Abstract
Inborn errors of CoA (coenzyme A) biosynthesis lead to neurodegenerative disorders in humans. PKAN (pantothenate kinase-associated neurodegeneration) manifests with damage to brain, retina and testis and is caused by mutations in PANK2, the gene encoding the mitochondrial form of pantothenate kinase, a key regulatory enzyme in CoA synthesis. Further attention has been focused on this pathway by the recent discovery that mutations in the gene encoding CoA synthase lead to a similar neurodegenerative disorder, raising the spectre of a common mechanism of pathogenesis. How do defects in CoA production result in neurodegeneration? Why are certain tissues and cell types selectively vulnerable? And what is the underlying neurodegenerative process? Answers to some of these questions have come from animal models of disease, including flies and mice, as well as directly from humans. The damaged tissue types share key features that are likely to contribute to their selective vulnerability. These include the presence of a blood–tissue barrier, the milieu with respect to oxidative stress, tissue metabolic demand, relative expression of genes encoding similar proteins in these tissues and cell membrane composition. Substantial progress in understanding these important neurometabolic disorders has been made since the first gene discovery more than a decade ago. With rational therapeutics now in development for PKAN, we foresee prevention of neurodegeneration and hope for neuroregeneration or neuro-rescue.
Keywords: coenzyme A (CoA), coenzyme A synthase, pantothenate kinase, pantothenate kinase-associated neurodegeneration (PKAN)
Introduction
Single gene disorders offer a direct view of the origins of complex disease processes. When the causative gene is known there is a clear starting point from which to delineate the molecular cascade that underlies disease pathogenesis. The inborn errors of CoA (coenzyme A) biosynthesis provide a unique view into neurodegeneration and selective vulnerability of certain cell types.
Neurodegenerative diseases are the manifestation of molecular defects that perturb myriad cellular processes leading to direct effects and adaptations that compromise neuronal function. These processes serve critical functions of nutrient sensing, energy balance, waste management and membrane repair, as examples. Although neuronal dysfunction is a common endpoint, the primary site of a defect may in fact reside within other cell types, including astrocytes, microglia or vascular endothelial cells. Thus the large number of biochemical pathways leading to neurodegeneration underscores the complexity of processes required for neuronal health.
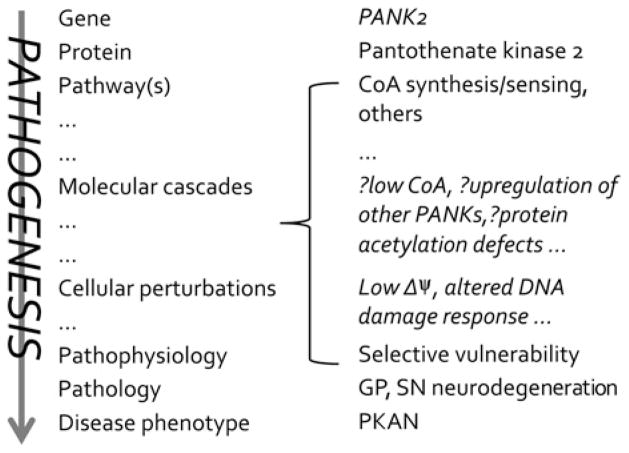
Delineating the pathogenesis of neurodegeneration in the CoA metabolic defects requires an iterative process. The gene, protein and pathway are known, as are many of the phenotypic features of the disorder. However, numerous steps in the molecular cascade of PKAN (pantothenate kinase-associated neurodegeneration) pathogenesis remain to be discovered (Figure 1). We face the challenge of discovering both the cellular perturbations arising directly from defects in PANK2 (pantothenate kinase 2) and distinguishing them from the adaptive responses that often contribute to human disease.
Figure 1.
PKAN pathogenesis from gene to disease
CoA biosynthesis, pantothenate kinase regulation and disease
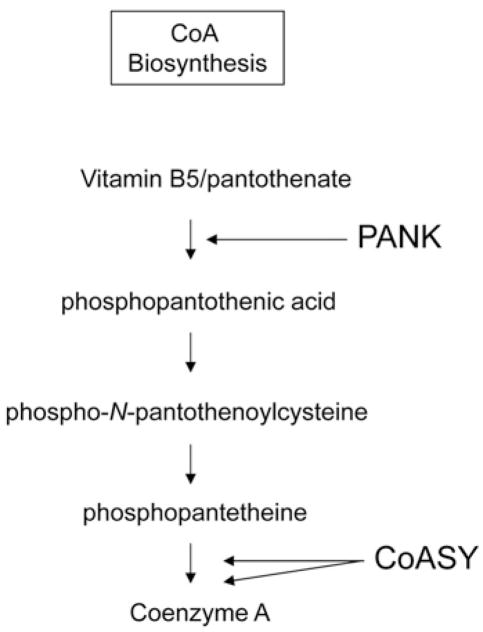
Pantothenate, or vitamin B5, forms the molecular core of CoA. CoA is required for hundreds of human biochemical reactions, underscoring the central role this cofactor plays in intermediary metabolism, neurotransmitter synthesis and other critical processes. It is therefore not surprising that the synthesis of CoA is tightly regulated. The first enzymatic step in CoA synthesis entails phosphorylation of pantothenate by pantothenate kinase (Figure 2). There are three human genes, PANK1, PANK2 and PANK3 that encode four functional pantothenate kinase proteins. PANK2, encoded by PANK2, is localized to mitochondria and is under exquisitely tight regulation [1–3]. PANK2 first transits to the nucleus before localizing to the intermembranous space of mitochondria [4]. Its nuclear role remains uncertain, but it may affect histone acetylation/deacetylation reactions [5]. In mitochondria, PANK2 serves a putative role as CoA sensor, signalling energy balance between the compartments housing the CoA synthetic enzymes in cytosol and mitochondria [3]. Although this hypothesized role explains many key aspects of PANK2 enzymatic regulation, it does not provide an explanation for the selective vulnerability of specific cell types to defects in PANK2. This is among the most intriguing aspects of PKAN.
Figure 2. CoA synthetic pathway.
PANK is pantothenate kinase, which is defective in PKAN. CoASY is CoA synthase, which is defective in CoPAN.
PKAN is an autosomal recessive disorder caused by mutations in PANK2, the human gene encoding PANK2 [6]. Classic disease manifests in childhood with dystonia (sustained contractions of opposing muscle groups), spasticity and pigmentary retinopathy [7]. Classic PKAN has its onset early in the first decade, with rapid progression leading to significant disability and loss of function. As is true for most autosomal recessive disorders, once the causative gene for PKAN was identified the phenotypic spectrum broadened [7]. Adults with dystonia and parkinsonism (slowed movements, postural instability) were also recognized to have mutations in PANK2, and their disease was generally classified as atypical PKAN [7]. In fact, the phenotype of PKAN extends along a continuum without clear delineation between the classic and atypical forms.
PKAN is characterized by unique neuroimaging features that suggest a pathogenic cascade. With more routine use of brain MRI in the early diagnostic evaluation of children and adults with dystonia, the distinctive pattern of PKAN is being recognized earlier in disease. Two key imaging features are evident: on T2-weighted imaging, the GP (globus pallidus) shows a region of hyperintense signal centrally surrounded by a region of hypointense signal [8]. The hypointense signal is known to arise from high levels of iron in this region of brain. The basis for the hyperintense signal is less certain but is likely to represent oedema based on the signal features and the anatomic pattern. The other possible origin of the hyperintense signal is gliosis, but this is less well supported by neuropathological evidence [9]. Early in disease, the hyperintense signal predominates, with a presumed cytotoxic process underway [10]. Although iron levels are increased early in disease, its signal dominates the imaging changes more so as disease progresses.
Whereas high levels of iron in certain brain regions are a key recognizable feature of PKAN, neither the mechanism of iron accumulation nor its relation to PANK2 dysfunction are understood. Similarly, the role of iron in the disease process is not clear [11,12]. What is clear is that there is a primary biochemical perturbation arising from defective PANK2 and causing disease manifestations.
Post-mortem brain pathological analysis has provided critical insights into PKAN pathogenesis. Most striking is the observation of an exquisitely circumscribed region of major pathological changes. The GP is severely affected in PKAN, with the focus of disease being the internal segment. The GP is almost entirely devoid of normal-appearing neurons. The neurons present show a progression of changes typical of cell death. What is distinctive is the persistence of these cells and their preservation in various stages of dying, as one might expect to occur if autophagy were impaired [9].
Selective vulnerability of basal ganglia structures
Although PANK2 is expressed in many regions of normal human brain, only the GP and SN (substantia nigra) pars reticulata show significant changes. Nigral involvement is seen later in disease and in only a subset of patients [10]. These regions of brain share important functions and features that are likely contributors to their selective vulnerability. The internal segment of the GP and the SN pars reticulata, both primarily GABAergic, serve as the primary inhibitory output nuclei to the thalamus. In brain circuitry diagrams, these two regions are considered as a single functional unit. Inhibitory neurons in GP are tonically active with high-energy requirements. In addition, these two regions of brain share other distinctive features, including being the most iron-rich in normal brain.
Iron is regionally distributed in mammalian brain, and levels increase with age. Those regions that are normally iron-rich contain levels that are among the highest of any tissue. These regions include GP, SN, red nucleus and dentate nucleus. The GP has as much iron per unit weight as the liver. The reason for this specific pattern of distribution is uncertain as is the mechanism by which normal iron levels are set in different brain regions. Although it is tempting to implicate iron in PKAN pathogenesis because of its role in generating ROS (reactive oxygen species), its role in disease remains unclear [11]. It may simply represent a ‘tombstone marker’ of a rampant advancing disease process in an iron-rich milieu.
Studies carried out on patients affected by neurodegenerative disorders with brain iron accumulation have directly informed our understanding of the role of pantothenate metabolism in neurodegeneration. The group of neurodegenerative disorders in which iron accumulates specifically in the GP and SN is called NBIA (neurodegeneration with brain iron accumulation). These disorders are clinically and genetically heterogeneous (Table 1). Although the culprit genes and defective pathways have been discovered for many of the different NBIA disorders, the intersection of their pathogeneses leading to iron accumulation remains to be discovered.
Table 1. NBIA disorders.
The NBIA disorders are genetically heterogeneous
| Gene | Protein | Disorder |
|---|---|---|
| PANK2 | Pantothenate kinase 2 | Pantothenate kinase-associated neurodegeneration (PKAN) |
| PLA2G6 | Calcium-independent phospholipase A2 group VIa (iPLA2β) | Phospholipase A2-associated neurodegeneration (PLAN), including infantile neuroaxonal dystrophy (INAD) |
| C19orf12 | C19orf12 | Mitochondrial membrane protein-associated neurodegeneration (MPAN) |
| WDR45 | WD40-repeat protein 45 | β-Propeller protein-associated neurodegeneration (BPAN) |
| CoASY | CoA synthase | CoA synthase protein-associated neurodegeneration (CoPAN) |
| ATP13A2 (PARK9) | Cation-transporting ATPase 13A2 | Kufor–Rakeb disease |
| FA2H | Fatty acid 2 hydroxylase | Fatty acid hydroxylase-associated neurodegeneration (FAHN) |
| CPL | Caeruloplasmin | Acaeruloplasminaemia |
| FTL | Ferritin light chain | Neuroferritinopathy |
Most people affected with one of the NBIA disorders receive a diagnosis as a result of brain MRI. The widespread use of this modality and the distinctive pattern resulting from high iron raises suspicion and enables swift diagnosis of an NBIA disorder [13]. Subsequent genetic testing typically reveals the specific disorder. Those patients without a known disease gene are providing new insight into NBIA as whole exome sequencing unmasks their causative genes. It was precisely this process that led to the recognition that mutations in CoA synthase cause an NBIA disorder [14].
A second CoA synthetic defects also causes NBIA
Whereas pantothenate kinase catalyses the first enzymatic step in CoA synthesis, CoA synthase catalyses the final two steps (Figure 2). This bifunctional enzyme generates CoA by coupling phosphopantetheine to ATP to form dephospho-CoA, which is then phosphorylated in the final step of CoA synthesis. The identification of a second inborn error of CoA biosynthesis has focused attention on the role of pantothenate metabolic defects in NBIA.
How does defective PANK2 lead to neurodegeneration?
Although PKAN was recognized as a defect in pantothenate metabolism more than a decade ago, the precise alteration in cell function remains to be delineated. There is still no direct evidence that the biochemical basis for disease in humans is deficient levels of CoA. In fact, several lines of evidence suggest that a simple product deficit is not the full explanation in PKAN, although it is likely to contribute to the disease mechanism. PANK2 is hypothesized to serve as a sensor of mitochondrial CoA needs through shifting levels of activator and repressor molecules [3,15]. As such, it does not function as the primary cellular phosphopantothenate synthetic enzyme. Therefore loss of PANK2 would not be predicted to cause a simple product deficit at the level of the whole cell. Instead, a sensing defect due to defective PANK2 would mimic the ‘off’ state of PANK2, erroneously signalling that mitochondrial CoA levels are sufficient when in fact there is demand to carry out fatty acid β-oxidation. Three other proteins with pantothenate kinase activity, including two isoforms of PANK1 and one of PANK3, may compensate for defective PANK2, at least in some cells and tissues [16]. PANK1 and PANK3 are cytosolic and seem to function as the main enzymes in the production of CoA. Therefore they may largely compensate for any decrease in CoA that might result from defective PANK2. These observations raise questions of whether significant CoA deficiency underlies PKAN, and data from studies of mutant human protein further challenge this notion.
Following the PANK2 gene discovery, the hypothesis was advanced that classic disease arose from complete loss of enzyme function, and atypical disease, with later onset and slower progression, is associated with partially functional enzyme [6,7]. This pattern would be typical for an autosomal recessive disorder associated with a defective metabolic enzyme. However, studies measuring the catalytic activity of mutant human PANK2 protein demonstrated normal and supranormal levels [17] for many of the mutations leading to severe disease. These results suggest that a loss of catalytic function is not the only mechanism for PANK2 dysfunction. Other possible mechanisms include defects that block dimerization or processing and localization of the PANK2 molecule.
Despite the lack of evidence for a CoA defect in the human disease, animal models of PKAN support this biochemical basis. Low CoA levels were measured in a Drosophila hypomorphic mutant, fumble [18,19]. Moreover, the Pank2−/− mouse has mitochondrial dysfunction [20], and newborn mouse pups show decreased brain total pantothenate kinase activity as well as low CoA levels, a difference that is no longer evident in brain from adult mice [16].
The recent discovery of a second inborn error of CoA biosynthesis has further implicated this pathway in neurologic health and disease. Mutations in the gene encoding CoA synthase lead to loss of function of this bi-functional enzyme that catalyses the final two steps in CoA synthesis and cause a disease that is being called CoPAN (CoA synthase protein-associated neurodegeneration). People with CoPAN have striking overlap in the region of brain demonstrating changes in MRI. The GP is the primary site of disease, as it is in PKAN, with most of the remaining brain tissue showing little or no radiographic evidence for disease. The precise localization of CoA synthase is in debate but there is general agreement that it associates with the mitochondria. As in PKAN, there is a presumption that cellular levels of CoA are decreased, perhaps only in certain compartments. Both CoA synthase and PANK2 are found in the nuclear compartment, at least transiently. The role of these proteins in the nuclear compartment and relevance of this function to disease is unclear. So, two CoA synthetic enzymes, both associated with the mitochondria, when defective lead to a GP defect with iron accumulation. Why should this tissue be so vulnerable?
Perspectives
PKAN and CoPAN are two of the seven NBIA disorders. Others are associated with mutations in genes that are not known to have a role in either CoA biosynthesis or iron metabolism and trafficking. Yet all NBIA disorders share the distinctive feature of selective vulnerability of primarily two regions of human brain, GP and SN. More specifically in PKAN, the regions where the disease process is centred are the GP interna and the SN pars reticulata. These two subregions of the basal ganglia are GABAergic and share the role of primary inhibitory output nuclei to the thalamus. They share other features as well, among them is their high iron content, high metabolic demand using primarily glucose, high relative expression of PANK2 compared with other PANKs, and a high tissue concentration of docosahexanoic acid.
The cell expression pattern of PANK2 sheds little light on this vulnerability. PANK2 is highly expressed in large neurons of these regions, but it is abundant in neurons in other regions where there is no disease pathology, including cortex, medullary inferior olivary nucleus and hippocampus, among others. If PANK2 is present in both protected and vulnerable neurons, what other explanations could account for this regional sensitivity?
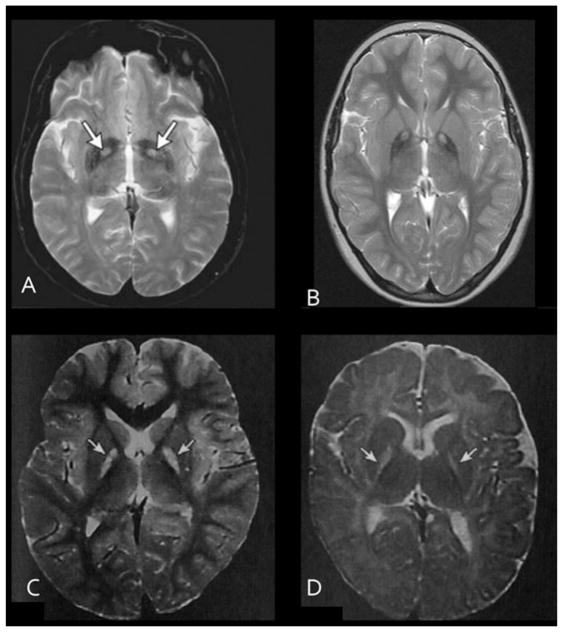
Several toxins and disorders, both exogenous and endogenous, selectively damage GP. These include carbon monoxide (CO), bilirubin and PDH (pyruvate dehydrogenase) deficiency. Brain MRIs of people who survive exposure to these agents or disorders, as in the case of PDH deficiency, demonstrate pallidal lesions similar to those seen in PKAN (Figure 3). On T2-weighted imaging, the pallidi show hyperintense signal in all of these disorders as a result of increased water, as occurs in oedema. Therefore a hypothesis can be developed of the pallidum as a high metabolic demand structure that is vulnerable to subacute oxidative stress from mitochondrial dysfunction caused by intrinsic defects (PDH deficiency, PKAN, CoPAN) or extrinsic factors (CO, bilirubin) [21]. As the precise molecular perturbations of these exposures are discovered, we will gain critical insight into how defects in pantothenate metabolism cause neurodegeneration.
Figure 3. Toxins that selectively damage the GP provide insight into the disease process.
T2-weighted brain MRI transverse sections through the GP show abnormal hyperintense signal (arrows) in (A) carbon monoxide poisoning; (B) PKAN; (C) hyperbilirubinaemia and kernicterus; (D) PDH.
International efforts to develop product replacement therapeutics are underway for PKAN. These promising compounds bypass the enzymatic block at various steps downstream in CoA synthesis. Along with their potential to treat PKAN, these compounds hold promise as tools to reveal new information about pantothenate and CoA biochemistry in normal and disease states.
Acknowledgments
I am grateful to all of the families worldwide with PKAN and related disorders and the physicians who care for them. Their ongoing support of our NBIA Research Programme is essential to its success.
Funding
This work was supported by the NBIA (Neurodegeneration with Brain Iron Accumulation) Disorders Association, Hoffnungsbaum e.V., Associazione Italiana Sindromi Neurodegenerative da Accumulo di Ferro (AISNAF), Association Internationale de Dystrophie Neuro Axonale Infantile (AIDNAI), National Eye Institute (NEI) [grant number 5R01EY012353], National Institute of Child Health and Human Development (NICHD) [grant number 5R01HD050832] and Retrophin Inc. This work was funded by the European Commission’s Seventh Framework Programme [grant number FP7/2007-2013, HEALTH-F2-2011, grant agreement number 277984, TIRCON]. This publication was supported by the Oregon Clinical and Translational Research Institute (OCTRI) [grant number UL1TR000128] from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Abbreviations
- CoPAN
CoA synthase protein-associated neurodegeneration
- GP
globus pallidus
- NBIA
neurodegeneration with brain iron accumulation
- PANK
pantothenate kinase
- PDH
pyruvate dehydrogenase deficiency
- PKAN
pantothenate kinase-associated neurodegeneration
- SN
substantia nigra
References
- 1.Hortnagel K, Prokisch H, Meitinger T. An isoform of hPANK2, deficient in pantothenate kinase-associated neurodegeneration, localizes to mitochondria. Hum Mol Genet. 2003;12:321–327. doi: 10.1093/hmg/ddg026. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MA, Kuo YM, Westaway SK, Parker SM, Ching KH, Gitschier J, Hayflick SJ. Mitochondrial localization of human PANK2 and hypotheses of secondary iron accumulation in pantothenate kinase-associated neurodegeneration. Ann NY Acad Sci. 2004;1012:282–298. doi: 10.1196/annals.1306.023. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi R, Rock CO, Jackowski S, Zhang YM. Activation of human mitochondrial pantothenate kinase 2 by palmitoylcarnitine. Proc Natl Acad Sci USA. 2007;104:1494–1499. doi: 10.1073/pnas.0607621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfonso-Pecchio A, Garcia M, Leonardi R, Jackowski S. Compartmentalization of mammalian pantothenate kinases. PLoS ONE. 2012;7:e49509. doi: 10.1371/journal.pone.0049509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siudeja K, Srinivasan B, Xu L, Rana A, de Jong J, Nollen EA, Jackowski S, Sanford L, Hayflick S, Sibon OC. Impaired coenzyme A metabolism affects histone and tubulin acetylation in Drosophila and human cell models of pantothenate kinase associated neurodegeneration. EMBO Mol Med. 2011;3:755–766. doi: 10.1002/emmm.201100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden–Spatz syndrome. Nat Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- 7.Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, Gitschier J. Genetic, clinical, and radiographic delineation of Hallervorden–Spatz syndrome. N Engl J Med. 2003;348:33–40. doi: 10.1056/NEJMoa020817. [DOI] [PubMed] [Google Scholar]
- 8.Sethi KD, Adams RJ, Loring DW, el Gammal T. Hallervorden–Spatz syndrome: clinical and magnetic resonance imaging correlations. Ann Neurol. 1988;24:692–694. doi: 10.1002/ana.410240519. [DOI] [PubMed] [Google Scholar]
- 9.Kruer MC, Hiken M, Gregory A, Malandrini A, Clark D, Hogarth P, Grafe M, Hayflick SJ, Woltjer RL. Novel histopathologic findings in molecularly-confirmed pantothenate kinase-associated neurodegeneration. Brain. 2011;134:947–958. doi: 10.1093/brain/awr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayflick SJ, Hartman M, Coryell J, Gitschier J, Rowley H. Brain MRI in neurodegeneration with brain iron accumulation with and without PANK2 mutations. AJNR Am J Neuroradiol. 2006;27:1230–1233. [PMC free article] [PubMed] [Google Scholar]
- 11.Hayflick SJ, Hogarth P. As iron goes, so goes disease? Haematologica. 2011;96:1571–1572. doi: 10.3324/haematol.2011.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:551–564. doi: 10.1038/nrn3453. [DOI] [PubMed] [Google Scholar]
- 13.Gregory A, Hayflick S. Neurodegeneration with brain iron accumulation disorders overview. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews®. University of Washington; Seattle: 2013. http://www.ncbi.nlm.nih.gov/books/NBK121988/ [PubMed] [Google Scholar]
- 14.Dusi S, Valletta L, Haack TB, Tsuchiya Y, Venco P, Pasqualato S, Goffrini P, Tigano M, Demchenko N, Wieland T, et al. Exome sequence reveals mutations in CoA synthase as a cause of neurodegeneration with brain iron accumulation. Am J Hum Genet. 2014;94:11–22. doi: 10.1016/j.ajhg.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallari DS, Jackowski S, Rock CO. Regulation of pantothenate kinase by coenzyme A and its thioesters. J Biol Chem. 1987;262:2468–2471. [PubMed] [Google Scholar]
- 16.Garcia M, Leonardi R, Zhang YM, Rehg JE, Jackowski S. Germline deletion of pantothenate kinases 1 and 2 reveals the key roles for CoA in postnatal metabolism. PLoS ONE. 2012;7:e40871. doi: 10.1371/journal.pone.0040871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YM, Rock CO, Jackowski S. Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration. J Biol Chem. 2006;281:107–114. doi: 10.1074/jbc.M508825200. [DOI] [PubMed] [Google Scholar]
- 18.Rana A, Seinen E, Siudeja K, Muntendam R, Srinivasan B, van der Want JJ, Hayflick S, Reijngoud DJ, Kayser O, Sibon OC. Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc Natl Acad Sci USA. 2010;107:6988–6993. doi: 10.1073/pnas.0912105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosveld F, Rana A, van der Wouden PE, Lemstra W, Ritsema M, Kampinga HH, Sibon OC. De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum Mol Gen. 2008;17:2058–2069. doi: 10.1093/hmg/ddn105. [DOI] [PubMed] [Google Scholar]
- 20.Leoni V, Strittmatter L, Zorzi G, Zibordi F, Dusi S, Garavaglia B, Venco P, Caccia C, Souza AL, Deik A, et al. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol Genet Metab. 2012;105:463–471. doi: 10.1016/j.ymgme.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston MV, Hoon AH., Jr Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–591. doi: 10.1177/088307380001500904. [DOI] [PubMed] [Google Scholar]