Abstract
Background
Aedes aegypti is a primary vector of dengue, chikungunya, Zika, and urban yellow fever viruses. Indoor, ultra low volume (ULV) space spraying with pyrethroid insecticides is the main approach used for Ae. aegypti emergency control in many countries. Given the widespread use of this method, the lack of large-scale experiments or detailed evaluations of municipal spray programs is problematic.
Methodology/Principal findings
Two experimental evaluations of non-residual, indoor ULV pyrethroid spraying were conducted in Iquitos, Peru. In each, a central sprayed sector was surrounded by an unsprayed buffer sector. In 2013, spray and buffer sectors included 398 and 765 houses, respectively. Spraying reduced the mean number of adults captured per house by ~83 percent relative to the pre-spray baseline survey. In the 2014 experiment, sprayed and buffer sectors included 1,117 and 1,049 houses, respectively. Here, the sprayed sector’s number of adults per house was reduced ~64 percent relative to baseline. Parity surveys in the sprayed sector during the 2014 spray period indicated an increase in the proportion of very young females. We also evaluated impacts of a 2014 citywide spray program by the local Ministry of Health, which reduced adult populations by ~60 percent. In all cases, adult densities returned to near-baseline levels within one month.
Conclusions/Significance
Our results demonstrate that densities of adult Ae. aegypti can be reduced by experimental and municipal spraying programs. The finding that adult densities return to approximately pre-spray densities in less than a month is similar to results from previous, smaller scale experiments. Our results demonstrate that ULV spraying is best viewed as having a short-term entomological effect. The epidemiological impact of ULV spraying will need evaluation in future trials that measure capacity of insecticide spraying to reduce human infection or disease.
Author summary
Aedes aegypti is a primary vector for medically important viruses that typically resides within houses. Indoor, ultra low volume (ULV) adulticide space spraying is considered to be more effective in controlling Ae. aegypti populations than outdoor spraying, and is widely used in tropical cities. Given the widespread use of indoor ULV spraying in emergencies by municipal control programs, the lack of large spatial scale evaluations is problematic. We conducted two large-scale experiments to evaluate indoor ULV pyrethroid spraying in the city of Iquitos, Peru in 2013 and 2014, and we also evaluated a municipal spraying effort. Our results demonstrate that densities of adults can be reduced by ULV spraying, but that adult densities in sprayed areas return to approximately pre-spray densities in less than a month. These findings agree with results from previous, smaller scale experiments, and confirm that ULV spraying should be viewed as causing a short-term decrease of Ae. aegypti populations. We provide extensive detail regarding our experimental design and data collection so that our results can assist in establishing best practices for future assessments of ULV spraying efforts, as well as aid in testing predictions of mathematical models of Ae. aegypti population dynamics.
Introduction
Aedes aegypti is a primary vector for dengue (DENV), chikungunya (CHIKV), Zika (ZIKV) and urban yellow fever viruses (YFV). Dengue has become the most important human arthropod-borne viral infection worldwide [1, 2]. Each of these pathogens can be associated with explosive epidemics, where high disease incidence and public fear combine to overwhelm health systems [3]. Such epidemics put intense pressure on public health departments to react with emergency vector control measures [4, 5].
Ae. aegypti adults are primarily diurnal and females take frequent blood meals, predominantly from humans [6–8]. These behaviors can in part explain why Ae. aegypti has been associated with epidemic virus transmission even when its population densities are low [9]. Because adults typically reside inside houses [8] where food, mates, and oviposition substrates are readily available, indoor adulticide space spraying has been more effective than outdoor spraying for suppressing Ae. aegypti populations in small scale evaluations [4, 10, 11].
When indoor space sprays are applied appropriately, in carefully controlled small-scale experiments, adult Ae. aegypti populations often decreased by >80%. Population densities typically recovered quickly, however, [12–15] due to emergence of nulliparous mosquitoes from larval aquatic habitats inside sprayed areas [11], through migration from locations outside of sprayed areas [15], or from females in sprayed houses that survived. In a systematic literature review, Esu et al. [4] found only six studies from 1970’s to 2010 that tested ultra-low volume (ULV) indoor space spraying under natural field conditions that met minimum standards for evaluating mosquito population suppression. None of the studies evaluated the impact of these methods on human virus infection or disease [4]. Results ranged from immediate reduction in biting by 99% and adult population reduction lasting six months [16], to a more common, modest control lasting 1–5 weeks [14, 15, 17]. Most studies were small scale, with each treatment typically including one replicate of less than 50 houses.
It is important to distinguish between indoor and outdoor space spraying, the latter usually delivered by vehicle-mounted machines and whose effectiveness is often limited by a failure of insecticide droplets to penetrate indoors where Ae. aegypti rest [11]. A more recent review of vector control effectiveness for dengue [18] concluded that “although space spraying is the standard public health response to a dengue outbreak worldwide, and is recommended by WHO [19] for this purpose, there is scant evidence available from studies to evaluate this method sufficiently.” In fact, Bowman et al. [18] could find no well-designed trial that assessed the impact of non-residual indoor or outdoor space spraying on human dengue infection or disease.
Ae. aegypti populations in the Amazonian city of Iquitos, Peru have been studied extensively since 1998. The spatial distribution of the species is highly clustered and does not have a consistent spatial or temporal structure [20, 21]. Adult and immature population indices are highly variable and subject to sampling error [22]. Evaluation of control measures for this species, therefore, requires large sample sizes and exhaustive sampling.
In addition to studying the mosquito itself, the Iquitos research program monitored DENV transmission through passive clinic-based febrile surveillance in health care facilities throughout the city [23] and a series of prospective cohort studies in targeted city neighborhoods [24–26]. The combination of longitudinal entomological and epidemiological studies created a database that could be used to examine, in real time, the impact of Ministry of Health (MoH) vector interventions on Ae. aegypti populations and human disease. During their interventions, the MoH sprayed non-residual insecticide inside homes three times over an approximately 3-week period [27]. Over a 10-year period, this kind of citywide municipal vector control program was associated with significant decreases in Ae. aegypti adult populations [28, 29] and when interventions were applied during the first half of the dengue transmission season, fewer dengue cases were detected and the transmission season was shorter [27]. While the qualitative results from that analysis of dengue are consistent with an expectation of a positive public health impact of intra-domicile ULV insecticide application on dengue incidence, more statistically robust epidemiological studies are needed [30].
Prevention of Aedes-transmitted viral disease will require integrated approaches; i.e., combinations of existing and/or novel vector control strategies as well as vaccination. Mathematical models provide a way to compare diverse strategies and identify the most promising approaches. For example, data on Ae. aegypti populations in Iquitos were used to develop a biologically detailed, spatially explicit, stochastic model that tracked Ae. aegypti dynamics and genetics in an 18-ha area of the city [31, 32]. Preliminary validation of the model using Iquitos data was carried out [31], but evaluation of its capacity to accurately predict the entomological outcome of a vector control perturbation had not been tested. The experiments described here were primarily designed to generate data that could be used to test the ability of the entomological model to predict impacts of suppression measures.
In this study, we carried out a large-scale evaluation of the entomological impact of a widely used emergency vector intervention of Aedes-transmitted viruses in a well-characterized study site. Our specific goal was to evaluate the impact of 6 cycles of indoor ULV pyrethroid spray applications (hereafter referred to as “spray applications”) on reductions of Ae. aegypti populations. Our experiments spanned periods of relatively low and high Ae. aegypti density in Iquitos, and compared the ULV application in experimental and public health settings. Our results constitute an important data set for development and validation of Ae. aegypti population dynamics models, and provide a detailed account of indoor space spray effects on Ae. aegypti populations.
Methods and materials
Ethics statement
The study protocol was approved by the Naval Medical Research Unit Six (Protocol #NAMRU6.2013.0001) Institutional Review Board, which included Peruvian representation, in compliance with all US Federal and Peruvian regulations governing the protection of human subjects. IRB authorization agreements were established between the Naval Medical Research Unit Six and the University of California at Davis and North Carolina State University. The protocol was reviewed and approved by the Loreto Regional Health Department, which oversees health research in Iquitos. In all instances consent from adult members of houses was obtained without written consent. Written information sheets were provided to study participants, providing a detailed overview of the experiment design, procedures, and study goals before initial pre-interventions surveys. Permission to enter houses was provided at each survey or spray application visit.
Study area
The studies were conducted in two neighborhoods in the Maynas district of Iquitos (Fig 1, Maps). Iquitos has a human population of ~380,000 (73.2°W longitude, 3.7°S latitude, 120 m above sea level). Located in the Amazon Basin of northeastern Peru, Iquitos is the largest urban center in the Department of Loreto, and has an average daily temperature of 25°C and an average annual precipitation of 2.7 meters. Dynamics of Ae. aegypti populations in Iquitos are described in detail in earlier publications [20–22, 24, 33–38]. These neighborhoods were selected because, historically, they consistently had high Ae. aegypti densities [33]. In addition, the area was spatially configured to meet our study design of a central spray area surrounded by buffer area that would serve as a control.
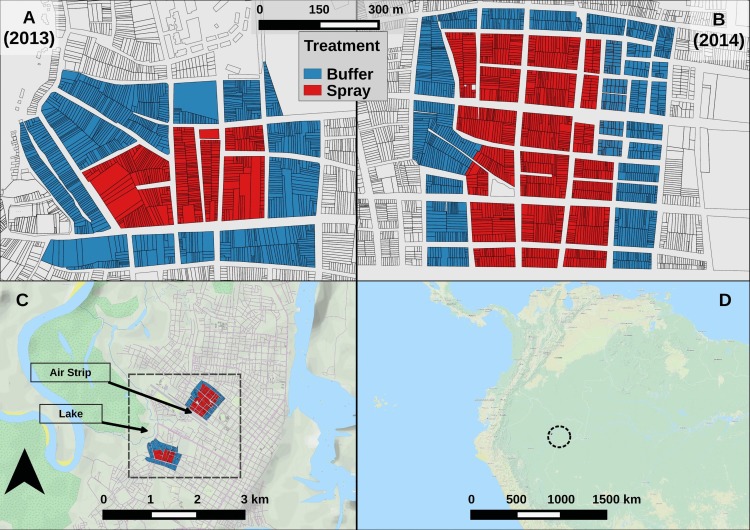
Fig 1. Map of experiment areas.
Detail for (A) S-2013 and (B) L-2014 experimental areas, showing individual houses. Color shows sector. (C) City of Iquitos. Black box highlights experimental areas. (D) Regional map. Black circle highlights Iquitos. See also S6 Fig.
Both experimental study neighborhoods were characterized by city blocks of row houses (dwellings that share walls). Most houses occupied lots that were narrow (3–10 m wide), but relatively deep (20–60 m long). The majority of houses served as family residences, often containing extended or multiple families. Some houses were used for small businesses or offices, and others were unoccupied. There were a small number of vacant lots containing no structures (<1%). Many study houses were mixed-purpose, sharing living areas with a small store (“bodega”), office, shop (e.g. carpentry or vehicle repair), or restaurant.
Vector control activities were ongoing in Iquitos. The MoH carried out regular entomological surveillance and larviciding activities with temephos (Abate) at ~3 month intervals. Since 2002, with few exceptions, MoH carried out 1–3 emergency indoor pyrethroid spray campaigns per year in response to dengue outbreaks, with variable success [27]. Our study was completed in 2014, while resistance bioassay profiles prior to January 2013 indicated Ae. aegypti populations in the city were susceptible to pyrethroids [39].
Fig 2 summarizes the design of our two separate experiments. The first and smaller of the two experiments (S-2013) ran for 16 calendar weeks and included an experimental buffer sector that was not sprayed, surrounding a central experimental sector that was sprayed. The buffer sector contained 765 houses and the spray sector had 398 houses (Fig 1A). The S-2013 study area was located on the western border of the city, proximal to Lake Moronacocha (Fig 1C).
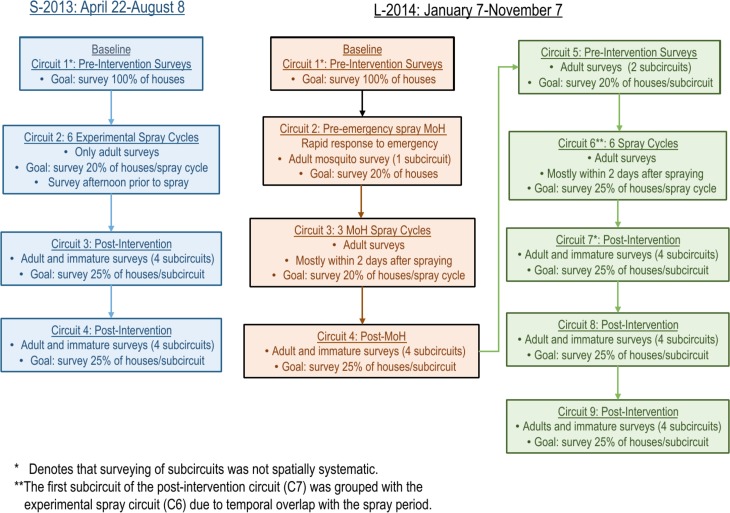
Fig 2. Experiment timeline.
Each box shows one circuit. With one exception (L-2014 C2), each house was visited (and possibly surveyed) at least once per circuit. Except where noted, each circuit consisted of one or more spatially systematic subcircuits. Each subcircuit lasted approximately one calendar week. See S7 Fig. for survey maps.
The larger second experiment (L-2014) ran for 44 calendar weeks, and included 1,051 houses in the surrounding buffer sector and 1,110 houses in the central spray sector (Fig 1B). L-2014 was carried out in a neighborhood several kilometers to the north of S-2013, centrally located in Iquitos, and bordered on the south by an abandoned airstrip (Fig 1C). The L-2014 study area was selected because the Ae. aegypti-free airstrip provided a physical barrier to Ae. aegypti dispersal on one of its four sides. This experimental structure of L-2014 was selected to test our mathematical model’s ability to capture any spatial features of the recovering mosquito population.
Entomological surveys
To monitor population densities and age structure of Ae. aegypti populations, standardized adult mosquito collections were carried out using Prokopack aspirators [40] (henceforth adult surveys) and standardized larval/pupal demographic surveys [41–43] (henceforth immature surveys) were undertaken, except when noted. Survey protocols are described in detail in previous publications [20–22, 35, 40].
Collected adults were immediately transported to a field laboratory in Iquitos for processing as described in Morrison et al. [33]. Adult mosquitoes were sedated by cold (4°C), identified, counted, and females separated. In most cases, female Ae. aegypti were scored as unfed, blood fed (full, half full, or trace amounts), or gravid. Females were also scored for parity [44].
Pyrethroid spray applications
Both the S-2013 and L-2014 experiments consisted of 6 cycles of spray applications, which were applied over 6 weeks. Spraying was done by MoH employees between 17:00–20:00 to avoid high temperatures and varying winds. Each spray team was comprised of 3 individuals: 2 MoH sprayers and 1 monitor from the research team. Each week, on the initial day of a spray cycle (usually Mondays), spraying was attempted in all houses in the spray sector. To improve spray coverage within each cycle, on subsequent days spray teams revisited houses that were not sprayed on the initial day of the spray cycle (a minimum of 2 and up to 10 visits, as needed) to conduct spraying. Pyrethroid insecticides were applied using Solo or Stihl backpack sprayers with settings adjusted for ULV application, or Colt hand-held ULV sprayers. Residents were instructed not to return to their houses for a minimum of 1 hour. See S1 Text for more details.
A MoH emergency intervention interrupted the L-2014 experiment with a set of spray applications (3 cycles over 2 weeks) that were applied similarly to the above experimental sprays. The principal differences were as follows: during the emergency intervention, MoH personnel generally sent an advance team with loudspeakers announcing the arrival of the spray teams; MoH personnel visited each house on a block a single time (they had no mechanism to spray houses missed on their initial visit); and pyrethroids were applied to both the spray and buffer sectors using only Solo or Stihl backpack aspirators.
Quality control for spray applications
As a quality control measure, for each spray cycle, 3 to 7 houses were selected to monitor efficacy of the insecticide spray. Operators did not know which houses would be selected for monitoring. For each monitored house, just after the spray operator had finished the application, a single screen cage containing adult mosquitoes was placed in each of the following locations: bedroom, living room, kitchen, and yard, based on standard WHO protocols [45–47]. Each cage contained 25 adult Ae. aegypti of age 24–36 hours from a pathogen-free laboratory colony [45, 46]. A separate laboratory colony was initiated for each experiment from mosquitoes collected from houses in Iquitos and held for 1–2 generations prior to use. One hour after spraying, all cages were retrieved and evaluated for knockdown (no movement), stored in a styrofoam cooler with moist paper towels for 24 hours, and then examined for mortality. When mortality was <80%, equipment was recalibrated to ensure proper spray function on subsequent days.
Droplet size
Teflon treated slides were placed in 2 randomly selected houses during each spray cycle and retrieved 1-hour post-spray. Droplet size was measured using a micrometer in Motic Images Plus 2.2. Droplets were counted and measured in a 1 cm2 square.
Experimental design
Experimental study sectors are depicted in Fig 1A & Fig 1B. We refer to the temporal sampling units as “circuits” because they were time periods when full survey routes through all of the blocks of houses in the spray and buffer sectors were completed (see Fig 2 for a flow chart of experimental design, and S7 Fig for survey maps). During each circuit, attempts were made to visit and survey 100% of the houses in the entire study area at least once (with one exception, L-2014 C2). The percentage of total houses successfully surveyed and/or sprayed in each circuit ranged from 67–90%, due to closed or unoccupied houses, or residents who chose not to participate in the study (see Fig 3B, S1 Table).
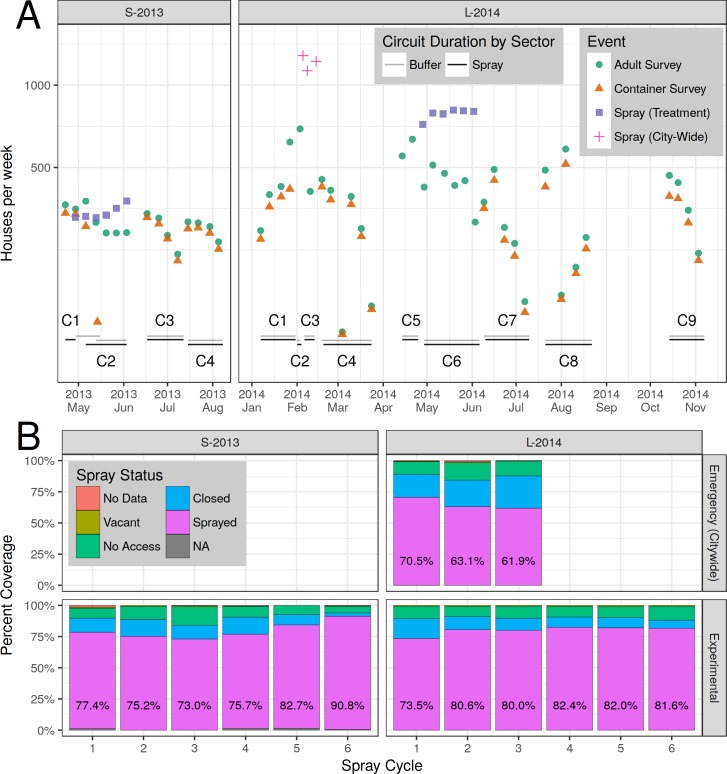
Fig 3. Sampling and spraying.
(A) Number of houses per week sprayed and/or surveyed. Circuits are labeled (e.g., C1), with date ranges shown by horizontal bars. Containers were not surveyed during spray periods. The first two emergency (citywide) spray events (red +) occurred within the same calendar week, but are plotted separately here. (B) Spray coverage by spray cycle. Percent houses sprayed is shown in text. Top row: emergency (citywide) spraying. Bottom row: experimental spraying.
Each circuit was divided into subcircuits that lasted approximately one week, but never more than 10 days. In general, subcircuit surveying was conducted systematically by block, with surveyors attempting to visit every 4th house (25% of the circuit) each week (see S2 Text for exceptions).
Both experiments consisted of 6 weekly cycles of ULV indoor spray applications (see above). Immature and adult surveys were carried out before (pre-intervention) and after (post-intervention) the spraying periods. During the experimental spray periods, only adult surveys were carried out (no immature surveys).
In the baseline pre-intervention circuit of each experiment (C1), 8 study teams surveyed the entire study area (2 people / team), proceeding by block until all houses in the study area were visited at least once (see below for details regarding L-2014). Houses that were not accessible on a day of a visit were revisited the next day and surveyed if open. After all study blocks were surveyed, houses that remained unsurveyed were visited a final time, and surveyed if possible. In subsequent circuits, similar spatially systematic surveying within subcircuits was carried out, and unsurveyed houses were visited a minimum of 3 times per circuit, or until access was obtained or refused.
Experiment 1 (S-2013)
The initial S-2013 baseline pre-intervention circuit (C1) was carried out from 22–29 April 2013 in the spray sector, and from 29 April-16 May 2013 in the buffer sector (C1, Fig 3A). During the experimental treatment circuit (C2), Alphacypermetrin 10% (Turbine 10%) was applied once per week for 6 consecutive weeks using Solo backpack sprayers (Cycles 1–6) or Colt hand-held sprayers (Cycles 4–6). Adult surveys were typically carried out during the spray period on Monday afternoons just prior to the initiation of each spray cycle, as described above. This design, therefore, measured adult densities up to 7 days after a previous spraying event. Post-intervention surveys (C3-C4) were initiated 10 days after completion of the last spray cycle (see S7A Fig and S8A Fig for detailed maps of surveys and sprays, respectively).
Experiment 2 (L-2014)
In contrast to S-2013, the L-2014 study teams worked in two groups during the pre-intervention baseline circuit (C1), with one group in each sector so as to survey both sectors simultaneously. Each group consisted of 4 two-person teams. Following the initial L-2014 baseline, pre-intervention circuit (C1), the experiment was interrupted by a MoH citywide emergency intervention in response to a dengue outbreak (see also S1 Text).
Citywide MoH intervention
In response to information from the MoH about their imminent emergency citywide spraying program (details above), we adapted our study design in 3 ways (see also S2 Text). First, we coordinated with the MoH to conduct adult surveys on a subset of L-2014 houses prior to (~20% of houses, C2) and during the emergency spray period (~20% of houses in each spray cycle, C3). No immature surveys were conducted during these circuits (for details see Fig 3A, S7B Fig and S1 Table). Second, we conducted independent monitoring of the 3 emergency citywide spray cycles (C3), along with standard quality control spraying procedures. We also added an additional circuit subsequent to the MoH emergency citywide intervention that consisted of four spatially systematic subcircuits of full surveys (immature and adults, C4). Third, we added an extra circuit of adult surveys (~25% of houses, C5) that preceded experimental intervention. Subsequent to Circuit 5 (11 weeks after 3 cycles of MoH spraying), we resumed our planned L-2014 experiment (See S7B Fig for a detailed map of survey locations).
The citywide MoH intervention consisted of 3 cycles of indoor cypermethrin 20% (SERPA ciper 20 EW) spray applied between 04:00–09:00 or 17:00–20:00 with Solo backpack sprayers applied to both the spray and buffer sectors (details above).
Experimental spraying
As in S-2013, 6 weekly cycles of ULV spraying were applied (C6). A different pyrethroid insecticide, cypermethrin 20% (ESTOQUE 20 E.C., Tecnologia Quimica y Comercio S.A.) was used. For each cycle, spraying began on Monday evening using Solo backpack sprayers. Attempts were made to spray all accessible houses. Follow-up spraying of houses missed during the first day was carried out Tuesday-Friday between 07:00 and 20:00 using Colt hand-held sprayers (see also S2 Text). In L-2014, adult surveys were typically carried out 1 to 4 days after a house was sprayed.
Data analysis
Unless otherwise noted, only Ae. aegypti data were analyzed, and houses were used as the basic spatial units of observation. A "spray status" indicator variable was assigned to each house survey conducted in the spray sector during experimental spray periods. "Prior spray" indicated that a spray application occurred in a surveyed house prior to the survey during the current or previous calendar week (otherwise, "no prior spray"). During L-2014, the relative timing between spray and subsequent survey was unclear for a limited number of surveys, which were designated as "timing unclear" (S4 and S5 Tables).
Statistical models
For each experiment, a suite of statistical models was developed to estimate the impact of spray treatment on mosquito densities, proportion of infested houses, and population age structure (as determined from parity examination). With one exception, all comparisons and significance tests were conducted within-experiment.
Two generalized linear model (GLM) specifications were employed, both of which used a log link. For all counts, a negative binomial GLM (NB-GLM) was used. Here, the response was the count of mosquitoes per house, and was assumed to follow a negative binomial distribution. The NB-GLM estimates the log of mean counts, and is akin to Poisson regression, while allowing for response over-dispersion (separate mean and variance) [48]. For all proportions, a logistic GLM (L-GLM, i.e., logistic regression) was used. Here, the response was the proportion of successes (out of total number of events), and was assumed to follow a binomial distribution. The choice of “success” was an arbitrary label applied to one of two mutually exclusive possibilities (presence or absence). The L-GLM estimates the log probability of success. For ease of interpretation, all model results were un-transformed after analysis and displayed in the original (unlogged) scale of observations.
To identify structural, pre-perturbation differences between sectors, an NB-GLM was used. This estimated the number of Ae. aegypti adults per house (AA/HSE) in the baseline circuit (C1) in response to physical characteristics of houses, including building, floor, and roof construction, as well as number of containers, rooms, and surveyed rooms.
To assess the effect of spraying, an NB-GLM was employed to estimate AA/HSE in response to circuit and spray sector. In addition, a companion L-GLM was used to predict Adult House Index (AHI: proportion of houses with 1 or more Ae. aegypti adults) in response to circuit and spray sector. Finally, the NB-GLM model formulation was tested with alternate responses: female Ae. aegypti adults per house, and non-Aedes adults per house.
An NB-GLM was also used to estimate the effect of study year and spray status on AA/HSE. This model included only surveys conducted in the spray sector during experimental spray periods.
Counts from immature surveys and parity surveys were converted to proportions: container surveys yielded per-house proportion of positive containers (henceforth called the PrPC), which is also referred to as the container index. Parity surveys yielded the per-house proportion of nulliparous females (henceforth called the PrNF). Each proportional measure (PrPC, PrNF, and PrIH) was analyzed using a pair of L-GLM, weighted by the number of observations, with a separate model for each study year. Predictors included circuit and sector. The response was the log proportion of “successful” events per house, i.e., detection of positive containers or nulliparous females. The container model estimated the log proportion positive containers per house, log(PrPC), and the reproductive status model estimated log proportion nulliparous females per house, log(PrNF). The total number of Ae. aegypti positive containers per house (PC/HSE) was modeled using an NB-GLM. Note that Breteau Index (BI) = 100*(PC/HSE).
To further evaluate the effect of spraying on mosquito densities, contrast analysis [49] was employed on the sector-by-circuit NB-GLM. Contrasts were made between circuits (spray sector only), and between sectors. The between-circuit contrast was complicated by temporal variation, either in extrinsic environmental factors, such as weather, or in intrinsic ecological processes, such as demographic stochasticity. The between-sector contrast was complicated by potential spatial ecological differences between sectors. More robust conclusions can be made if both types of contrasts provide similar assessments of the effect of spraying.
For the statistical models of adult, immature, and parity surveys, statistically indistinguishable groups and 95% confidence intervals (CI) of experimental group effects were estimated using least-squares means, also known as predicted marginal means, via the lsmeans R package [49]. Tukey's method was used to control the family-wise error rate [49].
Results
Overview
During S-2013, 1,860 ULV spray applications (6 weekly cycles) were carried out in 398 houses. During L-2014, 4,986 spray applications (6 weekly cycles) were carried out in 1,110 houses. During the MoH emergency spray campaign that interrupted the L-2014 experiment there were 3,502 spray applications in 1,617 houses over 10 calendar days. A total of 3,843 surveys over 16 weeks and 12,124 surveys over 44 weeks were carried out in S-2013 and L-2014 (including MoH emergency campaign), respectively (Fig 3A, Table 1). Adult Ae. aegypti densities were highly variable over space (S1 Fig) and time (S4 Fig) with highly skewed distributions. No adult mosquitoes were collected from most houses, and large numbers of adults were captured in very few houses (S1 Fig).
Table 1. Summary of Aedes aegypti collected in 2013 and 2014 experiments evaluating ultra-low-volume space sprays in Iquitos, Peru.
Observation counts include houses, surveys*, sampled adult mosquitoes, sampled containers, and adult female dissections** (parity).
| Experiment | Sector | Houses | Surveys | Adult Females | Total Adults | Positive Containers | Total Containers |
Nulliparous Females | Females Dissected |
|---|---|---|---|---|---|---|---|---|---|
| S-2013 | Buffer | 765 | 2,448 | 439 | 904 | 236 | 5,311 | 49 | 406 |
| S-2013 | Spray | 308 | 1,395 | 153 | 354 | 109 | 2,170 | 23 | 142 |
| L-2014 | Buffer | 1,051 | 5,810 | 1,585 | 3,165 | 251 | 6,811 | 81 | 1,444 |
| L-2014 | Spray | 1,110 | 6,314 | 2,092 | 4,244 | 278 | 7,454 | 191 | 2,004 |
*Note that houses were surveyed repeatedly
** Nearly all sampled adult females (column 5) were dissected to determine parity status (columns 9 & 10). See also S1 Table.
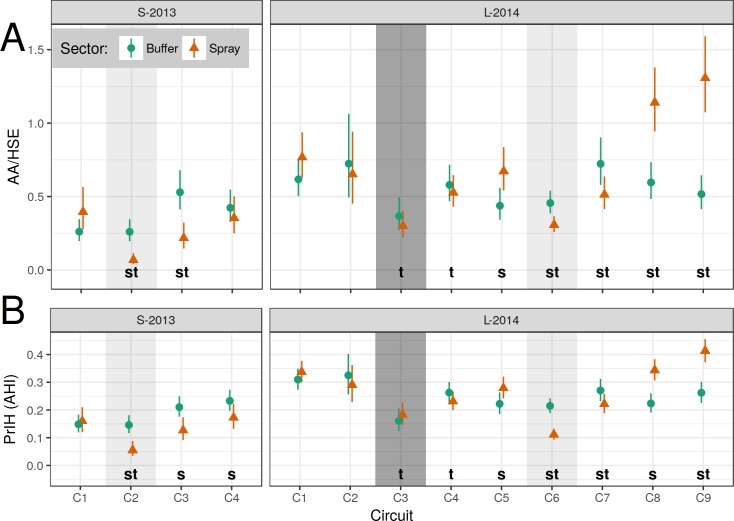
Model results (AA/HSE and AHI) are shown in Fig 4; model contrasts (AA/HSE) are shown in Fig 5; details of adult densities and house indices are shown in S6 and S7 Tables. Overall, adult densities in the S-2013 baseline circuit (early May, C1) were 0.26 and 0.40 Ae. aegypti per house (AA/HSE) in the buffer and spray sectors respectively. During this same baseline circuit, 15% and 16% of houses contained one or more Ae. aegypti adults (AHI) in the buffer and spray sectors, respectively (S7A and S7B Table). The L-2014 baseline circuit (January, C1) showed that Ae. aegypti adult densities were higher than in S-2013: 0.62 and 0.77 AA/HSE in the buffer and spray sectors, respectively. A later pre-intervention circuit in April (C5, prior to experimental spraying) yielded 0.44 and 0.67 AA/HSE in the buffer and spray sectors, respectively. The corresponding AHIs for these surveys were 31% and 34% in the spray and buffer sectors, respectively for January, C1, and 22% and 28% for April, C5.
Fig 4. Model estimates of Ae. aegypti adults per house (AA/HSE, top row) and proportion infested houses (PrIH = AHI).
A separate generalized linear model (GLM) was constructed for each experiment (column) and for each measured response (row). (A) AA/HSE, negative binomial GLM (NB-GLM). (B) PrIH, logistic GLM (L-GLM). Models describe response of measure (row) to time period (X-axis) and treatment sector (color & shape). Shading indicates spray events: experimental spraying (light) and citywide spraying (dark). Vertical bars show 95% CI; non-overlapping CI indicate highly significant difference. Letters (s, t) indicate significant differences between pairwise contrasts: s, between sector (within time, S2 Table); t, between time (within spray sector, relative to baseline C1, S3 Table). See also S6A and S6B Table, S7A and S7B Table, and S5 Fig.
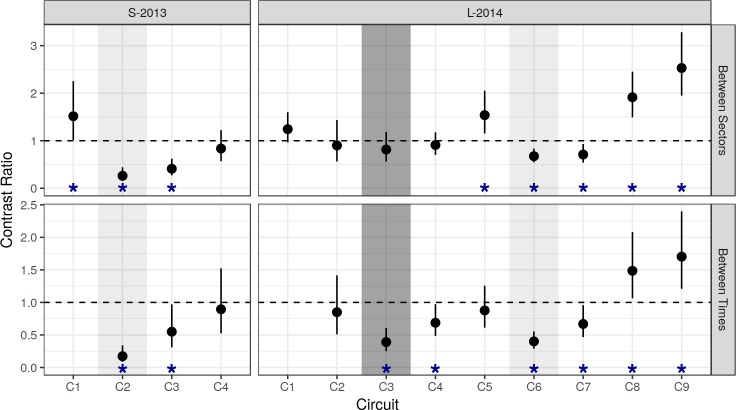
Fig 5. Contrast ratios of AA/HSE, based on NB-GLM.
Results from NB-GLM models (Fig 4A). Top row (between-sector): Spray/Buffer. Bottom row (between-time, within spray sector): contrast relative to baseline (C1). Vertical bars show 95% CI. Horizontal dashed line indicates null hypothesis of equality (H0: ratio = 1). Asterisks (*) indicate significant difference between pairwise contrasts (reject H0).
Adult Ae. aegypti densities and house indices within the spray sector during spray periods were also lower during S-2013 (0.07 AA/HSE; AHI 5.5%) compared to L-2014 (emergency spraying, C3: 0.30 AA/HSE; AHI 18%; experimental spraying, C6: 0.31 AA/HSE; AHI 11%).
In the S-2013 post-intervention circuits (C3-C4), Ae. aegypti adult densities in the spray sector achieved a maximum of 0.35 AA/HSE (AHI 23%). In L-2014 (C7-C9), Ae. aegypti adult densities in the spray sector reached a maximum of 1.31 AA/HSE (AHI 41%).
Meteorological conditions
Meteorological conditions were consistent between the two experiments, with average temperatures of 25.5°C (average minimum = 22.0°C, average maximum = 32.0°C) and 25.6°C (average minimum = 22.0°C, average maximum = 31.9°C) during the S-2013 and L-2014 experiments, respectively (National Climatic Data Center, https://www.ncdc.noaa.gov/cdo-web/). Precipitation during both years was approximately 0.84 cm per day. During the L-2014 entomological surveys for the MoH emergency citywide spray operation (January-March 2014), the temperatures were higher (average 25.9°C, average minimum = 23.3°C, average maximum = 32.6°C) and it was rainier (average 1.09 cm per day) than at other times during the S-2013 and L-2014 experiments.
Baseline surveys
Comparisons of spray and buffer sectors in both experiments indicated that the two sectors had similar housing characteristics. No household physical characteristic was a predictor of adult mosquito density. Consequently, these characteristics were not included in our statistical models. Overall, for both years baseline numbers of Ae. aegypti adults were comparable between spray and buffer sectors (S2 Table). During S-2013, however, a marginally significant difference was found between the buffer and spray sectors during the baseline (C1) circuit (0.26 vs 0.40 AA/HSE, resp.; Fig 5, S2 Table, p = 0.039), making some statistical analyses of spray impacts conservative. During L-2014, baseline densities (C1) were not significantly different between the buffer sector (0.62 AA/HSE, AHI = 31.1%) and spray sector (0.77 AA/HSE, AHI = 33.7%) (Fig 5, S2 Table, p = 0.09). No statistically significant baseline differences in adult female age structure were observed between buffer and spray sectors (PrNF, S8 Table). Baseline immature indices were similarly not different; for example, Breteau Indices (BI = 100 * PC/HSE) ranged from 9.4–10 in the buffer and spray sectors in both experiments (S9 Table). Container indices (i.e., percentage of water-holding containers infested with larvae or pupae, 100*PrPC) ranged from 3.9–4.1 in S-2013 and 3.1–3.3 in L-2014 (S10 Table).
Spray coverage
The average percent of houses sprayed was lowest during the 3 MoH citywide emergency spray cycles in L-2014, ranging from 71% during cycle 1 to 62% in cycle 3 (Fig 3B). For S-2013, coverage started at 77% in cycle 1, decreased to 73% in cycle 3, and then improving in each subsequent cycle to 90% (cycle 6). During L-2014 experimental spraying, coverage started at 74% in cycle 1, then modestly increased over time to approximately 82% in cycle 6 (Fig 3B).
In both experiments, most spray sector houses were sprayed in more than 3 out of 6 spray cycles, and more than half of target houses were sprayed in all 6 spray cycles (S2 Fig). The primary reasons for not spraying a house were: house closed when personnel visited (3–16% for S-2013 spray, 19–28% for L-2014 MoH emergency spray, 7–16% for L-2014 spray), or residents did not allow access to the house (6–14% for S-2013 spray, 9–11% for emergency spray, 8–11% for L-2014 spray). During the S-2013 experiment, but not in L-2014, the reasons given by residents for refusing access were recorded. In many cases, teams were allowed access on subsequent visits. In early cycles, about one-third of the refusals cited a direct objection to fumigation, saying they did not believe it was effective or that the teams were not really using insecticide. In other cases, the reason given was inconvenience to the residents: eating, bathing, working, selling food, or that a sick person or newborn was in the house and could not leave. In some instances, the homeowner was not present and consent could not be given.
Spray efficacy
During S-2013, 24-hour mortality of caged sentinel mosquitoes ranged from 87–97% with some variation across cycles (S3 Fig). Mean mortality was lower in L-2014, ranging from 53–87%. Overall, a significant decrease in spray efficacy was observed in L-2014 relative to S-2013 (Table 2). During S-2013, Colt hand-held ULV sprayers were used on 1/3rd of the blocks during spray cycles 4–6. Higher mortality and knockdown were observed in cycles 4–6, while less variation was observed in cycles 1–3, which employed only backpack sprayers.
Table 2. Generalized linear mixed model (L-GLMM) results: Ae. aegypti control cage mortality and knockdown.
| Experiment | Assay | nObs | Group | Est | SE | 95% CI |
|---|---|---|---|---|---|---|
| S-2013 | Kill (24 hours) | 112 | A | 0.94 | 0.01 | 0.90–0.96 |
| L-2014 | Kill (24 hours) | 76 | B | 0.75 | 0.05 | 0.62–0.84 |
| S-2013 | Knockdown (1 hour) | 112 | A | 0.94 | 0.02 | 0.86–0.97 |
| L-2014 | Knockdown (1 hour) | 76 | B | 0.65 | 0.10 | 0.41–0.83 |
L-GLMM results, fit for each assay, show a significant decrease in spray efficacy in L-2014. Experiment is a fixed effect. Spray cycle and house are nested random effects. Each cage contains 25 mosquitoes taken from a field-derived colony (one colony per experiment). Group: significance groups (Tukey HSD). See also S3 Fig.
Droplet size (mean±SD) varied between experiments and sprayer type. Colt sprayers had smaller and more consistent droplets (19.1±12.6 μm) than backpack sprayers (29.2±19.5 μm). During the L-2014 MoH emergency spray, backpack sprayers were not properly calibrated during the initial cycle, with an average droplet size of 39.8±25.8 μm. This improved to 20.6±14.1 μm in subsequent cycles. During the L-2014 6-cycle experiment, droplet size averaged 18.1±14.7 μm and 23.6±13.2 μm for Colt and backpack sprayers, respectively.
Experiment 1 (S-2013)
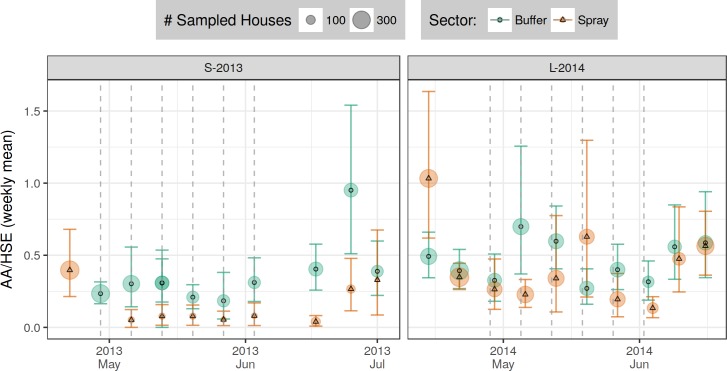
Surveys conducted during the 6-week spray period (C2) generally occurred about one week after spraying. During the spray period, ULV spraying reduced adult Ae. aegypti population densities rapidly and significantly from 0.40 to 0.07 AA/HSE after six cycles of spraying (Fig 4), yielding an 82.5% reduction relative to baseline (Fig 5, S3 Table, p<0.00001). The buffer sector, in contrast, had 0.26 AA/HSE both before (C1) and during (C2) the spray period.
Adult densities in the sprayed sector were 73.1% lower than in the buffer sector during the spray period (C2, Fig 5, S2 Table, p<0.00001). Ongoing surveys within the spray sector during the spray period ranged from 0.04–0.08 AA/HSE, and did not change significantly over the course of the six sprays (Fig 6). Spray sector AA/HSE remained 45% lower than baseline levels during the first post-intervention period (C3, Fig 5, S3 Table, p = 0.035), but densities increased from 0.04 to 0.27 AA/HSE between the first and second week post-spray. During the second post-intervention period (C4), spray sector adult densities returned close to baseline densities, increasing from 0.22 to 0.35 AA/HSE (S6A Table) which was 89% of baseline (Fig 5, S3 Table, p = 0.94) and 83.3% of the buffer sector density at that time (Fig 5, S2 Table, p = 0.36).
Fig 6. Detailed time series of AA/HSE response to ULV spraying, aggregated by week.
X-axis shows week start date. Color and symbol shape shows sector (orange triangle: spray sector). Point size shows number of surveyed houses. Vertical dashed lines show approximate dates of experimental spraying (spray sector only). Vertical colored bars show bootstrap 95% CI (1e+04 draws per circuit).
Adult house indices in the spray sector, by comparison, decreased from 16% during baseline surveys to 5.5% during the spray period (C2), then increased to 12.7% and 17.3% during the first and second post-intervention periods, respectively (C3-C4, S7A Table). In the buffer sector, AHIs were 15% during both baseline and spray periods, then increased to 21% and 23% in the first and second post-intervention evaluations (S7A Table).
During the S-2013 spray period (C2), only a small number of females (9 total) were collected in the spray sector (S8A Table). Therefore, no attempt was made to compare the age structure of Ae. aegypti populations before and after spray applications for this experiment. Model estimates of the proportion of nulliparous females (PrNF) showed accordingly high uncertainty (S5B Fig and S8A Table).
Results from pupal demographic surveys followed a pattern similar to that of adult house indices. Baseline BIs were 10.0 in both the buffer and control sectors (S9A Table). BIs were not measured during the spray period; during the first post-intervention period (C3), however, BI decreased slightly in the spray sector to 7.4 and increased to 16.1 in the buffer sector. During the second post-intervention period (C4), BIs were 15.1 and 22.3 in the buffer and spray sector, respectively. The post-treatment spray sector had statistically significantly higher PrPC than any other sector or time period (S5B Fig, S10A Table). In the spray sector during C4, PrPC reached approximately 0.11, significantly higher than seen in the spray sector during the baseline C1 (0.04) or in the buffer sector during C4 (0.06).
Experiment 2 (L-2014)
MoH citywide emergency spray
MoH ULV spray applications were carried out in both L-2014 sectors (spray, buffer) prior to initiation of L-2014 experimental spraying. In the baseline circuit (C1), AA/HSE ranged from 0.62–0.77 (S6B Table), and AHI ranged from 31–34% (S7B Table). During the subsequent citywide emergency spray period, AA/HSE decreased to 0.37 (AHI 16%) in the buffer sector and 0.30 AA/HSE (AHI 18%) in the spray sector, thus showing a modest 40–50% reduction in adult densities relative to the C1 baseline (Fig 5, S3 Table, p<0.0001). Ae. aegypti densities in the geographically central spray sector were more variable than for houses in the surrounding buffer sector. Ae. aegypti densities did show some recovery in the post-emergency circuit (C4), rising from 0.37 to 0.58 AA/HSE in the buffer sector and from 0.30 to 0.53 AA/HSE in the spray sector. There was also a small trend toward an increase in the proportion of nulliparous females (PrNF) between baseline and the emergency spray period, from 0.03 to 0.10 and from 0.07 to 0.11 in the buffer and spray sectors, respectively (C1 to C3, S8B Table).
Immature indices, which were measured at baseline (C1) and the post-emergency survey (C4), were similar over time. For example, the baseline spray sector BI (10.0) was not statistically different than in post-intervention surveys (6.3–11.9, S9B Table). The proportion of positive containers (PrPc) ranged from 0.4–0.5 across the baseline and post-emergency circuits (C1 and C4, S10B Table).
Experimental spray
For our experimental evaluation, a circuit of pre-intervention adult surveys was carried out during April (C5) before initiating 6 cycles of ULV spray applications. In both spray and buffer sectors, adult densities were consistent with the January baseline surveys (Fig 5, S3 Table p = 0.95). During C5, however, there were significantly higher adult densities in the spray sector (0.67 AA/HSE) relative to the buffer sector (0.44 AA/HSE) (Fig 5, S2 Table, p = 0.0034). During the experimental spray period (C6), AA/HSE decreased significantly in the spray sector (from 0.67 to 0.31) compared to the unsprayed buffer sector (from 0.44 to 0.46, S6B Table). In addition, AHI decreased significantly in the spray sector (from 28% to 11%) compared to the unsprayed buffer sector (from 22% to 21%, S7B Table).
Adult densities rebounded quickly after cessation of spraying (C7, Fig 4, Fig 6, S6B Table). AA/HSE increased from 0.31 during the spray period (C6) to 0.51 post-spray (C7). The latter (C7) was not statistically significantly different from the January baseline of 0.77 (C1) or from that of the April pre-intervention survey (C5, 0.67). During the L-2014 post-spray monitoring period (C7-C9), increases in adult densities were observed in the spray sector, with a 170% increase above January (C1) baseline levels in the final circuit (C9, S3 Table). In the buffer sector, from C6 to C9, AHI ranged from a low of 21% during the spray period (C6) to a high of 27% (C7). In contrast, in the spray sector, AHI increased during each post-intervention survey, ranging from 11% during the spray period (C6) to 41% during the final post-intervention period (C9) (Table 7B). Adult densities during the first post-intervention circuit (C7) remained significantly lower than baseline (C1) levels (Fig 5, S3 Table, p = 0.017). In C8-C9, however, densities were significantly higher than baseline levels (S3 Table, p≤0.01). When comparing the buffer and spray sector, a similar pattern was observed. Adult densities during C7 remained significantly lower in the spray sector compared to the buffer sector. During C8 and C9, however, the spray sector had significantly more adult Ae. aegypti than the buffer sector (Fig 5, S2 Table).
A strong effect of experimental spraying on parity was observed. During the spray period (C6), the proportion of youngest (nulliparous) females (PrNF) was significantly higher in the spray sector than in the buffer sector. Likewise, an approximate doubling of PrNF in the spray sector relative to baseline was observed (S8B Table).
Immature indices increased between the post-emergency spray survey (C4) and first post-experimental study survey (C7). For example, BI increased from 7.9 to 15.0 and from 8.3 to 11.9 in the buffer and spray sectors, respectively (S9B Table). Between the first and second post intervention surveys (C7-C8), BI dropped to 5.9 and 6.3 in the buffer and spray sectors, respectively. Two months later (C9), the BI decreased to 4.4 in the buffer sector and increased to 7.6 in the spray sector. Similar patterns were seen for the proportion of containers with immatures (S10B Table).
Comparison of sprayed and unsprayed houses
During the S-2013 experiment, entomological surveys were carried out during the afternoon before each ULV spray cycle was initiated. For the majority of S-2013 house surveys with a prior spray application, Ae. aegypti densities were measured 7 days after the previous spraying, and 308 out of 311 surveys with prior sprays occurred from 5 to 8 days after the spray application. In contrast, L-2014 house surveys with prior sprays were typically conducted 1 to 4 days after the surveyed house's prior spray. This difference was the result of logistical concerns, as L-2014 involved many more houses. For 164 of the 1,054 house surveys with prior sprays during L-2014 (16%), the exact timing of the spray event relative to the subsequent survey was not available. In addition, some houses within the spray sector were surveyed without a prior spray (S5 Table). Thus, the average interval between a house's spray application and subsequent survey was shorter in L-2014 than in S-2013 (median 2 days and 7 days, respectively). In both experiments, AA/HSE were lower in spray sector houses that had been sprayed prior to surveying compared to those that had not. In S-2013, AA/HSE was 0.06 and 0.11 in houses with prior spray and no prior spray, respectively, while L-2014 experienced 0.28 and 0.56 AA/HSE in houses with prior spray and no prior spray, respectively (S5 Table). A (marginally) significant difference in AA/HSE between spray status groups was observed only during 2014 (S4 Table, p = 0.047).
Discussion
Despite the lack of a well-informed evidence base [18], vector control of Ae. aegypti is often described as ineffective yet continues to be widely practiced by public health programs [11, 18, 50–52]. Increasing attention has been given to integrated vector management, community involvement, and sustainability [53]. There is increasing recognition, however, that programs lacking interventions specifically directed at adult mosquitoes are insufficient for suppression of dengue and other Aedes-borne diseases [10, 54]. A WHO dengue Scientific Working Group identified “analysis of the factors that contribute to the success or failures of national programs in the context of dengue surveillance and outbreak management”, including vector control, as a priority topic for future research [55].
Through two large-scale experimental studies and an assessment of a MoH emergency intervention campaign, our study evaluated an adulticiding strategy that is embedded in some national Aedes-transmitted virus control programs. We observed a clear Ae. aegypti population reduction during the extended period of repeated spray applications. These reductions were, however, not sustained after cessation of spraying.
Our study design could not logistically include randomized replicates [30, 51, 56] because we focused on monitoring spraying in large neighborhoods of houses. A review of previous Ae. aegypti space spray studies [4] shows that each replicate included 50 or fewer houses so that movement of adults from surrounding houses could have impacted results. In contrast, we monitored spraying in large numbers of houses: more than 1,100 houses (up to 2,100 houses) during the two experimental interventions, and a MoH citywide emergency spray program. Our experimental design reduced the potential impact of adults moving into the sprayed sector from unsprayed locations. In the citywide spraying, all areas of the city were expected to have about the same decrease in Ae. aegypti densities so adult movement should not have impacted the recovery at all. There is clearly a tradeoff between degree of replication possible and the size of experimental units.
In order to maintain study quality, our experimental interventions were supervised by trained entomologists. Our monitoring of the impacts of the L-2014 citywide emergency spraying provides a realistic and complimentary effectiveness assessment under practical, public health circumstances. It is also important to note that our study was primarily designed to provide data that could then be used to evaluate a computer simulation model [32] under extreme perturbation conditions, which was a major reason for evaluating a single centralized spray sector surrounded by a buffer sector. If viewed as an evaluation of a vector control measure, the design of our study has some important limitations: relatively short pre-intervention surveys, lack of randomization of intervention areas, and the absence of replicates in time. Cluster randomized trials (CRTs) are considered the gold standard for efficacy trials [30, 57]. Yet properly powered studies are large, expensive, and logistically challenging, especially when measuring an impact on disease. When CRTs are not possible, large, carefully monitored studies such as ours can reassure public health agencies that employ emergency indoor space spraying programs. In addition, studies such as this one can provide target coverage goals and help manage expectations about the real-world impacts of spraying. Furthermore, studies of similar rigor are needed to evaluate the more commonly employed outdoor space spray campaigns.
The effectiveness of pyrethroid applications varied between years, but was similar between citywide emergency sprays and experimental sprays in 2014. Interestingly, in all experiments adult Ae. aegypti densities decreased significantly after the first cycle of spraying then fluctuated at relatively low levels during the remaining spray cycles: that is, additional cycles did not lower mosquito densities further. In all three interventions, adult populations partially recovered within 2 weeks of spray cessation. The pattern of rapid recovery of the Ae. aegypti population in our study is consistent with several previous reports [5]. Studies by Perich et al. [13, 14] in Honduras and Costa Rica showed an approximately 90 percent reduction in adults one week after spraying, but the effect of the treatment was no longer significant after 6–7 weeks.
In the two experimental suppression trials we could not definitively determine if recovery of population densities was from adults migrating in from the surrounding buffer sector and/or from new adults emerging from development sites within the spray sector. However, in the emergency citywide spraying, the recovery was similar to that in the experimental trials. This suggests that movement of adults was not the key factor. Mosquito densities after the L-2014 experimental spray were monitored for a longer period of time: 23-weeks post-spray in L-2014 versus 9-weeks post-spray in S-2013. During L-2014, the density of adults in the spray sector increased to well above that in the buffer. In L-2014, ULV spraying resulted in a higher proportion of nulliparous females, indicating a shift to a younger adult female age distribution. This indicates that the spray sector continued to have active larval habitats that were producing new Ae. aegypti adults. In S-2013, for example, 22 Ae. aegypti positive containers were identified in a single house during a post intervention survey, whereas the baseline survey of that house revealed only three containers total, of which only one was positive. This kind of variation illustrates the stochastic and dynamic nature of Ae. aegypti larval habitats [20, 21]. The dramatic L-2014 post-treatment increase cannot, however, be explained by an outlier in the form of a “superproductive” household [33]. One possibility is compensation by the immature population due to a reduction in larval population densities, which led to reduced density dependent competition within containers and increased survival to adult emergence. This kind of rebound effect merits further investigation.
In L-2014, both emergency and experimental spraying had significant, but lesser impact on the adult densities than in S-2013, even though L-2014 spray sector surveys with prior sprays were conducted (on average) fewer days after spray applications. The L-2014 24-hour mortality of caged sentinel mosquitoes was lower than in S-2013, something that could be due to characteristics of the different insecticide used, changes in pyrethroid resistance levels in Iquitos mosquito populations between S-2013 and L-2014, and/or differences in spray quality between the two experiments. By the end of 2014, significant pyrethroid resistance was detected in Iquitos [39]. Although we did not detect pyrethroid resistance before the S-2013 experiment, we do not have similar assay information from populations evaluated just prior to the L-2014 experiment. It is possible, therefore, that the lower efficacy observed in the L-2014 experiment was due in part to resistance in the local Ae. aegypti population. By 2015 the MoH had abandoned use of pyrethroid insecticides for indoor spraying and switched to malathion in an effort to improve efficacy.
A strong argument can be made that logistical challenges associated with application of ULV spray over a larger sector in the L-2014 experiment contributed to lower efficacy. First, Colt hand-held sprayers were only used in L-2014 when initially unsprayed houses were revisited, whereas in S-2013 they were used on at least 33% of the houses. Colt sprayers had significantly better and consistent droplet sizes than backpack sprayers. The L-2014 experiment was a much larger effort with at least double the number of backpack machines and MoH fumigators participating, and droplets were only evaluated on a fraction of the machines used. In addition, during the L-2014 experiment coverage rates were lower overall.
Our results demonstrate that intensive, carefully administered space spraying can temporarily decrease the number and average age of female Ae. aegypti in houses. These results support smaller scale studies showing space spray induced reductions in Ae. aegypti density [12–15]. When, where, and how ULV mosquito control leads to meaningful reductions in disease remains a critical unanswered public health problem for policy makers. Computer simulation models have been employed to inform outcomes in limited situations, such as pathogen strain invasions (e.g. Newton and Reiter [58]). Certain tentative recommendations, however, can be made based on existing data. Emergency indoor ULV spray interventions have the potential to mitigate Ae. aegypti-transmitted viruses, but coverage must be maximized with multiple spray cycles per house; i.e., at least 3 spray cycles based on our experience in Iquitos [27]. Officials should have no expectations of sustained reductions in mosquito densities and must recognize that these sprays only have the potential to mitigate the immediate impact of an arbovirus outbreak. Quality control of spraying efforts and insecticide resistance testing must be an integrated component of national programs. Although these are not new messages [47, 59], our study adds new data to the vector control evidence base that we hope will better inform intervention programs and, thus, help refine policy for the application of space spray as a public health response to Ae. aegypti-transmitted viruses.
Supporting information
(PDF)
Rows show treatment sector. X-axis is sqrt-scaled. The majority of house surveys find no adults.
(PDF)
Summary of spray coverage in S-2013 (A) and L-2014 (B). In both years, most houses were sprayed in at least 5 out of 6 spray cycles, while a small number of houses were never sprayed. In L-2014, experimental spray coverage was much higher than emergency (citywide) spray coverage.
(PDF)
Insects were from a laboratory colony (one colony per year).
(PDF)
X-axis shows week start date. Color and line-type shows treatment sector (orange triangle: spray sector). Point size shows number of surveyed houses. Vertical lines show approximately spray dates: dashed, experimental spraying (spray sector only); dotted, citywide spraying (February 2014, all sectors). Vertical colored bars show bootstrap 95% CI (1e+04 draws per circuit). (A) Adult surveys. (B) Container (PrPC) and parity (PrNF) surveys.
(PDF)
All models include fixed effects of sector and circuit, with a separate model for each year. (A) Counts: negative binomial GLM (NB-GLM). (B) Proportions: logistic GLM (L-GLM). Note that Breteau Index (BI) = 100*PC/HSE. See also S2–S10 Tables.
(PDF)
Note the scale differs between experiments. See also Fig 1.
(PDF)
(A) S-2013. (B) L-2014.
(PDF)
(A) S-2013. (B) L-2014. During L-2014, in addition to experimental spraying, 3 cycles of emergency citywide spraying were conducted. Note the map scale differs between A and B. See also Fig 1.
(PDF)
Weeks: Week number from experiment start. Houses: number of unique houses surveyed. Surveys: total surveys (either adult, or combined adults and immature). Full surveys: surveys where both adult and immatures were surveyed. Buffer, Spray: surveys in buffer and spray sector, respectively.
(PDF)
Ratio of AA/HSE in spray sector relative to buffer sector (spray/buffer). Bold p-values: significant difference between sectors. In both years, the spray sector starts with more adults per house, and spraying reduces AA/HSE relative to buffer sectors. As in S3 Table, the effects of spraying are more pronounced in S-2013. See also Fig 4A.
(PDF)
Ratio of AA/HSE relative to baseline (C1, spray sector only). Bold p-values: significant difference from baseline circuit. In both years, spraying reduces AA/HSE relative to baseline (C1). The effects of spraying are most pronounced in S-2013, but are short-lived in both years. See also Fig 4A.
(PDF)
Ratio of AA/HSE in houses that were either sprayed or not sprayed prior to surveying (no prior spray/prior spray). Bold p-values: In L-2014, houses without prior spraying yielded significantly more adults than houses with prior spraying. In S-2013, most houses were sprayed in the prior week. In L-2014, the exact date of spraying was uncertain for a small number of houses. See S5 Table for details.
(PDF)
A single model (NB-GLM) includes both experiment year and spray status as predictors. Group: significance groups (Tukey HSD) compare among all rows. Only house surveys in the spray sector during experimental spraying are included (i.e., S-2013 C2 and L-2014 C6). Not all sprayed houses were subsequently surveyed. The average interval between each house's spray application and survey was shorter in L-2014 (median 2 days) than in S-2013 (median 7 days). See also S4 Table.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors; significance groups (Tukey HSD) compare among all rows. See Fig 4A for model description.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See Fig 4B for model description.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See S5 Fig.
(PDF)
(A) S-2013. (B) L-2014. Note that Breteau Index (BI) = 100*PC/HSE. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See S5 Fig.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. No container surveys were conducted during spraying See also S5 Fig.
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Brandon Hollingsworth, Jaye Sudweeks, Sumit Dhole, and Jennifer Baltzegar for helpful discussion. We are grateful to the Ministerio de Agricultura y Riego de Perú, Dirección General Forestal y de Fauna Silvestre for permission to conduct these studies under the auspices of Resolución Directoral No. 0406-2013-MINAGRI-DGFFS/DGEFFS. We thank the residents of Iquitos, Peru for allowing us to undertake this study in and around their homes. We greatly appreciate support of the Loreto Regional Health Department including Dra. Wilma Casanova and Cristiam Carey, who all facilitated our work in Iquitos. Gerson Perez Rodriguez supervised the collection and processing of mosquitoes. Entomological surveys were carried out by Jhon Bardales Cardenas, Cesar Campos Cardenas, Jimmy Maykol Castillo Pizango,Willy Chavez, Fernando Chota Ruiz, Guillermo Elespuru Hidalgo, Victor Elespuru Hidalgo, Fernando Espinoza Benavides, Rusbel Huinapi Tamani, Guillermo Inapi Huaman, Nestor Jose Nonato Lancha, Federico Reategui Viena, Edson Pilco Mermao, Angel Puertas Lozano, Juan Luiz Sifuentes Rios, Manuel Ruiz Rioja, and Abner Enrique Varzallo Lachi. Jimmy Roberto Espinoza Benavides carried out data entry. We thank Gabriela Vasquez de la Torre, Lorena Quiroz, Alfonso Vizcarra, Esther Jennifer Rios, and Jhonny Cordova Lopez, for their support in community engagement, project execution and monitoring of MoH space sprays. Drs. Robert Hontz, Christopher Mores, Frederick Stell, Craig Stoops, Diego Munoz, Cecilia Gonzales, Kyle Peterson, Adam Armstrong, Guillermo Pimentel, Zoe Moran, Toane Zuleta and Ms. Roxana Lescano of the U.S. Naval Medical Research Unit No. 6 in Lima, Peru were instrumental in facilitating these studies.
Disclaimer
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Copyright statement
Some authors of this manuscript are military service members and employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this Title is not available for any work of the United States Government”. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Data Availability
All data are available through DRYAD at https://doi.org/10.5061/dryad.160023v.
Funding Statement
This research was funded by the National Institutes of Health (NIH) grant R01-AI091980, the W. M. Keck Foundation, and the National Science Foundation (RTG/DMS - 1246991 and NSF-IGERT - 1068676). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. doi: 10.1038/nature12060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760 doi: 10.1371/journal.pntd.0001760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilder-Smith A, Byass P. The elusive global burden of dengue. Lancet Infect Dis. 2016;16(6):629–31. doi: 10.1016/S1473-3099(16)00076-1 . [DOI] [PubMed] [Google Scholar]
- 4.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15(5):619–31. doi: 10.1111/j.1365-3156.2010.02489.x . [DOI] [PubMed] [Google Scholar]
- 5.Horstick O, Runge-Ranzinger S, Nathan MB, Kroeger A. Dengue vector-control services: how do they work? A systematic literature review and country case studies. Trans R Soc Trop Med Hyg. 2010;104(6):379–86. doi: 10.1016/j.trstmh.2009.07.027 . [DOI] [PubMed] [Google Scholar]
- 6.Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am J Trop Med Hyg. 1997;57(2):235–9. doi: 10.4269/ajtmh.1997.57.235 . [DOI] [PubMed] [Google Scholar]
- 7.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman D, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37(1):89–101. doi: 10.1603/0022-2585-37.1.89 . [DOI] [PubMed] [Google Scholar]
- 8.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28(3):114–21. doi: 10.1016/j.pt.2012.01.001 . [DOI] [PubMed] [Google Scholar]
- 9.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17(2):321–35. doi: 10.1093/oxfordjournals.epirev.a036196 . [DOI] [PubMed] [Google Scholar]
- 10.Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5(3):e68 doi: 10.1371/journal.pmed.0050068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter P. Surveillance and Control of Urban Dengue Vectors In: Gubler DJ, Ooi EE, Vasudevan SG, Farrar J, editors. Dengue and dengue hemorrhagic fever. 2nd edition. ed Wallingford, Oxfordshire; Boston, MA: C.A.B. International; 2014. p. 484–521. [Google Scholar]
- 12.Perich MJ, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J Med Entomol. 2000;37(4):541–6. doi: 10.1603/0022-2585-37.4.541 . [DOI] [PubMed] [Google Scholar]
- 13.Perich MJ, Rocha NO, Castro AL, Alfaro AW, Platt KB, Solano T, et al. Evaluation of the efficacy of lambda-cyhalothrin applied by three spray application methods for emergency control of Aedes aegypti in Costa Rica. J Am Mosq Control Assoc. 2003;19(1):58–62. . [PubMed] [Google Scholar]
- 14.Perich MJ, Sherman C, Burge R, Gill E, Quintana M, Wirtz RA. Evaluation of the efficacy of lambda-cyhalothrin applied as ultra-low volume and thermal fog for emergency control of Aedes aegypti in Honduras. J Am Mosq Control Assoc. 2001;17(4):221–4. . [PubMed] [Google Scholar]
- 15.Koenraadt CJ, Aldstadt J, Kijchalao U, Kengluecha A, Jones JW, Scott TW. Spatial and temporal patterns in the recovery of Aedes aegypti (Diptera: Culicidae) populations after insecticide treatment. J Med Entomol. 2007;44(1):65–71. doi: 10.1093/jmedent/41.5.65 . [DOI] [PubMed] [Google Scholar]
- 16.Pant CP, Mathis HL, Nelson MJ, Phanthumachinda B. A large-scale field trial of ultra-low-volume fenitrothion applied by a portable mist blower for the control of Aedes aegypti. Bull World Health Organ. 1974;51(4):409–15. . [PMC free article] [PubMed] [Google Scholar]
- 17.Castro M, Quintana N, Quinones PM. Evaluating two pyrethroids in dengue vector control in Putumayo, Colombia. Rev Salud Publica (Bogota). 2007;9(1):106–16. . [DOI] [PubMed] [Google Scholar]
- 18.Bowman LR, Donegan S, McCall PJ. Is Dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10(3):e0004551 doi: 10.1371/journal.pntd.0004551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Regional Office for South-East Asia. Comprehensive guidelines for prevention and control of dengue and dengue haemorrhagic fever. Revised and expanded edition. 2011. Available from: http://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEAROTPS60/en/ [Google Scholar]
- 20.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69(5):494–505. doi: 10.4269/ajtmh.2003.69.494 . [PubMed] [Google Scholar]
- 21.LaCon G, Morrison AC, Astete H, Stoddard ST, Paz-Soldan VA, Elder JP, et al. Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8(8):e3038 doi: 10.1371/journal.pntd.0003038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison AC, Astete H, Chapilliquen F, Ramirez-Prada C, Diaz G, Getis A, et al. Evaluation of a sampling methodology for rapid assessment of Aedes aegypti infestation levels in Iquitos, Peru. J Med Entomol. 2004;41(3):502–10. doi: 10.1603/0022-2585-41.3.502 . [DOI] [PubMed] [Google Scholar]
- 23.Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4(8):e787 doi: 10.1371/journal.pntd.0000787 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison AC, Minnick SL, Rocha C, Forshey BM, Stoddard ST, Getis A, et al. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4(5):e670 doi: 10.1371/journal.pntd.0000670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha C, Morrison AC, Forshey BM, Blair PJ, Olson JG, Stancil JD, et al. Comparison of two active surveillance programs for the detection of clinical dengue cases in Iquitos, Peru. Am J Trop Med Hyg. 2009;80(4):656–60. doi: 10.4269/ajtmh.2009.80.656 . [PubMed] [Google Scholar]
- 26.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110(3):994–9. doi: 10.1073/pnas.1213349110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoddard ST, Wearing HJ, Reiner RC Jr., Morrison AC, Astete H, Vilcarromero S, et al. Long-term and seasonal dynamics of dengue in Iquitos, Peru. PLoS Negl Trop Dis. 2014;8(7):e3003 doi: 10.1371/journal.pntd.0003003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison AC, Astete H, Rocha C, Rodriguez H, Sifuentes GA, Alava F, et al. Evaluation of Emergency Vector Control Measures during a Dengue Epidemic in Iquitos, Peru 2002–2003. Am J Trop Med Hyg. 2003;69(S3):539 doi: 10.4269/ajtmh.2003.69.392 [Google Scholar]
- 29.Morrison A, Astete H, Rocha C, Lopez V, Olson J, Kochel T, et al. Impact of the dengue vector control system (DVCS) on Aedes aegypti populations in Iquitos, Peru 2004–2005. Am J Trop Med Hyg. 2005;73(S6):326 doi: 10.4269/ajtmh.2005.73.30116103599 [Google Scholar]
- 30.Reiner RC Jr., Achee N, Barrera R, Burkot TR, Chadee DD, Devine GJ, et al. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl Trop Dis. 2016;10(5):e0004588 doi: 10.1371/journal.pntd.0004588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legros M, Magori K, Morrison AC, Xu C, Scott TW, Lloyd AL, et al. Evaluation of location-specific predictions by a detailed simulation model of Aedes aegypti populations. PLoS One. 2011;6(7):e22701 doi: 10.1371/journal.pone.0022701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, et al. Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis. 2009;3(9):e508 doi: 10.1371/journal.pntd.0000508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, Focks D, et al. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41(6):1123–42. doi: 10.1603/0022-2585-41.6.1123 . [DOI] [PubMed] [Google Scholar]
- 34.Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, et al. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100 Suppl 1:S73–S86. doi: 10.1179/136485906X105534 . [DOI] [PubMed] [Google Scholar]
- 35.Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41(4):634–42. doi: 10.1603/0022-2585-41.4.634 . [DOI] [PubMed] [Google Scholar]
- 36.Hayes CG, Phillips IA, Callahan JD, Griebenow WF, Hyams KC, Wu SJ, et al. The epidemiology of dengue virus infection among urban, jungle, and rural populations in the Amazon region of Peru. Am J Trop Med Hyg. 1996;55(4):459–63. doi: 10.4269/ajtmh.1996.55.459 . [DOI] [PubMed] [Google Scholar]
- 37.Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, et al. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354(9188):1431–4. doi: 10.1016/S0140-6736(99)04015-5 . [DOI] [PubMed] [Google Scholar]
- 38.Paz-Soldan VA, Plasai V, Morrison AC, Rios-Lopez EJ, Guedez-Gonzales S, Grieco JP, et al. Initial assessment of the acceptability of a Push-Pull Aedes aegypti control strategy in Iquitos, Peru and Kanchanaburi, Thailand. Am J Trop Med Hyg. 2011;84(2):208–17. doi: 10.4269/ajtmh.2011.09-0615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palomino-Salcedo M. Estado de susceptibilidad de la población natural de Aedes aegypti a los insecticidas en Punchana-Iquitos, Región Loreto (Novimbre 2014) Instituto Nacional de Salud, Peru, 2014. [Google Scholar]
- 40.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol. 2009;46(6):1256–9. doi: 10.1603/033.046.0602 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000;62(1):11–8. doi: 10.4269/ajtmh.2000.62.11 . [PubMed] [Google Scholar]
- 42.Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997;56(2):159–67. doi: 10.4269/ajtmh.1997.56.159 . [DOI] [PubMed] [Google Scholar]
- 43.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): simulation results and validation. J Med Entomol. 1993;30(6):1018–28. doi: 10.1093/jmedent/30.6.1018 . [DOI] [PubMed] [Google Scholar]
- 44.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, et al. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37(1):77–88. doi: 10.1603/0022-2585-37.1.77 . [DOI] [PubMed] [Google Scholar]
- 45.Reiter P, Nathan MB. Guidelines for assessing the efficacy of insecticidal space sprays for control of the dengue vector Aedes aegypti Geneva, Switzerland: WHO, 2001. Available from: http://apps.who.int/iris/handle/10665/67047. [Google Scholar]
- 46.Reiter P, Nathan MB. Guías para la evaluación de la eficacia del rociado espacial de insecticidas para el control del dengue Aedes aegypti Geneva, Switzerland: WHO, 2003. Available from: http://apps.who.int/iris/handle/10665/68400. [Google Scholar]
- 47.World Health Organization. Guidelines for efficacy testing of insecticides for indoor and outdoor ground-applied space spray applications Geneva, Switzerland; WHO, 2009. Available from: http://apps.who.int/iris/handle/10665/70070. [Google Scholar]
- 48.Zeileis A, Kleiber C, Jackman S. Regression Models for Count Data in R. Journal of Statistical Software. 2008;27(1):1–25. doi: 10.18637/jss.v027.i08 [Google Scholar]
- 49.Lenth R. Least-Squares Means: The R Package lsmeans. J Statistical Software. 2016;69:1–33. doi: 10.18637/jss.v069.i01 [Google Scholar]
- 50.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–32. doi: 10.1056/NEJMra1110265 . [DOI] [PubMed] [Google Scholar]
- 51.James S, Simmons CP, James AA. Mosquito trials. Science. 2011;334(6057):771–2. doi: 10.1126/science.1213798 . [DOI] [PubMed] [Google Scholar]
- 52.Andersson N, Nava-Aguilera E, Arostegui J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J, et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ. 2015;351:h3267 doi: 10.1136/bmj.h3267 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–e6. doi: 10.1016/S1473-3099(16)30518-7 . [DOI] [PubMed] [Google Scholar]
- 54.Achee NL, Gould F, Perkins TA, Reiner RC Jr., Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9(5):e0003655 doi: 10.1371/journal.pntd.0003655 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runge-Ranzinger S, Kroeger A, Olliaro P, McCall PJ, Sanchez Tejeda G, Lloyd LS, et al. Dengue contingency planning: From research to policy and practice. PLoS Negl Trop Dis. 2016;10(9):e0004916 doi: 10.1371/journal.pntd.0004916 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, et al. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol. 2015;31(8):380–90. doi: 10.1016/j.pt.2015.04.015 . [DOI] [PubMed] [Google Scholar]
- 57.Wolbers M, Kleinschmidt I, Simmons CP, Donnelly CA. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Negl Trop Dis. 2012;6(11):e1937 doi: 10.1371/journal.pntd.0001937 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newton EAC, Reiter P. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg. 1992;47(6):709–20. doi: 10.4269/ajtmh.1992.47.709 . [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. 2009. Available from http://www.who.int/rpc/guidelines/9789241547871/en/. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Rows show treatment sector. X-axis is sqrt-scaled. The majority of house surveys find no adults.
(PDF)
Summary of spray coverage in S-2013 (A) and L-2014 (B). In both years, most houses were sprayed in at least 5 out of 6 spray cycles, while a small number of houses were never sprayed. In L-2014, experimental spray coverage was much higher than emergency (citywide) spray coverage.
(PDF)
Insects were from a laboratory colony (one colony per year).
(PDF)
X-axis shows week start date. Color and line-type shows treatment sector (orange triangle: spray sector). Point size shows number of surveyed houses. Vertical lines show approximately spray dates: dashed, experimental spraying (spray sector only); dotted, citywide spraying (February 2014, all sectors). Vertical colored bars show bootstrap 95% CI (1e+04 draws per circuit). (A) Adult surveys. (B) Container (PrPC) and parity (PrNF) surveys.
(PDF)
All models include fixed effects of sector and circuit, with a separate model for each year. (A) Counts: negative binomial GLM (NB-GLM). (B) Proportions: logistic GLM (L-GLM). Note that Breteau Index (BI) = 100*PC/HSE. See also S2–S10 Tables.
(PDF)
Note the scale differs between experiments. See also Fig 1.
(PDF)
(A) S-2013. (B) L-2014.
(PDF)
(A) S-2013. (B) L-2014. During L-2014, in addition to experimental spraying, 3 cycles of emergency citywide spraying were conducted. Note the map scale differs between A and B. See also Fig 1.
(PDF)
Weeks: Week number from experiment start. Houses: number of unique houses surveyed. Surveys: total surveys (either adult, or combined adults and immature). Full surveys: surveys where both adult and immatures were surveyed. Buffer, Spray: surveys in buffer and spray sector, respectively.
(PDF)
Ratio of AA/HSE in spray sector relative to buffer sector (spray/buffer). Bold p-values: significant difference between sectors. In both years, the spray sector starts with more adults per house, and spraying reduces AA/HSE relative to buffer sectors. As in S3 Table, the effects of spraying are more pronounced in S-2013. See also Fig 4A.
(PDF)
Ratio of AA/HSE relative to baseline (C1, spray sector only). Bold p-values: significant difference from baseline circuit. In both years, spraying reduces AA/HSE relative to baseline (C1). The effects of spraying are most pronounced in S-2013, but are short-lived in both years. See also Fig 4A.
(PDF)
Ratio of AA/HSE in houses that were either sprayed or not sprayed prior to surveying (no prior spray/prior spray). Bold p-values: In L-2014, houses without prior spraying yielded significantly more adults than houses with prior spraying. In S-2013, most houses were sprayed in the prior week. In L-2014, the exact date of spraying was uncertain for a small number of houses. See S5 Table for details.
(PDF)
A single model (NB-GLM) includes both experiment year and spray status as predictors. Group: significance groups (Tukey HSD) compare among all rows. Only house surveys in the spray sector during experimental spraying are included (i.e., S-2013 C2 and L-2014 C6). Not all sprayed houses were subsequently surveyed. The average interval between each house's spray application and survey was shorter in L-2014 (median 2 days) than in S-2013 (median 7 days). See also S4 Table.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors; significance groups (Tukey HSD) compare among all rows. See Fig 4A for model description.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See Fig 4B for model description.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See S5 Fig.
(PDF)
(A) S-2013. (B) L-2014. Note that Breteau Index (BI) = 100*PC/HSE. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. See S5 Fig.
(PDF)
(A) S-2013. (B) L-2014. Model estimates by circuit and treatment sector. Horizontal line separates treatment sectors, significance groups (Tukey HSD) compare among all rows. No container surveys were conducted during spraying See also S5 Fig.
(PDF)
(PDF)
(PDF)
Data Availability Statement
All data are available through DRYAD at https://doi.org/10.5061/dryad.160023v.