Abstract
1-Aminocyclopropane-1-carboxylic acid synthase (ACS) is one of the key regulatory enzymes involved in the synthesis of the hormone ethylene and is encoded by a multigene family containing at least eight members in tomato (Lycopersicon esculentum). Increased ethylene production accompanies ripening in tomato, and this coincides with a change in the regulation of ethylene synthesis from auto-inhibitory to autostimulatory. The signaling pathways that operate to bring about this transition from so-called system-1 to system-2 ethylene production are unknown, and we have begun to address these by investigating the regulation of ACS expression during ripening. Transcripts corresponding to four ACS genes, LEACS1A, LEACS2, LEACS4, and LEACS6, were detected in tomato fruit, and expression analysis using the ripening inhibitor (rin) mutant in combination with ethylene treatments and the Never-ripe (Nr) mutant has demonstrated that each is regulated in a unique way. A proposed model suggests that system-1 ethylene is regulated by the expression of LEACS1A and LEACS6. In fruit a transition period occurs in which the RIN gene plays a pivotal role leading to increased expression of LEACS1A and induction of LEACS4. System-2 ethylene synthesis is subsequently initiated and maintained by ethylene-dependent induction of LEACS2.
The plant hormone ethylene mediates plant responses to many developmental signals and environmental stimuli (Abeles et al., 1992). Tomato (Lycopersicon esculentum) fruit ripening represents just one example in plant development where ethylene synthesis and perception have been shown to be essential for the full completion of the ripening process (Oeller et al., 1991; Picton et al., 1993; Lanahan et al., 1994; Wilkinson et al., 1997). The pathway of ethylene synthesis is well established in higher plants (Yang and Hoffman, 1984), and regulatory control is achieved at two steps: the formation of 1-aminocyclopropane-1-carboxylic acid (ACC) from S-adenosyl-l-Met and the conversion of this intermediate to ethylene (Kende, 1993). The first step is catalyzed by the enzyme ACC synthase (ACS) and the second by ACC oxidase (ACO). In higher plants both of the enzymes are encoded by multigene families (Zarembinski and Theologis, 1994) generating the option of multiple control points at which ethylene synthesis may be regulated. Eight ACC-synthase genes have been identified in tomato (Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993; Olson et al., 1995; Nakatsuka et al., 1998; Shiu et al., 1998) along with four ACO genes (Holdsworth et al., 1988; Blume et al., 1997; Nakatsuka et al., 1998). Complexity in the regulation of ethylene synthesis in tomato fruit is emerging with no fewer than five ACS and three ACO genes reportedly expressed. The ripening-related expression of two ACS genes, LEACS2 and LEACS4, has been well documented in tomato (Olson et al., 1991; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993). Antisense inhibition of LEACS2 in transgenic plants caused down-regulation of endogenous LEACS2 and LEACS4 expression and reduced ripening-related ethylene synthesis to 0.1% of that produced by control fruit, thus demonstrating the importance of these isoforms during ripening (Oeller et al., 1991). In addition, in a more recent study, the expression of LEACS1A, LEACS3, and LEACS6 in tomato fruit has also been described (Nakatsuka et al., 1998). The importance of ACO in regulating ethylene synthesis was also demonstrated using antisense technology (Hamilton et al., 1990; Picton et al., 1993). Down-regulation of endogenous LEACO1 expression in transgenic plants caused reduced ethylene synthesis in transgenic fruit and retarded ripening (Picton et al., 1993). LEACO1 appears to be the major ACO gene expressed in tomato fruit (Barry et al., 1996; Blume and Grierson, 1997) but ripening-related expression of LEACO3 and LEACO4 has also been reported (Barry et al., 1996; Nakatsuka et al., 1998).
Two systems of ethylene regulation have been proposed to operate in higher plants (for review, see Lelièvre et al., 1998). System 1 is functional during normal vegetative growth, is ethylene auto-inhibitory, and is responsible for producing the basal levels of ethylene detectable in all of the tissues including non-ripening fruit. System 2 operates during the ripening of climacteric fruit and during petal senescence when ethylene is autostimulatory and requires the induction of both of the ACS and ACO. The signaling pathways that bring about the induction of these two enzymes through co-ordinated regulation of ACS and ACO gene families remain unknown, although a large amount of evidence is available that indicates that a combination of both ethylene and developmental factors are required. For example, analysis of tomato and melon fruit expressing an ACO antisense transgene showed that ACC accumulated despite greatly reduced ethylene synthesis indicating that ACC synthesis is under developmental regulation (Picton et al., 1993; Guis et al., 1997). Additionally, treatment of immature green fruit with ethylene is insufficient to induce ACS activity, indicating that ACS induction is dependent on the developmental stage of the fruit (Liu et al., 1985). However, ethylene has also been shown to stimulate ACS and ACO expression in tomato and other fruit (Maunders et al., 1987; Dong et al., 1992; Lincoln et al., 1993; Lasserre et al., 1996; Blume and Grierson, 1997). These results suggest that different ACS genes may be regulated by different mechanisms in tomato fruit.
In this study we have examined the regulation of the tomato ACS gene family during fruit ripening using a combination of ripening mutants and ethylene treatments. Four transcripts corresponding to LEACS1A, LEACS2, LEACS4, and LEACS6 were identified in tomato fruit, and we demonstrate that the corresponding genes are each regulated in a unique way. The results are discussed in terms of system-1 and -2 ethylene synthesis and a model of ACS gene regulation proposed.
RESULTS
ACS Gene Expression during Ripening of Wild-Type and Ripening Inhibitor (rin) Tomato Fruit
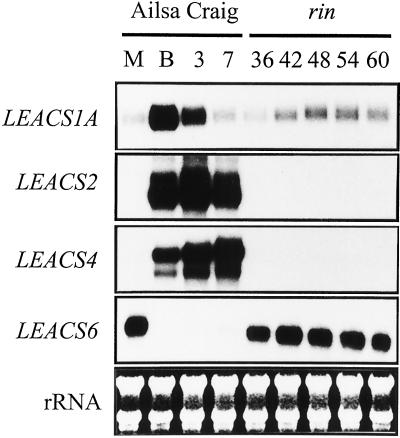
The expression of eight members of the tomato ACS gene family was analyzed during ripening of wild-type cv Ailsa Craig fruit. Transcripts from LEACS1A, LEACS2, LEACS4, and LEACS6 were detected in tomato fruit (Fig. 1), however no expression of LEACS1B, LEACS3, LEACS5, and LEACS7 was evident (data not shown). Three of the genes, LEACS1A, LEACS2, and LEACS4 showed a ripening-related increase in expression. Transcripts of these genes were low or undetectable in mature green fruit and increased at the breaker stage. As ripening progressed the expression levels of both of the LEACS2 and LEACS4 continued to rise, whereas the increase in LEACS1A transcripts was only transient with maximum abundance detected at the breaker stage. In contrast to the other three genes, LEACS6 transcripts were present in mature green fruit but declined rapidly as ripening was initiated. Based on relative exposure times to x-ray film (see legend to Fig. 1), both of the LEACS1A and LEACS6 represent lower abundance transcripts than LEACS2 and LEACS4.
Figure 1.
ACS gene expression in wild-type cv Ailsa Craig and rin fruit. Twenty-five micrograms of total RNA from cv Ailsa Craig mature green (M), breaker (B), breaker +3 (3), and breaker +7 (7) and rin fruit harvested at 36, 42, 48, 54, and 60 DPA was hybridized to radiolabeled RNA probes specific for tomato ACS genes and RPA analysis was performed as described in “Materials and Methods.” Results are shown only for the genes that showed a positive hybridization signal. Exposure times to x-ray film were as follows: LEACS1A, 7 d; LEACS2, 20 h; LEACS4, 20 h; and LEACS6, 16 d.
The expression of the ACS gene family was also examined in the rin mutant of tomato (Tigchelaar et al., 1978). The rin mutant produces fruit with severely reduced ripening, and one characteristic of these fruit is that the burst of ethylene production normally associated with the ripening of climacteric fruit (system 2) is absent. However, rin fruit still produce a low basal level of ethylene production (system 1), similar to wild-type green fruit, throughout their development (Herner and Sink, 1973; Lincoln and Fischer, 1988). Therefore, rin fruit represent an ideal system for identifying individual genes that are involved in either system-1 or -2 ethylene synthesis. All of the four ACS genes showed the same expression pattern in rin fruit throughout development as was observed in mature green cv Ailsa Craig wild-type fruit. Both of the LEACS1A and LEACS6 transcripts were present in rin fruit at comparable levels with those seen in wild-type mature green fruit. However, the ripening-related changes in expression of the four genes observed in wild-type fruit did not occur in rin.
ACS Gene Expression in Wild-Type and rin Fruit Treated with Ethylene
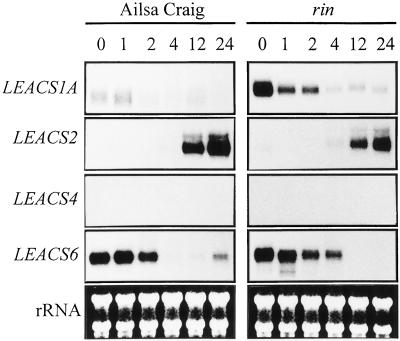
The role of ethylene in inducing ripening-related changes in ACS gene expression was investigated by incubating mature green wild-type fruit in 10 μL L−1 ethylene over 24 h (Fig. 2). Following the 1st h after ethylene application LEACS1A transcripts began to decline and remained low for the duration of the experiment. LEACS6 expression was also reduced by ethylene exposure, although kinetically these changes occurred slightly slower than for LEACS1A between 2 and 4 h after treatment. LEACS2 transcripts were undetectable until 4 h and were highly up-regulated by 12 h of incubation. No LEACS4 expression was detected throughout the duration of the experiment.
Figure 2.
ACS gene expression in response to ethylene. Mature green wild-type and rin tomato fruit (37 DPA) were treated with 10 μL L−1 ethylene as described in “Materials and Methods” and harvested at time zero (0), 1 h (1), 2 h (2), 4 h (4), 12 h (12), and 24 h (24) after treatment. Twenty-five micrograms of total RNA was hybridized with radiolabeled RNA probes specific for LEACS1A, LEACS2, LEACS4, and LEACS6 and subjected to RPA analysis as described in the “Materials and Methods.” Exposure times to x-ray film were as follows: LEACS1A, 20 d; LEACS2, 4 d; LEACS4, 4 d; LEACS6, 14 d (cv Ailsa Craig); LEACS1A, 4 d; LEACS2, 3 d; LEACS4, 4 d; and LEACS6, 2 d (rin).
An identical experiment was performed using rin fruit to ascertain whether the expression patterns detected during rin-fruit development (Fig. 1) were the result of the mutation per se or an indirect consequence brought about by reduced-ethylene synthesis. The changes in ACS gene expression that occurred in ethylene-treated wild-type fruit were also seen in rin with almost identical kinetics (Fig. 2). Both of the LEACS1A and LEACS6 transcripts declined throughout the duration of the experiment. LEACS2 expression was induced by ethylene treatment, and no induction of LEACS4 expression was evident.
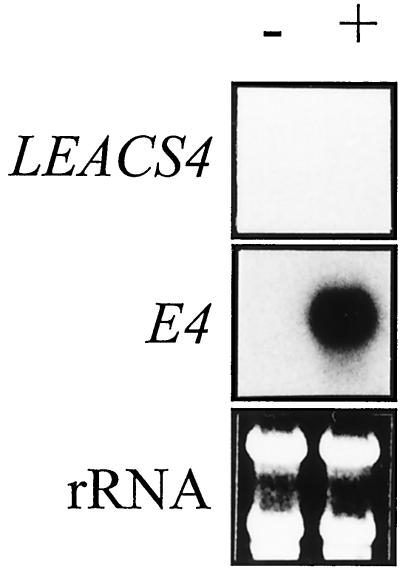
The lack of LEACS4 expression in wild-type and rin fruit treated with ethylene may be due to a lack of maturity and, therefore, competence to respond to ethylene. To test this possibility an additional experiment was performed using 60-d-old rin fruit that were incubated in either air or 10 μL L−1 ethylene for 4 d (Fig. 3). Following ethylene exposure, transcripts of E4, an ethylene-regulated gene (Lincoln et al., 1987), were induced in rin fruit, whereas no induction of LEACS4 was observed.
Figure 3.
LEACS4 expression in 60-DPA rin fruit treated with ethylene. Sixty-DPA rin fruit were held in air (−) or in 10 μL L −1 ethylene (+) for a further 4 d. Twenty-five micrograms of total RNA was hybridized with a radiolabeled RNA probe specific for LEACS4 and subjected to RPA analysis as described in the “Materials and Methods.” RNA gel-blot analysis of E4 expression is included as a positive control. Exposure times to x-ray film were as follows: LEACS4, 4 d; and E4, 16 h.
ACS Expression in Tomato Seedlings
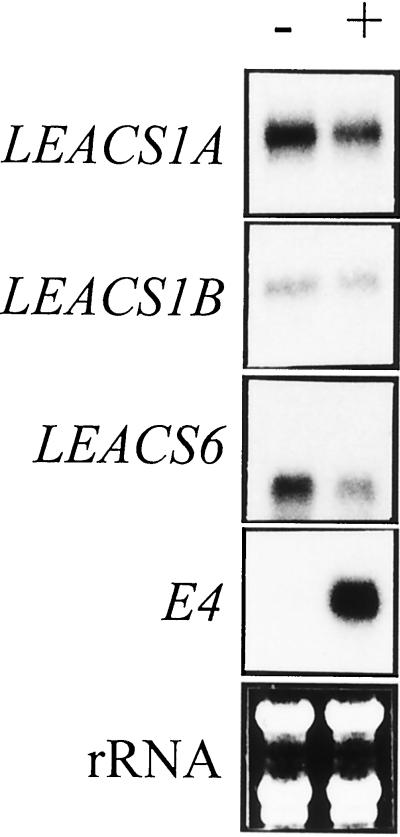
In fruit, ethylene negatively regulated LEACS1A and LEACS6 expression, whereas it induced LEACS2 expression (Fig. 2). To investigate ethylene regulation of ACS gene expression in a non-climacteric tissue, expression was analyzed in 10-d-old light-grown seedlings held in air or treated with 10 μL L−1 ethylene for 12 h (Fig. 4). Of the eight ACS genes only transcripts corresponding to LEACS1A, LEACS1B, and LEACS6 were detectable in air- and ethylene-treated tomato seedlings. Ethylene treatment caused a reduction in transcript abundance of LEACS1A and LEACS6 but had no effect on LEACS1B expression. E4 expression was measured as a positive control for ethylene response and showed clear ethylene induction in tomato seedlings.
Figure 4.
ACS expression in tomato seedlings. The expression of eight ACS genes was examined in 10-d-old light-grown tomato seedlings grown in air (−) or treated with 10 μL L −1 ethylene (+) for 12 h. Protection assays were carried out according to the “Materials and Methods” using 50 μg of total RNA per assay. RNA gel-blot analysis of E4 expression is included as a positive control. Exposure times to x-ray film were as follows: LEACS1A, 7 d; LEACS1B, 5d; LEACS6, 3 d; and E4, 16 h.
ACS Gene Expression Is Altered in Never-Ripe (Nr) Fruit
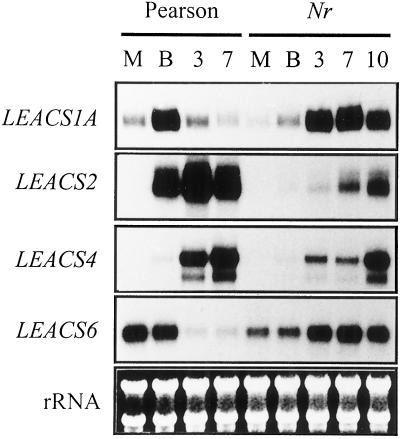
To investigate the role of ethylene in regulating ACS gene expression throughout ripening we have used the Nr mutant of tomato, which displays ethylene insensitivity due to a dominant mutation in a member of the ethylene receptor gene family (Lanahan et al., 1994; Wilkinson et al., 1995). The expression of each ACS gene followed the expected expression pattern in the cv Pearson control fruit (Fig. 5) except that the decline in LEACS6 expression took slightly longer than in cv Ailsa Craig fruit (Fig. 1). In Nr fruit the four genes displayed altered expression patterns. The transient increase in LEACS1A expression that occurred at the breaker stage of ripening in wild-type fruit was delayed in the Nr mutant and persisted for longer. Transcript levels increased at the onset of ripening and continued to rise, reaching a maximum 7 d after the onset of color change before declining slightly at 10-d post-breaker. Thus, although the Nr mutation appeared to affect the temporal expression of LEACS1A, transcript abundance ultimately reached the same levels as in wild-type fruit. The same effect was observed for LEACS4 expression. However, in contrast, LEACS2 transcript abundance was greatly reduced in Nr fruit and did not reach the levels seen in wild-type samples. In wild-type fruit LEACS6 transcripts declined as ripening progressed from a maximum abundance in mature green fruit, whereas in Nr fruit, transcript levels increased throughout fruit development, reaching the levels of wild-type mature green fruit at around breaker +7.
Figure 5.
ACS gene expression in Nr fruit. Total RNA was extracted from cv Pearson mature green (M), breaker (B), breaker +3 d (3), and breaker +7 d (7) and an isogenic line of the Nr mutant at mature green (M), breaker (B), breaker +3 d (3), breaker +7 d (7), and breaker +10 (10). The RNA was hybridized with radiolabeled RNA probes specific for LEACS1A, LEACS2, LEACS4, and LEACS6 and subjected to RPA analysis as described in the “Materials and Methods.” Exposure times to x-ray film were as follows: LEACS1A, 7 d; LEACS2, 20 h; LEACS4, 20 h; and LEACS6, 14 d.
DISCUSSION
Expression of ACS Gene Family Members in Tomato Fruit
We have undertaken a detailed examination of the regulation of ACS gene expression during tomato fruit ripening using a combination of ripening mutants and ethylene treatments. The results indicate that four ACS genes, LEACS1A, LEACS2, LEACS4, and LEACS6, are expressed in tomato fruit (Fig. 1), and our data suggest that each shows distinct regulation that until now has not been detected. Increased LEACS2 and LEACS4 expression during fruit ripening has been extensively characterized (Olson et al., 1991; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993; Nakatsuka et al., 1998). However, earlier studies have failed to distinguish differences in regulation of expression of these two genes, which were simply classed as ethylene- or ripening-related. In a more recent study the presence of LEACS1A, LEACS3, and LEACS6 transcripts in tomato fruit has been reported (Nakatsuka et al., 1998). These authors found that LEACS6 expression was negatively regulated by ethylene during ripening, which corresponds well with the findings of the present study. However, our data for LEACS1A and LEACS3 expression are clearly different. Nakatsuka et al. found that LEACS1A was expressed at a low constitutive level throughout fruit ripening, whereas our data suggests that it is highly regulated. Furthermore, in three independent experiments we failed to detect any LEACS3 transcripts in tomato fruit (data not shown), whereas data presented by Nakatsuka et al. described the expression of this gene as constitutive during fruit ripening. These discrepancies may be caused by the use of different cultivars or sampling techniques or alternatively may be due to the fact that we have used ribonuclease protection assays (RPA) as our preferred method for determining gene expression compared with RNA gel-blot analysis used by Nakatsuka et al. (1998). RPA analysis has the advantage of being more sensitive and offers a higher degree of specificity when analyzing transcripts of individual members of a gene family.
Differential ACS Expression during System-1 and System-2 Ethylene Synthesis
The use of the rin mutant has allowed us to gain new insights into ACS gene regulation. Throughout rin fruit development the expression profile of the ACS genes remained the same as in a wild-type mature green fruit with no ripening-related changes occurring (Fig. 1). This confirms, at the molecular level, previous findings that rin fruit are in a perpetual state of system-1 ethylene synthesis (Lincoln and Fischer, 1988) and indicates that LEACS6 together with the low levels of LEACS1A expression are likely to be responsible for system-1 ethylene synthesis in fruit. By analogy it follows that the ripening-related increases in LEACS1A, LEACS2, and LEACS4 expression, which occur only in wild-type fruit, are important for the transition to system-2 ethylene synthesis.
It has been proposed that system-1 ethylene synthesis is under negative regulation by ethylene (Lelièvre et al., 1998), and other studies have confirmed that ACS enzyme activity and gene expression in vegetative tissue can be negatively regulated by ethylene (Yoon et al., 1997; Peck and Kende, 1998). Treatment of mature green tomato fruit and seedlings with ethylene (Figs. 2 and 4) resulted in a decline in LEACS1A and LEACS6 transcript abundance, indicating that high ethylene levels exert a negative effect on regulation of these two genes. This suggests that at the molecular level system-1 ethylene synthesis involves the same two ACS genes in both of the vegetative and fruit tissues of tomato. Additionally, low levels of LEACS1B transcripts were also detected in seedlings, although the abundance was not influenced by ethylene. From this analysis it is not clear whether LEACS1B is also functioning in system-1 ethylene synthesis or in a separate developmental process associated with seedling development.
Differences between ACS Gene Regulation in Tomato Fruit
Our data clearly show that increased ethylene synthesis at the start of ripening, i.e. the transition to system 2, is correlated with increased LEACS1A, LEACS2, and LEACS4 expression (Fig. 1). Previous work has shown that LEACS2 and LEACS4 expression can be stimulated by ethylene (Lincoln et al., 1993; Nakatsuka et al., 1998), although differences between the regulation of these two genes were not highlighted. Ethylene treatment of rin fruit (Figs. 2 and 3) indicates differences are apparent between LEACS2 and LEACS4 regulation. The induction of LEACS4 expression has a strict requirement for the RIN gene product and is not influenced by ethylene treatment even in older mutant rin fruit treated for extended time periods. In contrast, the expression of LEACS2 could be induced purely by ethylene even in the rin background. This finding suggests that, whereas LEACS4 expression is directly influenced by RIN, the effect on LEACS2 expression is an indirect one brought about as a result of reduced ethylene synthesis in the mutant background. However, general induction of LEACS2 by ethylene throughout the plant does not occur as expression was not detected in ethylene-treated seedlings. This suggests that ethylene signal transduction, with respect to LEACS2 expression, differs between fruit and vegetative tissues.
Further differences between LEACS2 and LEACS1A and 4 were identified by expression analysis in Nr fruit (Fig. 5). LEACS2 transcripts show severely delayed accumulation in Nr fruit, indicating that LEACS2 expression has a strong ethylene requirement, which was confirmed by the results of the experiment shown in Figure 2. In contrast, only a slight delay to maximal expression of LEACS1A and LEACS4 was observed in Nr fruit, implying that ethylene may not have such an essential role in regulation of these genes. The regulation of LEACS6 expression by ethylene appears complex. Transcript levels declined rapidly in response to treatment with high levels of ethylene (Figs. 2 and 4) and during ripening when ethylene levels peak (Fig. 1). However, expression analysis in Nr fruit suggests that ethylene perception is important for maintaining low-level LEACS6 expression as transcript abundance was lower in Nr mature green fruit than in cv Pearson control fruit (Fig. 5). Furthermore LEACS6 expression increased as ripening was initiated in Nr fruit, and transcript levels eventually reached the same level as observed in cv Pearson mature green fruit. The significance of this is unclear but one possibility is that a critical level of LEACS6 expression may need to be achieved for ripening to be initiated and that this requires ethylene perception.
Model of ACS Gene Regulation during the Transition from System-1 to System-2 Ethylene Synthesis
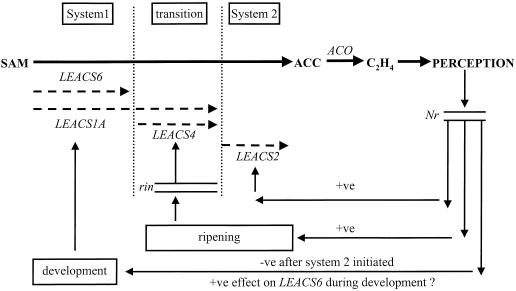
A model for the proposed interactions that regulate ACS gene expression to initiate the transition to autocatalytic ethylene synthesis in tomato fruit is shown in Figure 6. In green fruit and vegetative tissue, system-1 ethylene is regulated by developmental pathways with unknown components via LEACS1A and 6 expression. System 1 continues throughout fruit development until a competence to ripen is achieved at which point a transition occurs. This transition may be brought about by a change in ethylene sensitivity due to continual system-1 ethylene production. This is supported by the observation that the transition period, as evidenced by delayed LEACS6, LEACS1A, and LEACS4 expression, is extended in Nr fruit. The RIN protein plays an integral role within this transition period to cause an increase in LEACS1A expression and induce LEACS4. As a result of increased ethylene synthesis due to LEACS1A and LEACS4 activation, LEACS2 expression is induced and autocatalysis is initiated. High ethylene production occurs, resulting in negative feedback on the system-1 developmental pathway, resulting in reduced LEACS1A and LEACS6 expression.
Figure 6.
Model proposing the regulation of ACS gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. The symbols −ve (negative) and +ve (positive) refer to the action of ethylene on signaling pathways resulting in repression (−ve) or stimulation (+ve) of ACS gene expression.
CONCLUSIONS
The regulation of four members of the tomato ACS gene family during fruit ripening has been investigated. We have extended previous studies to show that each has a unique expression pattern. Additionally we have described for the first time the expression pattern of ACS gene family members in vegetative tissues of tomato. The use of ripening mutants and ethylene treatments has allowed us to propose a model that begins to explain system-1 and -2 ethylene synthesis at the molecular level. This model will be used as a basis to unravel the signaling pathways that cause changes in ACS gene expression during ripening.
MATERIALS AND METHODS
Plant Material and Treatments
Tomato (Lycopersicon esculentum Mill. cv Ailsa Craig and cv Pearson) plants were grown and maintained under standard greenhouse conditions. Fruit from wild type and mutants from the near-isogenic lines of cv Ailsa Craig homozygous for rin and near-isogenic lines of cv Pearson homozygous for Nr were picked at distinct stages of ripening or number of DPA. Ethylene measurements were performed as described below to confirm the developmental stage of fruit. Mature green fruit were classified as having well-formed locular gel but with no internal lycopene formation and producing less than 0.2 nL g−1 h−1 of ethylene. Breaker fruit showed a loss of chlorophyll and the first signs of lycopene accumulation and control fruit produced around 0.5 to 1.0 nL g−1 h−1 of ethylene. All of the harvested pericarp tissue was frozen immediately in liquid nitrogen and then stored at −70°C until required. Ethylene treatments were performed by incubating fruit in sealed jars of known volume and injecting ethylene to a final concentration of 10 μL L−1.
Experiments using tomato seedlings were performed as follows. Seeds were surface sterilized by soaking in 70% (v/v) ethanol for 10 min followed by 50% (v/v) bleach for a further 10 min. Bleach residues were removed by rinsing several times using sterile distilled water. Seeds were sown under sterile conditions on the surface of 1% (v/v) water agar containing Murashige and Skoog salts at a concentration of 3.8 g L−1 and were grown in controlled-environment conditions under a 16-h photoperiod at 23°C followed by an 8-h dark cycle at 16°C. Ten days after sowing, the seedlings were transferred to chambers of known volume and incubated in air or 10 μL L−1 ethylene for 12 h. The roots were excised, and the remaining tissue was frozen in liquid nitrogen and stored at −70°C.
Ethylene Measurements
Fruit were harvested and stored in open jars of known volume for 2 h to reduce the effect of wound ethylene caused by picking. The jars were then sealed for 2 h, and a 1-mL gas sample was withdrawn from the jar via a Subaseal. Ethylene concentration in the gas sample was measured by gas chromatography using an ATI UNICAM 610 series gas chromatograph linked to a PC with UNICAM 4880 chromatography data handling software. Column specifications were as follows: 150-mm length, 6-mm outer dimension, 4-mm inside dimension, and alumina F1 mesh-range 80 to 100 support. Temperatures were as follows: 110°C oven/column, 108°C injector, and 160°C detector.
RNA Isolation and Gel-Blot Analysis
RNA was extracted from 10 g of fruit pericarp tissue from a mixed pool of at least three individual fruit as previously described (Griffiths et al., 1999). Seedling RNA was extracted using the same protocol but from 2 g of frozen material. The RNA was quantified spectrophotometrically and 10 μg was fractionated on 1% (w/v) agarose gels containing 7.5% (v/v) formaldehyde to check for integrity and to compare sample concentration. Northern-blot analysis was performed as described by Griffiths et al. (1999).
ACS Probes and RNase Protection Analysis
Gene-specific probes for LEACS1A, LEACS1B, LEACS5, and LEACS6 were PCR amplified using primers previously described by Oetiker et al. (1997). The products were cloned into the pCR2.1 vector (Invitrogen, San Diego). LEACS2, LEACS3, LEACS4, and LEACS7 (Rottmann et al., 1991; Lincoln et al., 1993; Olson et al., 1995; Shiu et al., 1998) gene-specific probes were designed from the 3′ end of each gene based on sequences deposited within databases. Primer pairs were as follows: LEACS2, ACS2F 5′-ttaaaaggga-agaatttaatt-3′ and ACS2R 5′-taacaatataatcgagaaag-3′ generating a probe from nucleotides 2,702 through 2,957; LEACS3, ACS3F 5′-gtcattctccaagtgggttt-3′ and ACS3R 5′-gtagtagtttgaacatttcaag-3′ generating a probe from nucleotides 4,073 through 4,377; LEACS4, ACS4F 5′-ggagtca-tgaagaacaagcac-3′ and ACS4R 5′-aactatgttgggcccgtgct-3′ generating a probe from nucleotides 2,624 through 2,855; LEACS7, ACS7F 5′-gtctagtcatgtgaaagt-3′ and ACS7R2 5′-gcacttgtgcggtcacct-3′ generating a probe from nucleotides 4,066 through 4,335. PCR products were cloned into SmaI cut pBluescript II SK+ (Stratagene, La Jolla, CA) or pGEM-T Easy (Promega, Madison, WI). The identity of all of the clones was confirmed by DNA sequence analysis.
Single-strand-specific radiolabeled RNA probes were prepared by in vitro transcription from linearized plasmid template using either T3 or T7 RNA polymerase (Promega) according to the manufacturer's instructions. RNase-protec-tion assays were performed as described by Barry et al. (1996) using 25 μg of total RNA for fruit samples and 50 μg for seedlings. Digestions with RNase ONE (Promega) were performed at 28°C for 3 h using 3 units of enzyme per reaction. Protection assays were performed at least twice to confirm reproducibility.
ACKNOWLEDGMENTS
The authors would like to thank Paul Kasprzak for technical assistance and Dr. Jeremy Roberts for comments on the manuscript.
Footnotes
This work was supported by the European Union Fair Program (grant no. CT95 0225 to D.G.).
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. San Diego: Academic Press; 1992. [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Blume B, Barry CS, Hamilton AJ, Bouzayen M, Grierson D. Identification of transposon-like elements in non-coding regions of tomato ACC oxidase genes. Mol Gen Genet. 1997;254:297–303. doi: 10.1007/s004380050419. [DOI] [PubMed] [Google Scholar]
- Blume B, Grierson D. Expression of ACC oxidase promoter-GUS fusions in tomato and Nicotiana plumbaginifolia regulated by developmental and environmental stimuli. Plant J. 1997;12:731–746. doi: 10.1046/j.1365-313x.1997.12040731.x. [DOI] [PubMed] [Google Scholar]
- Dong JG, Fernández-Maculet JC, Yang SF. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA. 1992;89:9789–9793. doi: 10.1073/pnas.89.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A, Barry C, Alpuche-Solis AG, Grierson D. Ethylene and developmental signals regulate expression of lipoxygenase genes during tomato fruit ripening. J Exp Bot. 1999;50:793–798. [Google Scholar]
- Guis M, Bouquin T, Zegzouti H, Ayub R, Ben Amor M, Lasserre E, Botondi R, Raynal J, Latché A, Bouzayen M, Balagué C, Pech JC. Differential expression of ACC oxidase genes in melon and physiological characterization of fruit expressing an antisense ACC oxidase gene. In: Kanellis A, Chang C, Kender H, Grierson D, editors. Biology and Biotechnology of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 327–338. [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- Herner RC, Sink KC. Ethylene production and respiratory behavior of the rin tomato mutant. Plant Physiol. 1973;52:38–42. doi: 10.1104/pp.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Schuch W, Grierson D. Organisation and expression of a wound/ripening-related small multigene family from tomato. Plant Mol Biol. 1988;11:81–88. doi: 10.1007/BF00015661. [DOI] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. The Never ripe mutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserre E, Bouquin T, Hernandez JA, Bull J, Pech JC, Balagué C. Structure and expression of three genes encoding ACC oxidase homologs from melon (Cucumis melo L.) Mol Gen Genet. 1996;251:81–90. doi: 10.1007/BF02174348. [DOI] [PubMed] [Google Scholar]
- Lelièvre JM, Latché A, Jones B, Bouzayen M, Pech JC. Ethylene and fruit ripening. Physiol Plant. 1998;101:727–739. [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Lincoln JE, Cordes S, Read E, Fischer RL. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA. 1987;84:2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Fischer RL. Regulation of gene expression by ethylene in wild-type and rin tomato (Lycopersicon esculentum) fruit. Plant Physiol. 1988;88:370–374. doi: 10.1104/pp.88.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Hoffman NE, Yang SF. Promotion by ethylene of the capability to convert 1-aminocyclopropane-1-carboxylic acid to ethylene in preclimacteric tomato and cantaloupe fruit. Plant Physiol. 1985;77:407–411. doi: 10.1104/pp.77.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunders MJ, Holdsworth MJ, Slater A, Knapp JE, Bird CR, Schuch W, Grierson D. Ethylene stimulates the accumulation of ripening-related mRNAs in tomatoes. Plant Cell Environ. 1987;10:177–184. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, of 1-aminocyclopropane-1-carboxy-lateoxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller PW, Min-Wong L, Taylor LP, Pike DA, Theologis A. Reversible inhibition of tomato fruit senescence by antisense RNA. Science. 1991;254:437–439. doi: 10.1126/science.1925603. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Olson DC, Oetiker JH, Yang SF. Analysis of LE-ACS3, a 1-aminocyclopropane-1-carboxylic acid synthase gene expressed during flooding in the roots of tomato plants. J Biol Chem. 1995;270:14056–14061. doi: 10.1074/jbc.270.23.14056. [DOI] [PubMed] [Google Scholar]
- Olson DC, White JA, Edelman L, Harkins RN, Kende H. Differential expression of two genes for 1-aminocyclopropane-1-carboxylic acid synthase in tomato fruits. Proc Natl Acad Sci USA. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC, Kende H. Differential regulation of genes encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase in etiolated pea seedlings: effects of indole-3-acetic acid, wounding and ethylene. Plant Mol Biol. 1998;38:977–982. doi: 10.1023/a:1006033030081. [DOI] [PubMed] [Google Scholar]
- Picton S, Barton SL, Bouzayen M, Hamilton AJ, Grierson D. Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene-forming enzyme transgene. Plant J. 1993;3:469–481. [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Shiu OI, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar EC, McGlasson WB, Buescher RW. Genetic regulation of tomato fruit ripening. Hortscience. 1978;13:508–513. [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotech. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IS, Mori H, Kim JH, Kang BG, Imaseki H. VR-ACS6 is an auxin-inducible 1-aminocyclopropane-1-carboxylate synthase gene in mung bean (Vigna radiata) Plant Cell Physiol. 1997;38:217–224. doi: 10.1093/oxfordjournals.pcp.a029156. [DOI] [PubMed] [Google Scholar]
- Zarembinski T, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]