Abstract
Objective
The aim of this study was to conduct a meta-analysis of retrospective studies that investigated the association of preoperative C-reactive protein (CRP) levels with the overall survival (OS) of patients with bone neoplasms.
Methods
A detailed literature search was performed in the Cochrane Library, Web of Science, Embase and PubMed databases up to August 28, 2017, for related research publications written in English. We extracted the data from these studies and combined the hazard ratios (HR) and 95% confidence intervals (CIs) to assess the correlation between CRP levels and OS in patients with bone neoplasms.
Results
Five studies with a total of 816 participants from several countries were enrolled in this current meta-analysis. In a pooled analysis of all the publications, increased serum CRP levels had an adverse prognostic effect on the overall survival of patients with bone neoplasms. However, the combined data showed no significant relationship between the level of CRP and OS in Asian patients (HR = 1.73; 95% CI: 0.86–3.49; P = 0.125). Similar trends were observed in patients with bone neoplasms when stratified by ethnicity, histology, metastasis and study sample size.
Conclusions
The results of this meta-analysis suggest that increased CRP expression indicates a poorer prognosis in patients with bone neoplasms. More prospective studies are needed to confirm the prognostic significance of CRP levels in patients with bone neoplasms.
Introduction
Primary neoplasms of bone, namely osteosarcoma, chondrosarcoma, and the Ewing’s sarcoma family of tumors, are estimated to affect 3240 new patients and to cause 1550 deaths each year in the U.S. [1]. Osteosarcoma and Ewing’s sarcoma occur most commonly in teenagers, while chondrosarcoma occurs more frequently in older adults. Despite advances in surgical techniques and continuous efforts to improve therapy regimens, the 5-year relative survival rates of bone cancers have remained relatively stable since the 1980s. Therefore, multiple studies are being conducted to determine new markers for prognosis and targets for prospective treatments for those cancers [2].
C-reactive protein (CRP) is a common acute phase serum protein. It was first discovered in the plasma of patients with pneumonia and was named after its reactivity with the C polysaccharide derived from the pneumococcal cell wall [3]. CRP can interact with multiple ligands and receptors, including phosphocholine (PC) on pathogenic organism and damaged cell membranes, nuclear antigens, C1q in the classical complement pathway, and FcγRI and FcγRII on the surface of leukocytes, allowing it to play an important role in innate immunity [4]. CRP is produced by hepatocytes, mainly in response to interleukin-6 (IL-6) secreted by T cells and macrophages, which regulates CRP production at the transcriptional level [5]. In the circulating blood of healthy adults, CRP is only present in trace amounts, but its level rapidly rises within 2 hours of the onset of trauma, infection, and inflammation, and decreases quickly after the resolution of such conditions. Therefore, it has been used as a common marker for inflammation. Interestingly, CRP has been proven to be strongly associated with various cancers, exhibiting diagnostic or prognostic value [6–13]. While multiple studies have been carried out, the relationship between CRP and the prognosis of bone cancer remains controversial, with end results varying among studies. To evaluate the significance of the preoperative level of CRP for the outcome of bone cancer patients, we did a systematic review and meta-analysis using updated data on individual patients from all available trials.
Methods
Compliance with ethical standards
Ethical approval: All procedures performed in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or the national research committee and with the 1964 Helsinki declaration and its later amendment.
Search strategy
PubMed, Cochrane Library, Web of Science and Embase were thoroughly searched up to August 28, 2017 using the following key words: “C-reactive protein”, “bone neoplasms” and “bone cancer”. All studies identified in this manner were retrieved. The references of the selected studies were also searched for other relevant studies. The publication language was limited to English. The titles and abstracts of the selected studies were screened to filter appropriate studies, and the full texts were evaluated carefully. There were no restrictions on the number of patients in these published studies. This meta-analysis was registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO), and the registration number for this article is CRD42017075200.
Eligibility criteria
The following inclusion criteria were used: 1) patients included in studies were pathologically diagnosed as having bone neoplasms; 2) the level of CRP was evaluated before treatment; 3) the study provided HRs and 95% CIs for CRP in terms of OS or the data necessary to calculate them; 4) the publications were written in English. The exclusion criteria were as follows: 1) articles that were reviews, meeting abstracts, or letters, or lacking the full text in English; 2) nonhuman studies; 3) studies that did not provide the levels of CRP before treatment. If the data sets overlapped or were duplicated, only the most recent information was included in this meta-analysis. All identified studies were investigated independently for eligibility by two authors.
Data extraction
Two independent authors (W, L and X, L) independently extracted information from the eligible studies, and any disagreement between them was resolved by discussion and consensus. The following information was recorded from the 5 included studies: the surname of the first author, the study country, the year of publication, the sample size, the survival data and the detailed information regarding the bone neoplasms.
Quality assessment
The quality assessment of the primary studies was performed according to the Newcastle-Ottawa quality assessment scale (NOS). The maximum possible score is 9 points, and studies scoring ≥6 points were regarded as high-quality studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
Statistical analysis
All statistical analysis was carried out by STATA 14.0 (STATA Corp, College Station, TX) [14]. Statistical heterogeneity among the studies was evaluated with Q and I2 statistics, with the significance level set at p <0.05 [15]. If there was significant heterogeneity among the studies, the random effects model was used to calculate the pooled HR and 95% CI [16]. The potential publication bias was estimated using Begg’s test [17]. P< 0.10 was considered statistically significant. Sensitivity analyses were performed by excluding each study individually from the meta-analysis.
Result
The characteristics of the included studies
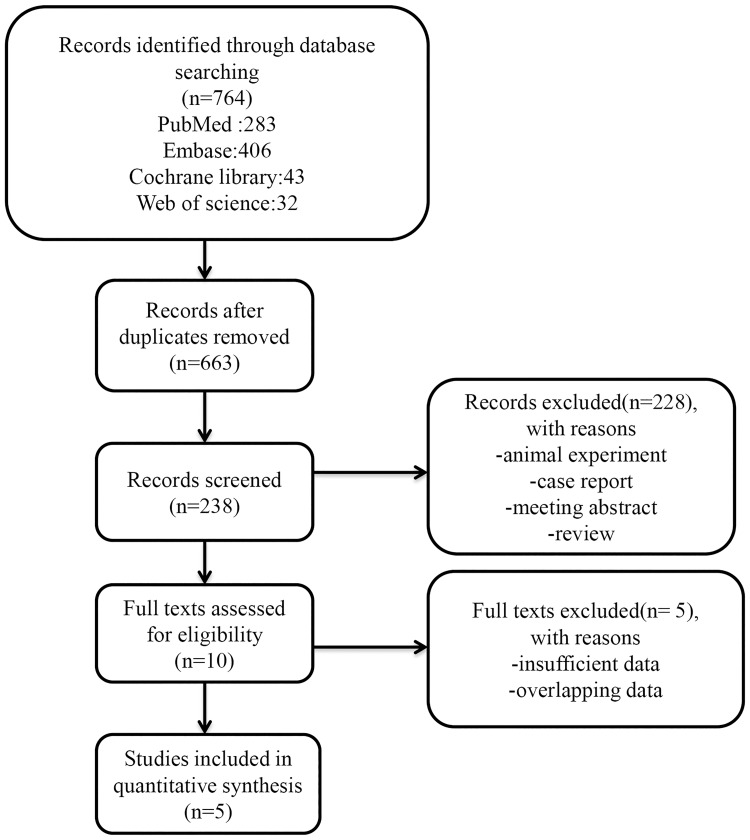
The flow diagram of the current study is presented in Fig 1. Five relevant studies with a total of 816 patients were selected for initial review by the search strategies described above [18–22]. The sample sizes ranged from 85 to 318 participants. All the enrolled studies were retrospective. Of these studies, three [18, 19, 22] were carried out in Europe, and the remaining studies were conducted in Asia. Only two publications [18, 22] involved patients with osteosarcoma and other kinds of bone neoplasms, and the other three studies focused on osteosarcoma [19–21]. An elevated level of CRP was defined as ≥8 mg/l or ≥75 nmol/l in one study [18]; otherwise, a normal level of CRP was defined as less than 10 mg/dl. Among the participants, there were 671 patients with distant metastasis, and 145 with no distant metastasis. All enrolled publications defined OS as the time from diagnosis to the day of death or the day of the last follow-up. The articles were published between 2011 and 2016, and the NOS scores of the included studies ranged from 6–9. The detailed information is shown in Table 1.
Fig 1. Flow chart of the study selection.
Table 1. Characteristics of all included studies.
| Study | Year | Location | Ethnicity | Follow-up | Sample size | Gender(M/F) | Type | Treatment | Outcomes | Metastasis | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu, B | 2016 | China | Asian | NA | 162 | 96/66 | OST | S,C and R | OS | 78/84 | 7 |
| Aggerholm-Pedersen, N. | 2016 | Denmark | European | 8.8 year(median) | 172 | 98/74 | CH = 62 EW/OST = 109 |
S and C | OS/disease-specific survival | NO | 8 |
| Li, X. C. | 2015 | China | Asian | NA | 85 | 43/42 | OST | C | OS | 37/48 | 6 |
| Nakamura, T. | 2013 | British | European | 40 month(mean) | 318 | 176/142 | OST = 112C H = 93 EW = 47 Other = 66 |
S,C and R | OS | NO | 8 |
| Funovics, P. T. | 2011 | Austria | European | 46 month(mean) | 79 | 42/37 | OST | S and C | OS/disease-specific survival | 30/49 | 8 |
Abbreviations: CH: chondrosarcoma; EW: Ewing’s sarcoma; OST: osteosarcoma; OS: overall survival; NOS: Newcastle-Ottawa quality assessment scale; S: surgery; C: chemotherapy; R: radiotherapy; NA: data were not provided in the publication.
Relationship between CRP and OS in bone neoplasms
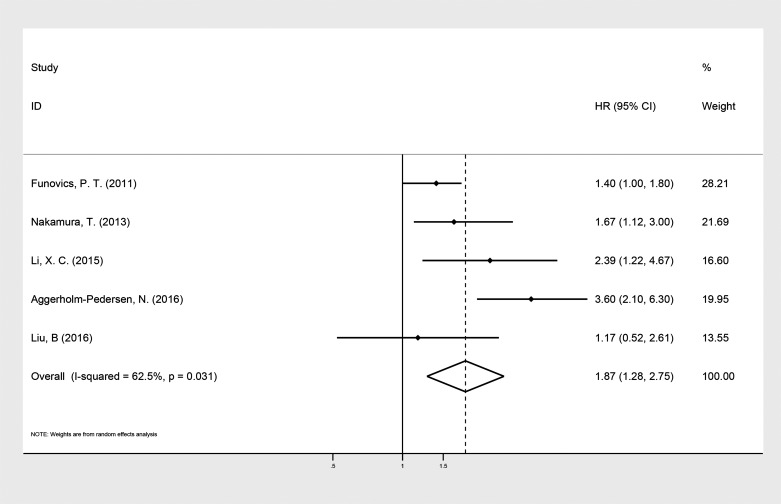
The five selected studies provided the levels of CRP before treatment and OS in patients with bone neoplasms. The random effects model showed a significant relationship between elevated levels of CRP and OS in patients with bone neoplasms (HR: 1.87; 95% CI: 1.28–2.75; P = 0.001), with heterogeneity (I2 = 62.4%, P = 0.031, Fig 2).
Fig 2. Forest plot of the association between the level of CRP and OS in patients with bone neoplasms.
Summary of estimated hazard ratios (HRs) and 95% CI for patients with bone neoplasms.
Subgroup analyses
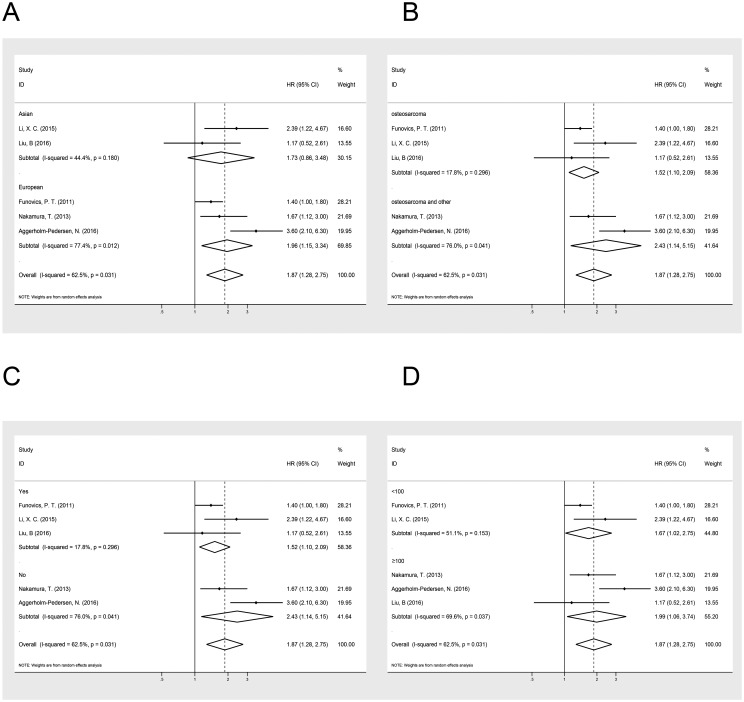
To detect the potential source of heterogeneity, the subgroup analyses were stratified by ethnicity, histology, metastasis and sample size (Table 2, Fig 3). As presented in Table 2, the relationship between the level of CRP and OS was not significant in Asian populations (HR = 1.73; 95% CI: 0.86–3.49; P = 0.125) (I2 = 44.4%; P = 0.18). However, the elevated CRP predict poorer OS in patients in Europe (HR = 1.96; 95% CI: 1.51–3.34; P = 0.013) (I2 = 77.4%; P = 0.01). We also performed subgroup analyses on histology, metastasis and sample size to further explain the results of this meta-analysis. Among patients with osteosarcoma, increased CRP was correlated with shortened OS (HR = 1.52; 95% CI: 1.10–2.09; P = 0.01) (I2 = 17.8%; P = 0.30), and the same was true for patients with other kinds of bone cancer (HR = 1.87; 95% CI: 1.28–2.75; P = 0.02) (I2 = 76.0%; P = 0.04). An increased level of CRP was correlated with decreased survival in patients regardless of metastasis (Table 2). Similar trends were also observed when stratified according to sample size (Table 2).
Table 2. A summary of HRs for the overall and subgroup analyses of CRP levels in patients with bone neoplasms.
| No. of studies | No. of participants | HR | 95%CI | P | I2 (%) | ||
|---|---|---|---|---|---|---|---|
| Overall | 5 | 816 | 1.87 | 1.28–2.75 | 0.001 | 62.4% | |
| Ethnicity | Asian | 2 | 247 | 1.73 | 0.86–3.49 | 0.125 | 44.4% |
| European | 3 | 569 | 1.96 | 1.51–3.34 | 0.013 | 77.4% | |
| Histology | osteosarcoma | 3 | 326 | 1.52 | 1.10–2.09 | 0.01 | 17.8% |
| Osteosarcoma and other kinds | 2 | 490 | 1.87 | 1.28–2.75 | 0.02 | 76.0% | |
| Metastasis | Yes | 3 | 326 | 1.52 | 1.10–2.09 | 0.01 | 17.8% |
| No | 2 | 490 | 1.87 | 1.28–2.75 | 0.02 | 76.0% | |
| Sample size | <100 | 2 | 164 | 1.67 | 1.02–2.75 | 0.04 | 51.1% |
| ≥100 | 3 | 652 | 1.99 | 1.06–3.74 | 0.03 | 69.6% |
Fig 3. Forest plot of the association between the level of CRP and OS in patients with bone neoplasms stratified by ethnicity (A), histology (B), metastasis (C) and sample size (D).
Summary of estimated hazard ratios (HRs) and 95% CI for patients stratified by (A) ethnicity, (B) histology, (C) metastasis and (D) sample size.
Publication bias and sensitivity analysis
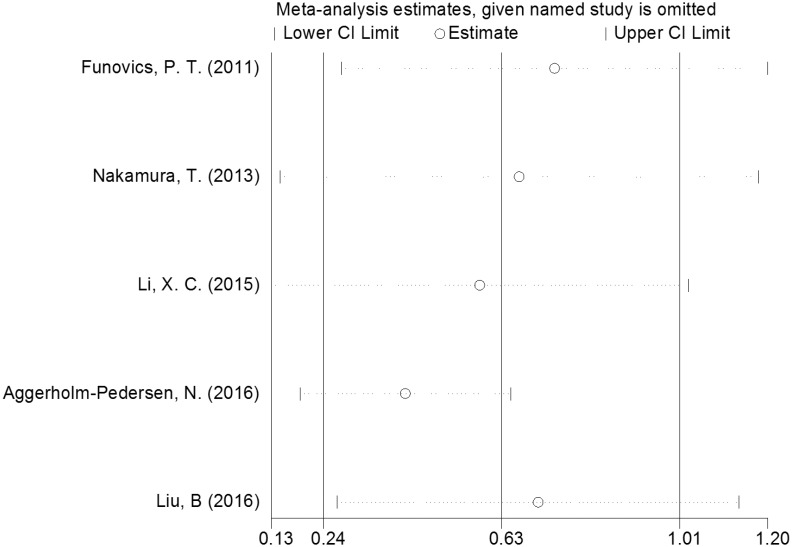
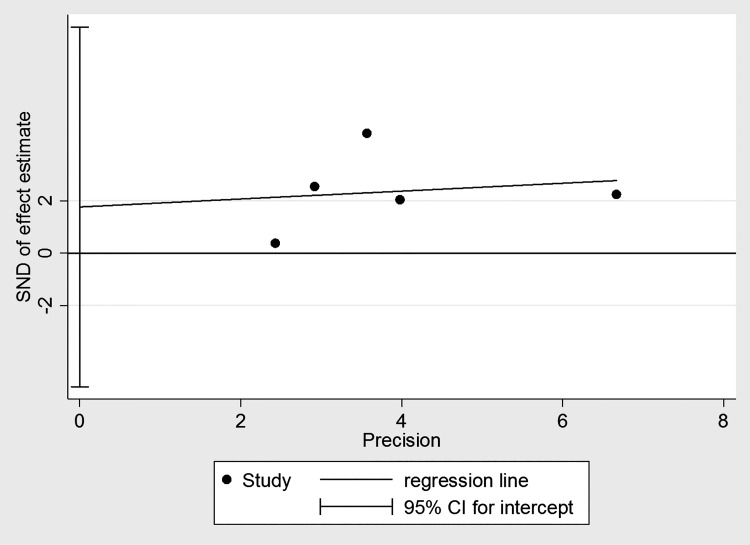
Significant heterogeneity was discovered among all studies (I2 = 62.4%, P = 0.031). The influence of each individual study on the combined HRs was evaluated by systematically deleting one included study at a time. The results showed that the pooled HRs for OS were robust in our study (Fig 4). Moreover, Egger’s test showed no evidence of obvious publication bias (P = 0.473) (Fig 5).
Fig 4. Sensitivity analysis of the relationship between CRP level and OS in bone neoplasms.
Sensitivity analyses were performed by excluding each study individually from the meta-analysis.
Fig 5. Begg’s funnel plot of the publication bias test for CRP level and OS in bone neoplasms.
Summary of funnel plots of publication bias for the included studies. They are funnel plots of the publication bias for this meta-analysis of hazard ratios (HRs).
Discussion
The current meta-analysis summarized the results of five retrospective studies, involving a total of 816 participants. By combining the HRs and 95% CIs from all studies, we showed the association between preoperational serum levels of CRP and the overall survival of patients with bone neoplasms. Our result revealed that higher levels of CRP are associated with shorter OS, with an HR of 1.87 (95% CI: 1.28–2.75; P = 0.001), indicating that high serum levels of CRP before treatment may be a negative prognostic factor for patients with bone cancers. However, stratified analysis by region showed no significant relationship between the level of CRP and OS in Asia (HR = 1.73; 95% CI: 0.86–3.49; P = 0.125). There might be several reasons for this result. First, the susceptibility genes for bone neoplasms in Asia are different from those in Europe, which might lead to different levels of CRP in patients with bone neoplasms. Second, the use of treatment regimens in Asia was significantly different from that in Europe, which might explain the lack of a correlation between the level of CRP and OS in Asia. Finally, the instruments measuring the levels of CRP were not the same in different places, which might cause the different results in Asia and Europe.
Inflammation has been proven to be closely related to all stages of cancer development. It may contribute to cancer initiation by supplying reactive oxygen and nitrogen species that damage DNA directly and it may alter DNA methylation and histone modification, thereby influencing gene expression [23]. Inflammation also facilitates tumor promotion by producing growth factors to sustain proliferation, survival factors to limit cell death, and proangiogenic factors to increase neovascularization [24]. Furthermore, inflammation can assist metastatic progression by providing inductive signals that activate epithelial–mesenchymal transition and extracellular matrix-modifying enzymes that aid tumor invasion, as well as by suppressing anti-tumor immune response [25]. Elevated levels of systemic inflammation have been indicated as being associated with worse survival in patients with solid tumors [26, 27]. Other inflammation biomarkers including the NLR (neutrophil to lymphocyte ratio), the PLR (platelet to lymphocyte ratio) and the mGPS (modified Glasgow prognostic score) could reflect the cancer-related inflammatory status and have been used as prognostic indicators in other cancers [28–32]. As an important biomarker of systemic inflammation, CRP is synthesized by liver cells in response to microbial invasion or tissue injury [33]. CRP is considered a non-specific but sensitive marker of inflammation. While it is well established that CRP levels rise rapidly during acute infection, inflammation, and tissue damage, elevated CRP levels are also seen as an important risk factor for atherosclerosis [34], stroke [35–37], and myocardial infarction [38]. Given that the inflammatory response plays a vital role in cancer, it is not surprising to find increased CRP levels in various cancers. In fact, the serum CRP level before treatment has been proven to be an independent prognostic factor in hepatocellular [39], esophageal [40, 41], renal [42, 43], bladder [44], prostate [45], colorectal [46], ovarian [47], pancreatic [48], and non-small cell lung cancer [49]. Large prospective studies looking for associations between circulating concentrations of CRP and cancer risks have produced conflicting results. Positive relationships were found between serum CRP levels and the increased risk of colorectal and lung cancers, while other studies indicated no relationship between CRP levels and breast, prostate or colorectal cancers [50]. Although the exact functional mechanism of CRP in the development of cancer remains obscure, several hypotheses have been proposed to explain this relationship. First, a causality model has been proposed wherein chronic inflammation causes the elevation of CRP levels, which initiates the formation of malignant tumors. Second, a reverse causality model has been proposed wherein tumor growth and invasion induces tissue inflammation, leading to the increase in CRP levels. A third proposed mechanism states that the body’s innate and adaptive immune systems may react to tumor antigens by increasing CRP levels. A fourth proposed mechanism cites the fact that tumor cells can produce CRP themselves, and they are also able to release cytokines such as IL-6 and IL-8, which contribute to the increase in CRP levels.
The results of this meta-analysis support increased levels of CRP as a prognostic factor for OS in bone cancer, which agrees with the results of most studies [18–20, 22]. We noticed that the previous study carried out by Yi, J. H. also evaluated whether the level of CRP was correlated with the outcome of patients with osteosarcoma [51]. The literature enrolled in that study only included studies published by 2013, and only two studies were included. Unlike that previous study, we had stricter inclusion criteria and enrolled 3 articles published in 2015 and 2016, including a relatively larger number of participants with detailed information. In addition, the research conducted by Yi, J. H. did not evaluate the association between CRP level and OS stratified by ethnicity, histology, metastasis or sample size, as we did. In addition, we concluded that there was no significant relationship between the CRP level and OS in Asian populations based on data from only two studies; therefore, the results should be interpreted with caution. Furthermore, three of the enrolled studies only included patients with osteosarcoma [19–21], although the remaining studies included patients with other kinds of bone cancer [18, 22]. As we know, different kinds of bone cancer might have different overall survival times, which may have contributed to the high heterogeneity in this meta-analysis. An elevated CRP level was defined as less than 10 mg/dl in all studies except one [18], which might also explain the high degree of heterogeneity. All survival data were extracted from multi-factor analyses adjusted for potential confounding factors, including gender, age, stage, treatment and other biomarkers. However, due to the limited information presented in the studies, it was not possible to perform a subgroup analysis according to all cofounding factors.
This research does have several limitations. First, obvious heterogeneity existed in this meta-analysis. Although the sensitivity analysis and the publication bias test indicated the credibility of the results, we could not rule out the cofounding factors or the study criteria that may have resulted in discrepancies between the studies. Second, all the studies included were retrospective studies instead of prospective ones, and therefore the result may represent reverse causation, survival bias or confounding. Third, this study only focused on the CRP level before treatment, and to further investigate its prognostic value, the levels of CRP after surgery and at recurrence should also be taken into consideration.
Generally, our meta-analysis demonstrated the prognostic value of increased preoperative levels of CRP for poorer OS in patients with bone cancer in Europe but not in Asia. However, given the limitations mentioned above, these findings should be treated with caution when applied to clinical practice. More prospective cohort studies are warranted to confirm our results.
Supporting information
(DOCX)
(DOC)
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No.81372180).
Data Availability
All articles enrolled in this current study were available from PubMed, Cochrane Library, Web of Science and Embase and all data used in this article were extracted from enrolled studies.
Funding Statement
This study was funded by the National Natural Science Foundation of China grant no. 81372180 to Z. Li. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2014 National Cancer Institute2017 [cited 2017 August 28]. https://seer.cancer.gov/csr/1975_2014/.
- 2.Evola FR, Costarella L, Pavone V, Caff G, Cannavò L, Sessa A, et al. Biomarkers of Osteosarcoma, Chondrosarcoma, and Ewing Sarcoma. Frontiers in pharmacology. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. Journal of Experimental Medicine. 1930;52(4):561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marnell L, Mold C, Du Clos TW. C-reactive protein: Ligands, receptors and role in inflammation. Clinical Immunology. 2005;117(2):104–11. doi: 10.1016/j.clim.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 5.Castell JV, Gómez-lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–86. [DOI] [PubMed] [Google Scholar]
- 6.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Critical reviews in clinical laboratory sciences. 2011;48(4):155–70. Epub 2011/11/01. doi: 10.3109/10408363.2011.599831 . [DOI] [PubMed] [Google Scholar]
- 7.Alifano M, Falcoz PE, Seegers V, Roche N, Schussler O, Younes M, et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142(5):1161–7. doi: 10.1016/j.jtcvs.2011.07.021 . [DOI] [PubMed] [Google Scholar]
- 8.Eggers H, Seidel C, Schrader AJ, Lehmann R, Wegener G, Kuczyk MA, et al. Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol. 2013;30(4):705 doi: 10.1007/s12032-013-0705-6 . [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita A, Onoda H, Takano K, Imai N, Saeki C, Fushiya N, et al. Pretreatment serum C-reactive protein level predicts poor prognosis in patients with hepatocellular carcinoma. Med Oncol. 2012;29(4):2800–8. doi: 10.1007/s12032-012-0220-1 . [DOI] [PubMed] [Google Scholar]
- 10.Li YJ, Li ZM, Xia Y, Huang JJ, Huang HQ, Xia ZJ, et al. Serum C-reactive protein (CRP) as a simple and independent prognostic factor in extranodal natural killer/T-cell lymphoma, nasal type. PLoS One. 2013;8(5):e64158 doi: 10.1371/journal.pone.0064158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao N, Cai Q. High pretreatment serum C-reactive protein level predicts a poor prognosis for combined small-cell lung cancer. Tumor Biology. 2015;36(11):1–6. [DOI] [PubMed] [Google Scholar]
- 13.Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Research. 2012;32(8):3535–8. [PubMed] [Google Scholar]
- 14.Higgins JP, Green S. Cochrane Handbook For Systematic Reviews Of Interventions Version 5.0.0 2009. [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke K, Shea B, Wells GA. Meta-Analysis of Clinical Trials: Springer; New York; 2001. 397–424 p. [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. Epub 1994/12/01. . [PubMed] [Google Scholar]
- 18.Aggerholm-Pedersen N, Maretty-Kongstad K, Keller J, Baerentzen S, Safwat A. The prognostic value of serum biomarkers in localized bone sarcoma. Translational Oncology. 2016;9(4):322–8. doi: 10.1016/j.tranon.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funovics PT, Edelhauser G, Funovics MA, Laux C, Berzaczy D, Kubista B, et al. Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. International orthopaedics. 2011;35(10):1529–36. Epub 2011/01/21. doi: 10.1007/s00264-011-1208-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Tian F, Wang F, Li Y. Serum C-reactive protein and overall survival of patients with osteosarcoma. Tumor Biology. 2015;36(7):5663–6. doi: 10.1007/s13277-015-3240-6 [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Huang Y, Sun Y, Zhang J, Yao Y, Shen Z, et al. Prognostic value of inflammation-based scores in patients with osteosarcoma. Scientific reports. 2016;6:39862 Epub 2016/12/23. doi: 10.1038/srep39862 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura T, Grimer RJ, Gaston CL, Watanuki M, Sudo A, Jeys L. The prognostic value of the serum level of Creactive protein for the survival of patients with a primary sarcoma of bone. Journal of Bone and Joint Surgery—Series B. 2013;95 B(3):411–8. [DOI] [PubMed] [Google Scholar]
- 23.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature reviews Cancer. 2013;13(11):759–71. Epub 2013/10/25. doi: 10.1038/nrc3611 . [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436 doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 25.Qian BZ. Inflammation fires up cancer metastasis. Seminars in cancer biology. 2017;47:170–6. doi: 10.1016/j.semcancer.2017.08.006 . [DOI] [PubMed] [Google Scholar]
- 26.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. European journal of cancer. 2011;47(17):2633–41. doi: 10.1016/j.ejca.2011.03.028 . [DOI] [PubMed] [Google Scholar]
- 27.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncology. 2010;6(1):149–63. doi: 10.2217/fon.09.136 . [DOI] [PubMed] [Google Scholar]
- 28.Su K, Wang X, Chi L, Liu Y, Jin L, Li W. High glasgow prognostic score associates with a poor survival in Chinese advanced non-small cell lung cancer patients treated with platinum-based first-line chemotherapy. International Journal of Clinical and Experimental Medicine. 2016;9(8):16353–9. [Google Scholar]
- 29.Wang X, Teng F, Kong L, Yu J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther. 2016;9:5761–70. doi: 10.2147/OTT.S106296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan H, Zhou L, Chi D, Zhou Q, Tang X, Zhu D, et al. Preoperative platelet to lymphocyte and neutrophil to lymphocyte ratios are independent prognostic factors for patients undergoing lung cancer radical surgery: A single institutional cohort study. Oncotarget. 2017;8(21):35301–10. Epub 2016/11/16. doi: 10.18632/oncotarget.13312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Salcedo P, de-Torres JP, Martinez-Urbistondo D, Gonzalez-Gutierrez J, Berto J, Campo A, et al. The neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers for lung cancer development. Lung Cancer. 2016;97:28–34. doi: 10.1016/j.lungcan.2016.04.010 . [DOI] [PubMed] [Google Scholar]
- 32.Kos M, Hocazade C, Kos FT, Uncu D, Karakas E, Dogan M, et al. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non-small cell lung cancer. Wien Klin Wochenschr. 2016;128(17–18):635–40. doi: 10.1007/s00508-015-0724-8 . [DOI] [PubMed] [Google Scholar]
- 33.Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. European Journal of Surgical Oncology (EJSO). 2008;34(7):727–9. doi: 10.1016/j.ejso.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 35.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma Concentration of C-Reactive Protein and Risk of Ischemic Stroke and Transient Ischemic Attack. The Framingham Study. 2001;32(11):2575–9. doi: 10.1161/hs1101.098151 [DOI] [PubMed] [Google Scholar]
- 36.Muir KW, Weir CJ, Alwan W, Squire IB, Lees KR. C-Reactive Protein and Outcome After Ischemic Stroke. Stroke. 1999;30(5):981–5. doi: 10.1161/01.str.30.5.981 [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, Zhou L, Guo T, Wang N, Hao H, Zhou Y, et al. Plasma proteomics reveals coagulation, inflammation, and metabolic shifts in H-type hypertension patients with and without acute ischemic stroke. Oncotarget. 2017;8(59):100384–95. Epub 2017/12/17. doi: 10.18632/oncotarget.22233 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Glynn RJ, Hennekens CH. C-Reactive Protein Adds to the Predictive Value of Total and HDL Cholesterol in Determining Risk of First Myocardial Infarction. Circulation. 1998;97(20):2007–11. doi: 10.1161/01.cir.97.20.2007 [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–64. Epub 2005/03/22. doi: 10.1002/cncr.20976 . [DOI] [PubMed] [Google Scholar]
- 40.Nozoe T, Saeki H, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. American journal of surgery. 2001;182(2):197–201. Epub 2001/09/28. . [DOI] [PubMed] [Google Scholar]
- 41.Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World journal of gastroenterology. 2006;12(23):3746–50. Epub 2006/06/15. doi: 10.3748/wjg.v12.i23.3746 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamb GW, McMillan DC, Ramsey S, Aitchison M. The relationship between the preoperative systemic inflammatory response and cancer-specific survival in patients undergoing potentially curative resection for renal clear cell cancer. British journal of cancer. 2006;94(6):781–4. Epub 2006/03/09. doi: 10.1038/sj.bjc.6603034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson T, Harris W, Watkins A, Michigan A, Ogan K, Pattaras J, et al. CRP- based classification of localized renal cell carcinoma into low, intermediate and high riskof mortality 2011. e667 p. [Google Scholar]
- 44.Gakis G, Todenhofer T, Renninger M, Schilling D, Sievert KD, Schwentner C, et al. Development of a new outcome prediction model in carcinoma invading the bladder based on preoperative serum C-reactive protein and standard pathological risk factors: the TNR-C score. BJU international. 2011;108(11):1800–5. Epub 2011/04/22. doi: 10.1111/j.1464-410X.2011.10234.x . [DOI] [PubMed] [Google Scholar]
- 45.Prins RC, Rademacher BL, Mongoue-Tchokote S, Alumkal JJ, Graff JN, Eilers KM, et al. , editors. C-reactive protein as an adverse prognostic marker for men with castration-resistant prostate cancer (CRPC): confirmatory results. Urologic Oncology: Seminars and Original Investigations; 2012: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen M, Kersten C, Sorbye H, Skovlund E, Glimelius B, Pfeiffer P, et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget. 2016;7(46):75013–22. Epub 2016/10/16. doi: 10.18632/oncotarget.12601 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(3):710–4. Epub 2008/02/05. doi: 10.1158/1078-0432.ccr-07-1044 . [DOI] [PubMed] [Google Scholar]
- 48.Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. British journal of cancer. 2014;110(1):183 doi: 10.1038/bjc.2013.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gagnon B, Abrahamowicz M, Xiao Y, Beauchamp ME, MacDonald N, Kasymjanova G, et al. Flexible modeling improves assessment of prognostic value of C-reactive protein in advanced non-small cell lung cancer. British journal of cancer. 2010;102(7):1113–22. Epub 2010/03/18. doi: 10.1038/sj.bjc.6605603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. Journal of epidemiology and community health. 2007;61(9):824–33. Epub 2007/08/19. doi: 10.1136/jech.2006.051292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi JH, Wang D, Li ZY, Hu J, Niu XF, Liu XL. C-reactive protein as a prognostic factor for human osteosarcoma: a meta-analysis and literature review. PLoS One. 2014;9(5):e94632 Epub 2014/05/08. doi: 10.1371/journal.pone.0094632 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All articles enrolled in this current study were available from PubMed, Cochrane Library, Web of Science and Embase and all data used in this article were extracted from enrolled studies.