [Google Scholar]
- 28.Kolínská R., Drevínek M., Jakubů V., and Zemlicková H. 2008. Species identification of Campylobacter jejuni ssp. jejuni and C. coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and PCR. Folia Microbiol. (Praha). 53:403–409 [DOI] [PubMed] [Google Scholar]
- 29.Muhamadali H., Weaver D., Subaihi A., AlMasoud N., Trivedi D.K., Ellis D.I., Linton D., and Goodacre R. 2016. Chicken, beams, and Campylobacter: rapid differentiation of foodborne bacteria via vibrational spectroscopy and MALDI-mass spectrometry. Analyst 141:111–122 [DOI] [PubMed] [Google Scholar]
- 30.Chen P.L., Lee T.F., Wu C.J., Teng S.H., Teng L.J., Ko W.C., and Hsueh P.R. 2014. Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate Aeromonas dhakensis from A. hydrophila, A. caviae, and A. veronii. J. Clin. Microbiol. 52:2625–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norström M., Johnsen G., Hofshagen M., Tharaldsen H., and Kruse H. 2007. Antimicrobial resistance in Campylobacter jejuni from broilers and broiler house environments in Norway. J. Food Prot. 70:736–738 [DOI] [PubMed] [Google Scholar]
- 32.Di Giannatale E., Di Serafino G., Zilli K., Alessiani A., Sacchini L., Garofolo G., Aprea G., and Marotta F. 2014. Characterization of antimicrobial resistance patterns and detection of virulence genes in Campylobacter isolates in Italy. Sensors (Basel). 14:3308–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mäesaar M., Kramarenko T., Meremäe K., Sõgel J., Lillenberg M., Häkkinen L., Ivanova M., Kovalenko K., Hörman A., Hänninen M.L., and Roasto M. 2016. Antimicrobial resistance profiles of Campylobacter spp. isolated from broiler chicken meat of Estonian, Latvian and Lithuanian origin at Estonian retail level and from patients with severe enteric infections in Estonia. Zoonoses Public Health 63:89–96 [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Boto D., Herrera-León S., García-Peña F.J., Abad-Moreno J.C., and Echeita M.A. 2014. Molecular mechanisms of quinolone, macrolide, and tetracycline resistance among Campylobacter isolates from initial stages of broiler production. Avian Pathol. 43:176–182 [DOI] [PubMed] [Google Scholar]
- 35.Raeisi M., Khoshbakht R., Ghaemi E.A., Bayani M., Hashemi M., Seyedghasemi N.S., and Shirzad-Askil H. 2017. Antimicrobial resistance and virulence-associated genes of Campylobacter spp. isolated from raw milk, fish, poultry, and red meat. Microb. Drug Resist. [Epub ahead of print]; DOI: 10.1089/mdr.2016.0183 [DOI] [PubMed] [Google Scholar]
- 36.Zhao S., Tyson G.H., Chen Y., Li C., Mukherjee S., Young S., Lam C., Folster J.P., Whichard J.M., and McDermott P.F. 2016. Whole-genome sequencing analysis accurately predicts antimicrobial resistance phenotypes in Campylobacter spp. Appl. Environ. Microbiol. 82:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferro I.D., Benetti T.M., Oliveira T.C., Abrahão W.M., Farah S.M., Luciano F.B., and Macedo R.E. 2015. Evaluation of antimicrobial resistance of Campylobacter spp. isolated from broiler carcasses. Br. Poult. Sci. 56:66–71 [DOI] [PubMed] [Google Scholar]
- 38.Son I., Englen M.D., Berrang M.E., Fedorka-Cray P.J., and Harrison M.A. 2007. Antimicrobial resistance of Arcobacter and Campylobacter from broiler carcasses. Int. J. Antimicrob. Agents 29:451–455 [DOI] [PubMed] [Google Scholar]
- 39.Woźniak A. 2011. Fluoroquinolones resistance of Campylobacter jejuni and Campylobacter coli isolated from poultry in 1994–1996 and 2005–2008 in Poland. Bull. Vet. Inst. Pulawy 55:15–20 [Google Scholar]
- 40.Woźniak A., and Wieliczko A. 2011. Tetracycline, erythromycin, and gentamicin resistance of Campylobacter jejuni and Campylobacter coli isolated from poultry in Poland. Bull. Vet. Inst. Pulawy 55:51–54 [Google Scholar]
- 41.Kittl S., Kuhnert P., Hächler H., and Korczak B.M. 2011. Comparison of genotypes and antibiotic resistance of Campylobacter jejuni isolated from humans and slaughtered chickens in Switzerland. J. Appl. Microbiol. 110:513–520 [DOI] [PubMed] [Google Scholar]
- 42.Boonmar S., Morita Y., Fujita M., Sangsuk L., Suthivarakom K., Padungtod P., Maruyama S., Kabeya H., Kato M., Kozawa K., Yamamoto S., and Kimura H. 2007. Serotypes, antimicrobial susceptibility, and gyrA gene mutation of Campylobacter jejuni isolates from humans and chickens in Thailand. Microbiol. Immunol. 51:531–537 [DOI] [PubMed] [Google Scholar]
- 43.Bachoual R., Ouabdesselam S., Mory F., Lascols C., Soussy C.J., and Tankovic J. 2001. Single or double mutational alterations of gyrA associated with fluoroquinolone resistance in Campylobacter jejuni and Campylobacter coli. Microb. Drug Resist. 7:257–261 [DOI] [PubMed] [Google Scholar]
- 44.Piddock L.J., Ricci V., Pumbwe L., Everett M.J., and Griggs D.J. 2003. Fluoroquinolone resistance in Campylobacter species from man and animals: detection of mutations in topoisomerase genes. J. Antimicrob. Chemother. 51:19–26 [DOI] [PubMed] [Google Scholar]
- 45.Pratt A., and Korolik V. 2005. Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J. Antimicrob. Chemother. 55:452–460 [DOI] [PubMed] [Google Scholar]
- 46.Gibreel A., Kos V.N., Keelan M., Trieber C.A., Levesque S., Michaud S., and Taylor D.E. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirz S.E., Overesch G., Kuhnert P., and Korczak B.M. 2010. Genotype and antibiotic resistance analyses of Campylobacter isolates from ceca and carcasses of slaughtered broiler flocks. Appl. Environ. Microbiol. 76:6377–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giacomelli M., Andrighetto C., Rossi F., Lombardi A., Rizzotti L., Martini M., and Piccirillo A. 2012. Molecular characterization and genotypic antimicrobial resistance analysis of Campylobacter jejuni and Campylobacter coli isolated from broiler flocks in northern Italy. Avian Pathol. 41:579–588 [DOI] [PubMed] [Google Scholar]