Abstract
Many murine and non-human primate animal models have been recently developed to understand Zika viral pathogenesis. However, a major limitation with these models is the inability to directly examine the human-specific immune response. Here, we utilized a BLT humanized mouse model endowed with a transplanted human immune system. Plasma viremia could be detected within 48 h after viral challenge and viremia persisted for as long as 220 days in some mice. Neutralizing human antibody was detected in infected mice and mouse sera showed reactivity with the viral envelope and capsid proteins in a radio-immunoprecipitation assay. Human monocytes/macrophages, B cells and hematopoietic stem cells in the bone marrow were found to be virus infected. These data establish that BLT mice are permissive for Zika viral infection and are capable of generating viral-specific human immune responses thus providing a human surrogate model for future testing of vaccine and antiviral therapeutic candidates.
Introduction
Zika virus (ZIKV) is a mosquito-borne flavivirus related to dengue (DEN), yellow fever, West Nile and Japanese encephalitis viruses. First isolated in 1947 from a sentinel rhesus monkey in the Zika forest of Uganda, ZIKV has been shown to be endemic to several African and Asian countries causing a rare mild syndrome in humans characterized by a self-limiting flu-like illness (Dick et al., 1952; Duffy et al., 2009; Simpson, 1964). It emerged from relative obscurity being responsible for a major 2007 outbreak in the small Micronesian island of Yap, and later spread to neighboring islands in the South Pacific (Duffy et al., 2009). A more catastrophic outbreak in Brazil during 2015 that rapidly spread to 46 countries and territories in the Western Hemisphere involved millions of people (Aliota et al., 2017; WHO, 2016).
In recent ZIKV outbreaks, there was a higher risk/incidence of Guillain-Barre syndrome (GBS) in adults (do Rosário et al., 2016; Paploski et al., 2016) and severe birth defects including fetal microcephaly (now collectively termed as Congenital Zika Syndrome (CZS)), which are directly attributed to ZIKV infection of pregnant women (Calvet et al., 2016; Mlakar et al., 2016; Oliveira et al., 2016; Schuler-Faccini et al., 2016; Vesnaver et al., 2017). Aedes aegypti and Aedes albopictus are the major mosquito vectors for viral transmission (Aliota et al., 2017). Unusual for a typical arbovirus, sexual transmission has been confirmed by observations of overseas travelers returning to non-endemic regions (Foy et al., 2011). Given the grave situation with the Zika epidemic and lack of adequate knowledge on its pathogenesis, there is an urgent need for the development of animal models that allow a greater understanding of disease pathogenesis, vaccine and therapeutic advancement. A number of murine models have been developed that mainly utilize neonatal mice, KO mice and interferon (IFN) receptor deficient mice (Aliota et al., 2017; Morrison and Diamond, 2017). Productive infection and various pathologies were observed, including neurological maldevelopment (Cugola et al., 2016; Govero et al., 2016; Miner et al., 2016; Rossi et al., 2016, Yockey et al., 2016). Sexual transmission was demonstrated and vaccine testing was conducted in the background of murine immune responses (Aliota et al., 2017; Fernandez and Diamond, 2017; Govero et al., 2016; Sumathy et al., 2017; Winkler et al., 2017; Yockey et al., 2016).
One major drawback with the IFN deficient mouse model is the lack of innate immune response, which serves as a first-line viral defense thus somewhat limiting pathogenesis studies. A variety of non-human primate (NHP) models have also been used for Zika studies yielding important information about various aspects of ZIKV pathogenesis (Aliota et al., 2016; Dudley et al., 2016; Koide et al., 2016; Li et al., 2016; Osuna et al., 2016). An advantage with the NHP models is their physiological similarities to humans. Many pathological lesions were recapitulated in this system that included congenital neurological abnormalities upon infection of pregnant animals (Adams Waldorf et al., 2016; Nguyen et al., 2017). T cell responses and production of neutralizing antibody were demonstrated in NHPs permitting subsequent vaccine testing studies (Aliota et al., 2016, 2017; Dowd et al., 2016; Morrison and Diamond, 2017).
A major deficiency with the above animal models has been the inability to directly evaluate human immune responses in the face of ZIKV infection. Humanized mice, with a transplanted human immune system, can potentially overcome these shortcomings. Among the new generation of humanized mouse (hu-mice) models, hu-HSC mice are prepared by transplanting human hematopoietic stem cells (HSC), while bone marrow, liver and thymus (BLT) mice are made by HSC transplantation and engraftment of autologous human fetal liver and thymus under the kidney capsule of immunodeficient mice (Akkina, 2013b; Lan et al., 2006; Melkus et al., 2006; Shultz et al., 2012; Wege et al., 2008).
Both primary and secondary lymphoid organs develop in these BLT mice with human cells being present in thymus, bone marrow, spleen, liver, lymph nodes, gut and reproductive organs (Akkina, 2013b; Shultz et al., 2012; Wege et al., 2008). Of particular significance, human immune responses can be generated due to the presence of human T cells, B cells, macrophages and dendritic cells, which are essential components of the human immune system (Akkina, 2013a, b; Ito et al., 2012; Traggiai et al., 2004; Wege et al., 2008). BLT mice permit both human antibody responses as well as HLA restricted cellular immune responses (Akkina, 2013b, 2014; Melkus et al., 2006; Shultz et al., 2012; Seung and Tager, 2013). Many previous studies, including ours, have successfully employed the hu-mice for studies with human specific pathogens such as human immunodeficiency (HIV) and dengue viruses (Reviewed in: Akkina, 2013b; Denton and Garcia, 2011; Ito et al., 2012; Seung and Tager, 2013; Shultz et al., 2012). Here we used BLT hu-mice to evaluate ZIKV infection and human immune responses. Our results show that these mice are readily permissive for ZIKV infection, sustaining viremia and giving rise to neutralizing human antibody responses.
RESULTS
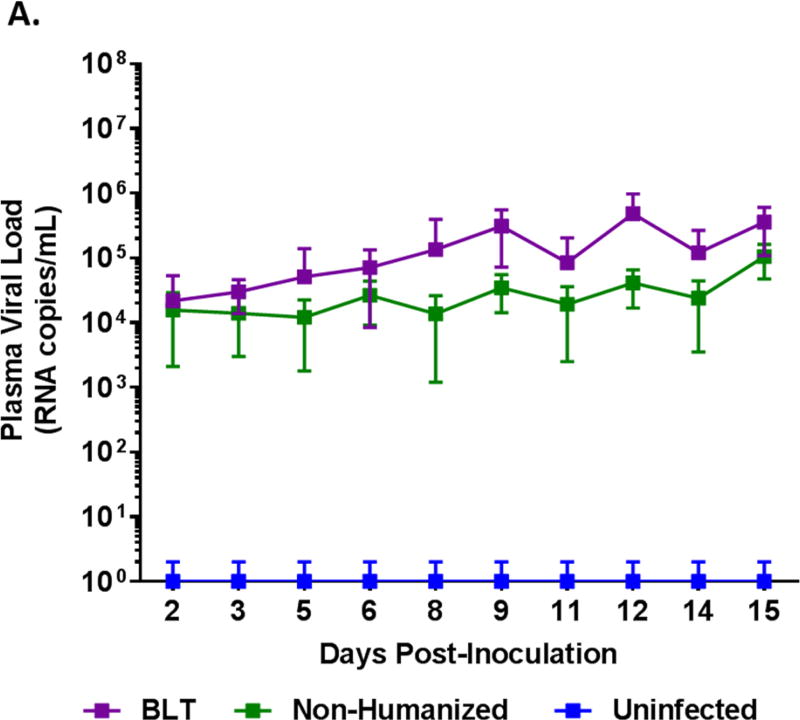
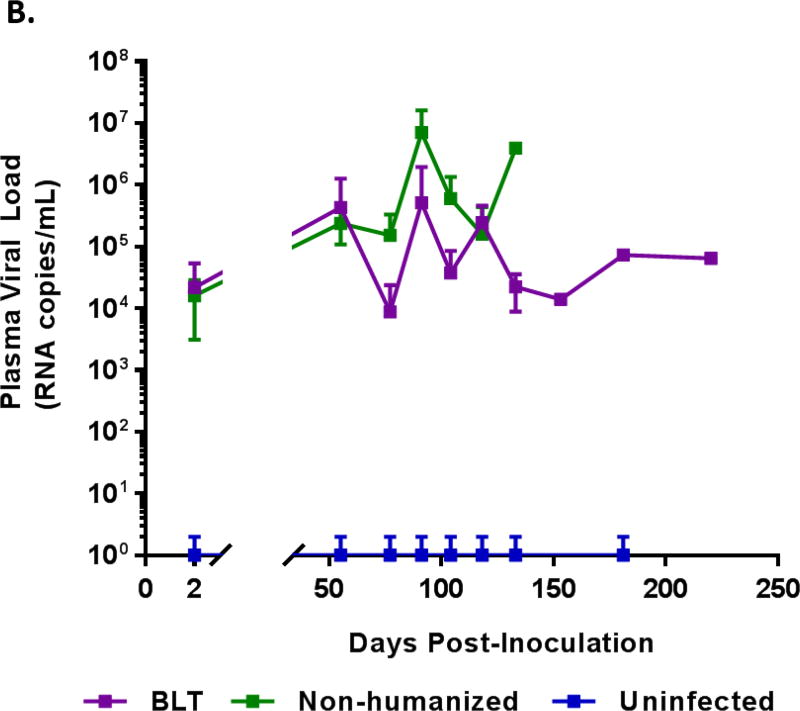
BLT mice support productive ZIKV infection resulting in chronic viremia
BLT mice were generated and verified for human cell engraftment prior to proceeding with ZIKV infection with the Puerto Rico strain PRVABC59. As a control, non-engrafted immunodeficient Rag2−/−γc−/− (non-hu) mice were similarly infected. Whole blood samples were collected and plasma viral loads were monitored by qRT-PCR every other day for 14 or 15 days and then weekly. All ZIKV challenged mice became readily infected and displayed viremia. Acute infection was followed for 2 weeks wherein viremia was evident by day 2 and the viral loads gradually increased during the 14–15 day period (Figure 1A, Supplemental Figure 1). Overall, BLT mice maintained significantly higher viral loads (p<0.05) than non-humanized mice and reached peak viral loads (4.84 × 105 RNA copies/mL) by day 12. These results demonstrated that BLT mice as well as non-humanized mice are permissive for ZIKV infection. We also followed the course of viremia in some mice to determine how long the virus can persist. BLT mice maintained productive infection for 133 days with one mouse showing plasma viral loads for as long as 220 days, the last time point evaluated. Not all the mice were followed long-term due to terminal tissue collection, age related mortalities and/or graft versus host disease (GvH). With regard to viral persistence, the non-humanized mice also showed chronic viremia for 125 days with one mouse for 139 days (Figure 1B). During the chronic phase of infection, no virus was detected in the plasma of some mice at various time points (Supplemental Figure 2). The above data showed the susceptibility of both BLT and non-humanized mice of Rag2−/−γc−/− genetic background for ZIKV infection and viral persistence.
Figure 1. ZIKV plasma viremia in BLT mice.
Plasma samples were collected at different times post-inoculation during the acute and chronic stages of infection and assayed by qRT-PCR to determine viral loads. A) Acute stage viral loads. Purple line, composite data for infected BLT (12 mice) and green line for infected non-humanized mice (11 mice) are shown. B) Chronic stage viral loads. The infected mice above were followed long-term for several weeks to determine how long the viremia could persist. Not all mice survived or maintained until the last collection point at day 220. The Purple line represents BLT mice and the Green line represents non-humanized mice. Only mice that were followed for both acute and chronic stage of infection are shown here and individual viral loads are shown in Supplementary Figure 1.
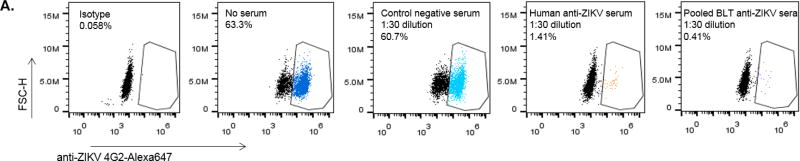
ZIKV human immune response and neutralizing antibody production in BLT mice
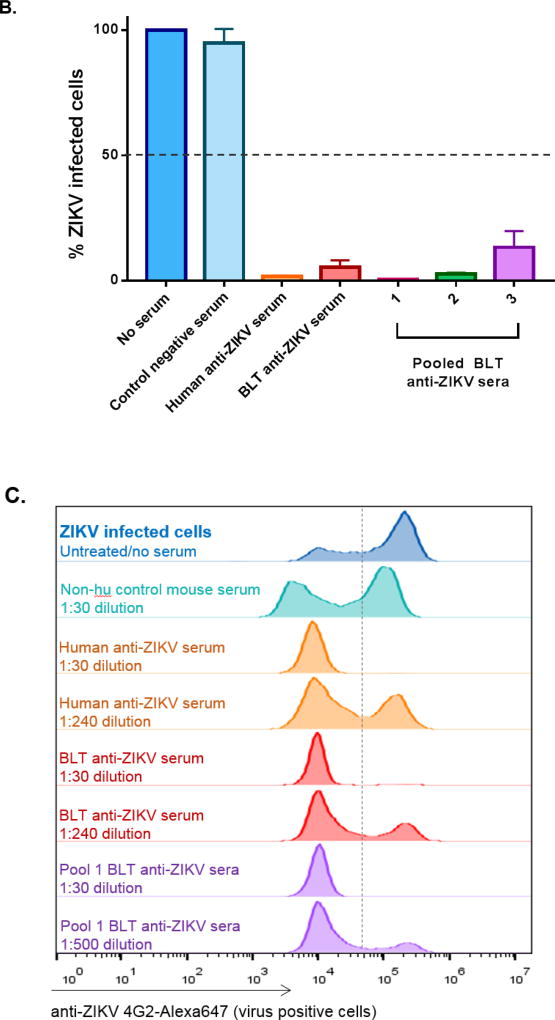
As hu-mice are capable of human immune responses and were previously shown to generate virus-specific antibodies to agents such as HIV and DEN (Reviewed in: Akkina, 2013b; Denton and Garcia, 2011; Ito et al., 2012; Seung and Tager, 2013; Shultz et al., 2012), we wanted to determine whether the productive ZIKV infection seen above leads to a virus-specific human antibody response. Accordingly, we assessed the capacity of sera from infected BLT mice to neutralize ZIKV using a FACS-based neutralization assay (FNT) (Lambeth et al., 2005; Kuruvilla et al., 2007) (Figure 2A, B). Sera that caused a 50% reduction in the number of infected cells detected relative to the number of infected cells seen in the untreated virus control were scored as neutralizing. For example, the virus alone untreated control Fig 2A, panel 2 showed 63.3% infected cells whereas the human Zika antisera and BLT mice pooled antisera treated samples showed only 1.41% and 0.40% infected cells demonstrating viral neutralization (panels 4 and 5; respectively).
Figure 2. Neutralizing antibody response to ZIKV in BLT mice.
Plasma samples collected from ZIKV infected BLT mice (at 12 weeks or later) were assayed by a FACS-based virus neutralization assay. Virus-Sera mixtures, as described in Methods, were plated on Vero cells and the percent ZIKV positive cells were determined by FACS at 24 h. A) FACS plots showing neutralization by representative samples of different sera. Panels 1 to 5 (left to right): Panel 1, isotype antibody. Panel 2, Virus alone and no sera. Panel 3, control negative serum. Panel 4, human anti-Zika serum. Panel 5, Pooled BLT anti-Zika sera. Note that in virus only plot 2, 63.3% of cells are virus positive whereas in plots 4 and 5, with human anti-ZIKV sera and BLT anti-ZIKV sera; respectively, showed only 1.4% and 0.4% of the virus positive cells, demonstrating neutralization. B) Bar graphs showing virus neutralization by BLT mouse sera. Infected BLT mouse sera were tested for neutralization as pools or individually as described in Methods. The percent of ZIKV infected Vero cells was calculated by normalizing the data to untreated virus infected cells (Virus alone control set as 100%) in the absence of serum and then calculating the percent of infected cells. Note that with different antisera, including those from BLT mice pooled sera samples 1, 2 and 3 as well as an individual BLT mouse sera sample, there was neutralization. With regard to the individual BLT sera, of the three tested, all showed neutralization. Only one of these is shown here as an example. C) Histograms showing endpoint titration of BLT mouse sera for virus neutralization. Infected BLT mouse and human ZIKV anti-sera were diluted and tested to determine the minimal dilution of antibody needed for ZIKV neutralization as described in the Methods. Sera that caused a 50% reduction in the number of infected cells relative to that seen in the untreated/no serum virus control were scored as neutralizing. Note that neutralizing activity was seen at a 1:240 dilution for the human anti-ZIKV serum positive control and the individual mouse BLT serum sample as well as at a 1:500 dilution for the BLT mice pooled sera sample 1. These histograms are representative examples of both individual and pooled ZIKV infected BLT antisera neutralization. All experiments were conducted in triplicate.
Overall, our results showed that all three pooled BLT antisera groups and a representative individual BLT mouse antiserum sample were able to neutralize ZIKV (0.41–21.1% infection) relative to the samples without serum (63.3% infection) (Figure 2A, B). The degree of viral neutralization by BLT mouse antisera was comparable to that of a human antiserum sample tested from an individual who recovered from a previous ZIKV infection (Figure 2B). In contrast, the uninfected control BLT mouse serum had no neutralizing activity. We also evaluated the endpoint neutralization titer of BLT mouse sera versus human ZIKV sera. Serum from an individual mouse had a titer of 1:240 similar to the human serum test sample (also 1:240) whereas a pooled BLT sera sample (pool 1) showed a higher titer of 1:500 (Figure 2C) indicating the capacity of hu-mice in generating comparable human neutralizing immune responses to ZIKV.
We also examined the specificity of the infected BLT mouse antisera by radioimmunoprecipitation to determine their reactivity to virus-specific proteins. As seen in Figure 3 (lanes h, j and l), three representative serum samples from ZIKV infected BLT mice showed immunoprecipitation of virus-specific envelope and capsid polypeptides in contrast to control uninfected mouse serum (Figure 3, lane f). Of interest, sera from the mouse with detectable viremia at 220 days (Figure 1B), demonstrated both neutralization and (Figure 2B, C) and immunoprecipitating antibodies (lane l, Figure 3). Collectively, these data showed that BLT mice are capable of generating ZIKV antibody responses.
Figure 3. Immunoprecipitation of ZIKV proteins by BLT mouse antisera.
Control uninfected and ZIKV infected Vero cells were radiolabeled with 35S-methionine/cysteine. Viral proteins were immunoprecipitated from the labeled cell lysates by using BLT mouse or human antisera and resolved on SDS-PAGE gels as described in Methods. Uninfected and infected ZIKV cell lysates immunoprecipitated by: lanes (a) and (b), uninfected and infected cell lysates with normal control human sera. Lanes (c) and (d), uninfected and infected cell lysates with ZIKV-positive human antisera. Lanes (e) and (f), uninfected and infected cell lysates with uninfected control mouse sera. Lanes (g, i, k) and (h, j, l), uninfected and infected cell lysates with three representative BLT mouse antiserum. ZIKV envelope protein (50 kDa) and capsid (12 kDa) polypeptides are indicated by *.
ZIKV Infection of human hematopoietic cells in BLT mice
Regarding the viral tropism, studies on NHP, murine models and human tissue sample have shown that ZIKV can infect a wide variety of cells/tissues such as the skin, testis, vaginal epithelium and uterine fibroblasts, placenta, brain and eye (reviewed in Aliota et al., 2017). While many in vitro studies have been done, data on what specific blood cells are infected in the human patient have been limited. Two studies have identified that human macrophages/monocytes support ZIKV in vivo in the human (Lum et al., 2017; Michlmayr et al., 2017). Here we determined which human cell populations in BLT mice can be infected with ZIKV. Cells from spleen, bone marrow and lymph nodes of chronically infected BLT mice at 16 weeks post-ZIKV infection were analyzed by FACS. Our results showed that human myeloid cells (4.83%), B cells (4.21%) and CD34+ HSC (4.34%) were infected with ZIKV in the bone marrow. However, no infected myeloid or B cells were found in either the spleen or lymph nodes (Figure 4A). No T cell infection could be detected in any of the above samples (Figure 4B).
Figure 4. ZIKV infected human cells cells BLT mice.
Spleen, LN, and bone marrow single cell suspensions from ZIKV infected and uninfected mice were stained with different labelled antibodies and analyzed. A) Flow cytometric analysis of B cells, myeloid cells, and hematopoietic stem cells (HSCs) from the bone marrow. Samples were pre-gated on indicated cellular markers from B cells (CD19+), myeloid (CD14+) or HSC (CD34+) and intracellular staining for ZIKV (4G2+) was examined within each gate. B) T cells (CD3+) from LN and spleen were analyzed by flow cytometry for presence of ZIKV infection. Representative flow plots are shown.
DISCUSSION
Animal model development to study ZIKV has advanced with impressive speed and rapid progress (Aliota et al., 2017; Morrison and Diamond, 2017). However, none of these models permit evaluation of human-specific immune responses to active ZIKV infection. Humanized BLT mice capable of generating a full repertoire of human immune cells consisting of T and B cells, monocytes/macrophages and dendritic cells, offer a novel avenue in this context. Here in our proof-of-concept studies, we showed that BLT mice could support active ZIKV infection and generate virus-specific neutralizing human antibody responses.
Infection of BLT mice gave rise to viremia within 48 h post-challenge. There was a steady increase in the viremia levels during the two-week acute infection period with peak viral loads apparent by the 12th day. Non-humanized immunodeficient mice that were used to construct BLT mice were similarly virally challenged. Surprisingly, even the non-humanized mice also showed viral infection with sustained viremia. However, the levels of viral loads were found to be significantly higher during the acute phase of infection in humanized BLT mice. This could be due to ZIKV having a greater affinity for the engrafted human cells. Previous mouse models have employed IFN receptor knockout, anti-IFNR antibody treated or neonatal mice to achieve a productive viral infection (Aliota et al., 2017; Morrison and Diamond, 2017; Rossi et al., 2016). A disadvantage of IFN deficient mice is the lack of an innate immune system, which is not the case with the Rag2−/−γc−/− mice we used here. In contrast to utilizing neonatal wild type mice for successful infection, even the adult Rag2−/−γc−/− mice readily supported ZIKV infection. Thus these mice can be put to use for future ZIKV infection studies that require an intact innate immune system in an adult animal. For example, in utero viral transmission to the developing fetus in pregnant animals can be studied more efficiently without compromising innate immunity.
In previous mouse model studies, infection kinetics and viremia have only been followed shortterm (Aliota et al., 2017; Govero et al., 2016; Lazear et al., 2016; Morrison and Diamond, 2017). To determine the extent of viremic persistence, we followed the infected mice for a much longer period. Our results showed that virus could be detected in the plasma for as long as 220 days in BLT mice and 139 days in non-humanized mice. While viremia levels mostly remained at steady state levels in mice studied long-term, plasma viral loads transiently fell below the detection limit in some mice (Supplemental Figure 2). This is similar to that seen during ZIKV infection of rhesus macaques wherein viral loads fell below detection at some points but returned to detectable levels later (Dudley et al., 2016; Hirsch et al., 2017). It is also of interest to determine which human cells are supporting viral replication during the long-term infection in BLT mice. Analysis of human cells in spleen, lymph nodes and bone marrow at 16 weeks post-infection revealed myeloid cells, B cells and hematopoietic stem cells (CD34+ HSC) being positive for virus in bone marrow (Figure 4). No virus could detected in T cells in any tissue compartment. As pointed out above, only limited studies have been done on the hematopoietic compartment of ZIKV infected individuals and the available data only points to infection of monocytes/macrophages (Lum et al., 2017; Michlmayr et al., 2017). Infection of human HSC and B cells, as noted here in BLT mice, poses new questions regarding the viral tropism to these cells and its biological significance for viral pathogenesis and persistence. HSC reside in a specialized microenvironment in the bone marrow and give rise to all blood cells; therefore, ZIKV infected HSC may have additional important implications and clues to Zika viral reservoirs. Future in depth studies need to tackle these important questions.
Neutralizing antibody plays a crucial role in affording protection. Our results showed that infected BLT mice produced neutralizing antibody. In addition, the human antibodies produced in this system reacted with ZIKV envelope glycoprotein as well as the capsid protein further confirming the viral-specific immune response. Endpoint neutralization tests showed that BLT mice are capable of producing antibody titers comparable to that seen with ZIKV infected human subjects.
It is puzzling that chronically infected BLT mice while producing neutralizing and immunoprecipitating antibodies also have detectable plasma viral loads by qRT-PCR. It is likely that viral replicating cell and tissue reservoirs contribute a low level of cell free virus (as shown by the presence of infected myeloid cells, B cells and HSC in bone marrow) which is neutralized by the antibody but viral RNA can still be detected in plasma due to the circulating virus-antibody complexes. This may not be unusual since it was previously observed that virus could be detected for as long as 70 days in rhesus macaques and up to 80 days in a pregnant human ZIKV study participant (Nguyen et al., 2017; Paz-Bailey et al., 2017). More in depth studies are needed to evaluate the dynamics of viral persistence in tissue sanctuaries and the interplay of the immune response.
On a practical front, currently a number of vaccine candidates are in the pipeline for ZIKV prophylaxis, and the BLT mouse model can now be exploited to determine which of these experimental vaccines is more effective in generating human neutralizing antibody (Aliota et al., 2017; Fernandez and Diamond, 2017). This will provide important pre-clinical data to inform field vaccine trials. In summary, our studies demonstrated the utility of BLT mice to study ZIKV infection and human response in a physiological setting. Small animal models, such as the humanized mice used here, will have many advantages over larger animal models, such as NHPs, due to low-cost and larger numbers that can be employed for experimental studies. Thus this current BLT mouse model would provide a unique platform to evaluate ZIKV immunity and test novel vaccine and therapeutic candidates.
Materials & Methods
Generation of BLT humanized mice
BLT mice were generated as previously described (Akkina, 2013b; Lan et al., 2006; Melkus et al., 2006; Shultz et al., 2012; Wege et al., 2008). Briefly, 5–8 week old BALB/c-Rag1−/−γc−/− or BALB/c-Rag2−/−γc−/− mice were preconditioned by irradiation at 350 rads and human fetal liver and thymic fragments were surgically implanted underneath the kidney capsule. The following day, these mice were injected intravenously with 0.5–1.0 × 106 autologous CD34+ hematopoietic stem cells. Mice were screened for human cell engraftment at 10–12 weeks post-reconstitution. Peripheral blood was collected in heparinized capillary tubes by tail vein puncture. Five µl of FcγR-block (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added to whole peripheral blood and then stained using mouse anti-human CD45-FitC (eBioscience), CD3-PE (eBioscience) and CD4-PE/Cy5 (eBioscience) antibodies. The red blood cells were lysed using the Whole Blood Erythrocyte Lysing Kit per the manufacturer’s instructions (R & D Systems, Minneapolis, MN). Samples were then washed and fixed in 1% paraformaldehyde. Stained cells were analyzed using a BD Accuri C6 flow cytometer and FlowJo v10 software. Mice were maintained at the Colorado State University Painter Animal Center and these studies have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
Human CD34 hematopoietic cells and cell culture
Human fetal liver-derived CD34+ hematopoietic stem cells were purified and cultured in media containing 10 ng/mL of IL-3, IL-6 and SCF (R & D Systems, Inc., Minneapolis, MN) as previously described (Akkina et al., 1994). Vero cells were cultured in DMEM containing 10% FBS (HI) supplemented with 1× antibiotic-antimycotic mix and 2 mM L-glutamine (Thermo Fischer Scientific). For Zika virus infections, 2% FBS (HI) DMEM supplemented with 1× antibiotic-antimycotic mix and 2 mM L-glutamine (Thermo Fischer Scientific) was used.
ZIKV propagation and mouse inoculations
The Puerto Rico ZIKV strain PRVABC59 used here was obtained from Dr. Rushika Perera at Colorado State University. Vero cells were infected at an MOI of 0.01 in DMEM containing 2% FBS (HI) supplemented with 1× antibiotic-antimycotic mix and 2 mM L-glutamine. Virus was harvested when cytopathic effect was severe with a 50% cell death.. Viral supernatant was 0.45 µm filtered and titered by qRT-PCR or plaque assay. Non-humanized and BLT engrafted mice were inoculated with 150 µl of 2 × 107 PFU/mL of low passage ZIKV both intraperitoneally (i/p) and subcutaneously (s/c).
Zika viral load determination by qRT-PCR
Following infection, during the first 14–15 days mice were divided into two groups for sample collection. For group one, the mice were bled on days 2, 5, 8, 11 and 14, while the mice from group two were bled on days 3, 6, 9, 12 and 15 (Supplemental Figure 1). Later, both groups were bled weekly until euthanasia. Viral RNA was extracted from plasma using the E.Z.N.A. ® Viral RNA kit (Omega bio-tek, Norcross, GA) and subjected to qRT-PCR using the iScript One-Step RT-PCR kit with SYBR Green per the manufacturer’s instructions (Bio Rad, Hercules, CA). Primers were designed and optimized based on a publication by Lanciotti and colleagues: forward (5’- CCGCTGCCCAACACAAG-3’) and reverse (5’- CCACTAACGTTCTTTTGCAGACAT-3’) (Lanciotti et al., 2008). Samples were run using BioRad C1000 Thermal Cycler with a CFX9 Real-Time System (BioRad, Hercules, CA) and the following cycling conditions: 50°C for 10 min, 95°C for 3 min, followed by 40 cycles of 95°C for 15 sec, and 58°C for 60 sec. The standard curve was prepared using a series of 10-fold dilutions of ZIKV PRVABC59 at a known concentration. The sensitivity of this assay was 1,000 RNA copies per ml. Graphs were made using the GraphPad Prism software.
FACS-based ZIKV neutralization assay
The ability of BLT mouse generated antibodies to neutralize ZIKV was determined by a FACS-based neutralization test (FNT) that both we and others have used to analyze dengue antibodies (Kuruvilla et al., 2007; Lambeth et al., 2005). To determine the neutralizing antibody titer by an endpoint assay infected and uninfected ZIKV BLT mouse sera and the human ZIKV sera were serially diluted ending at a 1:1,000 dilution. In brief, each diluted serum was mixed with ZIKV (MOI 3 to infect Vero cells seeded at 5× 104 per well in a 24-well plate) in a total volume of 150 µl. The virus-plasma mixture was incubated on ice for 30 min followed by 15 min at RT. This mixture was then used to infect the pre-seeded Vero cells. Plasma samples tested consisted of those from infected BLT mice (≥12 weeks post-viral challenge) either as pooled batches (Pools 1 to 3, with 4 mice per group) or as individual samples. Sera from uninfected mice and human Zika antisera were used as negative and positive controls, respectively. At 24 h post-infection, the cells were analyzed by FACS to detect the percentage of ZIKV infected cells. Briefly, single cell suspensions were fixed and permeabilized by using the BD Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) per the manufacturer’s instructions. All subsequent antibody steps were performed in the CytoPerm/Cytowash solution. First, cells were incubated with 1 µg/mL of the monoclonal anti-flavivirus group antigen 4G2 antibody (Millipore, Billerica, MA) or a mouse IgG2a isotype control antibody (Millipore, Billerica, MA) on ice for 1 h, followed by 2.5 µg/mL of Alexa Fluor™ 647 goat anti-mouse IgG2a (γ2a) (Invitrogen) secondary antibody for 1 h on ice. Cells were then washed, fixed in 1% paraformaldehyde and analyzed by using the BD Accuri C6 flow cytometer and FlowJo v10 software. The percent virus positive cells seen the in untreated virus control served as a baseline to determine the neutralization capacity of test sera by looking for reduction in the number of virus positive cells. Antisera that gave at least 50% reduction in the number of infected cells in the FACS plot relative to untreated virus controls were scored as neutralization positive. The degree of neutralization was calculated by normalizing the data to Vero infected cells in the absence of serum and then calculating the percent of infected cells. Graphs/histograms were created using the GraphPad Prism software. All neutralization experiments were performed in triplicate.
Detection of human anti-ZIKV antibodies in BLT mouse sera by radio-immunoprecipitation
To evaluate the capacity of ZIKV antisera from infected BLT mice in recognizing viral-specific proteins, a radio-immunoprecipitation assay was employed as described previously (Akkina et al., 1987). Briefly, ZIKV infected Vero cells at 24 h post-infection were pulse labeled for 3 h with 50 µCi/mL of 35S-methionine/cysteine (PerkinElmer, Boston, MA) after starving the cells for 45 min in DMEM media without methionine/cysteine. The cells were then washed 3× with PBS and lysed in cold HO buffer (pH 7.2) containing 10 mM Tris-HCl, 2 mM EDTA, 100 mM NaCl, 1% NP40 and 1% Halt Protease Inhibitor Cocktail (Thermo Scientific). The lysate was clarified by centrifugation at 14,000 rpm for 15 min at 4°C. Immunoprecipitation was carried out by adding 10 µl of BLT mouse or ZIKV positive human antisera to 0.5 ml lysate each. The BLT antisera from ZIKV infected mice are from ≥ 16 weeks post-viral challenge. The lysates were incubated for 1 h on ice. Later, to precipitate both IgM and IgG antibodies, Protein A/G Agarose (Thermo Scientific) beads (40 µl) were added and incubated for 10 min at 4°C. The beads were then spun down at 5,000 rpm for 5 min and washed first with IM buffer containing 10 mM Tris-HCl (pH 7.2), 1 M NaCl and 0.1% NP40, followed by two washes with RIPA buffer (10 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1% Na deoxycholate, 1% TritonX100 and 0.1% SDS). The washed and pelleted beads were resuspended in SDS-PAGE sample buffer and boiled for 2 min. The samples were electrophoresed on a 10% SDS-PAGE gel, fixed and dried. Radiolabeled viral protein bands were detected by using the Typhoon Trio Imager (GE Healthcare). All analysis was performed using the ImageQuantTL software.
FACS analysis of human hematopoietic cells from ZIKV infected BLT mice
To determine which of the human cells support ZIKV replication in chronically infected BLT mice, spleen, mesenteric LNs, and bone marrow were harvested and processed into single cell suspensions by collagenase (spleen) or physical dissociation (LN, bone marrow) at 16 weeks post-ZIKV inoculation. Isolated cells were treated with FcγR-block (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 5 min and stained with the antibodies indicated below in FACS buffer (1× PBS + 1% BSA + 0.05% sodium azide) at a 1:50 dilution. In brief, cell surface markers were stained on ice for 20 min. The cells were washed and fixed/permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) per the manufacturer’s instructions. The 4G2 intracellular staining was performed as described for FACS-based ZIKV neutralization assay. The anti-human primary antibodies used were: CD3-PE (BD Biosciences, San Jose, CA; clone HIT3a), CD19-PE/Cy7 (BD Biosciences, San Jose, CA; clone SJ25C1), CD14-FITC (BD Biosciences, San Jose, CA; clone M5E2), and CD34-PE (BD-Biosciences, San Jose, CA; clone 563). All experiments were run utilizing a BD Accuri C6 flow cytometer and FlowJo v10 software.
Supplementary Material
Supplemental Figure 1. Acute ZIKV viremia in BLT and non-humanized mice. Composite short-term plasma viral loads from ZIKV infected BLT and non-humanized mice were described in Figure 1A. Here data is presented for groups of mice which were bled on alternative days until days 14 to 15. For group 1, mice were bled on days 2, 5, 8, 11 and 14, while mice from group 2 were bled on days 3, 6, 9, 12 and 15, as detailed in the Methods.
Supplemental Figure 2. Long-term ZIKV viremia in BLT and non-humanized mice. Long-term individual plasma viral loads from ZIKV infected BLT mice and non-humanized mice described in Figure 1. Of the 22 BLT and 13 non-humanized mice used in this set of experiments, all mice became virus positive after challenge. Only mice showing both acute and long-term viremia are shown. A. Chronically infected BLT mice. Light blue lines indicate individual mouse viral load values over time and the dark blue line represents composite data for all the 12 mice. B. Chronically infected non-humanized mice. Light orange lines indicate individual mouse viral load values over time and the dark red line represents composite data for all the 11 mice. Not all the mice could be monitored to the last time point as detailed in Methods.
Highlights.
Modeling Zika viral infection and immune response in humanized mice
First study showing human neutralizing antibody production in an animal model
Human immune response animal model for Zika virus
Long-term Zika viral infection in a humanized mouse model
Huma Zika viral antibody reactivity with viral specific proteins
Acknowledgments
Work reported here was supported in part by the NIH, USA grants RO1 AI100845 and RO1 AI120021 to R. A. We would like to thank the Rushika Perera and Nunya Chotiwan for providing Zika viral stocks and reagents, Brian Foy for the Zika human antisera and Molly Price for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M, Jr, Rajagopal L. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22(11):1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013a;25(3):403–9. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology. 2013b;435(1):14–28. doi: 10.1016/j.virol.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina R. Humanized Mice for Studying Human Immune Responses and Generating Human Monoclonal Antibodies. Microbiol Spectr. 2014;2(2) doi: 10.1128/microbiolspec.AID-0003-2012. [DOI] [PubMed] [Google Scholar]
- Akkina RK, Chambers TM, Londo DR, Nayak DP. Intracellular localization of the viral polymerase proteins in cells infected with influenza virus and cells expressing PB1 protein from cloned cDNA. J Virol. 1987;61(7):2217–24. doi: 10.1128/jvi.61.7.2217-2224.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkina RK, Rosenblatt JD, Campbell AG, Chen IS, Zack JA. Modeling human lymphoid precursor cell gene therapy in the SCID-hu mouse. Blood. 1994;84(5):1393–8. [PubMed] [Google Scholar]
- Aliota MT, Bassit L, Bradrick SS, Cox B, Garcia-Blanco MA, Gavegnano C, Friedrich TC, Golos TG, Griffin DE, Haddow AD, Kallas EG, Kitron U, Lecuit M, Magnani DM, Marrs C, Mercer N, McSweegan E, Ng LFP, O'Connor DH, Osorio JE, Ribeiro GS, Ricciardi M, Rossi SL, Saade G, Schinazi RF, Schott-Lerner GO, Shan C, Shi PY, Watkins DI, Vasilakis N, Weaver SC. Zika in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antiviral Res. 2017;144:223–246. doi: 10.1016/j.antiviral.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Dudley DM, Newman CM, Mohr EL, Gellerup DD, Breitbach ME, Buechler CR, Rasheed MN, Mohns MS, Weiler AM, Barry GL, Weisgrau KL, Eudailey JA, Rakasz EG, Vosler LJ, Post J, Capuano S, 3rd, Golos TG, Permar SR, Osorio JE, Friedrich TC, O'Connor SL, O'Connor DH. Heterologous Protection against Asian Zika Virus Challenge in Rhesus Macaques. PLoS Negl Trop Dis. 2016;10(12):e0005168. doi: 10.1371/journal.pntd.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendonca MCL, de Oliveira L, Tschoeke DA, Schrago CG, Thompson FL, Brasil P, Dos Santos FB, Nogueira RMR, Tanuri A, de Filippis AMB. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653–660. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011;13(3):135–48. [PMC free article] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- do Rosário MS, de Jesus PA, Vasilakis N, Farias DS, Novaes MA, Rodrigues SG, Martins LC, Vasconcelos PF, Ko AI, Alcantara LC, de Siqueira IC. Guillain-Barre Syndrome After Zika Virus Infection in Brazil. Am J Trop Med Hyg. 2016;95(5):1157–1160. doi: 10.4269/ajtmh.16-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd JP, Tsybovsky Y, Harris A, Huang YS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong WP, Mascola JR, Pierson TC, Graham BS. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354(6309):237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD, Moncla LH, Post J, Schultz-Darken N, Schotzko ML, Hayes JM, Eudailey JA, Moody MA, Permar SR, O'Connor SL, Rakasz EG, Simmons HA, Capuano S, Golos TG, Osorio JE, Friedrich TC, O'Connor DH. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Diamond MS. Vaccination strategies against Zika virus. Curr Opin Virol. 2017;23:59–67. doi: 10.1016/j.coviro.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature. 2016;540(7633):438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LM, Ducore RM, Grigsby PL, Hennebold JD, Swanson T, Legasse AW, Axthelm MK, MacAllister R, Wiley CA, Nelson JA, Streblow DN. Zika Virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13(3):e1006219. doi: 10.1371/journal.ppat.1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9(3):208–14. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J. Development of a Zika Virus Infection Model in Cynomolgus Macaques. Front Microbiol. 2016;7:2028. doi: 10.3389/fmicb.2016.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(−/−)gamma(c)(−/−) (RAG-hu) mice. Virology. 2007;369(1):143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lambeth CR, White LJ, Johnston RE, de Silva AM. Flow cytometry-based assay for titrating dengue virus. J Clin Microbiol. 2005;43(7):3267–72. doi: 10.1128/JCM.43.7.3267-3272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–92. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–9. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19(5):720–30. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Dong HL, Huang XY, Qiu YF, Wang HJ, Deng YQ, Zhang NN, Ye Q, Zhao H, Liu ZY, Fan H, An XP, Sun SH, Gao B, Fa YZ, Tong YG, Zhang FC, Gao GF, Cao WC, Shi PY, Qin CF. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine. 2016;12:170–177. doi: 10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum FM, Lin C, Susova OY, Teo TH, Fong SW, Mak TM, Lee LK, Chong CY, Lye DCB, Lin RTP, Merits A, Leo YS, Ng LFP. A Sensitive Method for Detecting Zika Virus Antigen in Patients' Whole-Blood Specimens as an Alternative Diagnostic Approach. J Infect Dis. 2017;216(2):182–190. doi: 10.1093/infdis/jix276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- Michlmayr D, Andrade P, Gonzalez K, Balmaseda A, Harris E. CD14(+)CD16(+) monocytes are the main target of Zika virus infection in peripheral blood mononuclear cells in a paediatric study in Nicaragua. Nat Microbiol. 2017;2(11):1462–1470. doi: 10.1038/s41564-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165(5):1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–8. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Morrison TE, Diamond MS. Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J Virol. 2017;91(8) doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S, 3rd, Tarantal AF, Osorio JE, O'Connor SL, Friedrich TC, O'Connor DH, Golos TG. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13(5):e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF, Huang S, Vanlandingham DL, Higgs S, Perelson AS, Estes JD, Safronetz D, Lewis MG, Whitney JB. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22(12):1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paploski IA, Prates AP, Cardoso CW, Kikuti M, Silva MM, Waller LA, Reis MG, Kitron U, Ribeiro GS. Time Lags between Exanthematous Illness Attributed to Zika Virus, Guillain-Barre Syndrome, and Microcephaly, Salvador, Brazil. Emerg Infect Dis. 2016;22(8):1438–44. doi: 10.3201/eid2208.160496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, Perez-Padilla J, Medina FA, Waterman SH, Gubern CG, Alvarado LI, Sharp TM. Persistence of Zika Virus in Body Fluids - Preliminary Report. N Engl J Med. 2017 doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94(6):1362–9. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- Seung E, Tager AM. Humoral immunity in humanized mice: a work in progress. J Infect Dis. 2013;208(Suppl 2):S155–9. doi: 10.1093/infdis/jit448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–98. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DI. Zika Virus Infection in Man. Trans R Soc Trop Med Hyg. 1964;58:335–8. [PubMed] [Google Scholar]
- Sumathy K, Kulkarni B, Gondu RK, Ponnuru SK, Bonguram N, Eligeti R, Gadiyaram S, Praturi U, Chougule B, Karunakaran L, Ella KM. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep. 2017;7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Vesnaver TV, Tul N, Mehrabi S, Parissone F, Strafela P, Mlakar J, Pizem J, Korva M, Zupanc TA, Popovic M. Zika virus associated microcephaly/micrencephaly-fetal brain imaging in comparison with neuropathology. BJOG. 2017;124(3):521–525. doi: 10.1111/1471-0528.14423. [DOI] [PubMed] [Google Scholar]
- Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149–65. doi: 10.1007/978-3-540-75647-7_10. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Woods TA, Rosenke R, Scott DP, Best SM, Peterson KE. Sexual and Vertical Transmission of Zika Virus in anti-interferon receptor-treated Rag1-deficient mice. Sci Rep. 2017;7(1):7176. doi: 10.1038/s41598-017-07099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Zika situation report, June 16, 2016; The 3rd meeting of the emergency committee (EC) regarding microcephaly and other neurological disorders. [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166(5):1247–1256. e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Acute ZIKV viremia in BLT and non-humanized mice. Composite short-term plasma viral loads from ZIKV infected BLT and non-humanized mice were described in Figure 1A. Here data is presented for groups of mice which were bled on alternative days until days 14 to 15. For group 1, mice were bled on days 2, 5, 8, 11 and 14, while mice from group 2 were bled on days 3, 6, 9, 12 and 15, as detailed in the Methods.
Supplemental Figure 2. Long-term ZIKV viremia in BLT and non-humanized mice. Long-term individual plasma viral loads from ZIKV infected BLT mice and non-humanized mice described in Figure 1. Of the 22 BLT and 13 non-humanized mice used in this set of experiments, all mice became virus positive after challenge. Only mice showing both acute and long-term viremia are shown. A. Chronically infected BLT mice. Light blue lines indicate individual mouse viral load values over time and the dark blue line represents composite data for all the 12 mice. B. Chronically infected non-humanized mice. Light orange lines indicate individual mouse viral load values over time and the dark red line represents composite data for all the 11 mice. Not all the mice could be monitored to the last time point as detailed in Methods.