Abstract
Genetic elements compete for transmission through meiosis, when haploid gametes are created from a diploid parent. Selfish elements can enhance their transmission through a process known as meiotic drive. In female meiosis, selfish elements drive by preferentially attaching to the egg side of the spindle. This implies some asymmetry between the two sides of the spindle, but molecular mechanisms underlying spindle asymmetry are unknown. Here we found that CDC42 signaling from the cell cortex regulated microtubule tyrosination to induce spindle asymmetry. Non-Mendelian segregation depended on this asymmetry. Cortical CDC42 depends on polarization directed by chromosomes, which are positioned near the cortex to allow the asymmetric cell division. Thus, selfish meiotic drivers exploit the asymmetry inherent in female meiosis to bias their transmission.
Genetic conflict is inherent in any haploid-diploid life cycle because genetic elements compete for transmission through meiosis. Mendel’s Law of Segregation states that alleles of a gene are transmitted with equal probability, but this law can be violated by selfish genetic elements through meiotic drive, for example by eliminating competing gametes (e.g., sperm killing or spore killing) or by exploiting the asymmetry in female meiosis to increase transmission to the egg. Despite the impact of meiotic drive on evolution and genetics (1–4), the underlying mechanisms are largely unknown. Female meiosis provides a clear opportunity for selfish elements to cheat because only chromosomes that segregate to the egg can be transmitted to offspring, while the rest are degraded in polar bodies. Conceptually, female meiotic drive depends on three conditions: asymmetry in cell fate, a functional difference between homologous chromosomes that influences their segregation, and asymmetry within the meiotic spindle (5). The asymmetry in cell fate is well established (6), and chromosomal rearrangements and amplifications of repetitive sequences (e.g., centromeres) are associated with biased segregation (7–10). Asymmetry within the meiotic spindle was noted in grasshopper in 1976 (11) but not studied further.
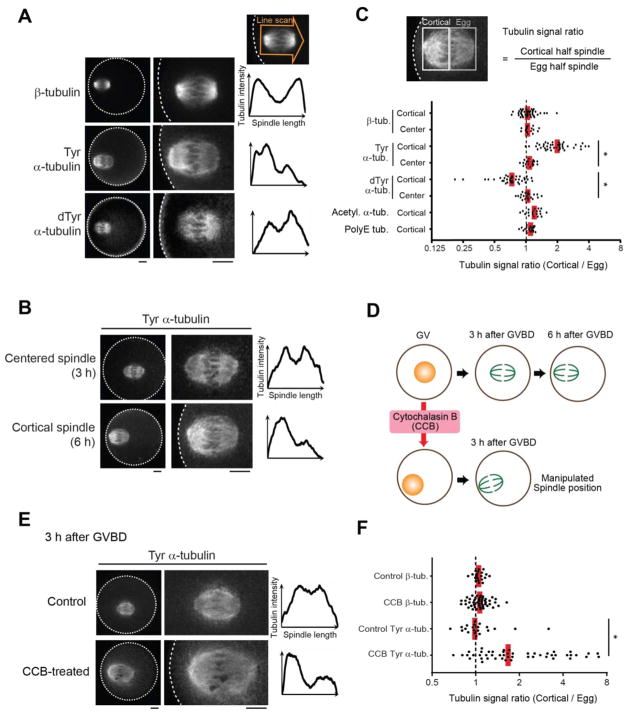
Oocyte spindles are positioned close to the cortex and oriented perpendicular to the cortex so that cytokinesis produces a large egg and a small polar body. A selfish element drives by preferentially attaching to the egg side of the spindle, implying some difference in microtubules (MTs) between the egg and cortical sides. To determine how such spindle asymmetry is regulated, using mouse oocytes as a model for meiotic drive (10, 12), we tested for asymmetry in post-translational modifications that functionally diversify MTs (Fig. S1A) (13–15). Only tyrosinated (Tyr) and detyrosinated (dTyr) α-tubulin showed asymmetry, with the cortical side enriched for Tyr α-tubulin and the egg side for dTyr α-tubulin (Fig. 1, A and C; Fig. S1B). Furthermore, we found that spindles were asymmetric late in metaphase of meiosis I (MI) when positioned near the cortex, but not earlier when positioned in the center of the oocyte (Fig. 1, B to D; Fig. S2). Because the MI spindle first forms in the center and then migrates towards the cortex (16–20), asymmetry might depend on either cortical proximity or time, or both. To distinguish between these possibilities, we manipulated spindle position by treating oocytes with cytochalasin B (CCB) before maturation to inhibit actin polymerization. The nucleus drifted to the cortex in 24% of these oocytes, with the spindle positioned near the cortex by 3 h after germinal vesicle breakdown (GVBD) vs. migration at 6 h under normal conditions (Fig. 1D; Fig. S3A). Cortical spindles in CCB-treated oocytes showed asymmetric Tyr α-tubulin staining at 3 h after GVBD, whereas β-tubulin staining remained symmetric (Fig. 1, E and F; Fig. S3B). Similar results were obtained with cytochalasin D (Fig. S3C). Asymmetry could be created by the spindle pole closer to the cortex generating higher levels of Tyr α-tubulin. However, mis-oriented spindles parallel to the cortex also had stronger Tyr α-tubulin signals on the cortical side, inconsistent with a difference between spindle poles (Fig. S4). Thus, the cortex directly regulates MTs to induce asymmetry within the spindle.
Fig. 1. Cortical proximity induces asymmetry within the mouse oocyte spindle.
(A–F) CF-1 oocytes were fixed at metaphase I and stained for the indicated post-translational modifications on tubulin. Cortical spindles (A–C) were examined at 6 h after GVBD, and centered spindles (B, C) at 3 h after GVBD. Cortical spindles in oocytes treated with cytochalasin B were examined at 3 h after GVBD (D–F). Images (A, B, E) are sum intensity z-projections showing the whole oocyte (left) or a magnified view of the spindle (right); dashed line, cortex; scale bars, 10 μm. Graphs are line scans of tubulin intensity across the spindle. Spindle asymmetry was quantified (C, F) as the ratio of the cortical half to the egg half (n > 18 spindles for each condition). Each dot represents a single spindle; red line, median; *p < 0.0001.
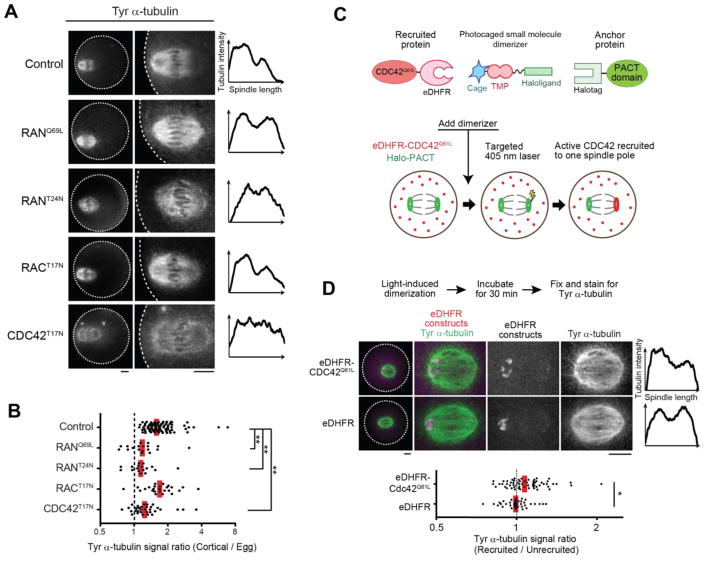
The cortex overlying the spindle is polarized through a chromatin-based gradient of RANGTP (21, 22) (Fig. S5A) and enriched in multiple signaling factors, including active CDC42 and RAC GTPases, and in polymerized actin (called the actin cap) (6, 23, 24) (Fig. S5A). To determine whether spindle asymmetry depends on cortical polarization, we expressed either constitutively-active (RANQ69L) or dominant-negative (RANT24N) RAN mutants. In each case, loss of polarization led to loss of spindle asymmetry (Fig. 2, A and B; Fig. S5B). We next tested CDC42 and RAC GTPases by expressing dominant-negative mutants. CDC42T17N diminished the Tyr α-tubulin signal overall and prevented asymmetry, whereas RACT17N did not affect asymmetry (Fig. 2, A and B; Fig. S5, B and C). Furthermore, expressing a constitutively-active CDC42 mutant with the plasma membrane targeting CAAX motif removed (CDC42Q61LΔCAAX) (25) significantly increased Tyr α-tubulin signal (Fig. S7). We next tested whether the actin cap, which depends on CDC42 activity (24) (Fig. S5A), contributes to spindle asymmetry. Inhibiting the actin nucleating ARP2/3 complex, using the small molecule inhibitor CK-666, abolished actin cap formation (26) but did not affect spindle asymmetry (Fig. S6). Thus, active CDC42 is sufficient to increase α-tubulin tyrosination and required for spindle asymmetry independent of actin cap formation.
Fig. 2. Cortical polarization and localized CDC42 signaling induce spindle asymmetry.
(A, B) CF-1 oocytes expressing the indicated GTPase mutant were fixed 6 h after GVBD and stained for Tyr α-tubulin. Images are sum intensity z-projections showing the whole oocyte (left) or a magnified view of the spindle (right), and graphs are line scans of tubulin intensity across the spindle. Spindle asymmetry was quantified (B) as the ratio of the cortical half to the egg half (n > 17 spindles for each condition). (C) Schematics of the light-induced dimerization experiment. The dimerizer is composed of a Halo ligand linked to the eDHFR ligand Trimethoprim (TMP), which is photocaged. The PACT domain, fused to EGFP and Halotag, localizes to spindle poles, and CDC42Q61LΔCAAX is fused to mCherry and eDHFR. The dimerizer covalently binds Halo-PACT at spindle poles, and eDHFR-CDC42Q61LΔCAAX is recruited to one pole by local uncaging with light. (D) Halo-EGFP-PACT was co-expressed with either mCherry-eDHFR-CDC42Q61LΔCAAX (top) or mCherry-eDHFR (bottom) in CF-1 oocytes. Recruitment of eDHFR fusion proteins was induced by uncaging at one spindle pole. 30 min after uncaging, oocytes were fixed and stained for Tyr α-tubulin. Images are maximum intensity z-projection showing whole oocytes (left) or magnified views of the spindle, and graphs are line scans of tubulin intensity across the spindle. Spindle asymmetry was quantified as the ratio of the recruited side to the unrecruited side (n > 39 spindles for each condition). Each dot represents a single spindle; red line, median; *p < 0.01; **p < 0.0001. Scale bars, 10 μm.
Our observations suggest that asymmetric localization of active CDC42 relative to the spindle is the mechanism underlying spindle asymmetry. To test this hypothesis, we developed an optogenetic strategy to target active CDC42 to one pole of a centered spindle, which is normally symmetric, using a photocaged small molecule that heterodimerizes Halotag and E. coli DHFR (eDHFR) fusion proteins (27, 28) (Fig. 2C). We used Halotag fused to a PACT domain, which localizes to spindle poles (29), to recruit eDHFR fusion proteins specifically to one pole by local uncaging of the dimerizer (Fig. S8A). Recruiting the constitutively-active CDC42Q61LΔCAAX mutant induced spindle asymmetry by increasing Tyr α-tubulin signals on the recruited side, whereas recruiting eDHFR alone had no effect (Fig. 2D; Fig. S8B). These results strongly support our model that cortically localized CDC42 activity induces asymmetry within the spindle. Several factors may contribute to the weaker asymmetry induced by our optogenetic approach, compared to that observed normally on spindles near the cortex. CDC42Q61LΔCAAX expression increased Tyr α-tubulin overall (Fig. S7), leaving less opportunity to create asymmetry by a further increase on one side. In addition, experimentally induced levels of CDC42 at spindle poles may be lower than normal levels at the cortex, and other cortical factors may also contribute to the asymmetry.
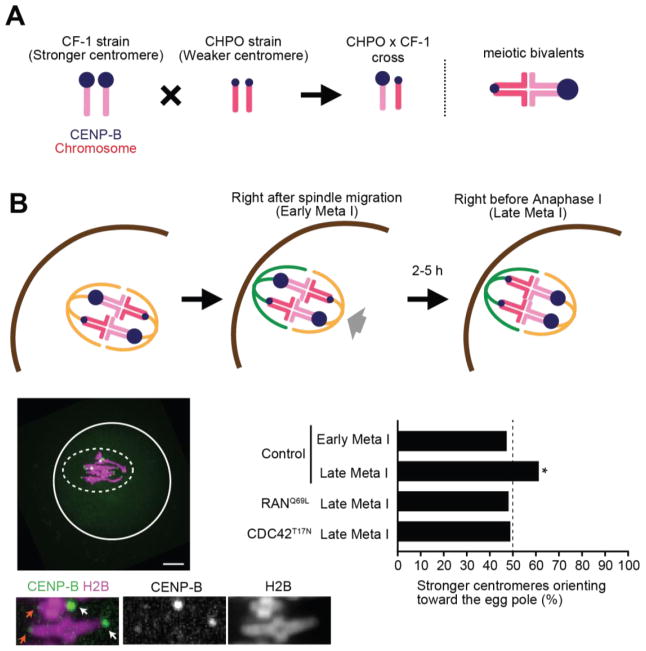
To determine the significance of spindle asymmetry for meiotic drive, we measured the biased orientation of selfish centromeres towards the egg pole in hybrid oocytes produced in a cross between two strains, CHPO and CF-1. Bivalents in these oocytes have both weaker and stronger centromeres, inherited from CHPO and CF-1, respectively (Fig. 3A). Stronger centromeres have higher levels of kinetochore proteins and more minor satellite DNA containing binding sites for the centromere protein CENP-B (10, 12). Using fluorescently-tagged CENP-B to distinguish stronger and weaker centromeres in live cells, we showed that stronger centromeres preferentially oriented towards the egg pole just before anaphase I (10) (Fig. 3B, late meta I). To abolish spindle asymmetry, which we also observed in this hybrid strain (Fig. S9), we expressed RANQ69L or CDC42T17N mutants. Biased orientation was lost in both cases (Fig. 3B), demonstrating that meiotic drive depended upon spindle asymmetry induced by cortical polarization.
Fig. 3. Spindle asymmetry is essential for biased orientation of selfish centromeres.
(A) Schematic of biased orientation assay. A strain with stronger centromeres (CF-1) is crossed to a strain with weaker centromeres (CHPO). Bivalents in the hybrid offspring contain both stronger and weaker centromeres, which can be distinguished by CENP-B levels. (B) CHPO x CF-1 hybrid oocytes expressing CENP-B-EGFP and H2B-mCherry were imaged live, either shortly after spindle migration to the cortex (within 30 min, early meta I), or shortly before anaphase onset (within 30 min, late meta I). Image is a maximum intensity z-projection showing late meta I; white line: oocyte cortex, dashed line: spindle outline; scale bar, 10 μm. Insets are optical slices showing two bivalents; arrows indicate stronger (white) and weaker (orange) centromeres. The fraction of bivalents with the stronger centromere oriented towards the egg was quantified; n=152 bivalents for early meta I, 204 for late meta I, 108 for RanQ69L and 143 for CDC42T17N. * indicates significant deviation from 50% (p < 0.005).
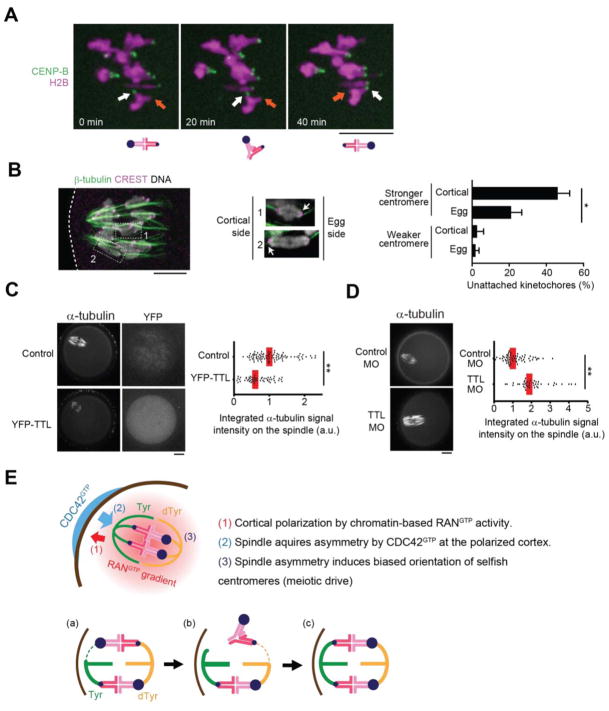
Initial MT attachments are established before spindle migration to the cortex (30), while the spindle is symmetric, and we did not find biased orientation shortly after migration in CHPO x CF-1 hybrid oocytes (Fig. 3B, early meta I). Thus, the bias arose from re-orientation or flipping of stronger centromeres from the cortical to the egg side of the spindle while it was cortically positioned and asymmetric. Hybrid oocytes remained in MI for 2–5 h after spindle migration, likely due to chromosomes positioned off-center on the spindle (12, 31) (Fig. 3B), which would provide time for these flipping events. Indeed, we found examples of bivalents flipping after spindle migration in hybrid oocytes (21 events in 23 cells) (Fig. 4A), consistent with previous observations (30). To establish a bias, flipping must preferentially occur in one direction, which suggests that one orientation is relatively more unstable than the other and implies difference between centromeres of homologous chromosomes and between the two sides of the spindle. To test for these differences in hybrid oocytes, we examined cold-stable kinetochore-MT fibers (32). Stronger centromeres had more unstable attachments compared to weaker centromeres, particularly when facing the cortical side of the spindle (Fig. 4B). Thus, stronger centromeres are more likely to detach, and the cortical side is more susceptible to detachment. To test whether the enrichment of Tyr α-tubulin makes the cortical side more unstable, we modulated the expression of Tubulin Tyrosine Ligase (TTL), which catalyzes α-tubulin tyrosination (33). TTL overexpression increased Tyr α-tubulin and destabilized spindle MTs based on sensitivity to low temperature (34), whereas depleting TTL decreased Tyr α-tubulin and stabilized spindle MTs (Fig. 4, C and D; Fig. S10, A and B). Thus, Tyr α-tubulin asymmetry allows stronger centromeres to interact differentially with the two sides of the spindle to preferentially orient towards the egg pole (Fig. 4E).
Fig. 4. MT tyrosination promotes unstable interactions between selfish centromeres and the cortical side of the spindle.
(A) CHPO x CF-1 oocytes expressing CENP-B-mCherry and H2B-EGFP were imaged live after spindle migration to the cortex (n = 23 cells). Time lapse images show an example of bivalent flipping; arrows indicate stronger (white) and weaker (orange) centromeres. (B) CHPO x CF-1 oocytes were analyzed for cold-stable MTs at 8 h after GVBD. Enlarged insets are optical slices showing individual bivalents with the stronger centromere (arrow) either facing the egg side and attached to cold-stable MTs (1) or facing the cortical side and not attached (2). Weaker centromeres are attached in both cases. Graph shows the average percentage of centromeres without cold-stable attachments. Error bars represent s.d. for 3 independent experiments (> 50 bivalents analyzed in each experiment). *p < 0.01. (C, D) CF-1 oocytes expressing YFP-TTL or microinjected with morpholino against TTL were analyzed for cold-stable MTs at 6 h after GVBD. Graphs show integrated α-tubulin intensity in the spindle (n > 41 spindles for each condition). Each dot represents a single spindle; red line, median; *p < 0.001. Images (A–D) are maximum intensity z-projections; scale bars, 10 μm. (E) Model for spindle asymmetry and meiotic drive. Top: cortical signals regulate MTs to induce tyrosination asymmetry within the spindle, and stronger centromeres (larger blue circles) orient preferentially to the egg side. Bottom: bivalent orientation is initially random (a), but attachment of a stronger centromere to the cortical side is unstable and tends to detach (b), followed by detachment of the weaker centromere, likely due to loss of tension across the bivalent, and re-orientation. This biased flipping of stronger centromeres to the egg side leads to biased orientation (c).
Here we have shown that asymmetry within the spindle is essential for meiotic drive. Because signals from the cell cortex regulate MTs to induce spindle asymmetry and the cortical side ultimately ends up in the polar body, our findings explain how spindle asymmetry is consistently oriented relative to cell fate, providing spatial cues to guide the segregation of selfish elements. Moreover, the cortical signals are a product of polarization directed by chromosomes positioned near the cortex. This chromosome positioning is crucial for female meiosis because it allows the highly asymmetric division that is a universal feature of sexual reproduction in animals (6, 21, 23, 35). Thus, selfish drive elements exploit the asymmetry inherent in female meiosis to bias their chances of transmission to the next generation.
Supplementary Material
Acknowledgments
We thank BE. Black and MT. Levine for comments on the manuscript, G. Halet for the CDC42 and RAC constructs and discussions, R. Li for the RAN constructs, BL. Prosser for the detyrosinated α-tubulin and TTL antibodies. The research was supported by NIH grants GM107086 (M.A.L. and R.M.S.) and GM122475 (M.A.L.), by Institut Curie, CNRS, INCA_6517, ANR-10-LBX-0038 part of the IDEX Idex PSL ANR-10-IDEX-0001-02 PSL (C.J.) and by JSPS postdoctoral fellowship for research abroad and Research fellowship from Uehara Memorial Foundation (T.A.). Data described can be found in the main figures and supplementary materials. The authors declare no conflict of interests.
Footnotes
REFERENCES AND NOTES
- 1.Werren JH. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc Natl Acad Sci U S A. 2011;108:10863–70. doi: 10.1073/pnas.1102343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice WR. Nothing in Genetics Makes Sense Except in Light of Genomic Conflict. Annu Rev Ecol Evol Syst. 2013;44:217–237. [Google Scholar]
- 3.Lindholm AK, et al. The Ecology and Evolutionary Dynamics of Meiotic Drive. Trends Ecol Evol. 2016;31:315–326. doi: 10.1016/j.tree.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Helleu Q, Gérard PR, Montchamp-Moreau C. Sex chromosome drive. Cold Spring Harb Perspect Biol. 2014;7:a017616. doi: 10.1101/cshperspect.a017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardo-Manuel de Villena F, Sapienza C. Nonrandom segregation during meiosis: the unfairness of females. Mamm Genome. 2001;12:331–9. doi: 10.1007/s003350040003. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 7.Didion JP, et al. A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2. PLOS Genet. 2015;11:e1004850. doi: 10.1371/journal.pgen.1004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman L, Saunders A. Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science. 2008;322:1559–62. doi: 10.1126/science.1161406. [DOI] [PubMed] [Google Scholar]
- 9.Pardo-Manuel de Villena F, Sapienza C. Female Meiosis Drives Karyotypic Evolution in Mammals. Genetics. 2001;159:1179–1189. doi: 10.1093/genetics/159.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata-Otsubo A, et al. Expanded Satellite Repeats Amplify a Discrete CENP-A Nucleosome Assembly Site on Chromosomes that Drive in Female Meiosis. Curr Biol. 2017;27:2365–2373.e8. doi: 10.1016/j.cub.2017.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitt GM. Meiotic drive for B-chromosomes in the primary oocytes of Myrmeleotettix maculatus (Orthopera: Acrididae) Chromosoma. 1976;56:381–391. doi: 10.1007/BF00292957. [DOI] [PubMed] [Google Scholar]
- 12.Chmátal L, et al. Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol. 2014;24:2295–300. doi: 10.1016/j.cub.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison P, et al. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science. 2016;352 doi: 10.1126/science.aaf0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barisic M, et al. Microtubule detyrosination guides chromosomes during mitosis. Science. 2015;348:799–803. doi: 10.1126/science.aaa5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janke C. The tubulin code: Molecular components, readout mechanisms, functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoury J, Lee KW, Georget V, Hikal P, Verlhac MH. Symmetry breaking in mouse oocytes requires transient F-actin meshwork destabilization. Development. 2011;138:2903–2908. doi: 10.1242/dev.060269. [DOI] [PubMed] [Google Scholar]
- 17.Almonacid M, Terret M-É, Verlhac M-H. Actin-based spindle positioning: new insights from female gametes. J Cell Sci. 2014;127 doi: 10.1242/jcs.142711. [DOI] [PubMed] [Google Scholar]
- 18.Schuh M, Ellenberg J. A new model for asymmetric spindle positioning in mouse oocytes. Curr Biol. 2008;18:1986–92. doi: 10.1016/j.cub.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Yi K, et al. Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J Cell Biol. 2013;200:567–76. doi: 10.1083/jcb.201211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azoury J, et al. Spindle Positioning in Mouse Oocytes Relies on a Dynamic Meshwork of Actin Filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase Mediates Chromatin Signaling to Control Cortical Polarity during Polar Body Extrusion in Mouse Oocytes. Dev Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Dehapiot B, Halet G. Ran GTPase promotes oocyte polarization by regulating ERM (Ezrin/Radixin/Moesin) inactivation. Cell Cycle. 2013;12:1672–1678. doi: 10.4161/cc.24901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halet G, Carroll J. Rac Activity Is Polarized and Regulates Meiotic Spindle Stability and Anchoring in Mammalian Oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Dehapiot B, Carrière V, Carroll J, Halet G. Polarized Cdc42 activation promotes polar body protrusion and asymmetric division in mouse oocytes. Dev Biol. 2013;377:202–212. doi: 10.1016/j.ydbio.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nat Rev Mol Cell Biol. 2011;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi K, et al. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol. 2011;13:1252–8. doi: 10.1038/ncb2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballister ER, Aonbangkhen C, Mayo AM, Lampson MA, Chenoweth DM. Localized light-induced protein dimerization in living cells using a photocaged dimerizer. Nat Commun. 2014;5:5475. doi: 10.1038/ncomms6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, et al. Optogenetic control of kinetochore function. Nat Chem Biol. 2017 doi: 10.1038/nchembio.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitajima TS, et al. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–81. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Chmátal L, Yang K, Schultz RM, Lampson MA. Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I. Curr Biol. 2015;25:1835–1841. doi: 10.1016/j.cub.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- 33.Prota AE, et al. Structural basis of tubulin tyrosination by tubulin tyrosine ligase. J Cell Biol. 2013;200:259–270. doi: 10.1083/jcb.201211017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue S. Cell Motility by Labile Association of Molecules: The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967;50:259–292. [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelick R, Carpinone J, Derraugh LJ. No universal differences between female and male eukaryotes: anisogamy and asymmetrical female meiosis. Biol J Linn Soc. 2016 doi: 10.1111/bij.12874. [DOI] [Google Scholar]
- 36.Stein P, Schindler K. Mouse oocyte microinjection, maturation and ploidy assessment. J Vis Exp. 2011 doi: 10.3791/2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–88. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 38.Dujardin D, et al. Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.