SUMMARY
Background
Non-alcoholic fatty liver disease (NAFLD) can lead to non-alcoholic steato-hepatitis (NASH) and cirrhosis. Fibrosis predicts worse outcomes and mortality. New treatments targeting fibrosis are being investigated to reverse disease progression.
Aim
To review the new pipeline therapeutic agents targeting fibrosis in NASH patients, with particular focus on clinical trials in which reversing fibrosis and portal hypertension are the primary outcomes.
Methods
The literature was searched in PubMed between January 2000 and January 2016 using search terms non-alcoholic fatty liver disease and NASH, with filters of ‘English language’. We focused on fibrosis improvement as the key outcome. We also searched the ClinicalTrials.gov for promising agents that target fibrosis in NASH patients.
Results
Significant advances have been made on approaches targeting fibrosis in NASH patients. Many therapeutic agents are already in development, some of which have shown promising results in preclinical and phase I studies. Novel therapies have entered phase II and III studies targeting fibrosis reversal and/or improvement in portal hypertension. Innovative studies have also started looking into combining these agents, aiming at different mechanisms to maximise therapeutic outcomes. We found five clinical trials in phase II and one in phase III focusing on fibrosis in NASH patients as key outcomes. One of the phase II trials is using combination therapy to target fibrosis.
Conclusions
Ongoing research studies are already investigating new pathways aimed at reversing fibrosis in NASH patients. Novel therapeutic agents are in development and are expected to offer unique options to NASH patients with advanced fibrosis.
INTRODUCTION
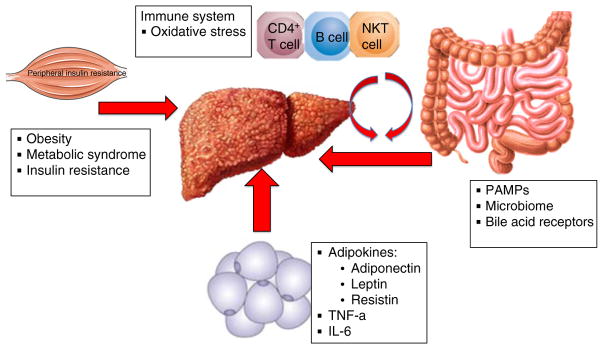
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in developed countries.1, 2 It may be broadly classified into two categories: non-alcoholic fatty liver (or simple steatosis) and non-alcoholic steatohepatitis (NASH). Although previously it was thought that steatosis was largely nonprogressive while NASH was the progressive form of NAFLD, recent evidence from serial biopsy studies demonstrates that both patients with steatosis or NASH have an increased risk of subsequent disease progression to advanced fibrosis and cirrhosis.3, 4 Indeed, longitudinal studies have shown that it is the presence and stage of fibrosis on index biopsy, rather than any distinction between steatosis and steatohepatitis, that is prognostic for increased risk of all-cause as well as liver-related mortality in patients with NAFLD.5, 6 The fibrosis progression rate in patients with NASH is estimated to be 7 years per stage of progression. Therefore, it may take up to 30–40 years to develop cirrhosis in the majority of individuals with NAFLD. However, a smaller subset of patients who are termed rapid progressors may proceed to develop cirrhosis in only a 10-year period.3 This inter-individual variability is due to various disease modifiers, either intrinsic (such as genetic variants, of which the best validated lie within the genes PNPLA37, 8 and TM6SF29, 10) or acquired (that include dietary factors and changes in the intestinal microbiota11). Understanding the pathogenetic mechanisms underlying the triggers that induce rapid fibrogenesis is a major unmet need in the field. Multiple factors, both intrahepatic and extra-hepatic, clearly influence fibrogenesis. Factors shown to be linked to NASH-associated fibrosis and disease progression, both directly and through synergistic interactions,12 include insulin resistance and diabetes,13, 14 obesity,15 oxidative stress,12 pro-inflammatory cytokines and adipokines,12, 16 and both innate17–20 and acquired21, 22 immune responses (Figure 1). Because fibrosis can ultimately lead to cirrhosis and liver-related mortality,23, 24 with the degree of fibrosis shown to be directly linked to disease progression and mortality,5, 6 it is crucial to identify the specific mechanisms and cells that can lead to fibrosis progression (fibrogenesis) in NASH and, through that, the potential therapies that might prevent this progression and even effect fibrosis regression (fibrolysis).
Figure 1.
Extrahepatic factors that drive liver fibrogenesis. The progression and resolution of fibrosis is a complex process that involves parenchymal and nonparenchymal liver cells in addition to infiltrating immune cells. The extrahepatic process in NASH involves many factors that are related to glucose and lipid metabolism and insulin resistance, gut microbiota and changes in the immune system along with production of oxidative stress. PAMAPs, pathogen-associated molecular patterns; TNF-α, tumour necrosis factor-α; IL6, interleukin 6.
The fibrosis scaffold
Fibrosis is a wound-healing process in which there is excessive deposition of extracellular matrix (ECM). ECM is composed of collagens, noncollagen glycoproteins, matrix bound growth factors, glycosaminoglycans, proteoglycans and matricellular proteins, which provide the scaffolding of both the normal and the fibrotic liver.25 However, as fibrosis develops, there are multiple changes in the specific contents of these, with a 3- to 10-fold increase in total collagen content26; an increase in several glycoproteins (cellular fibronectin, laminin, SPARC, osteonectin, tenascin and von Willebrand factor) and glycosaminoglycans (perlecan, decorin, aggrecan, lumican and fibromodulin); both an increase in proteoglycans and a shift from heparan sulphate-containing proteoglycans to those containing chondroitin and dermatan sulphates; and an increase in the fibril-forming collagens types I, III and IV and in some nonfibril-forming collagens (types IV and VI).27 With all of these changes, there is a transition from the low-density basement membrane-like matrix in the subendothelial space that is found in the normal liver to the interstitial type which is associated with hepatocyte dysfunction and activation of the hepatic stellate cells (HSCs), which are the primary source of ECM in both the normal and fibrotic liver. During activation, HSCs transition from their normal quiescent state to proliferative, fibrogenic and contractile myofibroblasts (Figure 2).28
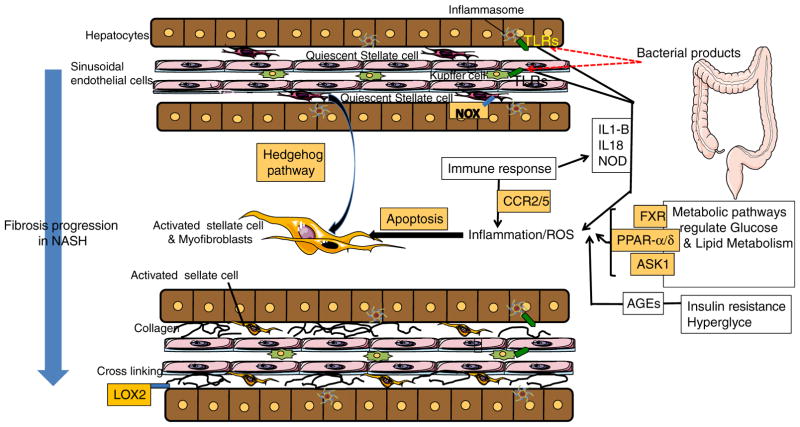
Figure 2.
Key mechanisms of fibrosis in NASH. Multiple pathways are involved in NASH-associated fibrosis including inflammasome-TLR activation and generation of the inflammatory cytokines, increased levels of hedgehog signalling, changes in lipid and glucose metabolism leading to oxidative stress, hepatocyte injury via apoptosis, cell death inducing inflammatory and pro-fibrogenic pathways in nonparenchymal cells and infiltrating immune cells. These processes lead to HSC activation which is the source of excessive deposition of extracellular matrix (ECM) in the parenchyma. TLR, toll-like receptor; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; IL1-B, interleukin 1-B; IL18, interleukin 18; NOD, nucleotide-binding oligomerization domain receptors; FXR, farnesoid X receptor; PPAR-α/δ, peroxisome proliferator-activated receptors α/δ; ASK1, apoptosis-signal-regulating kinase 1; AGEs, advanced glycation end products; CCR, chemokine receptors; LOX2, lysyl oxidase-like 2; HSC, hepatic stellate cell.
Key pathways in NASH fibrosis
Multiple signalling pathways play a role in the activation of HSCs in liver fibrosis in NASH, including transforming growth factor β (TGF-β), hedgehog (Hh), phos-phatidylinositol 3-kinase/protein kinase B (PI3K/Akt), Janus kinase/signal transducer and activator of transcription (JAK/STAT) and others.12 Two pathways appear to be particularly important in NASH-associated fibrosis: activation of the inflammasome, an intracellular protein scaffold that generates the inflammatory cytokines interleukin 1 beta (IL-1β) and IL-18, and its interaction with the microbiome; and progenitor cell amplification associated with increased levels of hedgehog signalling (Figure 2).29 The inflammasome is implicated in the pathogenesis of NASH by transducing signals that originate from gut-derived bacteria.30, 31 It appears that alterations in the composition of gut microbiota contribute to NASH pathogenesis and ultimately result in increased hepatic injury and fibrosis.30, 31 Increased hedgehog signalling in the liver and the appearance of cells that express progenitor cell markers have also been shown to be associated with fibrosis progression in NASH.32, 33 Another signalling network increasingly being recognised as important in NAFLD is the nuclear receptor family, which includes farnesoid X receptor (FXR), peroxisome proliferator-activated receptors (PPAR), vitamin D receptor, retinoid receptors, Reverbα and liver-X-receptor.34
Role of oxidative stress and metabolic signals
Oxidative stress is known to play a major role in the activation of HSCs in NASH,12, 35 and anti-oxidants not only exert a preventive effect on hepatocyte injury but may directly contribute to decreasing fibrogenesis,36–39 an effect supported by gene-association studies where variants affecting cellular anti-oxidant defences efficacy influence risk of NAFLD fibrosis.40 Multiple studies of NASH patients have also shown the association of insulin resistance with fibrosis.28 Hyperinsulinemia is known to promote steatosis and the generation of reactive oxygen species (ROS) and lipid peroxides, and the severity of insulin resistance has been shown to be associated with the degree of fibrosis.41 The lipotoxic effects of free cholesterol and the metabolic reprogramming that is fostered by it also contribute to HSC activation and NASH fibrogenesis.42–49 Advanced glycation end products (AGEs) may also stimulate profibrotic changes in NASH. In addition to the accumulation of AGEs through diet,50 they are produced in hyperglycaemia through the non-enzymatic glycation of serum proteins.51, 52 Because 28–55% of people with NAFLD and up to 75% of people with NASH are estimated to have diabetes mellitus, hyperglycaemia no doubt contributes substantially to increased AGE levels in NASH patients. Studies in mice have shown that increasing dietary AGEs stimulates proinflammatory and profibrotic changes, including increases in ROS, tumour necrosis factor-α (TNF-α) and TNF-α converting enzyme, and downregulation of Sirtu-in1/Timp3, the combination of which leads to HSC activation and fibrogenesis.51 These dietary effects in mice have been shown to be attenuated with AGE receptor (RAGE) or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) blockade.52 NOX is an enzyme system that catalyses the reduction of molecular oxygen to superoxide and generates ROS in HSCs. NOX receptors have been shown to locate on HSCs.
Role of the immune system and toll-like receptors
Recent studies have suggested that the activation of the innate immune system may play an important role in the pathogenesis of fibrosis in NAFLD. Toll-like receptors (TLRs), sensors that recognise bacterial and viral components, normally participate in host defence against pathogens by inducing proinflammatory cytokines in immune cells. In NASH, there is bacterial overgrowth and increased intestinal permeability that exposes the liver to a higher load of TLR ligands.53 There can be overactivation of TLR signalling and breakdown of TLR tolerance, with increased production of inflammatory cytokines leading to tissue injury.54 In mouse models, (i) a direct link has been established between TLR4 and the Kupffer cells responsible for clearing endotoxin in NASH pathogenesis, with TLR4 promoting inflammation and fibrosis55; (ii) TLR9 signalling has been shown to induce Kupffer cell production of IL-1β which also leads to inflammation and fibrosis20; (iii) TLR2 signalling has been shown to promote proinflammatory cytokines, including IL-1β and Nod-like receptor protein 3, an inflammasome component, thus promoting inflammation and fibrosis31; and (iv) TLR2 and TLR9 signalling act synergistically to induce production of the NASH-promoting inflammatory cytokines.31 Natural killer T (NKT) cells may also play an important role in fibrogenesis in NASH. Studies have found that NKT cells in the blood and liver are increased in patients with more advanced disease.56, 57 In mouse models of NASH, it was shown that in wild-type mice, hedgehog pathway activation leads to hepatic accumulation of NKT cells that contribute to fibrosis progression while CD1d-deficient mice that lack NKT cells are protected from fibrosis.22
EMERGING ANTI-FIBROTIC THERAPIES IN NASH
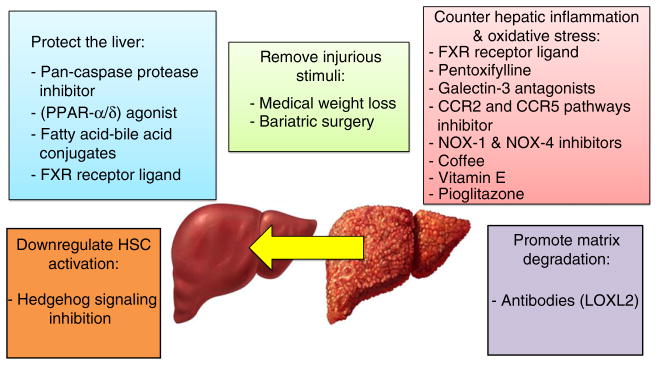
Just a few decades ago it was believed that cirrhosis was an irreversible condition. However, emerging data from treatment trials in chronic hepatitis B58–60 and C61, 62 have shown that cirrhosis can be reversed in a subset of patients, and improvement in hepatic fibrosis may be seen in the majority of successfully treated patients when you eliminate or remove the cause of the disease by achieving sustained virological response in patients with chronic hepatitis C and by sustained suppression of HBV DNA in the setting of chronic hepatitis B. Recent meta-analyses derived from randomised, controlled trials conducted in patients with biopsy-proven NASH suggest that improvement in hepatic fibrosis is feasible in NASH.63, 64 Experts have suggested that anti-fibrotic strategies could include (i) removing the injurious stimuli, (ii) suppressing or modulating hepatic inflammation, (iii) protecting the liver, (iv) downregulating stellate cell activation and (v) promoting matrix degradation65 (Figure 3). Some of these strategies may have direct effect on fibrosis pathway (e.g. matrix degradation), while others may have indirect effect through decreasing steatosis or resolving inflammation. In this review, we will focus on the emerging anti-fibrotic therapies for patients with NASH. We would refer the reader to other reviews on current therapies in NASH for further details.66, 67
Figure 3.
Anti-fibrotic strategies in NASH. Anti-fibrotic strategies in NASH could include (a) removing the injurious stimuli, (b) suppressing or modulating hepatic inflammation, (c) protecting the liver, (d) downregulating stellate cell activation and (e) promoting matrix degradation. FXR, farnesoid X receptor; CCR, chemokine receptors; NOX, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase; LOX2, Lysyl oxidase-like 2; PPAR-α/δ, peroxisome proliferator-activated receptors α/δ.
Remove injurious stimuli
Lifestyle interventions
There has been limited success to date with disease-specific therapies for NASH itself. Lifestyle interventions including restricting calories to induce weight loss and exercise to maintain weight loss have been shown to improve NASH. However, because for many patients, consistent exercise and weight loss are difficult, and many are unable to achieve or maintain the 10% weight loss required for histological improvement.66 In a recent Cuban study, a programme that included an aggressively hypocaloric diet (containing 750 kcal/day less than the calculated daily energy need), substantial amounts of exercise (200 min per week), and both individual and group counselling sessions to promote adherence to the programme resulted in significant weight loss in 30% of participants after 1 year, but fibrosis improvement in only 19% and fibrosis worsening in 16%.68 Both, aerobic and anaerobic exercises have been shown to decrease intrahepatic lipid accumulation with a greater effect seen with aerobic exercise.69 The detailed discussion of weight loss and exercise is beyond the scope of this review and can be found in a recent review by Marchesini et al.70
Bariatric surgery
Emerging studies in adults undergoing bariatric surgery have shown that it may result in resolution of NASH and improve hepatic fibrosis. Although worsening of fibrosis had been seen with ileojejunal bypass in the past,71 recent results have regenerated interest in bariatric surgery. In 109 morbidly obese patients with biopsy-proven NASH who underwent bar-iatric surgery using recent surgical techniques, NASH was no longer present in 85.4% of patients approximately 1 year after surgery. The results were considerably better in patients with mild NASH prior to surgery (response rate of 94.2%) compared with those with moderate or severe NASH (response rate of 70%).72 Significant improvements in fibrosis were noted in 33.8% of the total population and in 46.6% of patients with a fibrosis score ≥1 at baseline based on the Metavir score and in 46.3% and 51.4%, respectively, based on the Kleiner score. In noncirrhotic patients with a clinical indication for bariatric surgery with coexistent NASH, this may be considered as it might lead to collateral benefits in improving liver histology and fibrosis. However, any consideration of this approach must take into account the surgical risk, and much lengthier assessments through randomised, controlled trials will be required to determine long-term outcomes with such surgical procedures.
Counter hepatic inflammation and oxidative stress
Because oxidative stress12 and pro-inflammatory cytokines and adipokines12, 16 are known to be linked to NASH-associated fibrosis, and inflammatory mediators are involved in HSC activation, agents that may counter the inflammatory and oxidative stress components of NASH have been proposed as possibly being effective for prevention or improvement of fibrosis.65
Obeticholic acid
Obeticholic acid (OCA), 6-ethylcheno-deoxycholic acid, is a semisynthetic derivative of the primary human bile acid chenodeoxycholic acid (CDCA), which is the natural agonist of the farnesoid X receptor, a nuclear hormone receptor that regulates glucose and lipid metabolism.73 OCA is an approximately 100-fold more potent activator of the farnesoid X nuclear receptor than CDCA.74, 75 It ameliorates several metabolic pathways seen in NASH, such as hepatic steatosis, glucose tolerance, inflammation and even hepatic fibrosis by inhibiting HSC activity.76 In animal models, a broad range of anti-inflammatory and anti-fibrotic activities have been demonstrated, and it has been shown to reverse insulin resistance and reduce liver fat and fibrosis.74 In the multicentre Farnesoid X Receptor Ligand OCA in NASH Treatment (FLINT) trial, the results of treatment with a daily dose of 25 mg OCA were compared with placebo in patients with noncirrhotic NASH.77 Improvement in liver histology, defined as a decrease in the NAFLD activity score (NAS) by at least two points without worsening of fibrosis, was shown in 50 (45%) of 110 patients in the OCA group compared with 23 (21%) of 109 patients receiving placebo. There were improvements in NAS components (steatosis, hepatocellular ballooning and lobular inflammation) and a small improvement in fibrosis. The clinical significance of the small change in fibrosis will have to be examined in future trials. Pruritus occurred in 33 (23%) of 141 patients in the OCA group compared with nine (6%) of 142 in the placebo group. There were also statistically significant increases in triglycerides and low-density lipoprotein (LDL) and a decrease in high-density lipoprotein (HDL) in the OCA group compared with placebo. A phase III trial of OCA has been started and includes two primary endpoints measured, including fibrosis improvement with no worsening of worsening of NASH and NASH resolution with no worsening of fibro-sis (ClinicalTrials.gov Identifier: NCT02548351).
Other FXR agonists including intestinal-specific FXR are promising novel agents. Fang et al. showed that intestinal-specific FXR agonism can lead to activation of FXR in the small intestine and lead to reduction in inflammation in adipose tissue resulting in reduced free fatty acid influx to the liver.78 They reported that intestinal FXR agonism promoted browning of adipose tissue and led to reduction in obesity and improvement in both peripheral and hepatic insulin resistance. They also showed significant reduction in liver fat and decreased expression of SREPF1c, FAS and SCD1, thereby inhibiting fatty acid synthesis and in turn activating beta oxidation. These favourable effects were shown to reduce liver inflammation. It is plausible that intestinal-specific FXR may not have an associated increase in LDL and decrease in HDL cholesterol. Future studies are needed to assess preclinical and clinical efficacy of intestinal-specific FXR agonists in improving hepatic fibrosis in patients with NASH.
NOX-1 and NOX-4 inhibitors
In liver fibrosis, NADPH oxidase (NOX), an enzyme system that catalyses the reduction of molecular oxygen to superoxide, generates ROS in HSCs.79 NOX 1, NOX 2 and NOX4 are expressed on HSCs and have been shown in animal models to contribute to liver fibrosis.79–81 In a murine model, a novel, first-in-class NOX 1/4 inhibitor, GKT137831, suppressed ROS production and NOX and fibrotic gene expression, attenuated liver fibrosis and down-regulated markers of oxidative stress and inflammation in both wild-type and superoxide dismutase 1 G37R mutation mice.79 These data indicate that dual inhibition of Nox1 and Nox4 might provide a reasonable approach to improve fibrosis in NASH.
Pentoxifylline
Pentoxifylline, 5-oxohexyl-3, 7-dimethyl-xanthine, a xanthine derivative, is a nonspecific phosphodiesterase inhibitor which decreases TNF-α gene transcription, increases intracellular cyclic adenosine monophosphate (cAMP) and reduces inflammation.82, 83 The mechanisms by which it might have therapeutic effects on the damaged liver is not fully defined.84 It is known to inhibit lipid oxidation and decrease free-radical-mediated oxidative stress. Its impact on TNF-α might also be valuable since TNF-α activates the unfolded protein response,85 which has been shown to contribute to insulin resistance and inflammatory signalling and thus to potentially contribute to development of NASH.86–89 Pentoxifylline has been assessed as a treatment for NASH in several small studies.84, 90, 91 In a study with adults with NASH, dosing at 400 mg three times daily for 1 year resulted in statistically significant improvement compared with placebo in steatosis, lobular inflammation, and liver fibrosis (mean change in fibrosis score of −0.2 among those on pentoxifylline vs. +0.4 among those on placebo).91 In a follow-up to this study, it was shown that pentoxifylline was associated with a significant reduction of oxidised fatty acids.90 There were statistically significant correlations between decreases in certain specific oxidised fatty acids and outcomes, with decreases in hydroxyoctadecadienoic acids and oxo-octa-decadienoic acids (oxoODEs) shown to be correlated with the improved histological scores of fibrosis and decreases in hydroxyeicosatetraenoic acids correlated with decreased lobular inflammation. Thus, the improvements seen in NASH patients treated with pentoxifylline may be mediated at least in part through decreased lipid oxidation and, in particular, free-radical-mediated lipid oxidation. In another study, the same 1200 mg daily dose of pentoxifylline yielded significant improvements in steatosis and cellular ballooning but not in fibrosis scores.84 However, hepatic expression of collagen-1, a key gene involved in hepatic fibrogenesis, did decrease significantly and another fibrosis gene, TIMP-1, was reduced by 50% although this did not reach statistical significance. The authors noted that decreased expression of genes that are important in the development of fibrosis might herald changes before they become apparent on liver histological assessment. In a recent systematic review that assessed the comparative effectiveness of pharmacologic agents for the treatment of NASH, a direct meta-analysis found that only pentoxifylline and OCA were associated with improvement in fibrosis in NASH patients; with a Bayesian network meta-analysis, only pentoxifylline was associated with fibrosis improvement.92
Galectin-3 antagonists
Galectin-3 antagonists are being studied, with good preclinical results to date. Galectins are a family of proteins with a carbohydrate-binding domain that binds to terminal galactose residues on macromolecules such as glycoproteins.93, 94 Under normal physiological conditions, galectin-3 is widely expressed, particularly in immune cells, but only at low levels. However, in acute or chronic inflammation and fibrogenesis, its expression is markedly increased.93, 95 With repetitive tissue injury, galectin-3 is involved in the transition to chronic inflammation and the walling off of tissue injury with fibrogenesis.95 Galectin-3 null mice are known to have attenuated fibrosis after liver injury. GR-MD-02 (Galectin Therapeutics, Inc., Norcross, GA, USA) is a galactoarabino–rhamnogalacturonan polysaccharide polymer comprised predominantly of galacturonic acid, with ß-galactose and arabinose side chains, that is a galectin-3 inhibitor. Although not fully defined, the presumed proximate mechanism of action of GR-MD-02 is related to galectin-3 binding.96 The results of mouse studies suggest that macrophages, key cells in fibrogene-sis, may be a target for these complex carbohydrate drugs, but additional research will be needed to fully determine the mechanism(s).96 In a mouse model of streptozotocin-induced NASH, treatment with GR-MD-02 resulted in a reduction in galectin-3 expressed by activated macrophages which was associated with regression of NASH, including hepatocellular fat accumulation, hepatocyte ballooning, intra-portal and intra-lobular inflammatory infiltrate and deposition of collagen.96 In a model with thioacetamide-treated rats, treatment with GR-MD-02 reduced fibrosis, reversed cirrhosis, reduced galectin-3-expressing portal and septal macrophages and improved portal hypertension, indicating that this agent may not only effect regression of fibrosis and cirrhosis but also mitigate the pathophysiologic consequences of cirrhosis.97 Although the exact mechanism of GR-MD-02 on fibrosis is unknown, it seems to be a direct mechanism. A phase II clinical trial to evaluate the safety and efficacy of GR-MD-02 for the treatment of liver fibrosis and resultant portal hypertension in patients with NASH cirrhosis is ongoing (ClinicalTrials.gov Identifier: NCT02462967) (Table 1).
Table 1.
Ongoing NASH clinical trials with anti-fibrosis agents focusing on fibrosis outcomes
| Therapeutic agent | Molecular target | Study phase | Study type | Primary outcome | Secondary or other fibrosis outcome | Expected number of enrolled subjects |
|---|---|---|---|---|---|---|
| GR-MD-02 | antagonists Galectin-3 | Phase II | RCT | Reducing HPVG at 1 year | Changes in: oesophageal varices, digital collagen morphological change, Fibroscan, histological fibrosis | 156 |
| Simtuzumab (formerly GS-6624) | Monoclonal antibody against LOXL2 | Phase II | RCT | Reducing HPVG at 2 years | Oesophageal varices | 259 |
| Simtuzumab (formerly GS-6624) | Monoclonal antibody against LOXL2 | Phase II | RCT | Change from baseline in morphometric quantitative collagen on liver biopsy at year 1 | 222 | |
| Obeticholic acid | Agonist of FXR | Phase III | RCT | Fibrosis improvement with no worsening of NASH and NASH resolution with no worsening of fibrosis | Resolution of fibrosis | 2000 |
| Cenicriviroc | Inhibitor of the CCR2 and CCR5 pathways | Phase II | RCT | Improvement of NAS score with no concurrent worsening of fibrosis stage at 1 year | Changes in histological fibrosis, hepatic tissue fibrinogen protein, morphometric quantitative collagen (α-SMA), APRI, FIB-4, FibroTest, NFS, ELF | 289 |
| GS-4997 alone or in combination with Simtuzumab = | Inhibitor of ASK1 alone or with monoclonal antibody against LOXL2 and | Phase II | RCT | Adverse event profile of GS-4997 | Not provided | 70 |
RCT, randomised control trial; HPVG, hepatic portal-venous gradient; LOXL2, lysyl oxidase-like 2; FXR, agonist of the farnesoid X receptor; CCR2; chemokine receptor 2 antagonist, NAS, NAFLD activity score; APRI, AST/platelet ratio index; NFS, NAFLD fibrosis score; ELF, enhanced liver fibrosis score.
Cenicriviroc
Cenicriviroc (CVC) is a dual inhibitor of the CCR2 and CCR5 pathways, which have key roles in NASH-associated inflammation and fibrosis. CCR2 and/or CCR5 chemokine receptors are expressed on many types of cells, including monocytes, macrophages, Kupffer cells, HSCs, natural killer cells and T cells, and play a role in trafficking of these cells and in HSC activation and fibrosis progression.98 Therefore, CVC has a direct effect of fibrosis pathways. CVC has been shown to decrease fibrosis in a mouse model of diet-induced NASH and in a rat model of thioacetamide-induced liver injury.99, 100 The ongoing CENTAUR trial is evaluating whether cenicriviroc (150 mg daily) is effective and safe for the treatment of biopsy-proven NASH in adults with liver fibrosis (stage 1–3) (ClinicalTrials.gov Identifier: NCT02217475).
Vitamin E
Vitamin E is a chain-breaking anti-oxidant that is able to repair oxidising radicals and thus prevent the chain propagation step during lipid peroxidation.101 In addition, both the tocopherols and tocotrienols are essential components of biological membranes; the tocopherols, in particular, are thought to contribute to membrane stabilisation.102, 103 Vitamin E also regulates the activity of TGF-β1, PPARs and genes regulating apoptosis, inflammation and collagen deposition.104 Tocotrienols have been shown to improve insulin sensitivity through stimulating PPARs.105 Vitamin E and pioglitazone were studied in the Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Nondiabetic Patients with Non-alcoholic Steatohepatitis (PIVENS) trial. Although vitamin E therapy was associated with a significantly higher rate of improvement in NASH compared with placebo, pioglitazone was not.37 With both agents, there were significant reductions in serum alanine and aspartate aminotransferase levels and in hepatic steatosis and lobular inflammation but no significant improvement in fibrosis scores. While vitamin E showed a significant resolution of NASH in children, a sustained reduction in alanine aminotransferase was not achieved in the Treatment of NAFLD in Children (TONIC) trial.106 Although the use of vitamin E may be considered in nondiabetic adults with NASH, uniform adoption has been tempered by concerns regarding increased all-cause mortality107 and risk of prostate cancer.108
Pioglitazone
Pioglitazone, a derivative of thiazolidinedione (TZD), is a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist that is used to treat type 2 diabetes by increasing insulin sensitivity. In subjects with NAFLD, insulin resistance is associated with hyperinsulinemia, hyperglycaemia, high plasma free fatty acids and low plasma adiponectin levels. TZDs may reverse these abnormalities in NAFLD subjects. Pioglitazone has been shown to reduce liver fat content and liver enzymes and improve histology.109 However, as mentioned above, the PIVEN trial that compared treatment of vitamin E with pioglitazone and placebo showed that vitamin E therapy was associated with a higher rate of NASH improvement compared with placebo and pioglitazone. A pooled meta-analysis specifically focusing on pioglitazone suggests that pioglitazone may improve fibrosis.64
Coffee
Coffee has been shown to provide substantial anti-oxidant110, 111 and anti-inflammatory112, 113 effects in multiple studies. Because an earlier study had shown that higher caffeine consumption was associated with milder fibrosis in patients with chronic liver disease, particularly those with chronic hepatitis C,114 the association between coffee consumption and fibrosis in NAFLD and NASH patients has been assessed. In one study, there was significantly higher daily intake of coffee caffeine in patients with NASH stages 0–1 compared with those with NASH stages 2–4 and a negative relationship between coffee caffeine consumption and hepatic fibrosis.115 Two other retrospective studies have shown a beneficial effect of coffee on fibrosis in NASH patients; however, no prospective studies have been performed to date.116, 117
Probiotics and antibiotics
Gut microbiota has been associated with the development of obesity and hepatocellular injury.118, 119 Gut microbiota produces endotoxins, including lipopolysaccharides, which transmits to the liver and undergoes phagocytosis in Kupffer cells.120 The exposure to these endotoxins may lead to the progression to NASH and fibrosis. Few clinical trials have investigated the effect of probiotics and antibiotics in NASH patients and showed some improvement in liver enzymes, steatosis and inflammation.121, 122 A recent randomised trial using synbiotics vs placebo showed an improvement in liver enzymes and fibrosis measured non-invasively via transient elastography.123
Hepatoprotectants
Several agents in the class of drugs generally referred to as hepatoprotectants have been studied for possible effectiveness in NASH and associated fibrosis.
Anti-apoptosis. Emricasan
Emricasan (Conatus Pharmaceuticals, Inc., San Diego, CA, USA) is a pan-caspase protease inhibitor. Caspases play a key role in the induction of apoptosis and programmed cell death. They are responsible for completing the apoptotic pathways and for stimulation of cytokines such as IL-1β and IL-18 and are, therefore, an attractive therapeutic target. Previous pre-clinical124, 125 and clinical studies126 with a range of caspase inhibitors support this approach. Emricasan was shown in a preclinical model of NASH to inhibit fibrosis as well as apoptosis and inflammation associated with experimental NASH. In a phase II clinical trial in 38 patients with NAFLD, including a subset of NASH patients, emricasan treatment (25 mg orally, twice daily) resulted in significant reductions in ALT as well as in serum cCK18 levels, the latter demonstrating that emricasan reduces inflammation and elevated levels of apoptosis in NAFLD/NASH patients.127 The drug was safe and well tolerated, with no dose-limiting toxicities and no drug-related serious adverse events.
GFT505
GFT505 (Genfit, Loos, France) is a peroxisome proliferator-activated receptor alpha/delta (PPAR-α/δ) agonist. PPARα is highly expressed in the liver and regulates genes involved in lipid and lipoprotein metabolism, while PPARδ is expressed in various tissues and plays a central role in mitochondrial function, fatty acid oxidation and insulin sensitivity. In several animal models, GFT505 has been shown to have liver-protective effects on steatosis, inflammation and fibrosis and to improve liver dysfunction markers, decrease hepatic lipid accumulation and inhibit proinflammatory (IL-1β, TNF-α and F4/80) and profibrotic (transforming growth factor beta, tissue inhibitor of metalloproteinase 2, collagen type I, alpha 1, and collagen type I, alpha 2) gene expression.128 In a randomised controlled trial, 274 patients with histologically defined noncirrhotic NASH received GFT505 (80 or 120 mg) or placebo daily for 52 weeks.129 The primary endpoint, resolution of NASH without worsening of fibrosis, was not met. However, in a subanalysis of the population of NASH patients with an initial NAS score of ≥4, GFT505 at 120 mg/day led to a significant improvement in the primary endpoint. The results of this study have been described as suboptimal, and further studies are planned to clarify the effects of GFT505.
Aramchol
Aramchol (Trima Israel Pharmaceutical Products Ltd., Maabarot, Israel), a fatty acid-bile acid conjugate, is a synthetic lipid molecule that reduces hepatic fat content via inhibition of stearoyl coenzyme A desaturase 1 (SCD1) activity.130 SCD1 is a key enzyme that regulates fatty acid metabolism in the liver. SCD1 inhibition decreases fatty acid synthesis and increases β oxidation of fatty acids, resulting in decreased hepatic triglycerides and fatty acid contents. Aramchol has been studied in 60 patients with biopsy-confirmed NAFLD (six with NASH) who were given 100 or 300 mg once daily for 3 months.130 With the 300 mg dose, liver fat content was significantly reduced; because repeated liver biopsy within the short duration of the study was considered unwarranted, the possible effect on fibrosis was not determined. An ongoing phase IIb study is evaluating the efficacy for reducing liver fat and the safety of two higher Aramchol doses (400 and 600 mg) in 240 patients with biopsy-confirmed NASH; changes in inflammation and fibrosis biomarkers will be included in the assessments (ClinicalTrials.gov Identifier: NCT02279524).
ASK1 inhibitors
Apoptosis-signal-regulating kinase 1 (ASK1) is a kinase that is activated by various stimuli including hyperglycaemia, TGF-β and ROS.131 ASK1 induces apoptosis, fibrosis and metabolic dysfunction by activating the p38 and JNK1 pathways. The ASK1 pathway has been shown to be activated in human NASH liver biopsies.132 In a murine model, it was shown that in animals with established NASH (F1/2), a selective small molecule inhibitor of ASK1, GS-4997, significantly reduced hepatic steatosis and fibrosis and significantly improved key metabolic parameters associated with NASH.132 Treatment with GS-4997 resulted in a significant reduction in body weight; decreased fasting blood glucose and insulin levels (17% and 13%, respectively); reduction in plasma AST, ALT and cholesterol levels; a 68% reduction in hepatic steatosis; a 44% reduction in liver hydroxyproline; a reduction in alpha smooth muscle actin and p-P38 expression; an 84% reduction in fibrillar collagen area and reduced synthesis of both soluble and insoluble collagen. In a second mouse model study, ASK1 inhibition reduced hepatic fibrosis, steatosis and insulin resistance and normalised fatty acid synthesis and lipid metabolism.131 Animals treated with the ASK1 inhibitor had a 26% decrease in lipogenesis, a 21% decrease in stearoyl-CoA desaturase and a 78% increase in delta-5 desaturase. It was shown that gene networks regulating fatty acid synthesis, lipid metabolism and cholesterol biosynthesis were differentially expressed in ASK1 inhibitor–treated animals. Serum levels of osteopontin were reduced by 35%, hyaluronic acid by 33%, TIMP-1 by 41% and IL-6 by 50%. GS-4997 is currently being investigated in a phase II clinical trial of patients with NASH and moderate to severe liver fibrosis (Clini-calTrials.gov Identifier: NCT02466516).
Downregulate stellate cell activation
Hedgehog signalling inhibition with vismodegib has been shown in a nutrient excess murine model of NASH to reduce TNF-related apoptosis-inducing ligand (TRAIL)-mediated liver injury, thereby attenuating hepatic inflammation and fibrosis.133 In this model, treatment with vis-modegib resulted in (i) decreased hepatocyte injury despite hepatocyte lipid loading; (ii) reduced hepatic markers of macrophage accumulation and activation; (iii) inhibition of death receptor 5 (DR5) upregulation and DR5-mediated liver injury and (iv) decreased fibrosis. Since Hedgehog pathway is very important in NASH, trials using Hedgehog signalling inhibition are worth exploring.
Promote matrix degradation
Lysyl oxidase-like 2 (LOXL2) is an enzyme thought to promote cross-linking of interstitial collagen in the ECM, a process that renders it more resistant to degradation.134 LOXL2 is increased in fibrotic liver tissue. A monoclonal antibody to LOXL2 was shown to substantially attenuate fibrosis in several experimental models.135 An ongoing clinical trial is evaluating whether simtuzumab (formerly GS-6624; Gilead Sciences, Foster City, CA, USA), a monoclonal antibody against LOXL2, can prevent the histological progression of liver fibrosis and the clinical progression to cirrhosis in NASH patients (ClinicalTrials.gov Identifier: NCT01672866). A second ongoing trial is evaluating the safety and efficacy of simtuzumab for treatment of cirrhosis in patients with compensated cirrhosis secondary to NASH (ClinicalTrials.gov Identifier: NCT01672879). LOXL2 antibodies are considered to have a direct effect on fibrosis.
NEW DIRECTIONS: COMBINATION THERAPY
Hepatic fibrosis in NASH is driven by multiple risk factors that may interact with each other via several interrelated mechanistic pathways. It is plausible that the injurious stimuli may be heterogenous in NASH, but the resultant response in laying down of collagen and worsening of hepatic fibrosis may be a common response. Therefore, targeted therapies to multiple targets may eventually be required to reverse cirrhosis and hepatic fibrosis in NAFLD. Removal of cause would be the most efficient way to improve hepatic fibrosis. This has been supported by observations seen with other chronic diseases, including hepatitis C and B. It is unlikely that a single agent will effectively target all facets of pathogenesis and so the stage is set to move on towards combination therapies in the treatment of NASH.34, 136 In coming years, therapy may be decided based on stage of fibrosis. For example, stage 2 or 3 NASH fibrosis might require a combination of a hepatoprotectant with an agent countering hepatic inflammation and oxidative stress as well as hepatic steatosis, and improving cardiometabolic profile. On the other hand, compensated stage 4 fibrosis might require a more aggressive approach with a combination that includes a hepatoprotectant, an agent to counter hepatic inflammation and oxidative stress and an agent to promote matrix degradation. Early exploration of combination therapy has already begun. One example is an ongoing trial evaluating the safety and tolerability of the ASK1 inhibitor GS-4997 alone or in combination with simtuzumab (monoclonal antibody against LOXL2) in adults with NASH and fibrosis stages F2–F3 (ClinicalTrials.gov Identifier: NCT02466516). These are exciting times in the field of NASH and hepatic fibrosis. Anti-fibrotic therapies in NASH are evolving and will likely result in improved understanding of the pathogenesis of hepatic fibrosis in the coming years.
CONCLUSIONS
The recent progress that has been made in identifying the specific mechanisms that can lead to fibrosis progression in NASH has led to the development of targeted therapies that might not only prevent this progression but perhaps even lead to regression of fibrosis and cirrhosis. The greatest unmet need is the treatment of NASH patients who suffer from either bridging fibrosis or cirrhosis. Therefore, several anti-fibrotic approaches are being entertained both as mono and as combination therapies in NASH. There is considerable optimism that these anti- fibrotic strategies would greatly improve our understanding of fibrosis progression in NASH and will benefit patients suffering from liver disease in the near future.
Acknowledgments
Declaration of personal interests: Dr Loomba has served as a consultant to Gilead Sciences, Galmed, Arrowhead, Tobira, Alnylam, Zafgen, Metacrine, Viking, Celgene, Metacrine, BMS, Intercept, Shire, Boehringer Ingelheim, Eli Lily, DeutRx, Genkyotex, Nimbus, RuiYi, Enanta, Isis, Conatus, Scholar Rock, Merck, Profil Institute, Janssen, Corgenix, Roivant and Adheron. He has received research grant support from Daiichi Sankyo, Gilead Sciences, Merck, Promedior, Galectin, Immuron, Galmed, Tobira, Seimens, BMS, NGM, GE and Kinemed. Dr Anstee has served as a consultant to Intercept, Genfit, Pfizer, Inventiva, Abbott and Raptor on behalf of Newcastle University. He has received research grant funding from GSK, Vertex and Abbvie. Dr Noureddin has served on the advisory board for Abbott.
Declaration of funding interests: RL is supported, in part, by the American Gastroenterological Association (AGA) Foundation —Sucampo — ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; funding provided by Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors and the American Gastroenterological Association and grant K23-DK090303 and 1R01DK106419-01. QMA is the recipient of a Clinical Senior Lectureship Award from the Higher Education Funding Council for England (HEFCE) and is a member of the EPoS (Elucidating Pathways of Steatohepatitis) consortium funded by the Horizon 2020 Framework Program of the European Union under Grant Agreement 634413.
Footnotes
This uncommissioned review article was subject to full peer-review.
AUTHORSHIP
Guarantor of the article: Rohit Loomba.
Author contributions: Mazen Noureddin designed the study, performed the literature search, collected the data and wrote the paper. Rohit Loomba designed the study, performed the research and wrote the paper. Quentin M. Anstee reviewed the data collection and co-wrote the paper. All authors approved the final version of the article.
References
- 1.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–30e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–23. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. e9. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosingsteatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–55. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 7.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–17. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- 9.Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–6. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley EM, Monsour HP. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:262–9. doi: 10.1055/s-0035-1562946. [DOI] [PubMed] [Google Scholar]
- 12.Bian Z, Ma X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol. 2012;3:248. doi: 10.3389/fphys.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porepa L, Ray JG, Sanchez-Romeu P, et al. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ. 2010;182:E526–31. doi: 10.1503/cmaj.092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell PT, Newton CC, Patel AV, et al. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35:1835–44. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 16.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 17.Brun P, Castagliuolo I, Pinzani M, et al. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–8. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 18.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe A, Hashmi A, Gomes DA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–18. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 20.Miura K, Kodama Y, Inokuchi S, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34. e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa S, Ikejima K, Yamagata H, et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol. 2011;54:1195–204. doi: 10.1016/j.jhep.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Syn WK, Oo YH, Pereira TA, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Review Team. LaBrecque DR, Abbas Z, et al. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–73. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL. Pathogenesis of hepatic fibrosis. In: Post TW, editor. UpToDate. UpToDate; Waltham, MA: [Accessed on March 23, 2016]. [Google Scholar]
- 26.Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979;76:710–9. [PubMed] [Google Scholar]
- 27.Gressner AM. The cell biology of liver fibrogenesis – an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447–52. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- 28.Marra F, Aleffi S, Bertolani C, et al. Review article: the pathogenesis of fibrosis in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22(Suppl 2):44–7. doi: 10.1111/j.1365-2036.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 29.Friedman SL. Liver fibrosis in 2012: convergent pathways that cause hepatic fibrosis in NASH. Nat Rev Gastroenterol Hepatol. 2013;10:71–2. doi: 10.1038/nrgastro.2012.256. [DOI] [PubMed] [Google Scholar]
- 30.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura K, Yang L, van Rooijen N, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–89. doi: 10.1002/hep.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy CD, Suzuki A, Zdanowicz M, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nobili V, Carpino G, Alisi A, et al. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142–53. doi: 10.1002/hep.25742. [DOI] [PubMed] [Google Scholar]
- 34.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–41. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297–305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 36.McCarty MF. Full-spectrum antioxidant therapy featuring astaxanthin coupled with lipoprivic strategies and salsalate for management of non-alcoholic fatty liver disease. Med Hypotheses. 2011;77:550–6. doi: 10.1016/j.mehy.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 39.Serviddio G, Bellanti F, Vendemiale G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radic Biol Med. 2013;65:952–68. doi: 10.1016/j.freeradbiomed.2013.08.174. [DOI] [PubMed] [Google Scholar]
- 40.Al-Serri A, Anstee QM, Valenti L, et al. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: evidence from case-control and intra-familial allele association studies. J Hepatol. 2012;56:448–54. doi: 10.1016/j.jhep.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Bugianesi E, Manzini P, D’Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–87. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 42.Arsov T, Larter CZ, Nolan CJ, et al. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006;342:1152–9. doi: 10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 43.Chen A, Tang Y, Davis V, et al. Liver fatty acid binding protein (L-Fabp) modulates murine stellate cell activation and diet-induced nonalcoholic fatty liver disease. Hepatology. 2013;57:2202–12. doi: 10.1002/hep.26318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larter CZ, Yeh MM, Van Rooyen DM, et al. Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice. J Gastroenterol Hepatol. 2009;24:1658–68. doi: 10.1111/j.1440-1746.2009.05996.x. [DOI] [PubMed] [Google Scholar]
- 45.Teratani T, Tomita K, Suzuki T, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142:152–64. e10. doi: 10.1053/j.gastro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 46.Tomita K, Teratani T, Suzuki T, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59:154–69. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 47.Tomita K, Teratani T, Suzuki T, et al. Acyl-CoA:cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J Hepatol. 2014;61:98–106. doi: 10.1016/j.jhep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Van Rooyen DM, Gan LT, Yeh MM, et al. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J Hepatol. 2013;59:144–52. doi: 10.1016/j.jhep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 49.Van Rooyen DM, Larter CZ, Haigh WG, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–403. 1403 e1–5. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29:313–22. doi: 10.1007/s00726-005-0200-2. [DOI] [PubMed] [Google Scholar]
- 51.Jiang JX, Chen X, Fukada H, et al. Advanced glycation endproducts induce fibrogenic activity in nonalcoholic steatohepatitis by modulating TNF-alpha-converting enzyme activity in mice. Hepatology. 2013;58:1339–48. doi: 10.1002/hep.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung C, Herath CB, Jia Z, et al. Dietary glycotoxins exacerbate progression of experimental fatty liver disease. J Hepatol. 2014;60:832–8. doi: 10.1016/j.jhep.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Miura K, Seki E, Ohnishi H, et al. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847. doi: 10.1155/2010/362847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–35. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 55.Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tajiri K, Shimizu Y, Tsuneyama K, et al. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:673–80. doi: 10.1097/MEG.0b013e32831bc3d6. [DOI] [PubMed] [Google Scholar]
- 57.Adler M, Taylor S, Okebugwu K, et al. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011;17:1725–31. doi: 10.3748/wjg.v17.i13.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–93. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 59.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–51. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 60.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 61.D’Ambrosio R, Aghemo A, Rumi MG, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532–43. doi: 10.1002/hep.25606. [DOI] [PubMed] [Google Scholar]
- 62.Ng V, Saab S. Effects of a sustained virologic response on outcomes of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9:923–30. doi: 10.1016/j.cgh.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 63.Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology. 2015;62:1417–32. doi: 10.1002/hep.27999. [DOI] [PubMed] [Google Scholar]
- 64.Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman SL. Emerging therapies for hepatic fibrosis: uptodate.com. 2014. [Google Scholar]
- 66.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 67.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90:1233–46. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78. e5. doi: 10.1053/j.gastro.2015.04.005. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Bacha F, Hannon T, et al. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61:2787–95. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marchesini G, Petta S, Dalle Grave R. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2015 Dec 11; doi: 10.1002/hep.28392. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 71.Hocking MP, Duerson MC, O’Leary JP, et al. Jejunoileal bypass for morbid obesity. Late follow-up in 100 cases. N Engl J Med. 1983;308:995–9. doi: 10.1056/NEJM198304283081703. [DOI] [PubMed] [Google Scholar]
- 72.Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:379–88. doi: 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82. e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 74.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. doi: 10.3978/j.issn.2305-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pellicciari R, Costantino G, Camaioni E, et al. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559–69. doi: 10.1021/jm049904b. [DOI] [PubMed] [Google Scholar]
- 76.Fuchs M. Non-alcoholic fatty liver disease: the bile acid-activated farnesoid x receptor as an emerging treatment target. J Lipids. 2012;2012:934396. doi: 10.1155/2012/934396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–65. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aoyama T, Paik YH, Watanabe S, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–27. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paik YH, Iwaisako K, Seki E, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–41. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui W, Matsuno K, Iwata K, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949–58. doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 82.Doherty GM, Jensen JC, Alexander HR, et al. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–8. [PubMed] [Google Scholar]
- 83.Duman DG, Ozdemir F, Birben E, et al. Effects of pentoxifylline on TNF-alpha production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:2520–4. doi: 10.1007/s10620-006-9723-y. [DOI] [PubMed] [Google Scholar]
- 84.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. 2011;10:277–86. [PubMed] [Google Scholar]
- 85.Xue X, Piao JH, Nakajima A, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005;280:33917–25. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 86.Ozawa K, Miyazaki M, Matsuhisa M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 87.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 88.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–76. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 90.Zein CO, Lopez R, Fu X, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology. 2012;56:1291–9. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for non-alcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology. 2015;62:1417–32. doi: 10.1002/hep.27999. [DOI] [PubMed] [Google Scholar]
- 93.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 94.Di Lella S, Sundblad V, Cerliani JP, et al. When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry. 2011;50:7842–57. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–71. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 96.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Traber PG, Chou H, Zomer E, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–97. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lefebvre E, Hashiguchi T, Jenkins H, et al. Anti-fibrotic and anti-inflammatory activity of the dual CCR2 and CCR5 antagonist cenicriviroc in a mouse model of NASH. Hepatology. 2013;58:221A–2A. [Google Scholar]
- 100.Hong F, Chou H, Friedman SL. Significant anti-fibrotic a`activity of cenicriviroc, a dual CCR2/CCR5 antagonist, in a rat model of thioacetamide-induced liver fibrosis and cirrhosis. Hepatology. 2013;58:1381A–2A. [Google Scholar]
- 101.Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994;234:354–66. doi: 10.1016/0076-6879(94)34105-2. [DOI] [PubMed] [Google Scholar]
- 102.Kagan VE. Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. Ann N Y Acad Sci. 1989;570:121–35. doi: 10.1111/j.1749-6632.1989.tb14913.x. [DOI] [PubMed] [Google Scholar]
- 103.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brigelius-Flohe R. Vitamin E: the shrew waiting to be tamed. Free Radic Biol Med. 2009;46:543–54. doi: 10.1016/j.freeradbiomed.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Fang F, Kang Z, Wong C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol Nutr Food Res. 2010;54:345–52. doi: 10.1002/mnfr.200900119. [DOI] [PubMed] [Google Scholar]
- 106.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 108.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 110.Bakuradze T, Boehm N, Janzowski C, et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: results from an intervention study. Mol Nutr Food Res. 2011;55:793–7. doi: 10.1002/mnfr.201100093. [DOI] [PubMed] [Google Scholar]
- 111.Misik M, Hoelzl C, Wagner KH, et al. Impact of paper filtered coffee on oxidative DNA-damage: results of a clinical trial. Mutat Res. 2010;692:42–8. doi: 10.1016/j.mrfmmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 112.Kempf K, Herder C, Erlund I, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–7. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 113.Loftfield E, Shiels MS, Graubard BI, et al. Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiol Biomarkers Prev. 2015;24:1052–60. doi: 10.1158/1055-9965.EPI-15-0038-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Modi AA, Feld JJ, Park Y, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201–9. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Molloy JW, Calcagno CJ, Williams CD, et al. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology. 2012;55:429–36. doi: 10.1002/hep.24731. [DOI] [PubMed] [Google Scholar]
- 116.Anty R, Marjoux S, Iannelli A, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012;57:1090–6. doi: 10.1016/j.jhep.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 117.Bambha K, Wilson LA, Unalp A, et al. Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014;34:1250–8. doi: 10.1111/liv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 119.Anand G, Zarrinpar A, Loomba R. Targeting dysbiosis for the treatment of liver disease. Semin Liver Dis. 2016;36:37–47. doi: 10.1055/s-0035-1571276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iacono A, Raso GM, Canani RB, et al. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–5. [PubMed] [Google Scholar]
- 122.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–53. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 123.Eslamparast T, Poustchi H, Zamani F, et al. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–42. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 124.Anstee QM, Concas D, Kudo H, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542–50. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 125.Witek RP, Stone WC, Karaca FG, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–30. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 126.Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–28. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shiffman M, Freilich B, Vuppalanchi R, et al. A placebo-controlled, multicenter, double-blind, randomised trial of emricasan (IDN-6556) in subjects with non-alcoholic fatty liver disease (NAFLD) and raised transaminases. International Liver Congress 2015, the 50th Annual Meeting of the European Association for the Study of the Liver (EASL); Vienna, Austria. April 22–26, 2015. [Google Scholar]
- 128.Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–52. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 129.Ratziu V, Harrison SA, Francque SM, et al. An international, phase 2 randomized controlled trial of the dual PPAR α-δ agonist GFT505 in adult patients with NASH. Hepatology. 2015;62:261A–5A. [Google Scholar]
- 130.Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2085–91. e1. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 131.Karnik S, Charlton MR, Li L, et al. Efficacy of an ASK1 inhibitor to reduce fibrosis and steatosis in a murine model of NASH is associated with normalization of lipids and hepatic gene expression and a reduction in serum biomarkers of inflammation and fibrosis. The Liver Meeting 2015; San Francisco, CA. November 13–17; American Association for the Study of Liver Diseases; 2015. [Google Scholar]
- 132.Karnik S, Charlton M, Popov Y, et al. Pharmacological inhibition of apoptosis signal-regulating kinase 1 (ASK1) in a murine model of NASH with pre-existing disease blocks fibrosis, steatosis, and insulin resistance. The Liver Meeting 2014; Boston, Massachusetts. November 7–11; American Association for the Study of Liver Diseases; 2014. [Google Scholar]
- 133.Hirsova P, Ibrahim SH, Bronk SF, et al. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS ONE. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moon HJ, Finney J, Ronnebaum T, et al. Human lysyl oxidase-like 2. Bioorg Chem. 2014;57:231–41. doi: 10.1016/j.bioorg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 136.Torok NJ, Dranoff JA, Schuppan D, et al. Strategies and endpoints of antifibrotic drug trials: summary and recommendations from the AASLD Emerging Trends Conference, Chicago, June 2014. Hepatology. 2015;62:627–34. doi: 10.1002/hep.27720. [DOI] [PMC free article] [PubMed] [Google Scholar]