Abstract
Aim: Carbon monoxide (CO) functions as a therapeutic molecule in various disease models because of its anti-inflammatory and antiapoptotic properties. We investigated the capacity of CO to reduce hypoxia-induced islet cell death and dysfunction in human and mouse models.
Results: Culturing islets in CO-saturated medium protected them from hypoxia-induced apoptosis and preserved β cell function by suppressing expression of proapoptotic (Bim, PARP, Cas-3), proinflammatory (TNF-α), and endoplasmic reticulum (ER) stress (glucose-regulated protein 94, grp94, CHOP) proteins. The prosurvival effects of CO on islets were attenuated when autophagy was blocked by specific inhibitors or when either ATG7 or ATG16L1, two essential factors for autophagy, was downregulated by siRNA. In vivo, CO exposure reduced both inflammation and cell death in grafts immediately after transplantation, and enhanced long-term graft survival of CO-treated human and mouse islet grafts in streptozotocin-induced diabetic non-obese diabetic severe combined immunodeficiency (NOD-SCID) or C57BL/6 recipients.
Innovation: These findings underline that pretreatment with CO protects islets from hypoxia and stress-induced cell death via upregulation of ATG16L1-mediated autophagy.
Conclusion: Our results suggested that CO exposure may provide an effective means to enhance survival of grafts in clinical islet cell transplantation, and may be beneficial in other diseases in which inflammation and cell death pose impediments to achieving optimal therapeutic effects. Antioxid. Redox Signal. 28, 1309–1322.
Keywords: : carbon monoxide, autophagy, islet, apoptosis
Introduction
Carbon monoxide (CO) functions as a “protective molecule” in cellular processes based on its anti-inflammatory, antiapoptotic, and other protective properties. Salutary effects of CO exposure or administration of CO-releasing molecule (CORM)-A1 have been shown in models of endotoxic shock (53), uvetis (13), organ transplantation (16, 55), hyperoxic lung injury (64), vascular injury-induced arteriosclerosis (54), intimal hyperplasia (49), aeroallergen-induced airway inflammation (45), experimental allergic encephalomyelitis (14), sclerosis (12), and others (23, 32, 56).
Innovation.
That pretreatment with carbon monoxide (CO) protects islets from hypoxia and stress-induced cell death via upregulation of ATG16L1-mediated autophagy provides a novel mechanism of CO action. CO exposure may provide a unique and effective means to enhance survival of islet graft in clinical islet cell transplantation, and may be beneficial in other diseases in which inflammation and cell death pose impediments to achieving optimal therapeutic effects.
Pancreatic islet/β cell death is a major problem for patients with type 1 or type 2 diabetes. In the murine islet transplantation model, 50–60% of islet cells die of apoptosis 2–3 days post-transplantation; cell death is mainly caused by stresses including hypoxia, nutrient deprivation, inflammation, hyperglycemia, and lipotoxicity (28a, 35a, 63a). Islet cell death hinders the application of islet transplantation for the treatment of type 1 diabetes, in part because at least two donors are required to achieve normoglycemia in most islet allograft recipients. Although insulin independence may be observed 1 year post-transplantation, the insulin-independence rate is generally poor at 5 years post-transplantation (1, 52). Novel immunological and tissue protective strategies such as using monoclonal or polyclonal antibodies to target new costimulatory pathways, and the use of stem cells and regulatory T cells, have been developed and showed efficacy in improving islet transplantation outcomes (60, 61). In this context, CO exposure or pharmacological application of CO using CORM-A1 may enhance survival and function of transplanted islets by their abilities to ameliorate islet-directed autoimmunity and immune rejection. Previous studies reported that CO possessed anti-inflammatory, antiapoptotic, and immunodulatory effects and the potential induction of β cell regeneration (42, 43, 62).
Two major signaling pathways lead to apoptosis in cells: the intrinsic (mitochondrial-dependent) pathway, which is activated by intracellular signals such as the Bcl-2 family proteins, and the extrinsic (death receptor-initiated) pathway, which is initiated by cell surface death receptors (e.g., FasL, TNF-α receptor) (2, 17). Both pathways are involved in pancreatic β cell death (34, 57). Mitochondrial-dependent, intrinsic apoptotic pathway is mediated by changes in redox potential initiated by hypoxia; it plays a predominant role in the loss of isolated islets in vitro under various culture conditions (48). The death receptor-dependent, extrinsic apoptosis pathway also triggers selective apoptosis in human islet/β cells, leading to immune-mediated type 1 diabetes, as evident by Fas expression by β cells and FasL expression on the infiltrating cells in the pancreas of type 1 diabetic patients (38).
Autophagy is a lysosomal-dependent self-degradation process in cells, which disassembles unnecessary or dysfunctional cellular components by a regulated process, and helps balance sources of energy during development or under conditions of nutrient starvation (15). The autophagosome/lysosome system plays a housekeeping role in cellular adaptation to stress via clearance of misfolded proteins and damaged organelles as well as degradation of intracellular pathogens. A major step in the formation of an autophagosome is the covalent attachment of phosphatidylethanolamine (PE) to microtubule-associated protein 1 light chain 3 β (MAP1LC3B) to form LC3-PE, a common marker of autophagy (21, 25, 33). Autophagy plays a protective role in high-fat diet-induced β cell dysfunction, as well as in defending human islets from amyloid polypeptide-induced toxicity (10, 51). Rapamycin-stimulated autophagy induction delays diabetes and inhibits β cell apoptosis in the Akita diabetes mouse model (3).
We have previously shown in murine allogeneic islet transplantation that exposing donor islets ex vivo to CO increases survival and function of transplanted islets (16, 18, 62). Here, we have investigated the mechanisms contributing to CO-mediated protection from hypoxia-induced islet death, and have assessed effects of CO on autophagy. Using Food and Drug Administration-approved autophagy-inducing drugs (amiodarone HCl, trifluoperazine [TF]) and adenoviral vectors for expressing autophagy-related genes (ATG4B, Beclin-1, ATG7, and ATG16L1), we assessed the effects of CO exposure on human and mouse islet death under hypoxia in vitro, and we determined the short-term and long-term survival of CO-exposed human and mouse islets after transplantation into diabetic recipients.
Results
CO protects islets from hypoxia-induced apoptosis and dysfunction
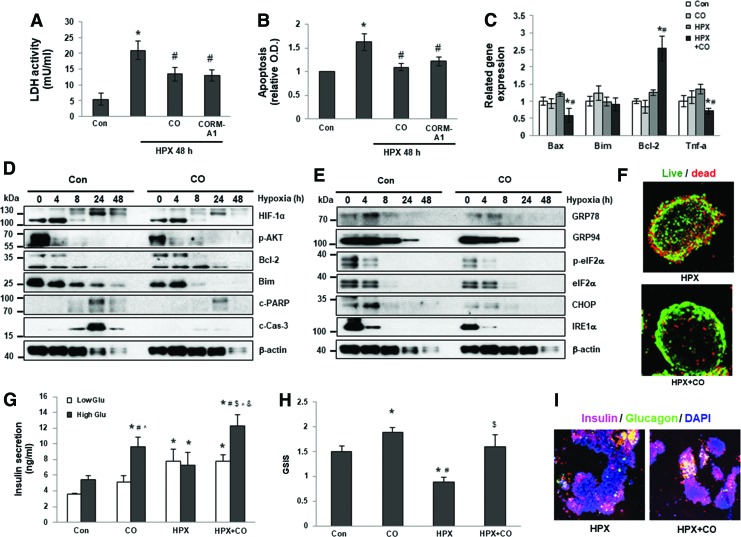
As we have previously shown, CO protects β cells and mouse islets from apoptosis post-transplantation (18, 62), and hypoxia is a major cause of islet death and dysfunction after transplantation. To assess the capacity of CO to protect human islets from hypoxia-induced apoptosis, we first compared apoptosis of human islets cultured in CO-saturated or control media. When exposed to hypoxic conditions (1% O2), control islets underwent dramatic cell death as indicated by elevated release of lactate dehydrogenase (LDH) (Fig. 1A) and of cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) (Fig. 1B). In contrast, islets cultured in CO-saturated medium (CO islets) or treated with the CORM-A1 underwent significantly reduced cell death when exposed to hypoxic conditions (Fig. 1A, B). We further measured expression of pro- and antiapoptotic genes and proinflammatory genes in control, CO only, control, and CO-islets exposed to hypoxic conditions. At the mRNA level, CO-islets showed reduced expression of Bax and TNF-α, and increased expression of Bcl-2 under hypoxic conditions (Fig. 1C). At the protein level, CO-islets showed reduced expression of hypoxia-induced HIF-1α, and of apoptotic markers including Bim, cleaved poly (ADP-ribose) polymerase (c-PARP), and cleaved Caspase 3 (c-Cas-3), and increased expression of Bcl-2 in a time-dependent manner (Fig. 1D and Supplementary Fig. S6A; Supplementary Data are available online at www.liebertpub.com/ars). In addition, CO exposure inhibited expression of endoplasmic reticulum (ER) stress-related genes including glucose-regulated protein 78 (GRP78), GRP94, and CHOP, indicating that CO suppressed hypoxia-induced ER stress in human (Fig. 1E and Supplementary Fig. S6B) and mouse islets (Supplementary Fig. S1).
FIG. 1.
CO-preincubation inhibits hypoxia-induced apoptosis and ER stress in human islets. Apoptosis in human islets cultured for 48 h under normoxia or hypoxia (HPX) with or without CO or CORM A1 was measured by LDH assay (A) or apoptosis ELISA kit (B). *p < 0.05 versus Con, #p < 0.05 versus HPX, one-way ANOVA. Messenger RNA (C) and protein (D, E) expression of specific genes were measured by qPCR and Western blot. *p < 0.05 versus Con, #p < 0.05 versus HPX, one-way ANOVA. (F) Representative confocal z-stacked images of islets, stained for live/dead cells (live, green; dead, red), after 48 h of culture in 1% O2 with or without CO. (G) Insulin secretion in islets cultured with or without CO under normal or hypoxic conditions. *p < 0.05 versus low-glucose Con, #p < 0.05 versus low-glucose CO, $p < 0.05 versus low-glucose HPX+CO, ^p < 0.05 versus high-glucose Con, &p < 0.05 versus high-glucose HPX, one-way ANOVA. (H) GSIS in islets cultured with or without CO under normal or hypoxic conditions. *p < 0.05 versus Con, #p < 0.05 versus CO, $p < 0.05 versus HPX, one-way ANOVA. (I) Insulin (red) and glucagon (green) costaining in control and CO islets cultured under hypoxic conditions. DAPI stains nuclei (blue). CO, carbon monoxide; CORM, CO-releasing molecule; ELISA, enzyme-linked immunosorbent assay; ER, endoplasmic reticulum; GSIS, glucose-stimulated insulin release; HPX, hypoxia; LDH, lactate dehydrogenase; qPCR, quantitative polymerase chain reaction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Hypoxic conditions lead to islet fragmentation and increased membrane permeability, which allows propidium iodide (PI) to enter the cell nucleus (46). Viability staining using Syto Green 13 and PI confirmed that CO exposure protected human islets from cell death under hypoxic conditions compared with control islets (Fig. 1F). Similar to human islets, exposure of mouse islets to CO protected those islets from hypoxia-induced cell death as indicated by decreases in LDH release and DNA fragmentation under hypoxic conditions (Supplementary Fig. S2A, B).
To examine whether CO exposure preserved or improved β cell function, glucose-induced insulin secretion assays were performed under normoxic or hypoxic conditions. CO islets responded significantly better than control islets after high-glucose stimulation under both conditions, shown by greater amounts of insulin secreted and increased insulin secretion indexes (Fig. 1G, H). CO islets exhibited more dense insulin staining under hypoxic conditions than control islets, suggesting better preservation of insulin+ β cells (Fig. 1I), which may have contributed to sustained insulin secretion upon glucose stimulation.
Induction of autophagy protects human islets from hypoxia-induced islet death
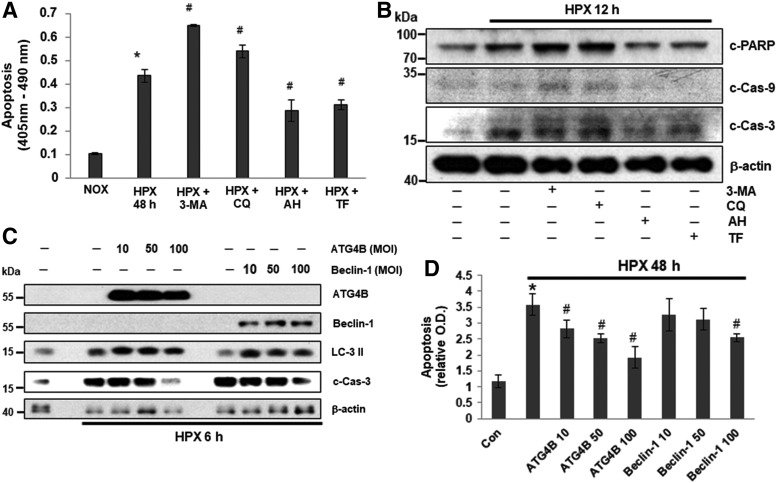
Hypoxia induces autophagy in some cell types, and autophagy induced by hypoxic stress may contribute, under different conditions, either to cell death or to survival. We used expression of LC-3II, a specific autophagy marker (36), to monitor autophagy induced by hypoxia in islets. Exposure to hypoxic conditions resulted in a time-dependent induction of autophagy as indicated by elevated expression of LC-3II, which was accompanied with changes in expression of apoptotic and ER stress-related genes in islets (Supplementary Figs. S1A, B and S11A, B). We then treated human islets with autophagy inhibitors, 3-methyladenine (3-MA) and the autophagosome-autolysosome fusion inhibitor, chloroquine (CQ), and measured hypoxia-induced cell death in the absence of autophagy. Islets treated with autophagy inducers, amiodarone hydrochloride (AH) and TF, were used as positive controls. After 24 h under hypoxic conditions, significantly more cell death was observed in islets pretreated with 3-MA and CQ than in islets cultured under normoxic conditions or under hypoxic conditions without inhibitors (Fig. 2A). In contrast, pretreatment with AH or TF significantly reduced hypoxia-induced islet death (Fig. 2A), which was confirmed by expression of death-related genes, including c-PARP, cleaved Cas-9, c-Cas-3 (Fig. 2B and Supplementary Fig. S7), and LC-3II (Supplementary Figs. S3 and S12). Together, these results showed that inhibition of autophagy increased hypoxia-induced apoptosis, whereas induction of autophagy reduced hypoxic-induced apoptosis, which suggests that autophagy may be an islet cell cytoprotective mechanism in response to hypoxia.
FIG. 2.
Effect of autophagy in hypoxia-induced human islet cell death. (A) Cell death in human islets challenged with hypoxia in the absence or presence of autophagy inhibitors 3-MA (5 mM), CQ (25 μM), autophagy inducers, AH (10 μM), or TF (25 μM) for 24 h. Cell death was measured by Apoptosis ELISA kit. *p < 0.05 versus Con, #p < 0.05 versus HPX, one-way ANOVA. (B) Western blot analysis of expression of c-PARP, c-Caspase 9, and c-Caspase 3 in human islets cultured under normoxic or hypoxic conditions in the absence or presence of autophagy inhibitors 3-methyladenine (3MA) (5 mM), CQ (25 μM), autophagy inducers, AH (10 μM), or TF (25 μM) for 12 h. (C–D) Human islets were infected with adenovirus expression constructs AdATG4B or AdBeclin-1 (10, 50, and 100 MOI), and exposed to hypoxia for 6 h. (C) Protein expression levels were examined by Western blot. (D) Apoptosis was measured by apoptosis ELISA kit. *p < 0.05 versus Con, #p < 0.05 versus HPX, one-way ANOVA. 3-MA, 3-methyladenine; AH, amiodarone hydrochloride; CQ, chloroquine; MOI, multiplicity of infection; TF, trifluoperazine.
ATG4B and Beclin-1 are essential autophagy genes that contribute to vesicle nucleation, an initial step for autophagosome formation in the process of autophagy (29, 35). To further confirm the role of autophagy in protecting islets from hypoxia-induced cell death, we tested whether overexpression of ATG4B or Beclin-1 reduced hypoxia-induced apoptosis in islet cells. Overexpression of ATG4B and Beclin-1 after infection with adenoviral expression constructs was verified by immunoblot analysis (Fig. 2C and Supplementary Fig. S8). Islets overexpressing ATG4B or Beclin-1 showed reduced apoptosis after 6 or 48 h under hypoxic conditions as indicated by decreased expression of c-Cas-3 and cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) compared with empty vector control islets. Expression levels of c-Cas-3 and elevated DNA fragments were inhibited in an ATG4B or Beclin-1 adenoviral multiplicity of infection (MOI)-dependent manner (Fig. 2C, D and Supplementary Fig. S8). These results indicate that induction of autophagy protects human islets from hypoxia-induced apoptosis.
CO protects human and mouse islets from hypoxia-induced apoptosis via upregulation of autophagy
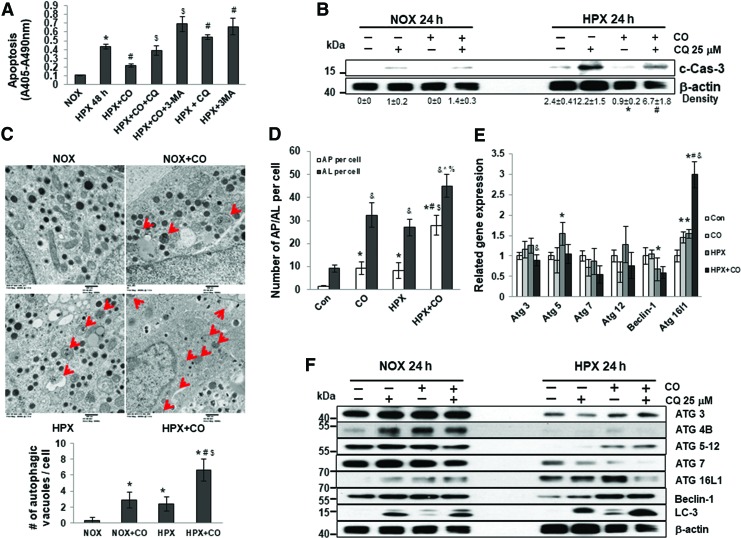
To determine whether CO protected islets from hypoxia-induced apoptosis through regulation of autophagy, islets were exposed to CO and challenged with hypoxia in the presence of autophagy inhibitors, CQ or 3-MA. The protective effects of CO on hypoxia-induced cell death were diminished when autophagy was blocked (Fig. 3A, B and Supplementary Fig. S9A), suggesting that autophagy induction is required for CO to protect islets from hypoxia-induced cell death. To verify that exposure to CO enhanced autophagy in human islets, we counted the number of autophagic vacuoles within islet cells using electron microscopy (EM). Few visible autophagic vacuoles were observed in human islet cells under normoxic conditions (0.25 ± 0.46 autophagic vacuoles per cell). More autophagic vacuoles were observed in cells exposed to CO under normoxic conditions (2.38 ± 0.92/cell) or to hypoxia alone (1.38 ± 0.92/cell). Considerably more autophagic vacuoles were seen in cells exposed to CO and hypoxia (6.38 ± 0.92/cell) (Fig. 3C). Finally, increased autophagic flux in response to CO was also demonstrated by accelerated degradation of GFP-LC-3, which is less stable than RFP-LC-3 in the acidic pH of the autolysosomes. A reduction of GFP-LC-3 relative to RFP-LC-3 autolysosome maker in CO-treated groups indicates an induction of autophagy in human islets under both normoxic and hypoxic conditions by CO (Fig. 3D and Supplementary Fig. 4).
FIG. 3.
CO-induced autophagy protects human islets from hypoxia-induced cell death. (A) Human islets were cultured for 48 h in normoxia or hypoxia (1% O2) with or without CO, CO+CQ, CO +3MA, CQ (25 μM), or 3-MA (5 mM). Cell death was measured by ELISA. *p < 0.05 versus Con, #p < 0.05 versus HPX, $p < 0.05 versus HPX+CO, one-way ANOVA. (B) Human islets were cultured for 24 h in normoxia or hypoxia with or without CO preincubation and CQ. Protein expression levels were examined by Western blot. Data are expressed as mean ± standard error of the mean; *p < 0.05 versus HPX, #p < 0.05 versus HPX+CO, one-way ANOVA. (C) Representative electron microscopy micrographs of cell-in-cell structure showing autophagic vacuoles (red arrow) in islets cultured in normoxia (NOX) and hypoxia (HPX) with or without CO. Results were quantified by counting the number of autophagic vacuoles per cell. *p < 0.05 versus NOX, #p < 0.05 versus NOX+CO, $p < 0.05 versus HPX, one-way ANOVA. (D) Quantification of autophagosomes (AP, overlap of GFP- and RFP-LC-3, yellow) and autolysosomes (AL, RFP-LC-3) per cell in different conditions. The results are represented as the average puncta fluorescence per cell from 20 cells per experiment. At least three experiments have been performed. *p < 0.05 versus AP per cell Con, #p < 0.05 versus AP per cell CO, $p < 0.05 versus AP per cell HPX, &p < 0.05 versus AL per cell Con, ^p < 0.05 versus AL per cell CO, %p < 0.05 versus AL per cell HPX, one-way ANOVA. (E) Relative mRNA expression of genes in human islets cultured in normoxia with or without CO preincubation for 24 h or hypoxia with or without CO preincubation for 6 h. *p < 0.05 versus Con, #p < 0.05 versus CO, &p < 0.05 versus HPX, one-way ANOVA. (F) Western blot analysis of autophagy-related genes in human islets treated with autophagy inhibitor CQ under normoxic or hypoxic conditions. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
We measured expression of autophagy-related genes in control and CO islets cultured under normoxic or hypoxic conditions in the presence or absence of CQ for 24 h. Similar mRNA expression levels of Atg3, 5, 7, 12, and Beclin-1 were observed in control and CO islets under hypoxic conditions, whereas expression of Atg16l1 was significantly increased in CO islets under hypoxic conditions (Fig. 3E). At the protein level under normoxic conditions, CQ treatment and/or CO exposure induced substantial increases in autophagy-related markers ATG4B, ATG16L1, and LC-3 II (Fig. 3F and Supplementary Fig. S9B). ATG3, ATG4B, ATG5-12, and Beclin-1 were downregulated in control islets under hypoxic conditions, whereas CO treatment reversed this reduction after hypoxia exposure. Expression of LC-3 II was increased in CO islets compared with control islets under either normoxic or hypoxic conditions, and the addition of CQ further enhanced the upregulation of LC-3 II in CO groups, indicating an induction of autophagy in CO islets (Fig. 3E). In addition, ATG16L1 expression was increased in both control and CO islets under hypoxic conditions compared with normoxic conditions (Fig. 3F), suggesting a potential role of ATG16L1 in CO-mediated islet protection. ATG7 was also downregulated by hypoxia and CO has no significant effect on ATG7 expression (Fig 3F).
ATG16L1 expression is essential for the antiapoptotic effects of CO
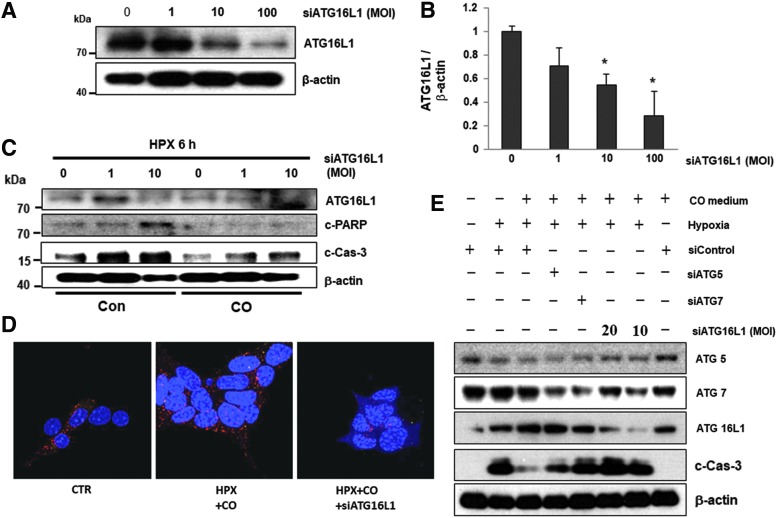
ATG16L1 is an important regulator of autophagy in hypoxia-induced osteoclast differentiation (58). To determine whether ATG16L1 mediated the antiapoptotic effects of CO, ATG16L1 expression was knocked down in human islets with adenovirus encoding siRNA against ATG16L1. At an MOI of 10, expression of ATG16L1 was substantially knocked down (Fig. 4A, B and Supplementary Fig. S10A). We then measured the effect of ATG16L1 knockdown on hypoxia-induced apoptosis in control and CO islets after 6 h under hypoxic conditions. Depletion of ATG16L1 resulted in islet cell death as indicated by increased expression of c-PARP and c-Cas-3 (Fig. 4C). Depletion of ATG16L1 attenuated autophagy and antiapoptotic effects of CO in islets, indicating that ATG16L1 is required for the protective effects of CO in hypoxia-induced autophagy and apoptosis (Fig. 4C–E and Supplementary Fig. S10B, C). Although CO failed to upregulate ATG5 and ATG7, we confirmed the positive effect of ATG5 or ATG7 on hypoxia-induced apoptosis in CO islets after 6 h under hypoxic conditions using ATG5 or ATG7 siRNAs. Against all expectations, the protective effects of CO diminished in cell lines depleted for ATG7 as indicated by expression of c-Cas-3, but not ATG5 (Fig. 4E and Supplementary Fig. S10C). These results indicate that inhibition of autophagy under hypoxic conditions leads to islet cell death, and CO protects islet cells from hypoxia-induced apoptosis through ATG7 and ATG16L1-mediated induction of autophagy.
FIG. 4.
ATG16L1 is required for CO-induced autophagy and prevention of islet death. (A) Mouse islets were infected with ATG16L1 siRNA AAV (MOI 1, 10, 100) for 48 h. Expression of ATG16L1 was measured by Western blot. (B) Expression of ATG16L1 quantified relative to β-actin. *p < 0.05 versus siATG16L1 0 MOI, one-way ANOVA. (C) Western blot analysis of expression of death-related factors in human islets exposed to hypoxia with or without CO for 6 h. (D) Autophagic flux was assessed by confocal microscopy. (E) βTC3 cells were transfected for 72 h with a control siRNA or specific siRNAs against ATG5, ATG7, and ATG16L1. After transfection, cells were incubated for 6 h under normoxia or hypoxia with or without CO. The immunoblot panels show the effect of ATG5, ATG7, or ATG16L1 knockdown on c-Cas-3 levels. AAV, adeno-associated virus; c-Cas-3, cleaved-Caspase 3. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Islet grafts exposed to CO exhibited reduced inflammation and elevated autophagy immediately after transplantation
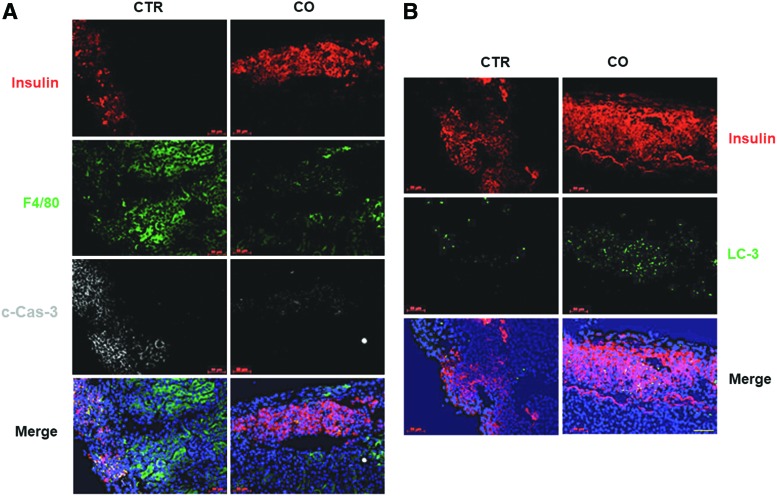
More than half of transplanted islets die immediately after transplantation, as their viability is severely compromised by hypoxia and inflammation associated with tissue trauma during islet isolation and after transplantation (5, 47). To determine whether CO protected islets from death via enhancing autophagy post-transplantation, we transplanted control or CO-treated human islets under the kidney capsules of streptozotocin (STZ)-induced, diabetic non-obese diabetic severe combined immunodeficiency (NOD-SCID) mice, and measured cell death, macrophage infiltration, and autophagy 3 days post-transplantation by immunofluorescent staining for c-Cas-3, F4/80, and LC3, respectively. Significantly fewer c-Cas-3+ and F4/80+ cells were observed in CO grafts than in control grafts (Fig. 5A), suggesting that CO treatment before grafting inhibited stress-induced apoptosis and macrophage infiltration after transplantation. Furthermore, increased autophagy was observed in CO-treated grafts compared with control grafts as indicated by increased LC-3+ cells (Fig. 5B), confirming our in vitro finding that CO protected islets from apoptosis at least, in part, via enhancing induction of autophagy.
FIG. 5.
Immunohistochemistry analysis of CO and control islet grafts 3 days post-transplantation. (A) Immunohistochemical staining of CTR or CO human islet grafts at 3 days post-transplantation using anti-insulin, anti-F4/80, and c-Cas-3 staining. Scale bar: 50 μM. (B) Immunohistochemical staining of human islet grafts using anti-insulin and anti-LC3 A/B antibodies. Scale bar: 50 μM. CTR, control. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
These findings were supported by RNAseq analysis of CO and control islet grafts retrieved from NOD-SCID recipients 3 days post-transplantation, which showed that 253 genes were differentially expressed (fold change >1.5; and p < 0.05) (Supplementary Fig. S5A). Through gene ontology analysis, we found that CO treatment inhibited genes critical for regulation of cytokine secretion (e.g., ANXA1, WNT5A, F2R, CD34, GAS6, and LRRC32) and for cytokine receptor binding (e.g., CRLF1, CXCL5, CXCL6, CXCL8 [IL8], ECM1, ITGA5, KITLG, LIF, and PXDN). We also performed canonical pathway enrichment analysis using ingenuity pathway analysis (IPA) tools and calculated significance for each pathway according to the fit of CO differential regulation data (https://apps.advaitabio.com/ipg/report/9901/contrast/11796) to the IPA database (Supplementary Fig. S5B). It has previously been shown that IL-8 was among the most highly induced genes after islet isolation and in islets cultured for 3 days without any treatment (40). Our data support the notion that the primary effect of CO is to downregulate inflammation-related pathways such as the IL-8 signaling pathway in transplanted islets (Supplementary Fig. 5B). By quantitative polymerase chain reaction (qPCR) analysis, we confirmed the RNAseq results that expression levels of CXCL5 and CXCL8 were downregulated in CO grafts compared with controls (Supplementary Fig. S5C).
Transplanted CO islets exhibit improved survival and function
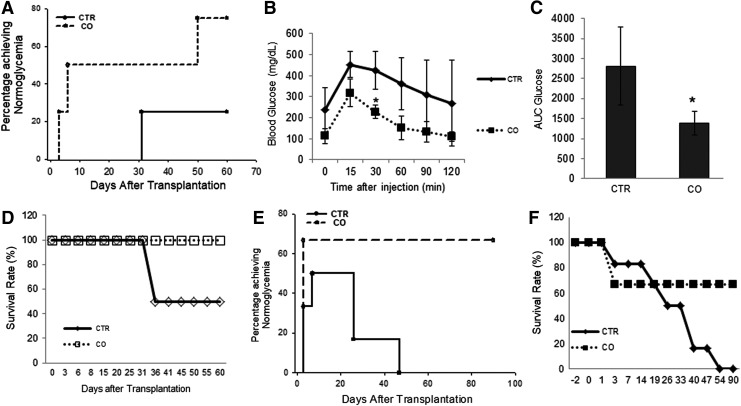
To assess the effects of CO exposure in long-term islet transplant survival and function, we performed islet transplantation using suboptimal number of islets. In the mouse model, 150 islets from C57BL/6 mice are a suboptimal number of islets that generally cannot reverse hyperglycemia in most syngeneic diabetic recipients. Indeed, when control islets were transplanted, only one of four (25%) recipients reached normoglycemia at 31 days post-transplantation; the other recipients remained hyperglycemic through the end of the study (day 60, Fig. 6A). In contrast, three of four (75%) recipients that received CO islets reached normoglycemia at day 50, and these remained normoglycemic through the end of the study (Fig. 6A). In addition, recipients that received CO islets exhibited better function in an intravenous glucose tolerance test (IVGTT) (Fig. 6B) and in area under the curve (AUC) at 45 days post-transplantation (Fig. 6C). Mice that received CO islets also exhibited better physical condition including slightly higher body weights and higher survival rate after surgery (Fig. 6D).
FIG. 6.
Survival and function of control and CO islets after islet transplantation. (A) Percentage of C57BL/6 mice transplanted with 150 CO (n = 4) or control mouse islets (n = 4) that achieved normoglycemia (<200 mg/dL). (B) Intravenous glucose tolerance test performed 45 days after transplantation in mice that received CTR (n = 4) or CO islets (n = 4). *p < 0.05, Student's t-test. (C) AUC for blood glucose levels during 120 min after glucose injection. *p < 0.05, Student's t-test. (D) Survival rate of C57BL/6 mice after receiving CO or control mouse islets. (E) Percentage of diabetic NOD-SCID mice receiving CO (n = 7) or control human islets (n = 8) that achieved normoglycemia. (F) Survival rate of diabetic NOD-SCID mice after receiving CO or control human islets. AUC, area under the curve; NOD-SCID, non-obese diabetic severe combined immunodeficiency.
When suboptimal number of human islets were transplanted under the kidney capsule of STZ-induced diabetic NOD-SCID mice, 66.7% of recipients that received CO islets reached normoglycemia at 25 days post-transplantation, whereas only 50% of recipients that received control grafts reached normoglycemia (Fig. 6E, p < 0.05, log rank test). More than 60% of mice that received CO islets survived until the end of the experiment (90 days post-transplantation), whereas all mice that received control islets died before the end of the experiment, including those reaching normoglycemia (Fig. 6F). These results again indicate that CO preincubation before transplantation enhances islet engraftment and survival.
Discussion
Despite strong demand from millions of patients with type 1 diabetes, islet transplantation has yet to become a major treatment option for this patient population. Stresses induced during islet isolation and/or after transplantation including hypoxia, cytokines, oxidative stress, and ER stress cause dramatic islet cell death and early phase nonimmune-related graft loss, which remain major hurdles for the clinical application of this otherwise promising treatment option (9, 11, 65). We previously showed that CO exposure protects βTC3 cells and mouse islets from apoptosis (18, 62). To further assess the possible clinical utility of CO in the prevention of transplant-related islet cell death in diabetes, we investigated the mechanisms of action of CO in protecting human and mouse islets from hypoxia-induced apoptosis in vitro and after transplantation.
We found that CO decreased the expression of proapoptotic (Bax, Bim), anti-inflammatory (TNF-α), and ER stress-related proteins, and increased the expression of antiapoptotic protein Bcl-2 and autophagy marker LC-3II in human and mouse islets. The short-term effects of CO preincubation in human islet/β cells included increased resistance to apoptosis and preservation of insulin secretory capacity when islets were challenged by hypoxia. CO treatment enhanced autophagy in islet cells when exposed to hypoxia in vitro and immediately after islet transplantation in vivo. The prosurvival effects of CO exposure resulted in improved engraftment and improved survival of transplanted islets in diabetic NOD-SCID or C57BL/6 mice. Furthermore, the immune modulation effects of CORM-A1 resulted in ameliorated islet-directed autoimmunity in animal models of type 1 diabetic NOD or C57BL/6 mice via the suppression of inflammation and apoptosis (42). Taken together, these observations highlight the potential clinical application of CO preincubation or CORM-A1 to augment human islets/β cell mass and function in the context of islet transplantation for treatment of diabetes.
CO has been shown to regulate autophagy in various cell types (8, 27, 28). In this regard, rapamycin-induced autophagy leads to β cell death via crosstalk with the apoptosis pathway in islets (59). We found that autophagy was increased in islet cells in response to hypoxic conditions. Inhibition of autophagy by chemical inhibitors (3-MA and CQ) increased apoptosis in islets cultured under hypoxic conditions. However, autophagy induced by CO preconditioning, by autophagy inducers, or by overexpression of ATG4B or Beclin-1 protected islets from apoptosis, which is consistent with other reports (63). These observations raise the question of why autophagy induced by hypoxia leads to cell death, whereas autophagy induced by CO or autophagy inducers is protective. It is reasonable to assume that although autophagy induced by hypoxia is a prosurvival mechanism, it is insufficient to protect islets from cell death, whereas autophagy induced by CO and autophagy inducers was sufficient to protect islets from hypoxia-induced cell death. Importantly, our study provides evidence that supports a protective role of autophagy during hypoxia-induced human and mouse islet cell death.
We found that enhancement of autophagy by CO was responsible for the increased survival of human islets challenged by hypoxia. Previous studies have shown that CO protects against hyperoxia-induced cell death in a variety of circumstances by enhancing Beclin-1-dependent autophagy and phagocytosis (8, 27, 28). The protective effect of Beclin-1-induced autophagy was confirmed in islets in our study; however, we found that ATG16L1, rather than Beclin-1, mediated the protective effects of CO in human and mouse islets. To further understand the mechanisms of how autophagy played a role in CO-dependent protection against hypoxia, we depleted ATG16L1 in human islets, and confirmed that ATG16L1 was, indeed, critical for CO-induced autophagy induction and islet protection. We also observed increased autophagy in transplanted islets 3 days post-transplantation, further indicating that the prosurvival effects of CO were mediated through ATG16L1-dependent induction of autophagy in islets. However, it is noteworthy that although autophagy induction by rapamycin could improve diabetes and prevent β cells apoptosis in Akita mice (41), many studies demonstrated that rapamycin had detrimental effects on β cell survival and function (4, 59). It is, therefore, possible that a lighter autophagy inducer such as CO exposure or CORM-A1 could induce beneficial effect of autophagy while avoiding the toxic effects of rapamycin in β cells. Further studies comparing the action of rapamycin with CO or CORM-A1 may be able to answer this question.
ER stress induced by hypoxia can cause massive islet loss in recipients after transplantation (7, 62, 65). In most cell types, the ER stress pathway interacts with the apoptotic pathway via Bcl-2 family proteins that tightly regulate the caspase cascade. For example, the BH3-only domain proteins PUMA and Bim are known to be activated by ER stress, and then convey the signal to the mitochondrial apoptotic machinery (7). CO has been shown to exert antiapoptotic effects against ER stress (24, 30). We found that CO exposure suppressed expression of ER stress proteins grp78, grp94, and CHOP, the proapoptotic gene Bim, and upregulated Bcl2. These results indicate that the antiapoptotic effect of CO is at least, in part, mediated via its ability to suppress ER stress.
There is ample evidence that islet grafts undergo inflammatory responses before and after transplantation (6, 20). In previous studies, we have shown that, by suppressing proinflammatory cytokine expression, exposure of donor islets to CO results in a higher percentage of islet allografts reaching normoglycemia in recipients (18, 62). Similarly, in this study, we found that islets preincubated in CO-saturated media survived and functioned better after transplantation. Immunohistological analysis showed that CO-treated islets were better preserved, and exhibited decreased Caspase 3 activation, reduced infiltrating mononuclear leukocytes, and increased autophagy in the islet graft. The RNAseq data further confirmed that the anti-inflammatory effects of CO, in addition to its antiapoptotic effects, contributed to improved islet survival after transplantation.
CO functions as a cellular signaling molecule in many cell types, including islet cells, wherein CO has been suggested to stimulate insulin and glucagon release (19). A number of the known effects of CO as a signaling molecule are believed to depend on stimulation of soluble guanylate cyclase (22, 37, 50). In this study, we found that CO enhanced insulin secretion by an effect exerted at the level of insulin granule exocytosis. Lundquist et al. reported that CO not only plays a regulatory role in glucose-stimulated insulin release through the guanylate cyclase-cGMP system but is also a trigger of intracellular calcium transients thought to coordinate the secretory activity of β cells (31). CO can strengthen the secretory activities of islets for longer times and over longer distances than nitric oxide, another critical gaseous signaling molecule (19, 44). Taken together, we suggest that CO improves β cell function through increased insulin secretion. Further studies will be required to address the exact mechanism(s) by which CO regulates insulin secretion.
CO and CORMs are gradually being accepted as a cytoprotective and homeostatic molecule in multiple preclinical models of organ transplantation and inflammatory disorders (39), and may have potential translational application in clinical islet transplantation. For example, we are currently testing the protective effect of isolating islets in CO-saturated medium in the prevention of islet death and surgical diabetes in chronic pancreatitis patients undergoing total pancreatectomy and islet autotransplantation (NCT02567240). In summary, we have found that CO pre-exposure inhibits hypoxia-induced mouse and human islet death via upregulation of autophagy. CO exposure quenches inflammation and cell death in vitro and after islet transplantation. We identify mechanisms by which CO protects islets from stress-induced apoptosis. CO exposure has potential as a simple and efficient approach to improve efficacy of human islet transplantation and to reduce stress-induced islet cell death in diabetes.
Materials and Methods
Animals
Male C57BL/6 and NOD-SCID mice at 6–8 weeks of age were purchased from the Jackson Laboratory (Bar harbor, ME). Mice were fed normal chow. All procedures and protocols were approved by the IACUC committee of the Medical University of South Carolina.
Exposure of islets to hypoxia and CO
Human or mouse islets were cultured at 37°C in an Oxycycler (Biospherix)-controlled incubator. Normoxic condition was 5% CO2 with normal air; hypoxic condition was 1% O2. CO exposure was in medium containing 1% CO. Bubbling preservation solution with 1% CO for 10 min resulted in a CO concentration of 13 ± 2.5 μmol/L in solution (26).
Real-time polymerase chain reaction analysis
RNA was extracted from cells and reverse transcribed into cDNA using a real-time polymerase chain reaction (RT-PCR) kit (Applied Biosystems). Expression levels of ATG3, ATG5, ATG7, ATG12, ATG16L1, Beclin-1, Bcl-2, Bax, Bim, and TNF-α mRNA were analyzed using commercially available primers from Applied Biosystems. PCRs were performed using the ABI 7700 sequence detection system (Perkin-Elmer, Applied Biosystems) as described previously (16). Fold changes in gene expression normalized to GAPDH expression were plotted, and compared between groups.
Isolation of mouse islets
Islets were excised and placed in normal or CO-saturated media. Islets were isolated from mice as described (62). In brief, pancreas was perfused with 5 mL of 0.6 mg/mL collagenase (Sigma) in Hanks' buffered saline solution (Hyclone) via the pancreatic duct, dissected, and digested at 37°C for 7 min; the digested pancrease was then passed through a 400-μm wire mesh. The digested pancreas was rinsed with Dulbecco's modified Eagle medium (DMEM) (Hyclone) containing 10% (v/v) bovine serum albumin (Hyclone), and islets were separated by density gradient centrifugation in Histopaque (Sigma). After several washes with DMEM, islets were handpicked under a dissecting microscope and cultured overnight in DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and streptomycin (Hyclone) in humidified 5% CO2 and 95% air at 37°C. Islets isolated with CO-bubbled mediums were cultured in Oxycycler containing 250 ppm of CO, 5% CO2, and 95% of air.
Human islets
Isolated human islets were obtained from Georgetown University. None of the donors had a previous history of diabetes or metabolic disorders. Islet purity was 90–95%, as assessed by dithizone staining. Islet viability was assessed by the Live/Dead viability/cytotoxicity kit for mammalian cells (Molecular Probe, L-3224). Human islets were cultured in normal or CO-saturated media.
Cell culture and transfection
βTC3 cells were cultured in DMEM with 10% FBS, 100 U/mL penicillin, and streptomycin (Hyclone) in humidified 5% CO2, 95% air at 37°C. Transfection of βTC3 using the ptfLC3 Plasmid (Addgen, plasmid #21074) was performed using LipofectAMINE2000 (Invitrogen) according to the manufacture's protocol. Stable transformants were selected in medium containing 500 μg/mL G418 (Invitrogen). In both ATG5 and ATG7 RNA knocking down experiments, cells were transfected with a 50 nM final concentration of corresponding siRNAs using LipofectAMINE2000 (Invitrogen).
Diabetes induction and islet transplantation
Diabetes was induced in male C57BL/6 mice or NOD-SCID mice by a single intraperitoneal injection of STZ (130 mg/kg; S-0130-1G, Sigma). Serum glucose levels were monitored using a FreeStyle Lite glucometer (FreeStyle). In cases wherein blood glucose levels were above the maximum meter reading, 500 mg/dL was recorded. Mice were considered diabetic when nonfasting blood glucose exceeded 300 mg/dL for at least 2 consecutive days. Islets were transplanted under the kidney capsule in recipients. For mouse islets, 150 C57BL/6 islets were transplanted per recipient; 300 human islets were transplanted per NOD-SCID mouse recipient. Mice with blood glucose levels <200 mg/dL were considered normoglycemic.
LDH and apoptosis enzyme-linked immunosorbent assay
Islets were incubated in a hypoxia chamber (1% O2, 37°C) for the indicated time. Apoptosis was measured using an LDH activity assay kit (Sigma) and an In Situ Cell Death Detection Kit (Sigma) according to the manufacturer's recommendation.
Intravenous glucose tolerance test
For IVGTT, mice were fasted overnight and injected with glucose solution (1 g/kg) via tail vein. Blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min after glucose injection. AUC from each mouse was calculated and used to compare efficacy of glucose disposal.
Gene expression profile by RNAseq analysis
Islet grafts under the kidney capsule were retrieved 3 days post-transplantation. RNA was extracted using the ISOLATE II RNA Mini kit (BIOLINE), and gene expression profiles were analyzed by RNAseq. Gene expression profiles were uploaded to the Ingenuity Analysis Software, and the differential expression of individual genes was used to obtain the most relevant biochemical networks with functional links.
Glucose-stimulated insulin release
Glucose-stimulated insulin release (GSIS) was performed by stimulating islets with 3 or 11 mM glucose. Insulin secretion was measured by an insulin chemiluminescence enzyme-linked immunosorbent assay (ELISA) kit (ALPCO, 80-INSHU-E01.1). A stimulation index was calculated using the ratio of insulin secreted at 11 mM glucose divided by insulin secreted at 3 mM glucose in each group.
Adenovirus construction and transfection
Adenovirus expression vectors for Beclin-1 (AdBeclin-1) or ATG4B (AdAT4B) were constructed with the corresponding complementary DNAs using the ViraPower Adenoviral Gateway Expression Kit (Invitrogen, Carlsbad, CA). ATG16L1 adeno-associated virus (AAV) siRNA pooled virus (serotype 2) was purchased from ABM (Applied Biological materials Inc.). Islets were infected with AdBeclin-1 or AdATG4B at 1–10 MOI in defined medium or DMEM, respectively. AdGFP or Adβ-galactosidase (AdLacZ) was used as empty vector controls.
Western blot
Control or CO islets were cultured under normoxic or hypoxic conditions for indicated times and washed with PBS. Total cell lysates (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and incubated with primary antibodies against AKT (#9272; Cell Signaling Technology), Atg3 (3415P; Cell Signaling Technology), ATG4B (13507S; Cell Signaling Technology), Atg5 (12994P; Cell Signaling Technology), ATG7 (8558P; Cell Signaling Technology), ATG16L1 (8089P; Cell Signaling Technology), β-actin (sc-492; Santa Cruz), Bax (sc-493; Santa Cruz), Beclin-1 (3495P; Cell Signaling Technology), Bcl-2 (sc-69879; Santa Cruz), Bim (#2933S; Cell Signaling Technology), CHOP (sc-575; Santa Cruz), c-Cas-3 (9664P; Cell Signaling Technology), cleaved-caspase-9 (7237P; Cell Signaling Technology), cleaved-PARP (5625P; Cell Signaling Technology), eIF2a (9722S; Cell Signaling Technology), GRP78 (sc-13968; Santa Cruz), GRP94 (ADI-SPA-850; Enzo lifesciences), HIF-1α (MAB5382; EMD Millipore), IRE1a (3294S; Cell Signaling Technology), LC3A/B (12741P; Cell Signaling Technology), p62 (Cell Signaling Technology), p-AKT (9272s; Cell Signaling Technology), p-eIF2a (35987S; Cell Signaling Technology), or p-PERK (sc-32577; Santa Cruz). Horseradish peroxidase-conjugated secondary antibodies were from Cell Signaling Technology. Signals were visualized using an enhanced chemiluminescence detection kit (34096; Thermo Scientific). Relative protein expression of genes was quantified using ImageJ software.
Assessment of islet cell death and inflammation
Immunohistochemistry of islet grafts was performed as described previously (62). Kidney carrying islet grafts was snap frozen in precooled 2-methylbutanol in liquid nitrogen. Sections (5 μm) were fixed in cold acetone for 3 min, followed by staining with anti-insulin (A0564; DAKO), anti-F4/80 (ab6640; Abcam), antic-Cas-3 (9661; Cell Signaling), and anti-LC3A/B antibodies (12741P; Cell Signaling); all antibodies were used at a 1:200 dilution. Positively stained cells were observed and quantified under a fluorescent microscope.
Preparation of cells for EM
For EM analysis, cells were pelletized and fixed in 2% phosphate buffered glutaraldehyde (EMS, 16536-10) for 1 h. Cell pellets were rinsed in 0.1 M phosphate buffered rinse, and then post fixed in 2% aqueous osmium tetroxide (75632; Sigma) for 30 min. After rinsing in distilled water, the pellets were dehydrated through a series of graded ethyl alcohol (32205-500ML; ETOH, Sigma) steps: 50% ETOH for 5 min, 70% ETOH for 5 min, 95% ETOH for 5 min, and twice with 100% ETOH for 5 min each. The dehydrant was removed using the intermediate fluid, propylene oxide (EMS, 20401), two changes of 10 min each. The pellets were infiltrated with a 1:1 solution of propylene oxide and EMbed 812 (EMS, 14120) for 1 h. The infiltration was continued using a 1:2 solution of propylene oxide and EMbed 812, overnight. The pellets were embedded in EMbed 812 the following day and polymerized in a 60°C oven for 48 h. Preliminary 1/2 μm sections were cut and stained with toluidine blue (EMS, 22050) and examined using a light microscope.
Statistical analyses
Percentage of recipients reaching normoglycemia after transplantation was compared by Kaplan–Meier survival curves plotted using StatView software. Statistical analyses were carried out by Student's t-test or one-way ANOVA followed by Turkey's post hoc test. A p value of <0.05 was taken as evidence of statistical significance.
Authors' Contribution
D.S.K. and H.W. designed the study, performed experiments, analyzed data, and wrote the article. L.S. and J.W. performed experiments. W.G. analyzed data, H.W. and J.S.K. generated adenoviruses. C.W. harvested human islets.
Supplementary Material
Abbreviations Used
- 3-MA
3-methyladenine
- AAV
adeno-associated virus
- AH
amiodarone hydrochloride
- AUC
area under the curve
- c-Cas-3
cleaved-Caspase 3
- CO
carbon monoxide
- CORM
CO-releasing molecule
- CQ
chloroquine
- CTR
control
- DMEM
Dulbecco's modified Eagle medium
- ELISA
enzyme-linked immunosorbent assay
- EM
electron microscopy
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GSIS
glucose-stimulated insulin release
- HPX
hypoxia
- IPA
ingenuity pathway analysis
- IVGTT
intravenous glucose tolerance test
- LDH
lactate dehydrogenase
- MOI
multiplicity of infection
- NOD-SCID
non-obese diabetic severe combined immunodeficiency
- NOX
normoxia
- PE
phosphatidylethanolamine
- PI
propidium iodide
- qPCR
quantitative polymerase chain reaction
- RT-PCR
real-time polymerase chain reaction
- STZ
streptozotocin
- TF
trifluoperazine
Acknowledgments
This study was supported, in part, by the National Institutes of Health (EB015744, DK097544, DK105183, and DK099696) to H.W. Dr. J.S.K. was supported by NIH DK079879 and DK090115 and National Institute on Aging AG028740. Dr. H.W. was supported by NIH grant DK107412. We thank the Reeves family for research support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andersson A, Carlsson PO, Carlsson C, Olsson R, Nordin A, Johansson M, Palm F, Tyrberg B, Kallskog O, Tillmar L, Welsh N, Mattsson G, and Jansson L. Promoting islet cell function after transplantation. Cell Biochem Biophys 40: 55–64, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A. and Dixit VM. Death receptors: signaling and modulation. Science 281: 1305–1308, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bachar-Wikstrom E, Wikstrom JD, Ariav Y, Tirosh B, Kaiser N, Cerasi E, and Leibowitz G. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes 62: 1227–1237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow AD, Nicholson ML, and Herbert TP. Evidence for rapamycin toxicity in pancreatic beta-cells and a review of the underlying molecular mechanisms. Diabetes 62: 2674–2682, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, and Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, and Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes 47: 316–323, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Carlsson PO. Influence of microenvironment on engraftment of transplanted beta-cells. Ups J Med Sci 116: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee SJ, and Choi ME. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. J Biol Chem 285: 37909–37919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donath MY, Storling J, Berchtold LA, Billestrup N, and Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev 29: 334–350, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, and Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 8: 325–332, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Eizirik DL, Miani M, and Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 56: 234–241, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Fagone P, Mangano K, Coco M, Perciavalle V, Garotta G, Romao CC, and Nicoletti F. Therapeutic potential of carbon monoxide in multiple sclerosis. Clin Exp Immunol 167: 179–187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagone P, Mangano K, Mammana S, Cavalli E, Di Marco R, Barcellona ML, Salvatorelli L, Magro G, and Nicoletti F. Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin Immunol 157: 198–204, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Fagone P, Mangano K, Quattrocchi C, Motterlini R, Di Marco R, Magro G, Penacho N, Romao CC, and Nicoletti F. Prevention of clinical and histological signs of proteolipid protein (PLP)-induced experimental allergic encephalomyelitis (EAE) in mice by the water-soluble carbon monoxide-releasing molecule (CORM)-A1. Clin Exp Immunol 163: 368–374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glick D, Barth S, and Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 221: 3–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg A, Parolini M, Chin BY, Czismadia E, Otterbein LE, Bach FH, and Wang H. Toll-like receptor 4 suppression leads to islet allograft survival. FASEB J 21: 2840–2848, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Green DR. and Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Gunther L, Berberat PO, Haga M, Brouard S, Smith RN, Soares MP, Bach FH, and Tobiasch E. Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes 51: 994–999, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Henningsson R, Alm P, Ekstrom P, and Lundquist I. Heme oxygenase and carbon monoxide: regulatory roles in islet hormone release: a biochemical, immunohistochemical, and confocal microscopic study. Diabetes 48: 66–76, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Johansson U, Olsson A, Gabrielsson S, Nilsson B, and Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun 308: 474–479, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, and Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaide JI, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, and Nasjletti A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest 107: 1163–1171, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashyap PC, Choi KM, Dutta N, Linden DR, Szurszewski JH, Gibbons SJ, and Farrugia G. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am J Physiol Gastrointest Liver Physiol 298: G1013–G1019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KM, Pae HO, Zheng M, Park R, Kim YM, and Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res 101: 919–927, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, Kitamura H, Niimura F, Matsusaka T, Soga T, Rakugi H, and Isaka Y. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902–913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohmoto J, Nakao A, Sugimoto R, Wang Y, Zhan J, Ueda H, and McCurry KR. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J Thorac Cardiovasc Surg 136: 1067–1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, Nakahira K, Ryter SW, and Choi AM. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal 20: 432–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, and Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol 45: 867–873, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes 56: 2295–301, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, and Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402: 672–676, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, and Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem 280: 872–877, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lundquist I, Alm P, Salehi A, Henningsson R, Grapengiesser E, and Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am J Physiol Endocrinol Metab 285: E1055–E1063, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Mackern-Oberti JP, Llanos C, Carreno LJ, Riquelme SA, Jacobelli SH, Anegon I, and Kalergis AM. Carbon monoxide exposure improves immune function in lupus-prone mice. Immunology 140: 123–132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T, Yamamoto A, Hamasaki M, Noda T, Isaka Y, and Yoshimori T. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J 32: 2336–2347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrup-Poulsen T. Beta-cell apoptosis: stimuli and signaling. Diabetes 50 Suppl 1: S58–S63, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Marino G, Uria JA, Puente XS, Quesada V, Bordallo J, and Lopez-Otin C. Human autophagins, a family of cysteine proteinases potentially implicated in cell degradation by autophagy. J Biol Chem 278: 3671–3678, 2003 [DOI] [PubMed] [Google Scholar]
- 35a.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med 2: a007823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizushima N, and Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542–545, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Morita T, Mitsialis SA, Koike H, Liu Y, and Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem 272: 32804–32809, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Moriwaki M, Itoh N, Miyagawa J, Yamamoto K, Imagawa A, Yamagata K, Iwahashi H, Nakajima H, Namba M, Nagata S, Hanafusa T, and Matsuzawa Y. Fas and Fas ligand expression in inflamed islets in pancreas sections of patients with recent-onset Type I diabetes mellitus. Diabetologia 42: 1332–1340, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Motterlini R. and Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 9: 728–743, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, and Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS One 7: e30415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoletti F, Fagone P, Meroni P, McCubrey J, and Bendtzen K. mTOR as a multifunctional therapeutic target in HIV infection. Drug Discov Today 16: 715–721, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Nikolic I, Saksida T, Mangano K, Vujicic M, Stojanovic I, Nicoletti F, and Stosic-Grujicic S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia 57: 980–990, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Nikolic I, Saksida T, Vujicic M, Stojanovic I, and Stosic-Grujicic S. Anti-diabetic actions of carbon monoxide-releasing molecule (CORM)-A1: Immunomodulation and regeneration of islet beta cells. Immunol Lett 165: 39–46, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Otterbein LE. and Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279: L1029–L1037, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, and Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med 9: 183–190, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Pedraza E, Coronel MM, Fraker CA, Ricordi C, and Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 109: 4245–4250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plesner A. and Verchere CB. Advances and challenges in islet transplantation: islet procurement rates and lessons learned from suboptimal islet transplantation. J Transplant 2011: 979527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran S, Desai NM, Goers TA, Benshoff N, Olack B, Shenoy S, Jendrisak MD, Chapman WC, and Mohanakumar T. Improved islet yields from pancreas preserved in perflurocarbon is via inhibition of apoptosis mediated by mitochondrial pathway. Am J Transplant 6: 1696–1703, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Raman KG, Barbato JE, Ifedigbo E, Ozanich BA, Zenati MS, Otterbein LE, and Tzeng E. Inhaled carbon monoxide inhibits intimal hyperplasia and provides added benefit with nitric oxide. J Vasc Surg 44: 151–158, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Raub JA, and Benignus VA. Carbon monoxide and the nervous system. Neurosci Biobehav Rev 26: 925–940, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Rivera JF, Costes S, Gurlo T, Glabe CG, and Butler PC. Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest 124: 3489–3500, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, and Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes 54: 2060–2069, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, and Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J 18: 854–856, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Siow RC, Sato H, and Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res 41: 385–394, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Song R, Kubo M, Morse D, Zhou Z, Zhang X, Dauber JH, Fabisiak J, Alber SM, Watkins SC, Zuckerbraun BS, Otterbein LE, Ning W, Oury TD, Lee PJ, McCurry KR, and Choi AM. Carbon monoxide induces cytoprotection in rat orthotopic lung transplantation via anti-inflammatory and anti-apoptotic effects. Am J Pathol 163: 231–242, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song R, Mahidhara RS, Liu F, Ning W, Otterbein LE, and Choi AM. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol 27: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Suarez-Pinzon W, Sorensen O, Bleackley RC, Elliott JF, Rajotte RV, and Rabinovitch A. Beta-cell destruction in NOD mice correlates with Fas (CD95) expression on beta-cells and proinflammatory cytokine expression in islets. Diabetes 48: 21–28, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Sun KT, Chen MY, Tu MG, Wang IK, Chang SS, and Li CY. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 73: 145–153, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Tanemura M, Ohmura Y, Deguchi T, Machida T, Tsukamoto R, Wada H, Kobayashi S, Marubashi S, Eguchi H, Ito T, Nagano H, Mori M, and Doki Y. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am J Transplant 12: 102–114, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Tezza S, Ben Nasr M, Vergani A, Valderrama Vasquez A, Maestroni A, Abdi R, Secchi A, and Fiorina P. Novel immunological strategies for islet transplantation. Pharmacol Res 98: 69–75, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Vergani A, Tezza S, Fotino C, Visner G, Pileggi A, Chandraker A, and Fiorina P. The purinergic system in allotransplantation. Am J Transplant 14: 507–514, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Lee SS, Gao W, Czismadia E, McDaid J, Ollinger R, Soares MP, Yamashita K, and Bach FH. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes 54: 1400–1406, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Crager M, and Pugazhenthi S. Modulation of apoptosis pathways by oxidative stress and autophagy in beta cells. Exp Diabetes Res 2012: 647914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63a.Wang X, Meloche M, Verchere CB, Ou D, Mui A, Warnock GL. Improving islet engraftment by gene therapy. J Transplant 2011: 594851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, and Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718–1726, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Zheng X, Zheng X, Wang X, Ma Z, Gupta Sunkari V, Botusan I, Takeda T, Bjorklund A, Inoue M, Catrina SB, Brismar K, Poellinger L, and Pereira TS. Acute hypoxia induces apoptosis of pancreatic beta-cell by activation of the unfolded protein response and upregulation of CHOP. Cell Death Dis 3: e322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.