Abstract
Pollination of many flowers leads to an increase in ethylene synthesis and flower senescence. We have investigated the regulation of pollination-induced ethylene synthesis in tomato (Lycopersicon esculentum) using flowers of the dialytic (dl) mutant, in which pollination can be manipulated experimentally, with the aim of developing a model system to study tomato flower senescence. Ethylene synthesis increased rapidly in dl pistils following pollination, leading to accelerated petal senescence, and was delayed in ethylene-insensitive Never-ripe (Nr) pistils. However, Nr pistils eventually produced more ethylene than dl pistils, suggesting the presence of negative feedback regulation of ethylene synthesis following pollination. LEACS1A expression correlated well with increased ethylene production in pollinated dl pistils, and expression in Nr revealed that regulation is via an ethylene-independent mechanism. In contrast, the induction of the 1-aminocyclopropane-1-carboxylic acid oxidases, LEACO1 and LEACO3, following pollination is ethylene dependent. In addition, the expression profiles of ACS and ACO genes were determined during petal senescence and a hypothesis proposed that translocated 1-aminocyclopropane-1-carboxylic acid from the pistil may be important for regulating the initial burst of ethylene production during petal senescence. These results are discussed and differences between tomato and the ornamental species previously studied are highlighted.
Pollination leads to the onset of fruit development and the senescence of floral organs that become obsolete after pollination has occurred. In many flowers, the initial response to pollination is an early increase in ethylene production by the stigma that is often followed by increased ethylene production from ovaries and petals. The pollination-induced ethylene produced by different floral organs is responsible for coordinating pollination-associated events such as ovary growth and senescence of the perianth (for review, see Larsen et al., 1993; Woltering et al., 1994). Ethylene is synthesized by two enzymes: 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), which catalyzes the conversion of S-adenosyl-l-Met into ACC, and ACC oxidase (ACO), which converts ACC into ethylene (Kende, 1993). These enzymes are encoded by multigene families in all species examined (Zarembinski and Theologis, 1994). The effect of pollination on the expression pattern of these genes has been studied in the ornamental species carnation, geranium, petunia, and orchid. Increased ethylene synthesis following pollination of these flowers is accompanied by increased ACS and ACO gene expression and elevated enzyme activities (Woodson et al., 1992; O'Neill et al., 1993; Tang et al., 1994; Tang and Woodson, 1996; Clark et al., 1997; Jones and Woodson, 1997; Bui and O'Neill 1998).
Tomato (Lycopersicon esculentum) has become one of the model species for studying the regulation of ethylene biosynthesis and perception. Tomato ACS is encoded by a multigene family containing at least eight members (LEACS1A, LEACS1B, and LEACS2-7; Zarembinski and Theologis, 1994; Oetiker et al., 1997; Shiu et al., 1998). The expression of some members of the ACS gene family has been investigated in fruit, roots, and leaves at different developmental stages and under various environmental conditions (Van der Straeten et al., 1990; Olson et al., 1991, 1995; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993; Spanu et al., 1993; Nakatsuka et al., 1998). However, little is known about the expression of members of the ACS gene family in tomato flowers and only the expression of LEACS2 has been characterized (Rottmann et al., 1991). LEACS2 transcripts accumulate in mature and senescent anthers and in fully senescent petals, but no expression could be detected in pistils.
Four ACO genes (LEACO1-4) have been identified in tomato and their expression has been analyzed in response to wounding and during flower development, leaf senescence, and fruit ripening (Holds-worth et al., 1988; Barry et al., 1996, Blume and Grierson, 1998; Nakatsuka et al., 1998). The studies of Barry et al. (1996) analyzed the spatial and temporal regulation of LEACO1, 2, and 3 gene expression in tomato flowers during development, although the effect of pollination on the expression was not examined. In addition, components of the ethylene perception and signal transduction pathway are beginning to be identified in tomato (Bleecker, 1999). The tomato Never-ripe (Nr) mutant displays ethylene insensitivity (Lanahan et al., 1994) and subsequent analysis has shown that it is defective in a member of the ethylene receptor gene family (Wilkinson et al., 1995). Nr plants have recently been successfully used to elucidate the role of ethylene in several processes (Aloni et al., 1998; Lund et al., 1998; Clark et al., 1999).
We have studied the physiological and molecular events associated with pollination-induced flower senescence in tomato and compared these with previous results from ornamental species. Our results show that LEACS1A, LEACO1, and LEACO3 appear to be important for pollination-induced ethylene synthesis in pistils. LEACS1A is regulated independently of ethylene, whereas LEACO1 and LEACO3 have a strong ethylene requirement. In addition, the differential expression of the ACS and ACO gene families during petal senescence is reported.
RESULTS
The Use of the dialytic Mutant as a Model to Study Pollination Events in Tomato
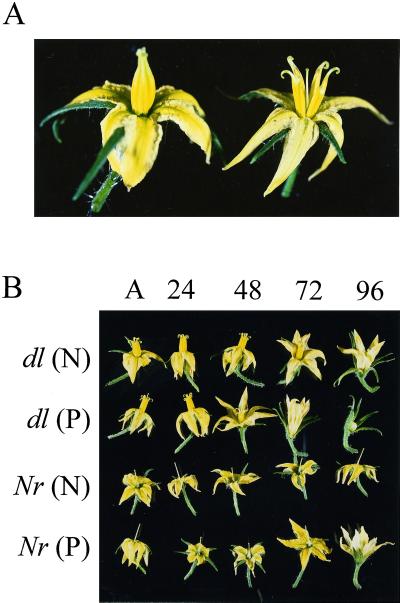
The flowers of tomato are comprised of six anthers fused to form a cone that is attached to the petals and surrounds a single enclosed pistil. The presence of the anther cone ensures that the flowers normally undergo self-fertilization. This makes pollination studies cumbersome, as emasculation must occur to prevent self-pollination and to allow access to the stigmatic surface. In turn, emasculation often leads to wound responses and severe damage of the flower petals, rendering them useless for further investigation. To circumvent these problems, we have utilized the flowers of the dialytic (dl) mutant (Darby et al., 1977), in which individual anthers are not fused to form a cone (Fig. 1A). This phenotype has two benefits for this study. First, the stigma is readily accessible without emasculation, and second, self-pollination is easily prevented as pollen does not collect around the stigmatic surface. Apart from the altered phenotype of the anthers, no other aberrant phenotype was evident; fertilization occurred normally and the rate of flower senescence was identical to wild-type cv Ailsa Craig plants. Therefore, dl flowers represent an ideal model for studying post-pollination events in tomato.
Figure 1.
A, Effect of the dl mutation in tomato flowers. Left, Wild-type tomato flower. Right, dl flower, in which individual anthers are not fused to form a cone. B, Natural and pollination-induced senescence of dl and Nr tomato flowers. Non-pollinated (N) and pollinated (P) dl or Nr flowers were examined at anthesis (A) and 24, 48, 72, and 96 h after anthesis. The Nr flowers were emasculated 2 d prior to anthesis.
Effect of Pollination on Flower Senescence
Physical changes in response to pollination were examined (Fig. 1B). Fully open dl flowers were hand-pollinated and collected after 24, 48, 72, and 96 h. Mock-pollinated flowers, in which the stigmatic surface was touched with a flat spatula free of pollen, were used as non-pollinated control. Whereas most of the non-pollinated flowers showed the first symptoms of corolla senescence at 72 h after anthesis, pollinated flowers presented visible wilting symptoms within 48 h. At 72 h after pollination, the perianth was clearly degraded, and by 96 h, had abscised.
The role of ethylene in mediating flower senescence in response to pollination was examined in the Nr mutant (Fig. 1B). Nr flowers have normal wild-type flower morphology, therefore, to avoid self-pollination, the anther cone was removed 2 d before anthesis and they were pollinated when the petals were fully reflexed. Mock-pollinated Nr flowers failed to show any visible signs of senescence during 96 h of observation. Senescence symptoms were visible in pollinated Nr flowers after 96 h.
Ethylene Production in Response to Pollination
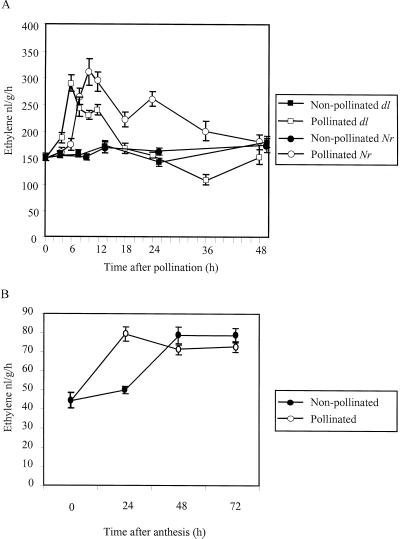
The rate of ethylene production was measured from pistils of dl flowers at different times after pollination (Fig. 2A). Ethylene production by pistils at anthesis was approximately 150 nL g−1 h−1. There was no detectable difference in the rate of ethylene production between mock-pollinated and pollinated pistils until 4 h post-pollination. At 4 h an increase was observed in pollinated pistils that continued to rise and peaked at 6 h after pollination when values were approximately double those measured at anthesis. The levels of ethylene decreased slowly to prepollination levels after 24 h. Pistils from non-pollinated flowers exhibited constant basal level of ethylene production during the experimental period.
Figure 2.
A, Ethylene production from pistils of dl and Nr in response to pollination. Individual pistils, isolated at anthesis (time 0) and at different times after pollination or mock-pollination, were enclosed in airtight vials and ethylene-sampled after 45 min. B, Ethylene production by petals isolated from pollinated and non-pollinated dl flowers. Flowers were pollinated or mock-pollinated at anthesis and collected after 24, 48, and 72 h. Isolated petals were enclosed in airtight vials for 45 min. Values represent means of at least 10 samples. Vertical bars represent se.
The rate of ethylene production was also analyzed in Nr pistils (Fig. 2A). The profile and level of ethylene production in Nr pistils was very similar to dl pistils, although the peak was delayed in Nr pistils, occurring approximately 10 h after pollination. Concomitant with the delayed increase, the decline to prepollination levels also took longer in Nr pistils and the total ethylene produced during the experimental period was substantially higher than that in dl pistils.
To investigate if the differences in the pattern of ethylene production were associated with different characteristics of pollen tube growth, pollinated pistils from dl and Nr flowers were stained with aniline blue and callose deposits visualized by fluorescence microscopy. In both cases, pollen grains germinated between 1 and 3 h after pollination and pollen tubes reached the base of the style within 8 h of pollination.
Ethylene production by dl petals was also examined (Fig. 2B). In petals from non-pollinated flowers, ethylene increased from approximately 44 nL g−1 h−1 at anthesis to 79 nL g−1 h−1 at 48 h after anthesis. Pollination accelerated the onset of ethylene production by petals by approximately 24 h. In both pollinated and non-pollinated flowers, the increase in ethylene production occurred before symptoms of senescence were apparent (compare Figs. 1B and 2B). No additional increase in ethylene production was detected in association with the progress of petal senescence.
ACS and ACO Expression in Tomato Pistils
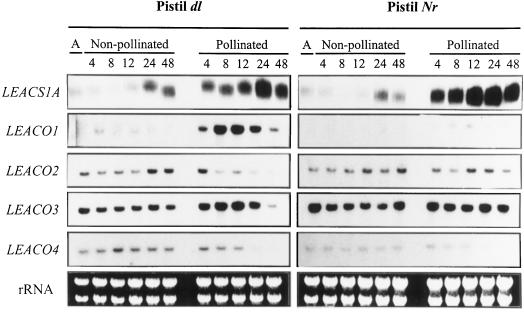
The abundance of eight ACS transcripts was measured by RNase protection assay (RPA) analysis in pistils of tomato flowers following pollination. The transcripts of LEACS1A, LEACS2, and LEACS6 genes were identified in pistils of dl flowers, but no signal was observed with the other probes (LEACS1B, LEACS3, LEACS4, LEACS5, and LEACS7). LEACS2 and LEACS6 transcripts were present at extremely low levels in dl pistils and no changes were observed in response to pollination (data not shown). LEACS1A transcripts were detectable in dl pistils at anthesis and remained constant in non-pollinated flowers for 24 h, when an increase in abundance was observed (Fig. 3). In pollinated dl pistils, an increase in LEACS1A expression occurred within 4 h and persisted through to 48 h post-pollination. Pollination of Nr pistils induced the same changes in LEACS1A expression with a similar kinetic profile.
Figure 3.
Accumulation of ACS and ACO transcripts in response to pollination in dl and Nr pistils. RNA was extracted from dl or Nr pistils at anthesis (A) and 4, 8, 12, 24, and 48 h after pollination. Pistils from mock-pollinated flowers (non-pollinated) were used as controls. Thirty micrograms of total RNA was hybridized to radiolabeled ACS gene-specific probes and used for RPA analysis. ACO gene expression was determined by RNA gel-blot analysis using 15 μg of total RNA. Gels were stained with ethidium bromide to ensure equal loading of the samples.
The expression of the four members of the tomato ACO gene family was assessed by northern-blot analysis with gene-specific probes. Our results indicated that all four ACO genes were expressed at constant levels in pistils from non-pollinated dl flowers, but they showed different regulation in response to pollination (Fig. 3). LEACO1 mRNA levels were very low in pistils of non-pollinated flowers, but accumulated dramatically after pollination, reached a maximum at around 12 h, and declined thereafter. LEACO3 expression also increased following pollination and showed the same pattern kinetically as LEACO1. Both LEACO2 and LEACO4 transcript abundance declined in response to pollination. The expression of ACO genes was examined in pistils from Nr flowers (Fig. 3). The increase in LEACO1 and LEACO3 and the decrease in LEACO2 in response to pollination did not occur in Nr pistils. In contrast, the pattern of expression of LEACO4 in pistils from Nr flowers was identical to that observed in dl pistils.
Expression of ACS and ACO Genes in dl Petals
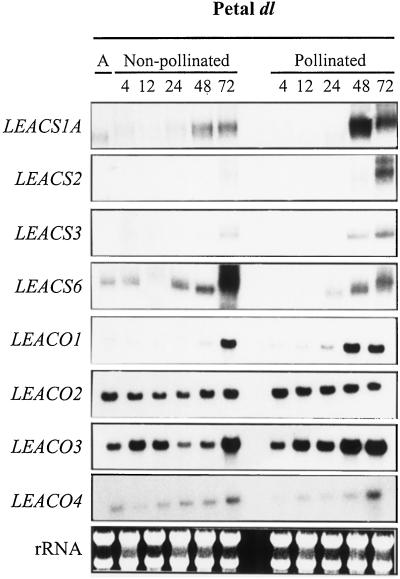
Petals were collected from dl tomato flowers at anthesis and at 4, 12, 24, 48, and 72 h after pollination. Mock-pollinated flowers were used as controls. Expression analysis of the eight members of the tomato ACS gene family indicated that only transcripts corresponding to LEACS1A, LEACS2, LEACS3, and LEACS6 were present in petals (Fig. 4). Increased expression of LEACS1A and LEACS6 was evident in petals of both non-pollinated and pollinated flowers at around 48 h post-anthesis. The expression of LEACS2 and LEACS3 increased at 72 h in non-pollinated flowers and 24 h earlier in pollinated flowers. All four ACO genes were expressed in petals, but different expression patterns were evident (Fig. 4). The expression of LEACO1 and LEACO3 was up-regulated in petals from non-pollinated flowers at 72 h after anthesis and 1 d earlier in petals from pollinated flowers. In contrast, the expression of LEACO2 and LEACO4 remained constant in all samples examined except that an increase was observed in the latter at 72 h in non-pollinated and pollinated samples.
Figure 4.
Analysis of the expression ACS and ACO genes in petals from dl flowers. Total RNA was obtained from petals collected from flowers at anthesis (A) and from pollinated and non-pollinated flowers at 4, 12, 24, 48, and 72 h after anthesis. For ACS gene expression, RPA analysis was performed using 30 μg of total RNA per sample. The accumulation of ACO transcripts was examined by RNA gel-blot analysis using 15 μg of total RNA. Gels were stained with ethidium bromide to ensure equal loading of the samples.
DISCUSSION
In this study, we have examined the effect of pollination in regulating flower senescence in tomato and analyzed the role of ethylene in mediating post-pollination events. In the absence of pollination, tomato flowers showed visible signs of senescence, as indicated by initial petal curling, approximately 72 h after anthesis. This was accelerated by approximately 24 h in pollinated flowers, indicating that pollination affects the rate of senescence (Fig. 1B). The rapid rate of tomato flower senescence in the absence of pollination is different from other species that have previously been studied. For example, flower longevity in carnation, petunia, and orchids can range from a couple of weeks to several months in the absence of pollination (Nichols, 1977; Pech et al., 1987; Singh et al., 1992; Stead, 1992; O'Neill et al., 1993). In many flowers pollination is followed by a rapid increase in ethylene production by the pistil, and this is accompanied by increased ACS and ACO expression and enzyme activity (O'Neill et al., 1993; Tang et al., 1994; Tang and Woodson, 1996; Jones and Woodson, 1997, 1999; Bui and O'Neill, 1998). Our results indicate that ethylene biosynthesis by tomato pistils starts to increase 4 h after pollination, peaks at 6 h, is elevated up to 12 h, and slowly declines thereafter. This increase in ethylene production is first detected after the pollen grains have germinated and when the pollen tubes have penetrated approximately one-quarter of the style length. This result contrasts with those reported in other flowers in which the increase in ethylene production occurs either simultaneously or before the germination of the pollen grains (Zhang and O'Neill, 1993; Larsen et al., 1995; Tang and Woodson, 1996).
In an attempt to understand the molecular basis for the increase in ethylene production following pollination of tomato pistils and to determine the role of ethylene, the expression of the genes involved in ethylene biosynthesis were analyzed in both dl and Nr pistils (Fig. 3). Pollination induced an increase in LEACS1A expression in dl pistils and exactly the same pattern was seen in Nr pistils, indicating that this change occurs independently of ethylene. All four ACO genes were expressed in dl pistils. LEACO1 and LEACO3 showed increased expression in response to pollination, whereas transcripts corresponding to LEACO2 and LEACO4 declined following pollination. The changes in expression of LEACO1, LEACO2, and LEACO3 following pollination did not occur in Nr pistils, indicating that they are ethylene dependent. However, LEACO4 expression was similar in both dl and Nr pistils, suggesting that expression is regulated independently of ethylene. Furthermore, increased expression of LEACS1A, LEACO1, and LEACO3 in dl pistils following pollination correlated well with the increased ethylene production (compare Figs. 2A and 3).
Examples of ethylene-independent induction of ACS gene expression in response to pollination have previously been reported for the Phal-ACS2 and Phal-ACS3 genes from orchid and the DCACS3 from carnation (Jones and Woodson, 1997, 1999; Bui and O'Neill, 1998). It has been suggested that these genes respond to primary signals derived from pollen and are, therefore, responsible for initiating ethylene synthesis in pistils. The expression pattern of LEACS1A suggests that it may also fall into the same category. Additionally, in orchid and carnation, secondary ethylene-dependent induction of ACS gene expression has been observed in pistils in response to pollination brought about by increased expression of Phal-ACS1 and DCACS1 and DCACS2, respectively (Jones and Woodson, 1997, 1999; Bui and O'Neill, 1998). In tomato, we did not detect any secondary ethylene-dependent increase in ACS gene expression, indicating differences occur in the regulation of ethylene synthesis between these species. The hypothesis that increased ethylene production in tomato pistils following pollination is due to elevated LEACS1A expression alone and not to any other secondary ethylene-dependent ACS expression is supported by comparison of ethylene production in dl and Nr pistils (Fig. 2A).
Disruption of ethylene perception in Nr did not lead to a reduction in ethylene production by pistils in response to pollination, although a delay of several hours occurred until maximal levels were attained and the total amount of ethylene produced was higher. This suggests that ethylene perception affects the timing and the extent of ethylene biosynthesis after pollination. This contrasts with previous studies using carnation and petunia flowers. Treatment of petunia styles with the ethylene action inhibitor 2,5-norbornadiene did not affect the timing and extent of ethylene production during the first 8 h after pollination, but inhibited the production of ethylene after 24 h (Tang and Woodson, 1996). In carnation the timing of ethylene production was unaltered, but the level of ethylene biosynthesis was lower in 2,5-norbornadiene- and diazocyclopentadiene-treatedflowers compared to non-treated flowers (Jones and Woodson, 1997).
The delay in ethylene production in Nr pistils may be attributed to a reduction in total ACO protein due to reduced expression of LEACO1 and LEACO3 (Fig. 3). This suggests an important role for ACO in regulating the timing of ethylene synthesis in tomato pistils in response to pollination. Although maximal ethylene synthesis was delayed in Nr pistils, they eventually produce higher levels of ethylene than dl pistils due to a reduction in the rate of decline following the peak of synthesis (Fig. 2A). This suggests the operation of a negative feedback mechanism by which ethylene can auto-inhibit its own synthesis following pollination. This is consistent with the results of Wilkinson et al. (1997) which showed that ethylene-insensitive petunia flowers produced more ethylene than wild-type flowers following pollination. Higher levels of LEACO2 and LEACO3 transcripts in pollinated Nr pistils at later time points may account for this increase (Fig. 3). Alternatively, in wild-type flowers, ethylene may induce ACC conjugation or cause inactivation of either ACS or ACO, leading to feedback inhibition.
Pollination of tomato flowers results in increased ethylene synthesis and an acceleration of petal senescence. However, petal senescence occurred in the absence of pollination, but with a 24-h delay (Fig. 1B). This indicates that the floral organ is already programmed to undergo senescence and that pollination simply accelerates the process. The fact that senescence is accelerated in tomato and other flowers by pollination suggests that following pollination, signals derived from the pistil are translocated to the perianth to induce ethylene production and senescence. The nature of the signal that mediates interorgan communication is not clear. ACC or ethylene itself have been proposed to be the translocated signal in orchids (O'Neill et al., 1993; Woltering et al., 1995; Bui and O'Neill, 1998) and carnation (Reid et al., 1984; Woltering, 1990; Have and Woltering, 1997). Alternatively, other factors such as auxin, short-chain fatty acids, or electrical signals have been suggested as mobile senescence signals (Burg and Dijkman, 1967; Linskens and Spanjers, 1973; Fromm et al., 1995; Halevy et al., 1996). In tomato, it is possible that ACC may be the signal that is translocated from pistils to petals. Indirect evidence to support this hypothesis comes from the observation that ethylene synthesis increases in petals of pollinated flowers prior to a de novo increase in ACS gene expression (compare Figs. 2B and 4). An increase in ACC may be produced via elevated LEACS1A expression in pistils at between 24 and 48 h (Fig. 3) at a time when ACO expression (Fig. 3) and ethylene synthesis (Fig. 2) are declining. Translocated ACC may then be converted to ethylene by the relatively high ACO that is already present in petals. Subsequent maintenance of ethylene production in petals through 72 h and complete senescence may then be achieved by increased ACS and ACO expression. The role of multiple ACS and ACO genes in petals remains unclear. However, as well as senescence, other biological phenomena occur in senescent petals. These include abscission, subsequent wounding at the abscission zone, wilting, and cell death, some of which are known to be regulated, at least in part, by ethylene (He et al., 1996; O'Donnell et al., 1996; González-Carranza et al., 1998).
CONCLUSIONS
We have investigated the role of pollination in regulating flower senescence in tomato both at the physiological and molecular level. Pollination causes an increase in ethylene synthesis, an enhanced rate of senescence, and is accompanied by changes in ACS and ACO gene expression. LEACS1A appears to be the sole ACS gene responsible for increased ethylene production in pistils and is regulated in an ethylene-independent way. Disruption of ethylene perception alters the timing of ethylene production in response to pollination as a delay occurs in Nr. This delay may occur as a result of reduced ethylene-dependent expression of LEACO1 and LEACO3 early after pollination. In addition, ethylene perception is required for the decrease in ethylene production after maximum levels have been reached, indicating a negative feedback control of ethylene synthesis following pollination. These data suggest that both ACS and ACO are important for regulating ethylene synthesis in tomato in response to pollination.
MATERIALS AND METHODS
Plant Material
Tomato (Lycopersicon esculentum Mill.) plants homozygous for the dl and Nr mutations contained within the Ailsa Craig genetic background were grown under standard greenhouse conditions using routine horticultural practices. Fully open dl flowers (with petals fully reflexed) were hand-pollinated by touching the stigmatic surface with a flat spatula loaded with pollen. Mock-pollinated flowers, in which the stigma was touched with a flat spatula free of pollen, were used as non-pollinated controls. Nr flowers were emasculated 2 d prior to anthesis to avoid self-pollination. Pollination and mock pollination were carried out as for dl flowers.
Ethylene Measurements
Ethylene production by the different floral organs was measured at different times after pollination. The samples (pistils or petals) were detached and enclosed in airtight vials and incubated at 25°C for 45 min following which 1 mL of the headspace was withdrawn. Ethylene concentration in the gas sample was measured by gas chromatography using an ATI UNICAM 610 series gas chromatograph (Unicam Analytical, Cambridge, UK) linked to a PC with UNICAM 4880 chromatography data handling software (Unicam Analytical). Column specifications were: length, 150 mm; outer dimension, 6 mm; inside dimension, 4 mm; support, alumina F1 mesh range 80 to 100. Temperatures were as follows: oven/column, 110°C; injector, 108°C; detector, 160°C.
Analysis of Pollen Tube Growth
Stigma/style from pollinated flowers were collected at 1-h intervals after pollination. Samples were prepared as described previously by Clark et al. (1997) and stained with 0.1% (w/v) aniline blue in 0.1 m K2HPO4. Samples were squashed on a slide with a coverslip and growth of pollen tubes was visualized using a LEITZ DMR microscope (Lieca Microsystems, Wetzlar, Germany) provided with a UV lamp. Pollen tube lengths were measured using an eyepiece graticule.
ACS and ACO Gene-Specific Radiolabeled Probes
Gene-specific probes for LEACS1A, LEACS1B, LEACS5, and LEACS6 were PCR-amplified using primers previously described by Oetiker et al. (1997). The products were cloned into the pCR2.1 vector (Invitrogen, San Diego). LEACS2, LEACS3, LEACS4, and LEACSS7 (Rottmann et al., 1991; Lincoln et al., 1993; Olson et al., 1995; Shiu et al., 1998) gene-specific probes were designed from around the 3′-untranslated region of each gene. Primer pairs were as follows: LEACS2, ACS2F: 5′-ttaaaagggaagaatttaatt-3′ and ACS2R: 5′-taacaatataatcgagaaag-3′ generating a probe from nucleotides 2,702 to 2,957; LEACS3, ACS3F: 5′-gtcattctccaagtgggttt-3′ and ACS3R: 5′-gtagtagtttgaacatttcaag-3′ generating a probe from nucleotides 4,073 to 4,377; LEACS4, ACS4F: 5′-ggagtcatgaagaacaagcac-3′ and ACS4R: 5′-aactatgttgggcccgtgct-3′ generating a probe from nucleotides 2,624 to 2,855; LEACS7, ACS7F: 5′-gtctagtcatgtg-aaagt-3′ and ACS7R2: 5′-gcacttgtgcggtcacct-3′ generating a probe from nucleotides 4,065 to 4,335. PCR products were cloned into SmaI cut pBluescript II SK+ (Stratagene, La Jolla, CA) or pGEM-T Easy (Promega, Madison, WI).
LEACO1-, LEACO2-, and LEACO3-specific probes have previously been described (Barry et al., 1996). A LEACO4-specific probe was designed from the 3′-untranslated region of the cDNA sequence reported by Nakatsuka et al. (1998) using the following primers: ACO4F, 5′-ggacacta-attaagaggattaaag-3′, and ACO4R, 5′- ccccatagagaacaacctc-3′. The resultant 137-bp fragment was cloned in to pGEM-T Easy (Promega). The identity of all clones was confirmed by DNA-sequence analysis.
Single-strand specific radiolabeled RNA probes were prepared by in vitro transcription from linear plasmid template using either T3 or T7 RNA polymerase (Promega) according to the manufacturer's instructions.
RNA Extraction and Analysis
RNA was extracted from frozen pistils and petals using the protocol described by Griffiths et al. (1999), except that the initial volume of extraction buffer was reduced to 2 mL to accommodate the reduced fresh weight of tissue used. On average, 40 flowers were used for each extraction. RNA gel-blot analysis was performed as described by Griffiths et al. (1999). RPA was used as previously described (Barry et al., 1996) with the following modifications. All hybridizations were carried out using 30 μg of total RNA and digestions with RNase ONE (Promega) were performed at 28°C for 3 h using 3 units of enzyme per reaction.
ACKNOWLEDGMENTS
We would like to thank Dr. Roger Chetelat (University of California-Davis) and Dr. Ian Taylor (University of Nottingham) for helpful discussions at the onset of this project and for providing seed stocks of the dl mutant. We would also like to thank Dr. Jeremy Roberts (University of Nottingham) for helpful comments on the manuscript.
Footnotes
This work was supported by the European Union (grant nos. FAIR–96–5069 and FAIR CT 95–0225 to D.G.).
LITERATURE CITED
- Aloni R, Wolf A, Feigenbaum P, Avni A, Klee HJ. The Never ripe mutant provides evidence that tumor-induced ethylene controls the morphogenesis of Agrobacterium tumefaciens-induced crown galls on tomato stems. Plant Physiol. 1998;117:841–849. doi: 10.1104/pp.117.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1-amino-cyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Bleecker AB. Ethylene perception and signaling: an evolutionary perspective. Trends Plant Sci. 1999;4:269–274. doi: 10.1016/s1360-1385(99)01427-2. [DOI] [PubMed] [Google Scholar]
- Blume B, Grierson D. Expression of ACC oxidase promoter-GUS fusions in tomato and Nicotiana plumbaginifolia regulated by developmental and environmental stimuli. Plant J. 1998;12:731–746. doi: 10.1046/j.1365-313x.1997.12040731.x. [DOI] [PubMed] [Google Scholar]
- Bui AQ, O'Neill SD. Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol. 1998;116:419–428. doi: 10.1104/pp.116.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Dijkman MJ. Ethylene and auxin participation in pollen induced fading of Vanda orchids. Plant Physiol. 1967;49:1648–1650. doi: 10.1104/pp.42.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. Root formation in ethylene-insensitive plants. Plant Physiol. 1999;121:53–59. doi: 10.1104/pp.121.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DG, Richards C, Hilioti Z, Lind-Iverson S, Brown K. Effect of pollination on accumulation of ACC synthase and ACC oxidase transcripts, ethylene production and flower petal abscission in geranium (Pelargonium × hortorum L.H. Bailey) Plant Mol Biol. 1997;34:855–865. doi: 10.1023/a:1005877809905. [DOI] [PubMed] [Google Scholar]
- Darby LA, Ritchie DB, Taylor IB. Isogenic lines of the tomato ‘Ailsa Craig.’ Annual Report Glasshouse Crops Research Institute. 1977. pp. 168–184. [Google Scholar]
- Fromm J, Hajirezaei M, Wilke I. The biochemical response of electrical signaling in the reproductive system of Hibiscus plants. Plant Physiol. 1995;109:375–384. doi: 10.1104/pp.109.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Lozoya-Gloria E, Roberts JA. Recent developments in abscission: shedding light on the shedding process. Trends Plant Sci. 1998;3:10–14. [Google Scholar]
- Griffiths A, Barry C, Alpuche-Solis AG, Grierson D. Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. J Exp Bot. 1999;50:793–798. [Google Scholar]
- Halevy AH, Porat R, Spiegelstein H, Borochov A, Botha L, Whitehead CS. Short-chain fatty acids in the regulation of pollination-induced ethylene sensitivity of Phalaenopsis flowers. Physiol Plant. 1996;97:469–474. [Google Scholar]
- Have A, Woltering EJ. Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol. 1997;34:89–97. doi: 10.1023/a:1005894703444. [DOI] [PubMed] [Google Scholar]
- He CJ, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 1996;112:463–472. doi: 10.1104/pp.112.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Schuch W, Grierson D. Organisation and expression of a wound/ripening-related small multigene family from tomato. Plant Mol Biol. 1988;11:81–88. doi: 10.1007/BF00015661. [DOI] [PubMed] [Google Scholar]
- Jones ML, Woodson WR. Pollination-induced ethylene in carnation: role of stylar ethylene in corolla senescence. Plant Physiol. 1997;115:205–212. doi: 10.1104/pp.115.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Woodson WR. Differential expression of three members of the 1-aminocyclopropane-1-carboxylate-synthase gene family in carnation. Plant Physiol. 1999;119:755–764. doi: 10.1104/pp.119.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. The Never-ripe mutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Ashworth EN, Jones ML, Woodson WR. Pollination-induced ethylene in carnation: role of pollen tube growth and sexual compatibility. Plant Physiol. 1995;108:1405–1412. doi: 10.1104/pp.108.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Woltering EJ, Woodson WR. Ethylene and interorgan signaling in flowers following pollination. In: Raskin I, Schultz J, editors. Plant Signals in Interactions with Other Organisms. Rockville, MD: American Society of Plant Physiologists; 1993. pp. 112–122. [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Linskens HF, Spanjers AW. Changes in the electrical potential in the transmitting tissue of petunia styles after cross- and self-pollination. Incompatible Newslett. 1973;3:81–85. [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. Sites of ethylene production in the pollinated and unpollinated senescing carnation (Dianthus caryophyllus) inflorescence. Planta. 1977;135:155–159. doi: 10.1007/BF00387165. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Olson DC, Oetiker JH, Yang SF. Analysis of LE-ACS3, a 1-aminocyclopropane-1-carboxylic acid synthasegene expressed during flooding in the roots of tomato plants. J Biol Chem. 1995;270:14056–14061. doi: 10.1074/jbc.270.23.14056. [DOI] [PubMed] [Google Scholar]
- Olson DC, White JA, Edelman L, Harkins RN, Kende H. Differential expression of two genes for 1-amino-cyclopropane-1carboxylate synthase in tomato fruits. Proc Natl Acad Sci USA. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell. 1993;5:419–432. doi: 10.1105/tpc.5.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech JC, Latché A, Larrigaudière C, Reid MS. Control of early ethylene synthesis in pollinated petunia flowers. Plant Physiol Biochem. 1987;25:431–437. [Google Scholar]
- Reid MS, Fujino DW, Hoffman NE, Whitehead CS. 1-Aminocyclopropane-1-carboxylic acid (ACC): the transmitted stimulus in pollinated flowers. J Plant Growth Regul. 1984;3:189–196. [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Shiu Y, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclo-propane-1-carboxylate synthase gene of the tomato, is tagged by Sol3 transposon. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Evensen KB, Kao T-H. Ethylene synthesis and floral senescence following compatible and incompatible pollinations in Petunia inflata. Plant Physiol. 1992;99:38–45. doi: 10.1104/pp.99.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P, Boller T, Kende H. Differential accumulation of transcripts of 1-aminocyclopropane-1-carboxylate synthase genes in tomato plants infected with Phytophthora infestans and in elicitor-treated tomato cell suspensions. J Plant Physiol. 1993;141:557–562. [Google Scholar]
- Stead AD. Pollination-induced flower senescence: a review. Plant Growth Regul. 1992;11:13–20. [Google Scholar]
- Tang X, Gomes AM, Bhatia A, Woodson WR. Pistil-specific and ethylene-regulated expression of 1-amino-cyclopropane-1-carboxylate oxidase genes in petunia flo-wers. Plant Cell. 1994;6:1227–1239. doi: 10.1105/tpc.6.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Woodson WR. Temporal and spatial expression of 1-aminocyclopropane-1-carboxylate oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol. 1996;112:503–511. doi: 10.1104/pp.112.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D, Van Wiemeersch L, Goodman HM, Van Montagu M. Cloning and sequence of two different cDNA encoding 1-aminocyclopropane-1-carb-oxylate synthase in tomato. Proc Natl Acad Sci USA. 1990;87:4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotech. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Woltering EJ. Inter-organ translocation of 1-amino-cyclopropane-1-carboxylic acid and ethylene coordinates senescence in emasculated Cymbidium flowers. Plant Physiol. 1990;91:837–845. doi: 10.1104/pp.92.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering EJ, Somhorst D, Van der Veer P. The role of ethylene in inter-organ signaling during flower senescence. Plant Physiol. 1995;109:1219–1225. doi: 10.1104/pp.109.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering EJ, Ten Have A, Larsen PB, Woodson WR. Ethylene biosynthetic genes and inter-organ signaling during flower development. In: Scott RJ, Stead AD, editors. Molecular and Cellular Aspects of Plant Reproduction. Cambridge, UK: Cambridge University Press; 1994. pp. 285–307. [Google Scholar]
- Woodson WR, Park KY, Drory A, Larsen PB, Wang H. Expression of ethylene biosynthetic pathway transcripts in senescing carnation flowers. Plant Physiol. 1992;99:526–532. doi: 10.1104/pp.99.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W-K, Moore T, Yang SF. Differential accumulation of transcripts for four 1-aminocyclopropane-1-carb-oxylate synthase homologs under various conditions. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski T, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- Zhang XS, O'Neill SD. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell. 1993;5:403–418. doi: 10.1105/tpc.5.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]