Abstract
Background
We assessed the outcomes of patients with unresected anaplastic thyroid carcinoma (ATC) in the National Cancer Data Base (NCDB) and explored potential relationships between radiation therapy (RT) dose and overall survival (OS).
Methods
The study group was composed of patients who received either no surgery or grossly incomplete resection. Correlates of OS were explored using univariate and multivariable analysis (MVA) analyses.
Results
A total of 1,288 patients were analyzed. Mean age was 70.2 years; 59.7% were female; and 47.6% received neck RT. Median OS was 2.27 months, with 11% alive at one year. A positive RT dose-survival relationship was seen for the entire study cohort, for those who received systemic therapy, and for those with stage IVA/IVB and IVC disease. On MVA, older age (HR: 1.317, CI: 1.137–1.526), ≥1 comorbidity (HR: 1.587, CI: 1.379–1.827), distant metastasis (HR: 1.385, CI: 1.216 –1.578), receipt of systemic therapy (HR: 0.637, CI: 0.547–0.742), and receipt of RT as compared with no RT (HR <45 Gy: 0.843, CI: 0.718–0.988; HR 45-59.9 Gy: 0.596, CI: 0.479–0.743; HR 60-75 Gy: 0.419, CI: 0.339 – 0.517) correlated with OS. The RT dose-survival relationship for those who received higher (60-75 Gy) vs. lower (45-59.9 Gy) therapeutic dose was confirmed by propensity score matching.
Conclusions
Survival was poor in this cohort of patients with unresected ATC and more effective therapies are needed. However, the association of RT dose with OS highlights the importance of identifying patients with unresected ATC who may still yet benefit from multi-modal local-regional treatment incorporating higher dose RT.
Keywords: Anaplastic thyroid carcinoma, Radiation therapy, National Cancer Data Base, Head and Neck, Propensity-score matching
Introduction
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive malignancies in the head and neck. While numerically rare, comprising 1-2% of all thyroid cancers, it has a grim prognosis, as ATC accounts for approximately 40% of all thyroid cancer-related deaths.1,2,3 Median survival ranges from 3-10 months, with long-term survival rates of <20%, as patients routinely present with advanced disease.4,5 Patients with ATC often present with rapidly growing and immediately threatening local tumor not amenable to meaningful resection. There are as yet no standard, highly effective treatment regimens for ATC, but treatment is generally multi-modal.6–8 Fit patients presenting without sign of distant metastases are evaluated for surgery and adjuvant therapy, to provide the best chance for a favorable outcome. However, since complete surgical resection is rarely feasible in ATC, radiation therapy (RT), often with concurrent chemotherapy, is considered in an attempt to induce local tumor regression (and achieve interim local control), to avoid or delay local progression, preventing or deferring airway obstruction, severe dysphagia, and/or death secondary to overwhelming local tumor burden, scenarios unfortunately common in these patients, despite prompt tracheostomy.9–13
Because presentation with or development of subsequent distant metastases is also common, clinicians are faced with the challenge of how to prioritize local therapies and their intent or aggressiveness.14,15,16 At our center, the presence of distant metastases does not necessarily preclude the use of upfront locally palliative therapy to either treat or prevent impending symptoms or death from tracheal or esophageal compromise, as medically fit patients with smaller volume distant disease may still benefit from more durable local tumor control through the use of RT.
Despite the poor overall prognosis of patients with unresected/unresectable local or distant metastatic disease and the palliative nature of treatment in these scenarios, we hypothesize those treatment regimens incorporating higher doses of neck RT may at least delay local tumor progression and thus translate to improved patient survival, given the rapidity of mortality for progressive disease. Given the rarity of this disease, and thus the inability of any single-center (even a high-volume tertiary center) to accrue sufficient numbers for statistical validity, we evaluated the survival of patients with unresected ATC within the National Cancer Data Base (NCDB).
The specific goals of the present study are to:
Characterize survival outcomes for patients with unresected ATC (i.e. those who received either no surgery or had grossly incomplete resection);
Explore patient, tumor, and treatment specific correlates of overall survival;
Assess RT dose-survival relationships, and define clinical subgroups of interest for future analyses
Methods
Dataset
The NCDB is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB, established in 1989, is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that captures 70% of all newly diagnosed malignancies in the United States annually, including 92% of all thyroid cancers.17 These cases come from approximately 1,500 CoC-accredited cancer programs. It is the world’s largest oncology outcomes database and contains over 30 million historical records. The data used in the study is derived from a de-identified NCDB file. Access to this Health Insurance Portability and Accountability (HIPAA)-compliant data was provided to the author CP as part of the NCDB’s Participant Use File (PUF) program.
Clinical variables of patients with ATC reported to the NCDB from 1998 to 2012 were retrieved from the NCDB using the International Classification of Disease, 3rd edition (ICD-O-3) code 8021/3. Those diagnosed after 2011 were excluded due to lack of available survival data at the time of data extraction and this analysis. As the purpose of this study was to focus on ATC patients with locally advanced or unresected/unresectable tumors, patients with tumor size <1cm and those who underwent complete surgical resection of macroscopic disease (classified as either a “R0” or “R1” resection) were excluded. Those who had gross residual tumor following attempted surgery (classified as a “R2” resection), or who did not undergo surgery were included.
Specific data examined included patient age at diagnosis, gender, race/ethnicity, presence of comorbidities, tumor size, lymph node involvement, evidence of distant metastasis, AJCC TNM category and stage grouping, type of surgery performed, surgical margin status, receipt of chemotherapy as part of the first course of treatment, and patient survival. RT details collected were: treatment site, modality, dose, number of fractions, overall treatment time, and interval from surgery to RT start. Details regarding type of systemic therapy and patterns of disease recurrence (local, regional or distant relapse) are not available within the NCDB.
Overall survival (OS) was compared by patient, tumor, and treatment groups of interest, including by neck RT dose. In order to explore the neck RT dose-survival relationship, we considered those who received 60-75 Gy to have received a higher therapeutic dose and those 45-59.9 Gy a lower, potentially therapeutic dose. Those who received <45 Gy were considered to have received palliative intent RT and are described here for completeness. Those who received >75 Gy were excluded from survival comparison analyses as they were considered to have received doses beyond the usual therapeutic range for this disease and anatomic site.
Statistical analysis
ANOVA and Pearson chi-square tests were used for continuous and categorical data. Median follow-up for surviving patients was calculated using the reverse Kaplan-Meier estimate. Survival curves were generated using the Kaplan-Meier product-limit method and were compared using the log-rank test. Time-to-event was indexed to the date of diagnosis. Correlates of OS were explored using univariate and multivariable analyses (MVA) via Cox proportional hazards approach for non-matched comparison. Propensity score matching was subsequently performed using 1:1 nearest neighbor method without replacement, for patients receiving higher versus lower therapeutic dose of neck RT.18 Balance was assessed using mean standardized differences. Univariate Weibull parametric survival analysis using the propensity match score as a stratifier was implemented to calculate the β-coefficient and hazard ratio of death as a function of RT dose. Propensity score adjusted p-value < 0.05 was considered significant. Analyses were performed using SPSS 23.0 (IBM Corp., Armonk, NY).
Results
Patients
Of the 355,028 cases of thyroid malignancy registered in the NCDB from 1998-2012, 3,266 (0.92%) were ATC. Of these, 2,987 were diagnosed between 1998 and 2011 and were also ≥1cm in size. 1,727 of these did not receive any surgery or had grossly incomplete surgical resection (i.e. R2 resection). Of these, 439 patients were excluded: 202 for having no documented target of RT, 110 for having a distant RT target, and 127 for incomplete RT dose information. The most common distant RT targets were documented as, “Other NOS,” “Lung/Chest,” and “Brain.” The 1,288 remaining patients who had either received documented RT to the neck, or received no RT, comprised the final study cohort. Of these, 674 (52.3%) received no neck RT, 294 (22.8%) <45 Gy, 134 (10.4) 45–59.9 Gy, 178 (13.8%) 60–75Gy, and 8 (0.6%) received >75 Gy (Figure 1). Of the 614 patients who received RT, details regarding dose per fraction were available for 544 (88.6%), and of these, 80 (14.7%) received ≤1.5Gy/fraction, a dose per fraction commonly used in hyperfractionation schedules. However, details RT fractionation schedules are not specifically recorded in the NCDB.
Figure 1.
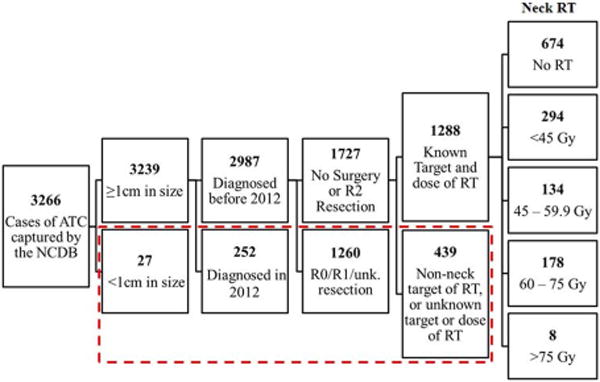
Study cohort selection from the National Cancer Data Base (1998-2012). Those in the dashed red box were excluded from this study. (ATC: Anaplastic thyroid carcinoma; RT: Radiation therapy; Unk: Unknown; Gy: Gray)
Patient, tumor, and treatment characteristics by neck RT dose group are shown in Table 1. For the study cohort, treatment was provided at 627 unique institutions. The mean number of cases treated per institution over the study period was 2.0 and the maximum at any single facility was 21 cases. Overall, 47.2% had distant metastases at the time of diagnosis, 45.8% regional lymph node involvement (N+), and 52.6% T4b tumors. When considering the neck RT groups of interest, the group that received 45–59.9 Gy, in comparison to the group that received 60–75 Gy was no different in mean age, gender, ethnicity, comorbidities, mean tumor size, lymph node involvement, receipt of surgery, and receipt systemic therapy, but were more likely to have had distant metastasis at diagnosis (48% vs. 34%, p = 0.011) and to have received intensity modulated radiation therapy technique (43.8% vs. 27.6%, p = 0.003). Of the 674 that received no neck RT, 582 (84%) also received no systemic therapy.
Table 1.
Patient, tumor, and treatment characteristics in unresected anaplastic thyroid cancer by neck radiation therapy group.
| No neck RT | <45 Gy | 45–59.9 Gy | 60–75 Gy | p-value* | |
|---|---|---|---|---|---|
| n=674 | n=294 | n=134 | n=178 | ||
| Average age, yrs. | 73.8 | 71.6 | 68.5 | 67.7 | 0.509 |
| Female gender (%) | 66.3 | 55.8 | 43.3 | 52.8 | 0.096 |
| White race (%) | 83.4 | 86.1 | 86.6 | 85.4 | 0.768 |
| Charlson Deyo score = 0 (%) | 70.7 | 71.8 | 78.0 | 78.6 | 0.898 |
| Mean tumor size (cm) | 6.88 | 8.16 | 6.52 | 6.81 | 0.632 |
| N+ (%) | 39.8 | 52.0 | 55.2 | 50.6 | 0.414 |
| M1 (%) | 48.5 | 52.0 | 48.5 | 34.3 | 0.011 |
| Receipt of any neck surgery (%) | 12.9 | 8.5 | 11.9 | 12.9 | 0.795 |
| Receipt of systemic therapy (%) | 13.6 | 46.6 | 73.9 | 80.9 | 0.139 |
| Days from diagnosis to RT start, median | - | 11 | 14 | 16 | 0.200 |
| RT dose (Gy), mean | - | 25.1 | 52.3 | 65.7 | - |
| RT number of fractions, median | - | 10 | 28 | 33 | - |
| RT technique, % IMRT | - | 11.9 | 27.6 | 43.8 | 0.003 |
comparison between 45–59.9 Gy and 60–75 Gy subgroups only
RT: Radiation therapy; Gy: Gray; IMRT: Intensity modulated radiation therapy
Survival Analyses
Median follow-up for surviving patients was 5.5 years. The median survival for the cohort overall was 2.27 months (standard error [SE] +/- 0.102), with 11.29% and 6.55% of patients alive at one and two years, respectively (see Supplementary Figure). The survival curves by neck RT group for the study cohort overall, those who received systemic therapy, and those with stage IVA/IVB and IVC disease are shown in Figure 2. A positive dose-survival relationship was demonstrated for each aforementioned group of interest on this univariate analysis, although differences for those with stage IVC disease were small. For those receiving systemic therapy and RT and for those with stage IVA/IVB disease, visible separation in the tail region of the curve shows extended survival in the higher RT dose group, with relative plateau of the curve after 2 years. For the cohort overall, median survival in months (+/- SE) was 1.31 (+/- 0.08) for those who did not receive neck RT, 1.97 (+/- 0.127) for the 1-44.9 Gy group, 4.240 (+/- 0.355) for the 45-59.9 Gy group, and 6.77 (+/- 0.391) for the 60-75 Gy group.
Figure 2.
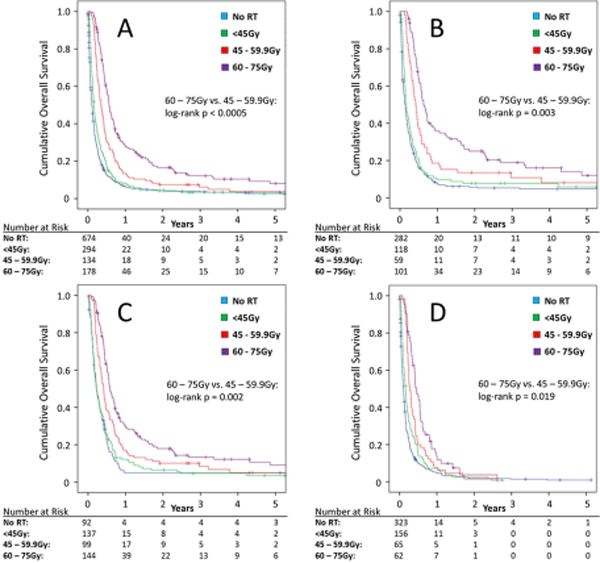
Actuarial survival in unresected anaplastic thyroid carcinoma by neck radiation therapy group for the overall study cohort (A), those with stage IVA/IVB disease (B), those who received systemic therapy (C), and those with stage IVC disease (D). (RT: Radiation therapy; Gy: Gray)
Correlates of survival
Results from the univariate and multivariable analysis are shown in Table 2. Variables included in the multivariable model were those statistically significant on univariate analysis, or established as a clinically important prognostic factor. The only variable included in the MVA that was not statistically significant on univariate testing was presence of nodal metastases, an established AJCC staging variable. On MVA, advanced age (HR: 1.317, CI: 1.137–1.526), ≥1 comorbidity (HR: 1.587, CI: 1.379 –1.827), distant metastasis (HR: 1.385, CI: 1.216 –1.578), receipt of systemic therapy (HR: 0.637, CI: 0.547–0.742), and receipt of RT as compared with no RT group (HR <45Gy: 0.843, CI: 0.718–0.988; HR 45–59.9Gy: 0.596, CI: 0.479–0.743; HR 60-75Gy: 0.419, CI: 0.339 – 0.517) correlated with patient survival.
Table 2.
Correlates of survival in patients with unresected anaplastic thyroid cancer: results of univariate and multivariable model.
| Univariate | Multivariable | ||||
|---|---|---|---|---|---|
| p-value | p-value | HR | 95% CI | ||
| Lower | Upper | ||||
| Age ≥65 vs Age <65 | <0.001 | <0.001 | 1.317 | 1.137 | 1.526 |
| Female vs. male | 0.008 | 0.696 | 1.026 | 0.902 | 1.167 |
| White vs. not white | 0.124 | - | - | - | - |
| 1 or more comorbidity vs. none | <0.001 | <0.001 | 1.587 | 1.379 | 1.827 |
| T4b vs. T4a | 0.609 | - | - | - | - |
| N+ vs. N0 | 0.811 | 0.324 | 1.066 | 0.939 | 1.210 |
| M1 vs. M0 | <0.001 | <0.001 | 1.385 | 1.216 | 1.578 |
| R2 resection vs. no surgery | 0.004 | 0.019 | 0.786 | 0.643 | 0.962 |
| Chemotherapy vs. none | <0.001 | <0.001 | 0.637 | 0.547 | 0.742 |
| RT dose (reference: no RT [0 Gy]) | - | - | - | - | - |
| <45 Gy | 0.001 | 0.035 | 0.843 | 0.718 | 0.988 |
| 45-59.9 Gy | <0.001 | <0.001 | 0.596 | 0.479 | 0.743 |
| 60-75 Gy | <0.001 | <0.001 | 0.419 | 0.339 | 0.517 |
HR: Hazard ratio; CI: Confidence interval; RT: Radiation therapy; Gy: Gray
Propensity Score Matched Analysis
To further explore the neck RT dose-survival relationship and to reduce the influence of patient selection bias and other confounding factors, propensity score matching was performed on the 312 patients who received neck RT within the specified therapeutic range (45-75 Gy). They were matched via propensity score according to age, gender, T-category, presence of nodal involvement, presence of metastasis at diagnosis, receipt of systemic therapy, and receipt of R2 surgery. We considered the treatment group to be those who received higher therapeutic dose (60-75 Gy [n = 178]), and the control group to be those who received lower, potentially therapeutic dose (45-59.9 Gy [n = 134]). Eventually, 125 patients in each RT dose cohort matched successfully. Balance between variables in both cohorts is shown in the Supplementary Table. Patients who received 60–75 Gy had significantly better OS compared with patients who received 45-59.9 Gy (31% vs 16% at 1-year, stratified log-rank p=.008, Figure 3). Univariate Weibull parametric survival analysis using the propensity match score as a stratifier showed that patients who received lower dose RT had higher risk of death compared to those who received higher dose RT (β-coefficient of 1.16, 95% CI 1.02-1.30; equivalent to a hazard ratio of 1.2, 95% CI 1.02 – 1.54; p = 0.027).
Figure 3.
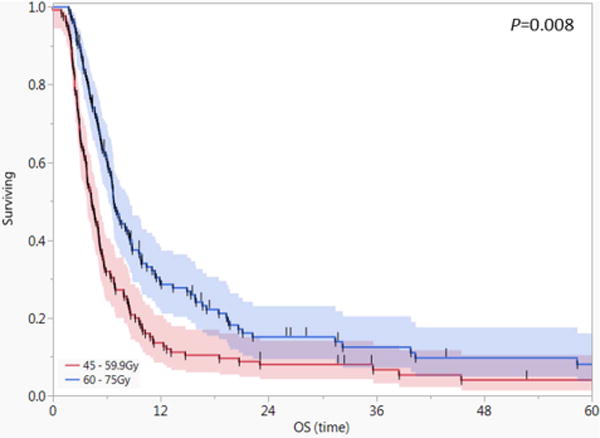
Actuarial survival curves for the propensity score matched cohorts with anaplastic thyroid carcinoma (n=250). Shaded areas represent 95% confidence intervals and short vertical lines censored data.
Discussion
Patients with unresected/unresectable ATC remain a significant challenge for clinicians due in part to the extremely aggressive local-regional behavior and frequent synchronous or rapid development of distant metastases, resulting in unsatisfying survival and often unsuccessful palliative efforts. We observed, in this large-scale, multi-site dataset, that survival of patients with unresected ATC within the NCDB was, as expected, poor. Nonetheless, patients who received treatment that incorporated higher dose neck RT showed incontrovertibly extended survival, though it remains unclear if, as we suspect, this is potentially due to more durable local tumor control, or uncollected confounders hidden within this large-scale registry. This dose-survival relationship was maintained when restricting the comparison to only those selected to receive more than traditional palliative doses of neck RT (≥45 Gy), suggesting that this was not likely to present a discrimination between dose as a surrogate short-course temporizing palliative radiotherapy (e.g. the classic 30 Gy in 10 fraction or “quad-shot” 14 Gy in four fraction regimens routinely used for pain control without expectation of local response).19 In order to better isolate a potential dose-survival relationship, we elected to compare these two groups using propensity score matching with the hypothesis being, accounting for differentials in other variables, patients treated in the lower dose range could have also been candidates for higher dose RT.
Regarding the patterns of care observed within this unresected/unresectable cohort, the majority of patients did not receive any neck RT, and within in this “no neck RT” group, only ~14% received any systemic therapy. This coupled with the strikingly short median survival in the no neck RT group (<2 months) suggests these patients may have been either judged to be unfit or unlikely to benefit from any cancer-directed therapy. Of those who did receive neck RT, 49% received what would be considered non-therapeutic (e.g. pain reduction/palliative) doses of RT (<45 Gy) and the observed median survival in this group was also less than 2 months. Overall, only 24% received RT to the neck in what we specified to be in the usual therapeutic range (45-75 Gy); this suggests that a minority of patients are being selected for more definitive RT schedules, and points to the utility of population level databases, such as NCDB to benchmark patterns of care and outcomes in a group so rare that neither institutional or cooperative group studies will likely provide Level 1 evidentiary guidance. Alternatively, it also points to a potential window for improved outcomes with more routine incorporation of RT, a widely available therapy, which may currently be underutilized in this population.
Glaser et al. recently queried the NCDB for all patients with ATC, and noted an association between survival and the following features: absence of lymph node metastases, no distant metastasis, tumors <=6 cm, R0 resection, total thyroidectomy and receipt of high dose RT (>59.4 Gy).20 The observation of comparatively longer survival associated with complete surgery and higher dose RT is consistent with multiple single institutional series.21–24 Unfortunately, per Glasser et al., only 13% of patients with ATC in the NCDB received an R0 resection and only ~22% higher dose neck RT, suggesting a limited minority were/are selected for or eligible for more aggressive local therapy approaches, which is also supported by our study.
The present study focuses on a single “intact disease” cohort, specifically, in order to assess dose-response signal in the NCDB, in an effort to restrict the potential confounding effects of surgical therapy. Thus, the observation of substantively improved survival in a cohort with intact disease suggest that, rather than intractable cases with no indication for local therapy, in fact, these cases are potentially candidates even in scenarios when complete resection is not an availed option. Additionally, a statistically significant dose-survival relationship was also maintained, albeit of small magnitude, even for patients with distant metastatic disease (stage IVC), suggesting a competing risk of death from local progression and distant metastatic progression might be altered by sufficient local therapy. Similar to Glaser’s study we found multiple patient, disease, and treatment characteristics correlated with ATC patient survival. Taken together, the results of our study and Glaser’s study may help inform clinicians as to who might benefit most from therapy, and alludes to need for patients to be evaluated for RT at therapeutic doses not only post-operatively, but in fit patients with unresectable disease.
As with any large-scale database, the standard caveats apply regarding data quality and generalizability.25 For example, any palliative benefit of RT or other treatments received could not be quantified and toxicity data is not directly available within the NCDB. Given that most patients with ATC have incurable disease, the preservation of quality of life and side effects of available therapies must be carefully balanced, but is veiled in this dataset. Although the NCDB represents the largest repository of data on ATC patients, and is thus a reasonable and justifiable approximation of the national outcomes for this disease, the incidence of tracheostomy, RT fractionation scheme, planned versus delivered RT dose, patient performance status, time from symptoms to diagnosis, clinical tumor kinetics (rate of tumor growth), patterns of disease recurrence, local-regional control rates, and disease-specific survival data are not available and would add much desired nuance to our findings. Lack of central pathology review raises the specter of misattribution of diagnosis for rare tumors, and conceivably those with more extended survival could have had a more differentiated carcinoma or differentiated component, as they can have a more favorable prognosis that those with “pure” ATC.26 Additionally, survivorship bias could contribute to the magnitude of the observed outcome effect in those treated with higher RT doses.
However, given the sparse numerical prevalence of ATC, single institution reports of such limited power as to render conclusions, if not suspect in terms of statistical validity, at least preclude ready generalization outside of the treating institution. Multicenter studies and population-based datasets such as the NCDB represent a cost-effective, readily available mechanism to characterize patterns of care and outcomes for such rare malignancies. That said, and despite attempts to control for inherent biases using a propensity-matching, our results should be considered hypothesis-generating and indicative, rather than definitive in scope.
Clearly more effective therapies for ATC are needed and treatment on clinical trial at centers with multidisciplinary expertise should be prioritized. A number of targeted agents and immunotherapy are being investigated in ATC, but how to integrate these with local therapies is yet to be defined.27 Durable local tumor control may become even more important if systemic therapy for the treatment of distant metastatic disease becomes more effective, as we are still likely on the proximal end of the local therapy benefit curve described by Punglia et al.28 With the ultimate goal to improve patient outcomes and in order to (1) more rapidly identify and evaluate patients with localized and potentially resectable tumors for upfront surgery, (2) initiate timely non-surgical therapies for the rest with unresectable or distant disease, and (3) prioritize palliation and symptom support for those medically unfit for cancer-directed treatment, we have initiated a fast track program at our institution for patients with (or suspected to have) ATC.29 Based on our institutional experience, current clinical guidelines, and the supportive findings from this and other retrospective analyses, we continue to consider upfront local regional therapy incorporating higher dose neck RT for those with localized disease at presentation and for select fit patients with smaller volume distant metastatic disease.30
Conclusion
Higher therapeutic dose of RT was associated with improved survival for unresected/unresectable ATC in the NCDB database. Overall, survival was poor as anticipated, and more effective systemic and local therapies are urgently needed. While the utilization of RT was relatively low in this cohort, these results highlight the importance of ensuring patients are evaluated by multi-disciplinary teams including radiation oncologists in order to identify more patients with ATC who may still yet benefit from combined modality local-regional treatment, particularly regimens incorporating higher dose neck RT.
Supplementary Material
Supplementary Figure: Actuarial survival in unresected anaplastic thyroid carcinoma.
Acknowledgments
None
Grant: P30CA016672.
Footnotes
Funding & Conflicts: The authors have no conflicts of interest or funding to disclose
Author Contributions: All authors assisted in study design, drafting, and final approval of the manuscript. Authors T Pezzi, A Mohamed, T Sheu, P Blanchard, CD Fuller, and GB Gunn also assisted in statistical analysis.
References
- 1.Kebebew E, Greenspan F, Clark O, Woeber K, McMillian A. Anaplastic Thyroid Carcinoma: Treatment Outcome and Prognostic Factors. Cancer. 2004;103:1330–1335. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- 2.Haymart MR, Banerjee M, Yin H, Worden F, Griggs JJ. Marginal Treatment Benefit in Anaplastic Thyroid Cancer. Cancer. 2013;119(17):3133–3139. doi: 10.1002/cncr.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–1153. doi: 10.1016/j.surg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Besic N, Hocevar M, Zgajnar J, Grazio-Frkovic S, Auersperg M. Prognostic factors in anaplastic carcinoma of the thyroid — a multivariate survival analysis of 188 patients. Arch Surg. 2005;390:203–208. doi: 10.1007/s00423-004-0524-5. [DOI] [PubMed] [Google Scholar]
- 5.Pezzi T, Sandulache V, Pezzi C, et al. Treatment and Survival of Patients with Insular Thyroid Carcinoma: 508 Cases from the National Cancer Data Base. Head Neck. 2015;38(6):906–912. doi: 10.1002/elan. [DOI] [PubMed] [Google Scholar]
- 6.Tennvall J, Lundell G, Hallquist A, Wahlberg P, Wallin G, Tibblin S. Combined doxorubicin, hyperfractionated radiotherapy, and surgery in anaplastic thyroid carcinoma. Cancer. 1994;74(4):1348–1354. doi: 10.1002/1097-0142(19940815)74:4<1348::aid-cncr2820740427>3.0.co;2-d. http://www.ncbi.nlm.nih.gov/pubmed/8055459. [DOI] [PubMed] [Google Scholar]
- 7.Stavas MJ, Shinohara ET, Attia A, Ning MS, Friedman JM, Cmelak AJ. Short Course High Dose Radiotherapy in the Treatment of Anaplastic Thyroid Carcinoma. J Thyroid Res. 2014;2014:1–7. doi: 10.1155/2014/764281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haigh PI, Ituarte PH, Wu HS, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91(12):2335–2342. doi: 10.1002/1097-0142(20010615)91:12<2335::AID-CNCR1266>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and Radiotherapy Improves Survival in Patients With Anaplastic Thyroid Carcinoma. Am J Clin Oncol. 2008;31(5):460–464. doi: 10.1097/COC.0b013e31816a61f3. [DOI] [PubMed] [Google Scholar]
- 10.Levy A, Leboulleux S, Lepoutre-Lussey C, et al. 18F-fluorodeoxyglucose positron emission tomography to assess response after radiation therapy in anaplastic thyroid cancer. Oral Oncol. 2015;51(4):370–375. doi: 10.1016/j.oraloncology.2014.12.014. http://dx.doi.org/10.1016/j.oraloncology.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Sherman EJ, Lim SH, Ho AL, et al. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: A critical re-evaluation including uniform pathologic review. Radiother Oncol. 2011;101(3):425–430. doi: 10.1016/j.radonc.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Tsang R, Asa S, Dickson B, Arenovich T, Brierley J. Clinical outcome of anaplastic thyroid carcinoma treated with radiotherapy of once- and twice-daily fractionation regimens. Cancer. 2006;107(8):1786–1792. doi: 10.1002/cncr.22203. [DOI] [PubMed] [Google Scholar]
- 13.Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic Thyroid Cancer: A Review of Epidemiology, Pathogenesis, and Treatment. J Oncol. 2011;2011:2–13. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130(6):1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell G, Huddart R, Harmer C. Phase II evaluation of high dose accelerated radiotherapy for anaplastic thyroid carcinoma. Radiother Oncol. 1999;50(1):33–38. doi: 10.1016/s0167-8140(98)00102-9. http://www.ncbi.nlm.nih.gov/pubmed/10225555. [DOI] [PubMed] [Google Scholar]
- 16.Goffredo P, Thomas SM, Adam MA, Sosa JA, Roman SA. Impact of Timeliness of Resection and Thyroidectomy Margin Status on Survival for Patients with Anaplastic Thyroid Cancer: An Analysis of 335 Cases. Ann Surg Oncol. 2015;22(13):4166–4174. doi: 10.1245/s10434-015-4742-6. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum BYPR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 19.Corry J, Peters LJ, Costa ID, et al. The “QUAD SHOT ”— a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137–142. doi: 10.1016/j.radonc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S. Anaplastic thyroid cancer: Prognostic factors, patterns of care, and overall survival. Head Neck. 2016:1–8. doi: 10.1002/HED. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia A, Rao A, Ang K, et al. Anaplastic Thyroid Cancer: Clinical Outcomes with Conformal Radiotherapy. Head Neck. 2010 Jul;:829–836. doi: 10.1002/hed. [DOI] [PubMed] [Google Scholar]
- 22.De Crevoisier R, Baudin E, Bachelot A, et al. Combined Treatment of Anaplastic Thyroid Carcinoma With Surgery, Chemotherapy, and Hyperfractionated Accelerated External Radiotherapy. IJROBP. 2004;60(4):1137–1143. doi: 10.1016/j.ijrobp.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Foote R, Molina J, Kasperbauer J, Lloyd R. Enhanced Survival in Locoregionally Confined Anaplastic Thyroid Carcinoma: A Single-Institution Experience Using Aggressive Multimodal Therapy. Thyroid. 2011;21:25–30. doi: 10.1089/thy.2010.0220. [DOI] [PubMed] [Google Scholar]
- 24.Mohebati A, Dilorenzo M, Palmer F, et al. Anaplastic Thyroid Carcinoma : A 25-year Single-Institution Experience. Ann Surg Oncol. 2014;21:1665–1670. doi: 10.1245/s10434-014-3545-5. [DOI] [PubMed] [Google Scholar]
- 25.Garden AS, Fuller CD, Rosenthal D, et al. Reply to Radiotherapy for Human Papillomavirus-Positive Oropharyngeal Cancers in the National Cancer Data Base. Cancer. 2016:1–2. doi: 10.1002/cncr.30193. [DOI] [PubMed] [Google Scholar]
- 26.Sun XS, Sun SR, Guevara N, et al. Chemoradiation in anaplastic thyroid carcinomas. Crit Rev Oncol Hematol. 2013;86(3):290–301. doi: 10.1016/j.critrevonc.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Cabanillas ME, Zafereo M, Gunn GB, Ferrarotto R. Anaplastic Thyroid Carcinoma: Treatment in the Age of Molecular Targeted Therapy. J Oncol Pract. 2016;12(6) doi: 10.1200/JOP.2016.012013. [DOI] [PubMed] [Google Scholar]
- 28.Punglia R, Morrow M, Winer E, Harris J. Local Therapy and Survival in Breast Cancer. NEJM. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 29.Cabanillas ME, Busaidy N, Khan S, Gunn GB, Dadu R. Molecular diagnostics and anaplastic thyroid carcinoma: the time has come to harvest the high hanging fruit. Int J Endocr Oncol. 2016;3(3):221–233. [Google Scholar]
- 30.Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association for Management of Patients with Anaplastic Thyroid Cancer. Thyroid. 2012;22(11):1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: Actuarial survival in unresected anaplastic thyroid carcinoma.


