Abstract
We report the Ni-catalyzed Suzuki–Miyaura coupling of aliphatic amide derivatives. Prior studies have shown that aliphatic amide derivatives can undergo Ni-catalyzed carbon–heteroatom bond formation but that Ni-mediated C–C bond formation using aliphatic amide derivatives has remained difficult. The coupling disclosed herein is tolerant of considerable variation with respect to both the amide-based substrate and the boronate coupling partner and proceeds in the presence of heterocycles and epimerizable stereocenters. Moreover, a gram-scale Suzuki–Miyaura coupling/Fischer indolization sequence demonstrates the ease with which unique polyheterocyclic scaffolds can be constructed, particularly by taking advantage of the enolizable ketone functionality present in the cross-coupled product. The methodology provides an efficient means to form C–C bonds from aliphatic amide derivatives using nonprecious-metal catalysis and offers a general platform for the heteroarylation of aliphatic acyl electrophiles.
Keywords: nickel, catalysis, cross-coupling, aliphatic, amides
Graphical abstract
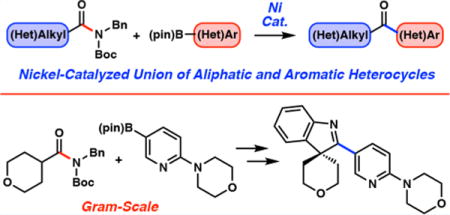
The facile unification of molecular fragments via C–C bond formation represents an important and challenging objective in transition-metal catalysis.1 Although the field has been largely dominated by the coupling of aryl electrophiles, there has been a recent resurgence in developing analogous methods using stable acyl electrophiles. More specifically, esters and amides have emerged as useful synthetic building blocks in a variety of acyl cross-coupling manifolds. Recent breakthroughs in the area include the Suzuki–Miyaura coupling of phenyl esters reported independently by Newman and Szostak, which proceeds using palladium catalysis,2,3 in addition to numerous amide C–N bond activation studies using either palladium or nickel.4–9
We and others have been especially interested in using nickel catalysis to enable facile C–C bond formation from amide derivatives. Such methods provide new strategies for the synthesis of ketones which complement Weinreb’s methodology10 but importantly avoid the use of highly basic or pyrophoric reagents. Previously, we have shown that nickel catalysis can promote the cross-coupling of Ts- or Boc-activated benzamide derivatives in C–C bond forming reactions.4b,d,k These cross-coupling platforms have allowed for the efficient coupling of aryl amide electrophiles; however, the corresponding activation of aliphatic amides is more challenging. Prior computational studies suggest that the use of aliphatic amides is inherently more difficult because of the high kinetic barrier of activation associated with oxidative addition into the resonance-stabilized C–N bond.4a Indeed, achievements in cross-couplings of aliphatic amides using Ni catalysis is limited to carbon–heteratom bond formation.4h,o Molander and coworkers have also reported an elegant coupling of N-acyl succinimides with alkyl trifluoroborate salts employing a dual-metal photoredox approach using nickel and an iridium photocatalyst,9 which nicely complements the method described herein.11,12
With the aim of developing a general cross-coupling manifold to build C–C bonds from aliphatic amides, we targeted the Suzuki–Miyaura coupling shown in Figure 1. From the outset, we opted to focus our efforts on the coupling of heterocyclic fragments due to their prevalence in bioactive molecules. Certain heterocycles can be challenging to employ in metal-mediated cross-couplings, as they are known to ligate metal catalysts and inhibit reactivity.1b Moreover, only a handful of isolated examples of heteroarylative Suzuki–Miyaura couplings of aliphatic acyl electrophiles exist13 (i.e., anhydrides,13a,b thioesters,13c,d and acid chlorides13e,f), and a general platform for the heteroarylation of aliphatic acyl electrophiles has not been developed. In this paper, we describe the nickel-catalyzed Suzuki–Miyaura coupling of aliphatic amide derivatives. Importantly, this methodology provides rapid access to functionalizable heterocyclic scaffolds while expanding the scope of synthetically useful transformations involving amide derivatives and nonprecious-metal catalysis.
Figure 1.
Suzuki–Miyaura heteroarylation of aliphatic amides to construct polyheterocyclic scaffolds.
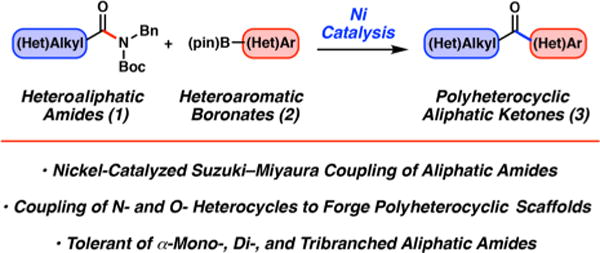
To initiate our study, we examined the coupling of piperidine derivative 414 with N-methylpyrrole-2-boronic acid pinacol ester (5), as shown in Figure 2. Our initial attempts to employ the N-heterocyclic carbene (NHC) ligand SIPr (7), which we had previously shown to be competent in the Suzuki–Miyaura coupling of aromatic amide derivatives,4b were met with difficulty, as no trace of the desired ketone product 6 was formed at 50 °C (entry 1). Moreover, increasing the temperature to 120 °C only led to partial decomposition of substrate 4 (entry 2). Next, we screened several ligand frameworks that have been used in the context of nickel-catalyzed couplings. Interestingly, efforts to utilize the ligand terpyridine (8), which had been shown to facilitate the nickel-catalyzed esterification of aliphatic amide derivatives,4h were also unfruitful (entry 3). Gratifyingly, however, use of the NHC precursor ICy·HBF4 (9) was found to promote the desired Suzuki–Miyaura coupling and delivered ketone 6 in 95% yield (entry 4). Ligand 9 has been used in other nickel-catalyzed processes,5b,f,15 including in the Heck reaction of benzamide derivatives.4k Finally, the related NHC precursor Benz-ICy·HCl (10) was evaluated and found to give similarly useful results (entry 5). As NHC precursor 10 was found to be broadly effective in subsequent scouting experiments, it was used in our further studies.16 Finally, although we focus on the use of N-Bn, Boc amides in this study, it should be noted that the methodology is not limited to the use of the N-benzyl group. For example, coupling of N-iPr, Boc cyclohexamide with boronate 5 under the optimized conditions gave the corresponding ketone in 72% yield.
Figure 2.
Evaluation of reaction conditions for the nickel-catalyzed coupling of aliphatic amide 4 with boronate 5 to furnish ketone 6. Legend: (a) Ni(cod)2 (5 mol %), 7–10 (10 mol %), substrate 4 (1.0 equiv), boronate 5 (2.5 equiv), K3PO4 (4.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at the indicated temperature for 16 h. Yields were determined by 1H NMR analysis using hexamethylbenzene as an internal standard.
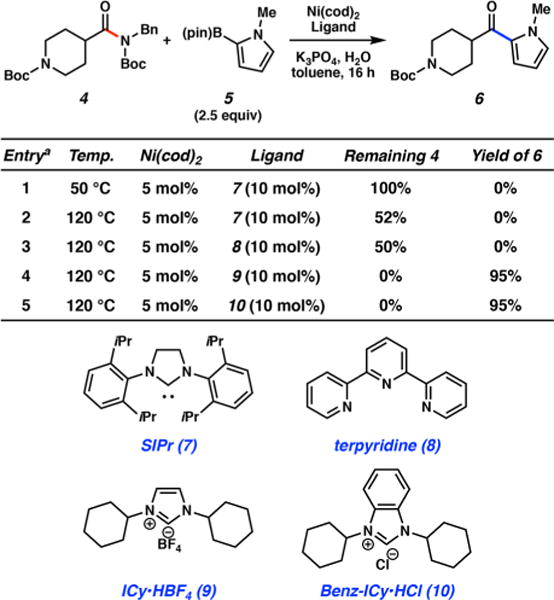
With the optimized conditions in hand, we explored the scope of the coupling with respect to both the heteroaliphatic amide-derived substrate and the heteroaryl boronate to afford a variety of bis-heterocyclic ketone products (Figure 3). The reaction was found to be widely tolerant of N-heterocyclic boronate nucleophiles, including pyrrole, quinoline, indole, pyrazole, and morpholino-pyridine moieties, as demonstrated by the formation of 6 and 11–16, all in good yields. Moreover, an isomeric piperidine amide substrate could be utilized, allowing for the formation of pyrrolo- and pyrazolo-ketones 17 and 18, respectively. Alternatively, the pyrrolidine heterocycle could also be employed to generate ketones 19 and 20 in 82% and 90% yields, respectively. Finally, substrates derived from both 4- and 3-isomers of tetrahydropyrancarboxylic acid were shown to be competent in the coupling, furnishing ketones 21–25 in good to excellent yields. The formation of 25 highlights the use of an oxygen-containing heterocyclic boronate in the coupling reaction. It is also worth noting that nonheterocyclic aryl boronates, such as 2-naphthyl and phenyl boronic esters, could be employed in the Suzuki–Miyaura coupling, as demonstrated by the formation of 26 and 27, respectively. In addition, o-Me, p-CF3, and p-CO2Me substituents were tolerated on the phenyl boronate, giving rise to ketones 28–30, respectively.17
Figure 3.
Scope of the Suzuki–Miyaura coupling with heteroaliphatic amide substrates and aryl boronates. Conditions: Ni(cod)2 (5 mol %), 10 (10 mol %), substrate (1.0 equiv), boronate (2.5 equiv), K3PO4 (4.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at 120 °C for 16 h. Unless otherwise noted, yields reflect the average of two isolation experiments. Legend: (a) reaction run using 3.3 equiv of the boronate; (b) yield determined by 1H NMR analysis using hexamethylbenzene as an external standard; (c) reaction run for 24 h using 5.0 equiv of the boronate.
The scope of the heteroarylative coupling with boronate 5 was also evaluated with respect to several nonheterocyclic aliphatic amide derivatives (Figure 4). Substrates derived from dihydrocinnamic and decanoic acids coupled in high yields to furnish ketones 31 and 32, respectively. Additionally, α-branched carbocyclic amides also underwent efficient couplings, providing pyrrolo-ketones 33 and 34. Finally, sterically encumbered carboxamides could also be employed in the coupling, as demonstrated by the production of tert-butyl ketone 35 in excellent yield.
Figure 4.
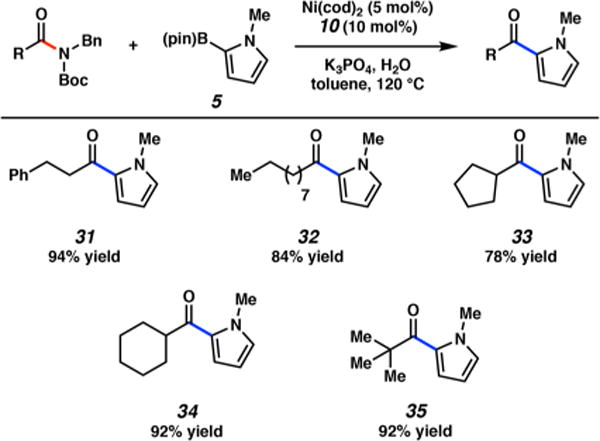
Scope of the coupling with nonheterocyclic aliphatic amide substrates and boronate 5. Conditions: Ni(cod)2 (5 mol %), 10 (10 mol %), substrate (1.0 equiv), boronate 5 (2.5 equiv), K3PO4 (4.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at 120 °C for 16 h. Yields reflect the average of two isolation experiments.
Although our paper focuses on aliphatic amides for the reasons mentioned earlier, we were curious if our optimal reaction conditions could be applied to a benzamide substrate (Figure 5). We have reported earlier the coupling of N-Bn, Boc benzamide 36 with phenylboronic acid pinacol ester 37 using a Ni/SIPr system at 50 °C. This gives ketone 38 in 96% yield (entry 1).4b We performed the corresponding coupling of 36 and 37 using the Ni/Benz-ICy catalyst system. At 50 °C, we obtained only a 14% yield of the cross-coupled product, 38 (entry 2). We also performed the cross-coupling using the Ni/Benz-ICy catalyst system at 120 °C, which furnished 38 in 60% yield (entry 3).18 As such, for practioners of this methodology, we recommend the use of Ni/SIPr at 50 °C to achieve the Suzuki–Miyaura coupling of benzamide-type substrates4b and the use of the conditions reported herein (i.e., Ni/Benz-ICy at 120 °C) for aliphatic amides.
Figure 5.
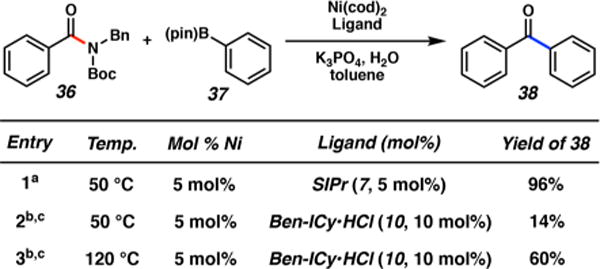
Suzuki–Miyaura coupling of amide 36 with boronate 37 using Ni/SIPr and Ni/Benz-ICy catalyst systems. Legend: (a) Ni(cod)2 (5 mol %), 7 (5 mol %), substrate (1.0 equiv), boronate 5 (1.2 equiv), K3PO4 (2.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at 50 °C for 24 h; (b) Ni(cod)2 (5 mol %), 10 (10 mol %), substrate (1.0 equiv), boronate 5 (2.5 equiv), K3PO4 (4.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at the indicated temperature for 16 h; (c) yields reflect the average of two experiments. Yields were determined by 1H NMR analysis using hexamethylbenzene as an external standard.
We also questioned if the methodology would be amenable to the coupling of an amide substrate containing a defined chiral center α to the carbonyl. As such, we attempted the coupling between amide 39 and boronate 5 (Figure 6). Although the use of standard conditions (i.e., 120 °C for 16 h) gave the desired ketone product 40 in 68% yield, roughly 20% epimerization was also observed. We found that, by carrying out the reaction at 90 °C for 2 h, the epimerization could be avoided. Thus, ketone 40 was obtained in 70% yield, without observable formation of the syn diastereomer. Moreover, the tolerance of the ester (and other functional groups)19 underscores the complementarity of this methodology to the Weinreb ketone synthesis,10 where such electrophilic functional groups typically do not withstand the use of highly basic and nucleophilic organometallic reagents. Importantly, this result provides the first example of an amide or ester Suzuki–Miyaura coupling that proceeds smoothly in the presence of an epimerizable stereocenter α to the amide carbonyl. The tolerance of the method to defined stereocenters α to the carbonyl was also evaluated using enantioenriched cyclohexenyl amide 41. Using standard conditions (i.e., 120 °C for 16 h), the desired ketone 42 was obtained in 81% yield, but only in 14% ee. When the temperature of the reaction was lowered to 70 °C, the desired coupling of 41 with boronate 5 proceeded in good yield and with significant preservation of stereochemical information. We hypothesize that the observed epimerization stems from the basicity of the deprotonated Benz-ICy·HCl (10). In fact, subjection of enantioenriched 42 to the free NHC in toluene at 120 °C for 4 h led to complete racemization of the substrate. In contrast, the corresponding experiments performed with Benz-ICy·HCl (10) or K3PO4 led to no or minimal observable loss in ee, respectively. It should also be noted that ketone product 42 was observed to racemize more readily in comparison to amide 41 under the standard reaction conditions (see the Supporting Information for details). Nonetheless, these results demonstrate the mildness of the reaction conditions and bode well for future synthetic applications.
Figure 6.
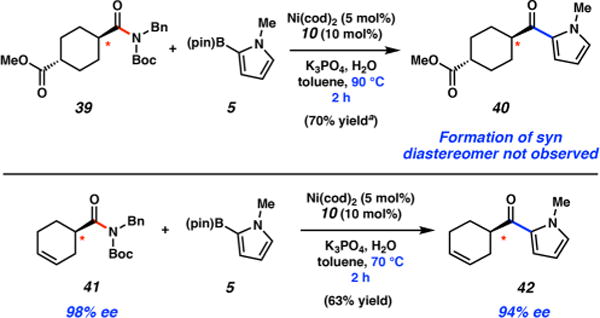
Stereoretentive Suzuki–Miyaura couplings of amide 39 and enantioenriched amide 41. Conditions: Ni(cod)2 (5 mol %), 10 (10 mol %), substrate (1.0 equiv), boronate 5 (2.5 equiv), K3PO4 (4.0 equiv), toluene (1.0 M), and H2O (2.0 equiv) heated at 70 °C for 2 h. Yield reflects the average of two isolation experiments. Legend: (a) reaction run at 90 °C for 2 h. Yield determined by 1H NMR analysis using hexamethylbenzene as an external standard.
In comparison to more classical aryl–aryl couplings, the products obtained from this methodology possess enolizable ketones, which serve as valuable synthetic handles. As a demonstration of this benefit, we performed a gram-scale Suzuki–Miyaura coupling and subsequent Fischer indolization reaction to construct a polyheterocyclic spiroindolenine scaffold (Figure 7). Spiroindolenines are commonly seen in bioactive molecules20 and also serve as valuable synthetic intermediates.21 In this case, Suzuki–Miyaura coupling of tetrahydropyran carboxamide 43 with boronate 44 took place on a gram scale under conditions employing reduced boronate, catalyst, and ligand loadings (1.2 equiv, 2.5 and 5 mol %, respectively) to furnish ketone 45 in 82% yield. Next, ketone 45 was transformed into spirocycle 47 in 61% yield by reaction with phenylhydrazine (46) in the presence of TFA by way of a Fischer indolization.22 The rapid construction of polyheterocyclic spiroindolenine 47,23 hinging upon the classical reactivity of enolizable ketones, underscores the utility of the Suzuki–Miyaura coupling of aliphatic amides and further demonstrates the ease with which a variety of unique heterocyclic compounds can be fashioned.
Figure 7.
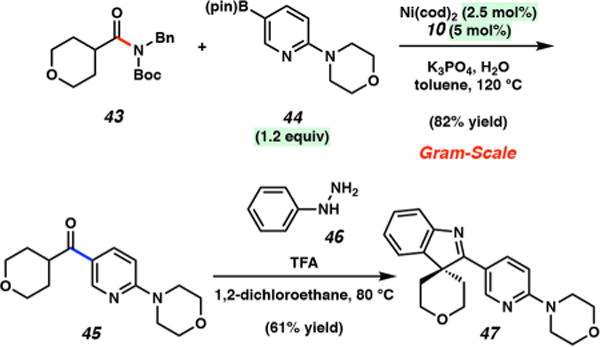
Sequential gram-scale Suzuki–Miyaura coupling and Fischer indolization to provide 47.
We have developed the nickel-catalyzed Suzuki–Miyaura coupling of aliphatic amides. The coupling was found to be tolerant of variation in both coupling partners and can be employed in the presence of heterocycles, epimerizable stereocenters, and sensitive functional groups (e.g., esters). The synthetic utility of this methodology was further demonstrated on a gram scale via a Suzuki–Miyaura coupling/Fischer indolization sequence to form polyheterocyclic spiroindolenine 47. These studies offer a general platform for the heteroarylation of aliphatic acyl electrophiles, while contributing to the repertoire of synthetic transformations involving amide derivatives and nonprecious-metal catalysis. Moreover, given their stability toward a variety of conditions, we view amides as having significant potential utility as synthons in the derivatization of biomolecules and multistep synthetic efforts.
Supplementary Material
Acknowledgments
The authors are grateful to the NIH-NIGMS (R01 GM117016), the UCLA Gold Shield Alumnae, and the University of California, Los Angeles, for financial support. K. Holman (Amgen Scholars Program) is acknowledged for experimental assistance, and we thank the Nelson laboratory (UCLA) for use of instrumentation. N.A.W. acknowledges the National Science Foundation (DGE-1144087) and the Foote Family, and T.B.B. acknowledges the University of California, Los Angeles, for fellowship support. These studies were also supported by shared instrumentation grants from the NSF (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscatal.7b03688.
Complete experimental procedures, analytical, and spectral data, including control experiments and robustness screen (PDF)
ORCID
Neil K. Garg: 0000-0002-7793-2629
Notes
The authors declare no competing financial interest.
References
- 1.(a) Miyaura N. In: Metal-Catalyzed Cross-Coupling Reactions. 2nd. de Meijere A, Diederich F, editors. Vol. 1. Wiley-VCH; Weinheim, Germany: 2004. pp. v–vi.pp. 41 [Google Scholar]; (b) Kotschy A, Timári T. Heterocycles from Transition Metal Catalysis: Formation and Functionalization. Springer; Dordrecht, The Netherlands: 2005. p. 176. [Google Scholar]; (c) Littke A. In: Modern Arylation Methods. Ackermann L, editor. Wiley-VCH; Weinheim, Germany: 2009. p. 29. [Google Scholar]; (d) Schröter S, Stock C, Bach T. Tetrahedron. 2005;61:2245–2267. [Google Scholar]
- 2.Recently, Szostak and Newman independently reported the Pd-catalyzed Suzuki–Miyaura coupling of phenol-derived esters. Both methods are highly focused on aryl esters, although four examples involving nonbranched aliphatic substrates were disclosed in total between the two studies. One example utilizing an α-branched substrate was reported, albeit with some challenges noted by Newman:; (a) Ben Halima T, Zhang W, Yalaoui T, Hong X, Yang YF, Houk KN, Newman SG. J Am Chem Soc. 2017;139:1311–1318. doi: 10.1021/jacs.6b12329. [DOI] [PubMed] [Google Scholar]; (b) Lei R, Meng G, Shi S, Ling Y, An J, Szostak R, Szostak M. Chem Sci. 2017;8:6525–6530. doi: 10.1039/c7sc02692g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For additional nondecarbonylative transition-metal-catalyzed reactions involving cleavage of the ester C–O bond, see:; (a) Tatamidani H, Kakiuchi F, Chatani N. Org Lett. 2004;6:3597–3599. doi: 10.1021/ol048513o. [DOI] [PubMed] [Google Scholar]; (b) Hie L, Fine Nathel NF, Hong X, Yang Y-F, Houk KN, Garg NK. Angew Chem Int Ed. 2016;55:2810–2814. doi: 10.1002/anie.201511486. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yu B, Sun H, Xie Z, Zhang G, Xu LW, Zhang W, Gao Z. Org Lett. 2015;17:3298–3301. doi: 10.1021/acs.orglett.5b01466. [DOI] [PubMed] [Google Scholar]; (d) LaBerge NA, Love JA. Eur J Org Chem. 2015;2015:5546–5553. [Google Scholar]; (e) Tatamidani H, Yokota K, Kakiuchi F, Chatani N. J Org Chem. 2004;69:5615–5621. doi: 10.1021/jo0492719. [DOI] [PubMed] [Google Scholar]; (f) Desnoyer AN, Friese FW, Chiu W, Drover MW, Patrick BO, Love JA. Chem - Eur J. 2016;22:4070–4077. doi: 10.1002/chem.201504959. [DOI] [PubMed] [Google Scholar]; (g) Shi S, Szostak M. Organometallics. 2017;36:3784–3789. [Google Scholar]; (h) Dardir AH, Melvin PR, Davis RM, Hazari N, Beromi MM. J Org Chem. 2017 doi: 10.1021/acs.joc.7b02588. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a recent review, see; (i) Takise R, Muto K, Yamaguchi J. Chem Soc Rev. 2017;46:5864–5888. doi: 10.1039/c7cs00182g. [DOI] [PubMed] [Google Scholar]; (j) Cheung CW, Ploeger ML, Hu X. Nat Commun. 2017;8:14878–14887. doi: 10.1038/ncomms14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For nickel-catalyzed reactions involving cleavage of the amide C–N bond, see:; (a) Hie L, Fine Nathel NF, Shah T, Baker EL, Hong X, Yang Y-F, Liu P, Houk KN, Garg NK. Nature. 2015;524:79–83. doi: 10.1038/nature14615. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weires NA, Baker EL, Garg NK. Nat Chem. 2016;8:75–79. doi: 10.1038/nchem.2388. [DOI] [PubMed] [Google Scholar]; (c) Baker EL, Yamano MM, Zhou Y, Anthony SM, Garg NK. Nat Commun. 2016;7:11554–11558. doi: 10.1038/ncomms11554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Simmons BJ, Weires NA, Dander JE, Garg NK. ACS Catal. 2016;6:3176–3179. doi: 10.1021/acscatal.6b00793. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Dander JE, Weires NA, Garg NK. Org Lett. 2016;18:3934–3936. doi: 10.1021/acs.orglett.6b01758. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shi S, Szostak M. Org Lett. 2016;18:5872–5875. doi: 10.1021/acs.orglett.6b02952. [DOI] [PubMed] [Google Scholar]; (g) Shi S, Szostak M. Chem - Eur J. 2016;22:10420–10424. doi: 10.1002/chem.201602202. [DOI] [PubMed] [Google Scholar]; (h) Hie L, Baker EL, Anthony SM, Desrosiers JN, Senanayake C, Garg NK. Angew Chem Int Ed. 2016;55:15129–15132. doi: 10.1002/anie.201607856. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Dey A, Sasmal S, Seth K, Lahiri GK, Maiti D. ACS Catal. 2017;7:433–437. [Google Scholar]; (j) Ni S, Zhang W, Mei H, Han J, Pan Y. Org Lett. 2017;19:2536–2539. doi: 10.1021/acs.orglett.7b00831. [DOI] [PubMed] [Google Scholar]; (k) Medina JM, Moreno J, Racine S, Du S, Garg NK. Angew Chem Int Ed. 2017;56:6567–6571. doi: 10.1002/anie.201703174. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Hu J, Wang M, Pu X, Shi Z. Nat Commun. 2017;8:14993–14999. doi: 10.1038/ncomms14993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Weires NA, Caspi DD, Garg NK. ACS Catal. 2017;7:4381–4385. doi: 10.1021/acscatal.7b01444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Shi S, Szostak M. Synthesis. 2017;49:3602–3608. [Google Scholar]; (o) Dander JE, Baker EL, Garg NK. Chem Sci. 2017;8:6433–6438. doi: 10.1039/c7sc01980g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Huang PQ, Chen H. Chem Commun. 2017;53:12584–12587. doi: 10.1039/c7cc07457c. [DOI] [PubMed] [Google Scholar]; (q) Deguchi T, Xin HL, Morimoto H, Ohshima T. ACS Catal. 2017;7:3157–3161. [Google Scholar]; Fora recent review, see:; (r) Dander JE, Garg NK. ACS Catal. 2017;7:1413–1423. doi: 10.1021/acscatal.6b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For nickel-catalyzed decarbonylative coupling reactions of amides, see:; (a) Shi S, Meng G, Szostak M. Angew Chem, Int Ed. 2016;55:6959–6963. doi: 10.1002/anie.201601914. [DOI] [PubMed] [Google Scholar]; (b) Hu J, Zhao Y, Liu J, Zhang Y, Shi Z. Angew Chem, Int Ed. 2016;55:8718–8722. doi: 10.1002/anie.201603068. [DOI] [PubMed] [Google Scholar]; (c) Srimontree W, Chatupheeraphat A, Liao HH, Rueping M. Org Lett. 2017;19:3091–3094. doi: 10.1021/acs.orglett.7b01194. [DOI] [PubMed] [Google Scholar]; (d) Liu C, Szostak M. Angew Chem Int Ed. 2017;56:12718–12722. doi: 10.1002/anie.201707102. [DOI] [PubMed] [Google Scholar]; (e) Yue H, Guo L, Liao HH, Cai Y, Zhu C, Rueping M. Angew Chem Int Ed. 2017;56:4282–4285. doi: 10.1002/anie.201611819. [DOI] [PubMed] [Google Scholar]; (f) Chatupheeraphat A, Liao HH, Lee SC, Rueping M. Org Lett. 2017;19:4255–4258. doi: 10.1021/acs.orglett.7b01905. [DOI] [PubMed] [Google Scholar]; (g) Yue H, Guo L, Lee SC, Liu X, Rueping M. Angew Chem Int Ed. 2017;56:3972–3976. doi: 10.1002/anie.201612624. [DOI] [PubMed] [Google Scholar]; (h) Lee S-C, Guo L, Yue H, Liao H-H, Rueping M. Synlett. 2017;28:2594–2598. [Google Scholar]; (i) Hu J, Wang M, Pu X, Shi Z. Nat Commun. 2017;8:14993–14999. doi: 10.1038/ncomms14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For palladium-catalyzed C–C bond forming reactions of amides, see:; (a) Li X, Zou G. Chem Commun. 2015;51:5089–5092. doi: 10.1039/c5cc00430f. [DOI] [PubMed] [Google Scholar]; (b) Yada A, Okajima S, Murakami M. J Am Chem Soc. 2015;137:8708–8711. doi: 10.1021/jacs.5b05308. [DOI] [PubMed] [Google Scholar]; (c) Meng G, Szostak M. Org Biomol Chem. 2016;14:5690–5705. doi: 10.1039/c6ob00084c. [DOI] [PubMed] [Google Scholar]; (d) Meng G, Szotak M. Angew Chem Int Ed. 2015;54:14518–14522. doi: 10.1002/anie.201507776. [DOI] [PubMed] [Google Scholar]; (e) Meng G, Szostak M. Org Lett. 2015;17:4364–4367. doi: 10.1021/acs.orglett.5b02209. [DOI] [PubMed] [Google Scholar]; (f) Liu C, Meng G, Liu Y, Liu R, Lalancette R, Szostak R, Szostak M. Org Lett. 2016;18:4194–4197. doi: 10.1021/acs.orglett.6b01836. [DOI] [PubMed] [Google Scholar]; (g) Lei P, Meng G, Szostak M. ACS Catal. 2017;7:1960–1965. [Google Scholar]; (h) Liu C, Liu Y, Liu R, Lalancette R, Szostak R, Szostak M. Org Lett. 2017;19:1434–1437. doi: 10.1021/acs.orglett.7b00373. [DOI] [PubMed] [Google Scholar]; (i) Liu C, Meng G, Szostak M. J Org Chem. 2016;81:12023–12030. doi: 10.1021/acs.joc.6b02294. [DOI] [PubMed] [Google Scholar]; (j) Meng G, Shi S, Szostak M. ACS Catal. 2016;6:7335–7339. [Google Scholar]; (k) Cui M, Wu H, Jian J, Wang H, Liu C, Stelck D, Zeng Z. Chem Commun. 2016;52:12076–12079. doi: 10.1039/c6cc06428k. [DOI] [PubMed] [Google Scholar]; (l) Wu H, Li Y, Cui M, Jian J, Zeng Z. Adv Synth Catal. 2016;358:3876–3880. [Google Scholar]; (m) Shi S, Szostak M. Org Lett. 2017;19:3095–3098. doi: 10.1021/acs.orglett.7b01199. [DOI] [PubMed] [Google Scholar]; (n) Lei P, Meng G, Ling Y, An J, Szostak M. J Org Chem. 2017;82:6638–6646. doi: 10.1021/acs.joc.7b00749. [DOI] [PubMed] [Google Scholar]; (o) Meng G, Szostak R, Szostak M. Org Lett. 2017;19:3596–3599. doi: 10.1021/acs.orglett.7b01575. [DOI] [PubMed] [Google Scholar]; (p) Meng G, Lalancette R, Szostak R, Szostak M. Org Lett. 2017;19:4656–4659. doi: 10.1021/acs.orglett.7b02288. [DOI] [PubMed] [Google Scholar]; (q) Osumi Y, Szostak M. Org Biomol Chem. 2017;15:8867–8871. doi: 10.1039/c7ob02269g. [DOI] [PubMed] [Google Scholar]; (r) Lei P, Meng G, Ling Y, An J, Nolan SP, Szostak M. Org Lett. 2017;19:6510–6513. doi: 10.1021/acs.orglett.7b03191. [DOI] [PubMed] [Google Scholar]
- 7.For examples of Pd-catalyzed Suzuki–Miyaura coupling of aliphatic amides, see:; Li X, Zou G. J Organomet Chem. 2015;794:136–145. [Google Scholar]
- 8.The field of nickel-catalyzed cross-couplings itself has gained tremendous interest in recent years due not only to the high natural abundance, low cost, and low CO2 footprint of nickel but also its ability to effect new or challenging transformations, including those involving activation of the amide C–N bond. For pertinent reviews on nickel catalysis, see:; (a) Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita AM, Garg NK, Percec V. Chem Rev. 2011;111:1346–1416. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mesganaw T, Garg NK. Org Process Res Dev. 2013;17:29–39. [Google Scholar]; (d) Ananikov VP. ACS Catal. 2015;5:1964–1971. [Google Scholar]
- 9.Amani J, Alam R, Badir S, Molander GA. Org Lett. 2017;19:2426–2429. doi: 10.1021/acs.orglett.7b00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahm S, Weinreb SM. Tetrahedron Lett. 1981;22:3815–3818. [Google Scholar]
- 11.As Molander’s methodology utilizes alkyl boron reagents (see ref 9), and ours uses aryl boron reagents, the two methods offer different strategic approaches to access ketone products.
- 12.The less activated N-Bn, Boc amides used in the present study are not competent substrates in the Ir/Ni photoredox-mediated couplings of N-acyl succinimides (see ref 9).
- 13.(a) Gooßen LJ, Ghosh K. Angew Chem Int Ed. 2001;40:3458–3460. doi: 10.1002/1521-3773(20010917)40:18<3458::aid-anie3458>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]; (b) Gooßen LJ, Ghosh K. Eur J Org Chem. 2002;2002:3254–3257. [Google Scholar]; (c) Yang H, Li H, Wittenberg R, Egi M, Huang W, Liebeskind LS. J Am Chem Soc. 2007;129:1132–1140. doi: 10.1021/ja0658719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lovell KM, Vasiljevik T, Araya JJ, Lozama A, Prevatt-Smith KM, Day VW, Dersch CM, Rothman RB, Butelman ER, Kreek MJ, Prisinzano TE. Bioorg Med Chem. 2012;20:3100–3110. doi: 10.1016/j.bmc.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Haddach M, McCarthy JR. Tetrahedron Lett. 1999;40:3109–3112. [Google Scholar]; (f) Nique F, Hebbe S, Triballeau N, Peixoto C, Lefrancois JM, Jary H, Alvey L, Manioc M, Housseman C, Klaassen H, Van Beeck K, Guedin D, Namour F, Minet D, Van Der Aar E, Feyen J, Fletcher S, Blanque R, Robin-Jagerschmidt C, Deprez P. J Med Chem. 2012;55:8236–8247. doi: 10.1021/jm300281x. [DOI] [PubMed] [Google Scholar]
- 14.The N-Bn, Boc amide derivatives employed in this study can be readily prepared from the corresponding carboxylic acids (i.e., benzyl amide formation, followed by treatment with Boc2O) or by direct means from acid chlorides (i.e., using HN(Bn)Boc).
- 15.For use of the Ni/ICy system in the Stille coupling of quaternary ammonium salts, see:; Wang DY, Kawahata M, Yang ZK, Miyamoto K, Komagawa S, Yamaguchi K, Wang C, Uchiyama M. Nat Commun. 2016;7:12937–12945. doi: 10.1038/ncomms12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.We attribute the improved competency of ligand 10 to its electron-rich nature, which ultimately renders oxidative addition more facile. For discussion of NHC ligands in transition-metal catalysis, see:; Hopkinson MN, Richter C, Schedler M, Glorius F. Nature. 2014;510:485–496. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]
- 17.For the relative nucleophilicities of substituted aryl boronates, see:; (a) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]; (b) Billingsley KL, Buchwald SL. Angew Chem Int Ed. 2008;47:4695–4698. doi: 10.1002/anie.200801465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The underpinnings behind the modest catalyst turnover (i.e., Figure 5, entries 2 and 3) is not presently understood.
- 19.In addition to esters, ketones, tertiary alcohols, secondary amides, carboxylic acids, and epoxides are tolerated in this methodology, as determined by a robustness screen; see the Supporting Information for details.
- 20.For bioactive spiroindolenines, see:; (a) Weisbach JA, Macko E, De Sanctis NJ, Cava MP, Douglas B. J Med Chem. 1964;7:735–739. doi: 10.1021/jm00336a012. [DOI] [PubMed] [Google Scholar]; (b) Fourtillan JB, Violeau B, Karam O, Jouannetaud MP, Fourtillan M, Jacquesy JC. Melatoninergic Agonist Spiro[indolepyrrolidine] Derivatives. Process for Their Preparation and Their Use as Medicinal Products U.S Patent US576347. 1998; (c) Chowdhury S, Fu J, Liu S, Qi J. Spiro-Condensed Indole Derivatives as Sodium Channel Inhibitors World Patent WO2010/53998. 2010; (d) Li XN, Cai XH, Feng T, Li Y, Liu YP, Luo XD. J Nat Prod. 2011;74:1073–1078. doi: 10.1021/np2000254. [DOI] [PubMed] [Google Scholar]; (e) Xu YJ, Pieters L. Mini-Rev Med Chem. 2013;13:1056–1072. doi: 10.2174/1389557511313070009. [DOI] [PubMed] [Google Scholar]; (f) Sweis RF, Pliushchev M, Brown PJ, Guo J, Li F, Maag D, Petros AM, Soni NB, Tse C, Vedadi M, Michaelides MR, Chiang GG, Pappano WN. ACS Med Chem Lett. 2014;5:205–209. doi: 10.1021/ml400496h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Manske RHF. The Alkaloids, Chemistry and Physiology. Academic Press; New York: 1981. [Google Scholar]; (b) Cordell GA. The Alkaloids: Chemistry and Biology. Academic Press; San Diego, CA: 1998. [Google Scholar]; (c) Chadha N, Silakari O. Eur J Med Chem. 2017;134:159–184. doi: 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 22.For the classic Fischer indole synthesis, see:; (a) Fischer E, Jourdan F. Ber Dtsch Chem Ges. 1883;16:2241–2245. [Google Scholar]; (b) Fischer E, Hess O. Ber Dtsch Chem Ges. 1884;17:559–568. [Google Scholar]
- 23.Indolino-spirotetrahydropyrans have been pursued as kinase inhibitors of the P13K-Akt pathway, ion channel modulators, 5-HT2c receptor agonists, HDAC inhibitors, and inhibitors of CMV DNA polymerase.; (a) Gonzalez-Lopez de Turiso F, Shin Y, Brown M, Cardozo M, Chen Y, Fong D, Hao X, He X, Henne K, Hu Y-L, Johnson MG, Kohn T, Lohman J, McBride HJ, McGee LR, Medina JC, Metz D, Miner K, Mohn D, Pattaropong V, Seganish J, Simard JL, Wannberg S, Whittington DA, Yu G, Cushing TD. J Med Chem. 2012;55:7667–7685. doi: 10.1021/jm300679u. [DOI] [PubMed] [Google Scholar]; (b) Brown M, Shin Y, Cushing TD, Gonzalez-Lopez de Turiso F, He X, Kohn T, Lohman JW, Pattaropong V, Seganish J, Shin Y, Simard JL. Heterocyclic Compounds and Their Uses World Patent WO2010/151737. 2010; (c) Chen G, Cushing TD, Fisher B, He X, Li K, Li Z, McGee LR, Pattaropong V, Faulder P, Seganish JL, Shin Y. Alkynyl Alcohols as Kinase Inhibitors World Patent WO2009/158011. 2009; (d) Padiya K, Kamlesh J, Nair PS, Rivindra RR, Chaure GS, Gudade GB, Parkale SS, Manojkumar VL, Swapnil RB, Smita AB. Spirocyclic Compounds as Voltage-Gated Sodium Channel Modulators World Patent WO2012/49555. 2012; (e) Semple G, Behan DP, Feichtinger K, Glicklich A, Grottick AJ, Kam MMS, Kasem M, Lehmann J, Ren AS, Schrader TO, Shanahan WR, Wong AST, Zhu X. 5-HT2c Receptor Agonists and Compositions and Methods of Use World Patent WO2015/66344. 2015; (f) Ng PY, Davis H, Bair KW, Millan DS, Rudintskaya A, Zheng X, Han B, Barczak N, Lancia D., Jr 3-Spiro-7-Hydroxamic Acid Tetralins as HDAC Inhibitors World Patent WO2016/168660. 2016; (g) Thibeault C, Rancourt J, Beaulieu PL, Dec A, Grand-Maitre C, Kuhn C, Villemure E, Leblanc M, Lacoste J-E, Moreau B, Jolicoeur E, Surprenant S, Hucke O. Inhibitors of Cytomegalovirus World Patent WO2014/70976. 2014
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


