Abstract
Objective
We evaluated the ability of third trimester ultrasound to diagnose disorders of fetal growth among women with diabetes mellitus.
Study design
This is a retrospective cohort study of women with diabetes who delivered term singleton neonates at a single academic medical center who had an ultrasound within 5 weeks of delivery. We characterized the sensitivity, specificity, positive predictive value and negative predictive value of ultrasound to detect large-for-gestational age (LGA) and small-for-gestational age (SGA) infants. LGA or SGA were defined as an ultrasound estimated fetal weight > 90% or < 10% based on the Hadlock formula, respectively; ultrasound estimates of LGA or SGA were compared to postnatal findings of LGA or SGA based on gestational age-based weight percentiles. Test characteristics were analyzed for the total cohort and by type of diabetes. We compared the area under the curve for receiver-operating characteristic (ROC) curves for different types of diabetes.
Results
Of 521 women, 3 (0.6%) screened positive for SGA and 64 (12.3%) delivered an SGA neonate. In contrast, 129 (24.8%) screened positive for LGA and 61 (11.7%) delivered an LGA neonate. The ROC curves did not differ significantly for different types of DM (p = 0.68).
Conclusion
Ultrasound in women with diabetes and term or late preterm pregnancies has a high specificity but poor sensitivity for SGA, and a low positive predictive value for LGA. Diagnostic capability of ultrasound to detect fetal growth abnormalities did not differ significantly by type of diabetes.
Keywords: ultrasound, diabetes, fetal growth disorders
Introduction
Due to the obesity epidemic, the proportion of pregnant women with either pre-gestational or gestational diabetes in pregnancy has increased in recent years.1,2 Women with gestational and pre-gestational diabetes are at increased risk of delivering a neonate that is large-for-gestational age (LGA).3 Diabetes and LGA fetuses are independent risk factors for a number of obstetric complications at the time of delivery, including shoulder dystocia,4 higher-order perineal lacerations,5 and cesarean delivery.6 Thus, a majority of women with diabetes undergo an ultrasound to assess fetal weight in the third trimester to assist with delivery planning.
Several technical challenges to ultrasound accuracy often exist in the diabetic pregnant patient. Ultrasound is known to be less accurate with increasing fetal weight7 and at later gestational ages.8 Many women with diabetes are also obese; maternal adiposity poses a challenge to an accurate ultrasonographically estimated fetal weight (US-EFW).9 Much previous literature on ultrasound in the diabetic population focuses on detection of macrosomia, with most studies showing that ultrasound performs relatively poorly in accurately detecting macrosomia.7 Overdiagnosis of macrosomia can lead to unindicated elective cesarean delivery or other interventions.10 Moreover, the diagnostic ability of ultrasound to detect SGA status among women with diabetes remains unclear.
Given these challenges but yet the common frequency of ultrasound in this population, it is important to understand the test characteristics of ultrasound for estimated fetal weight in this population. Additionally, it is unclear from previous studies whether the type of diabetes (gestational vs. pre-existing) affects the diagnostic accuracy of ultrasound to detect macrosomia. Thus in this paper, we used a large, tertiary care center cohort to characterize the diagnostic capability of a third trimester ultrasound to detect LGA and SGA fetuses in pregnant women with diabetes and to determine if there are differences in test characteristics by type of maternal diabetes.
Materials and methods
This is a retrospective cohort study of women aged 18 or greater with gestational or pre-gestational diabetes who delivered term (37.0 weeks' gestation or greater), singleton gestations and received sonographic examinations at Northwestern Memorial Hospital in Chicago, IL between 1/1/2010 and 12/31/2015. Women were included in the study if they had an ultrasound performed for fetal weight estimate within 5 weeks of delivery. Women were excluded if they had a fetus with a major anomaly, as this may impair accurately measuring fetal weight. Women who had ultrasounds performed solely for biophysical profile, fluid volume, or fetal presentation, but without assessment of estimated fetal weight, were excluded. Women who had informal “bedside” ultrasounds not read by the Division of Maternal-Fetal Medicine were additionally excluded. Clinical and demographic data were abstracted from the electronic medical record and ultrasound database, including information on estimated fetal weight and ultimate birthweight.
All ultrasounds were performed by trained sonographers calculating estimated fetal weight using the Hadlock formula incorporating head circumference, biparietal diameter, abdominal circumference, and femur length.11 For the purposes of consistent terminology, the phrases SGA and LGA refer to both fetuses and neonates at the extremes of growth, as terms such as “macrosomia” do not have consistently accepted definitions. We defined fetuses as SGA if the US-EFW was < 10% for gestational age at the time of ultrasound using the percentiles generated by Brenner et. al.12 and embedded into the AS ultrasound software package (AS Software Inc., Englewood Cliffs, NJ), and the corresponding neonate as SGA if birthweight was < 10% for gestational age at birth, using standardized growth curves for U.S. birthweights generated by Oken et. al.13 Similarly, we defined fetuses as LGA if the US-EFW was > 90% for gestational age at the time of ultrasound, and the corresponding neonate as LGA if birthweight was > 90% for gestational age at birth, again using the Brenner et. al. values for fetal growth curves and the standardized values generated by Oken et. al. for neonatal growth percentiles.12,13
We defined different types of gestational diabetes using the White criteria, with A1 gestational diabetics being those who were diet-controlled during pregnancy and A2 gestational diabetics those women that required medication.14 Women with pre-gestational diabetes were classified as either Type 1 or Type 2 using the diagnoses made prior to pregnancy according to patients' previous primary care physicians or endocrinologists; however, our institution follows recommended guidelines for early screening and diagnosis of type 2 diabetes during pregnancy.15
Patient demographic and clinical characteristics were compared by type of diabetes. We calculated the sensitivity, specificity, positive predictive value, and negative predictive value of an ultrasound performed within 5 weeks of delivery to detect SGA and LGA neonates for the overall sample, as well as for each type of diabetes. We also calculated the receiver-operating characteristic curve (ROC) associated with each type of diabetes, comparing the area under the curve (AUC) to see whether diagnostic performance varied across types of diabetes. The AUC values associated with different types of diabetes were compared for equality using the methods suggested by DeLong et. al.16
In order to determine the consequences of antenatal LGA and SGA diagnoses based on US-EFW, we also examined neonatal outcomes, including admission to the neonatal intensive care unit (NICU), shoulder dystocia, and third and fourth degree perineal laceration. We performed bivariable comparisons of neonatal outcomes based on weight category (SGA, appropriate for gestational age [AGA], or LGA) using chi square analysis and Fisher's exact test. Comparisons were considered statistically significant at the p < 0.05 level for two-sided hypotheses. All analyses were carried out in STATA (version 14.2, StataCorp, College Station, TX). Approval for this study was obtained from the Northwestern Institutional Review Board with a waiver of informed consent.
Results
Of 877 women with diabetes who otherwise met the study inclusion criteria, 59.5% (N=521) had an ultrasound within 5 weeks of delivery and comprised the population of interest. In this population, 0.6% (N=3) screened positive for SGA and 12.3% (N=64) delivered an SGA neonate. Thus, the overall sensitivity of ultrasound for SGA was 4.7%, and specificity was 100%. In contrast, 24.8% (N=129) screened positive for LGA and 11.7% (N=61) delivered an LGA neonate. The overall sensitivity of ultrasound for LGA was 80.3% and the specificity was 82.6%. The overall AUC was 0.82 for LGA and 0.52 for SGA. Table 1 shows there are no significant differences in demographic or obstetric characteristics between women whose fetuses were diagnosed accurately as compared to those who ultimately delivered a fetus in a different birthweight class than that predicted by ultrasound.
Table 1. Cohort characteristics.
| Variable | Accurately predicted birthweight category c (N = 368) | Inaccurately predicted birthweight category (N = 153) | P value |
|---|---|---|---|
| Maternal age (years) | 31.5 ± 5.4 | 31.0 ± 5.4 | 0.29 |
| Maternal race/ethnicity: | 0.20 | ||
| Non Hispanic white | 123 (38.4) | 45 (33.1) | |
| Non Hispanic black | 45 (14.1) | 30 (22.1) | |
| Hispanic | 76 (23.8) | 32 (23.5) | |
| Other | 76 (23.8) | 29 (21.3) | |
| Maternal BMI at delivery (kg/m2) | 0.40 | ||
| < 30 | 143 (38.9) | 69 (45.1) | |
| 30 – 39.9 | 151 (41.0) | 58 (37.9) | |
| ≥40 | 74 (20.1) | 26 (17.0) | |
| Type of diabetes: | 0.64 | ||
| A1 GDMb | 193 (52.5) | 86 (56.2) | |
| A2 GDM | 94 (25.5) | 40 (26.1) | |
| Type 2 DM | 39 (10.6) | 15 (9.8) | |
| Type 1 DM | 42 (11.4) | 12 (7.8) | |
| Gestational age at delivery (weeks) | 39.0 ± 1.0 | 39.0 ± 1.1 | 0.44 |
| Gestational age at ultrasound (weeks) | 37.1 ± 1.4 | 37.2 ± 1.4 | 0.24 |
| Time between last ultrasound and delivery (weeks) | 1.7 ± 1.3 | 1.5 ± 1.3 | 0.06 |
| Birthweight category at delivery: | < 0.001 | ||
| Small for gestational age | 3 (0.82) | 61 (39.9) | |
| Appropriate for gestational age | 316 (85.9) | 80 (52.3) | |
| Large for gestational age | 49 (13.3) | 12 (7.8) |
Numbers presented are either mean ± standard deviation or N(%).
GDM: gestational diabetes mellitus
‘Accurate’ and ‘inaccurate’ here refer to whether the predicted birthweight category (SGA, AGA, LGA) based on the ultrasound-estimated fetal weight was concordant with the actual birthweight category (SGA, AGA, LGA).
In assessing test characteristics by type of diabetes (Table 2), we identified that the sensitivity of ultrasound for the detection of LGA was lowest for women with type 2 diabetes mellitus (57.1%) and highest for women with type 1 diabetes mellitus (94.7%). In contrast, the specificity of ultrasound for LGA was greatest for women with GDMA1 (85.6%) and lowest for women with type 1 diabetes (68.6%). The positive predictive value of ultrasound for LGA was below 40% for all women except for those with type 1 diabetes, whereas the negative predictive value for LGA approached 100% for all women. With regard to SGA status, the sensitivity of ultrasound for SGA was universally poor, whereas specificity was universally 100%. The positive and negative predictive values for SGA were high for women with all types of diabetes.
Table 2. Diagnostic performance of ultrasound in the diabetic population.
| Any DM (N = 521) | A1 GDM (N = 279) | A2 GDM (N = 134) | Type 2 DM (N = 50) | Type 1 DM (N = 50) | |
|---|---|---|---|---|---|
| Large for gestational age: | |||||
| Screen positive | 129 | 53 | 34 | 13 | 29 |
| True positive | 61 | 22 | 13 | 7 | 19 |
| Sensitivity | 80.3% | 72.7% | 84.6% | 57.1% | 94.7% |
| Specificity | 82.6% | 85.6% | 81.0% | 80.9% | 68.6% |
| Positive predictive value | 38.0% | 30.2% | 32.4% | 30.8% | 62.1% |
| Negative predictive value | 96.9% | 97.3% | 98.0% | 92.7% | 96.0% |
| AUCa | 0.82 | 0.79 | 0.83 | 0.69 | 0.82 |
| Small for gestational age: | |||||
| Screen positive | 3 | 2 | 0 | 1 | 0 |
| True positive | 64 | 45 | 15 | 4 | 0 |
| Sensitivity | 4.7% | 4.4% | 0.0% | 25.0% | - |
| Specificity | 100% | 100% | 100% | 100% | - |
| Positive predictive value | 100% | 100% | - | 100% | - |
| Negative predictive value | 88.2% | 84.5% | 88.8% | 94.3% | - |
| AUC | 0.52 | 0.52 | - | 0.63 | - |
AUC: area under the curve
Figure 1 shows the receiver – operating characteristic for each type of diabetes in the detection of LGA. The ROC curves for the detection of LGA did not differ significantly by type of diabetes (p = 0.68). Further, adding type of diabetes to the predictive model did not improve the ability of ultrasound to detect LGA (p = 0.07). Given the overall poor performance of ultrasound to diagnose SGA, we did not compare ROC curves across types of diabetes for SGA.
Figure 1.
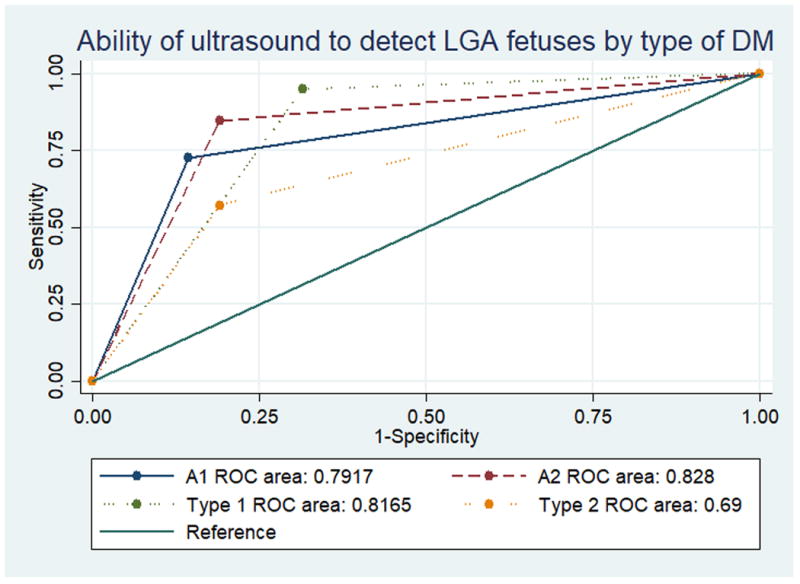
AUC values for different types of diabetes were not found to be significantly different, using the methods of DeLong et. al.16
Of the 389 women with fetuses determined to be appropriate for gestational age (AGA) on ultrasound, 15.7% (N=61) ultimately delivered an SGA neonate and 3.1% (N=12) delivered an LGA neonate. Of the 61 SGA neonates incorrectly identified as AGA on ultrasound, 31.2% (N=19) were admitted to the NICU compared to 21.2% (N=67) of the appropriately grown neonates (p = 0.09). Of the 12 women whose fetuses were identified as AGA but ultimately delivered LGA neonates, 5 delivered vaginally. Of these 5 women, 40.0% (N=2) experienced a third degree laceration, compared to 4.3% (N=8) of 185 women who were identified on ultrasound as having an AGA fetus and ultimately delivered an AGA fetus (p < 0.001). Of these 5 women, 20.0% (N=1) experienced a shoulder dystocia, compared to 3.6% (N=7) of women with AGA fetuses/neonates (p = 0.07). Of the 5 women who delivered vaginally, all 5 delivered an infant weighing > 4,000 grams, but only one woman delivered a neonate weighing > 4500 grams.
Discussion
Women with diabetes often undergo a growth ultrasound in the third trimester to aid in glycemic control and delivery planning.17 However, the diagnostic accuracy of ultrasound for estimated fetal weight in women with diabetes, for whom there is greater risk of LGA status but also potentially greater risk of error in ultrasound measurements, has not been well described. We characterized the diagnostic accuracy of ultrasound to detect fetal growth disorders, namely SGA and LGA, in a diverse population of women with both gestational and pre-gestational diabetes. Similar to other studies in older cohorts and in women without diabetes,7,18-20 the positive predictive value of a growth ultrasound for LGA in this population was low. This study confirms that the primary value of a third trimester growth ultrasound may be to rule out LGA. Women in this study who delivered an LGA fetus that was missed on prenatal diagnosis were more likely to experience shoulder dystocia and a third degree perineal laceration, although only one of these infants would have met the antepartum criteria of 4,500 grams for a primary cesearean delivery.21
A novel finding in this analysisis the low sensitivity of ultrasound for SGA in this sample of diabetic patients, which is considerably worse than in nondiabetic pregnancies.22-24 The underdiagnosis of SGA neonates in this population is concerning. While maternal diabetes is classically associated with fetal overgrowth, women with diabetes, particularly those with longstanding vascular disease, remain at risk of developing intrauterine growth restriction (IUGR),25 leading to a small for gestational age (SGA) neonate. Such risk can be compounded in the context of other risk factors for growth restriction, including inadequate getational weight gain,26 hypertension, or renal disease.27 These infants are at higher risk of stillbirth, neonatal morbidity, and neonatal mortality.28 A recent study among women with uncomplicated pregnancies indicates that SGA fetuses that are not properly diagnosed and monitored are at higher risk of stillbirth, neonatal mortality, and neonatal morbidity.28 While the majority of women in this population are already undergoing antenatal testing with either biophysical profiles or nonstress tests due to maternal diabetes, monitoring specific to intrauterine growth restricted fetuses, such as Dopplers of the umbilical artery, would not be performed. As clinicians likely have a higher index of suspicion for macrosomia, rather than IUGR, in this population, a substantial number of SGA infants may be missed despite frequent growth ultrasounds, an assertion that could be tested in other diabetic cohorts
The strengths of this analysis include study of a large cohort of women with multiple subtypes of diabetes. All ultrasounds were performed at a single institution by a small number of highly trained obstetric sonographers with ultrasounds read by a discrete group of maternal-fetal medicine physicians. However, this paper also has several limitations. First, the number of fetuses screening positive for SGA is low, which may make estimates of test characteristics unstable. Second, not all diabetic patients at our institution undergo third trimester growth studies (especially women with A1 gestational diabetes); thus, there may be selection bias in who received an ultrasound, although presumably women at greatest risk of LGA fetsues were the most likely to undergo a scan. Third, these results may not be generalizable to a broader population, as this study was performed at a tertiary care academic medical center, with highly trained obstetric sonographers. Finally, our study was underpowered to detect significant differences in rare adverse outcomes such as shoulder dystocia, as well as differences in the AUC for different ROC curves based on different subtypes of diabetes.
In summary, although ultrasound for estimated fetal weight in the third trimester is commonly performed for women with gestational or pre-gestational diabetes, the test characteristics of such an ultrasound suggest these estimates are far from perfect. Specifically, while ultrasound had a high negative predictive value for LGA, the sensitivity, specificity and positive predictive value for LGA are suboptimal. Moreover, ability to detect LGA did not differ by type of diabetes, although again, our study is likely underpowered to detect such differences. Further work is required to understand how clinicians can optimize the performance of ultrasound – such as by specific growth curves or other alterations in ultrasound performance – for this population.
Acknowledgments
LMY is supported by the NICHD K12 HD050121-11. Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A version of this paper was presented at the 64th Society for Reproductive Investigation Annual Scientific Meeting, Orlando, FL, March 15-18, 2017.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–813. doi: 10.1111/1471-0528.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng TY, Ehrlich SF, Crites Y, et al. Trends and racial and ethnic disparities in the prevalence of pregestational type 1 and type 2 diabetes in Northern California: 1996-2014. Am J Obstet Gynecol. 2017;216(2):177 e171–177 e178. doi: 10.1016/j.ajog.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191(3):964–968. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 4.Palatnik A, Grobman WA, Hellendag MG, Janetos TM, Gossett DR, Miller ES. Predictors of shoulder dystocia at the time of operative vaginal delivery. Am J Obstet Gynecol. 2016;215(5):624 e621–624 e625. doi: 10.1016/j.ajog.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Melamed N, Yogev Y, Meizner I, Mashiach R, Ben-Haroush A. Sonographic prediction of fetal macrosomia: the consequences of false diagnosis. J Ultrasound Med. 2010;29(2):225–230. doi: 10.7863/jum.2010.29.2.225. [DOI] [PubMed] [Google Scholar]
- 6.Scifres CM, Feghali M, Dumont T, et al. Large-for-Gestational-Age Ultrasound Diagnosis and Risk for Cesarean Delivery in Women With Gestational Diabetes Mellitus. Obstet Gynecol. 2015;126(5):978–986. doi: 10.1097/AOG.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Haroush A, Yogev Y, Hod M. Fetal weight estimation in diabetic pregnancies and suspected fetal macrosomia. J Perinat Med. 2004;32(2):113–121. doi: 10.1515/JPM.2004.021. [DOI] [PubMed] [Google Scholar]
- 8.Pressman EK, Bienstock JL, Blakemore KJ, Martin SA, Callan NA. Prediction of birth weight by ultrasound in the third trimester. Obstet Gynecol. 2000;95(4):502–506. doi: 10.1016/s0029-7844(99)00617-1. [DOI] [PubMed] [Google Scholar]
- 9.Paladini D. Sonography in obese and overweight pregnant women: clinical, medicolegal and technical issues. Ultrasound Obstet Gynecol. 2009;33(6):720–729. doi: 10.1002/uog.6393. [DOI] [PubMed] [Google Scholar]
- 10.Blackwell SC, Refuerzo J, Chadha R, Carreno CA. Overestimation of fetal weight by ultrasound: does it influence the likelihood of cesarean delivery for labor arrest? Am J Obstet Gynecol. 2009;200(3):340 e341–343. doi: 10.1016/j.ajog.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181(1):129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 12.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126(5):555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White P. Classification of obstetric diabetes. Am J Obstet Gynecol. 1978;130(2):228–230. doi: 10.1016/0002-9378(78)90373-3. [DOI] [PubMed] [Google Scholar]
- 15.Acog Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105(3):675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 17.Nelson L, Wharton B, Grobman WA. Prediction of large for gestational age birth weights in diabetic mothers based on early third-trimester sonography. J Ultrasound Med. 2011;30(12):1625–1628. doi: 10.7863/jum.2011.30.12.1625. [DOI] [PubMed] [Google Scholar]
- 18.Moore GS, Post AL, West NA, Hart JE, Lynch AM. Fetal weight estimation in diabetic pregnancies using the gestation-adjusted projection method: comparison of two timing strategies for third-trimester sonography. J Ultrasound Med. 2015;34(6):971–975. doi: 10.7863/ultra.34.6.971. [DOI] [PubMed] [Google Scholar]
- 19.Phillips AM, Galdamez AB, Ounpraseuth ST, Magann EF. Estimate of fetal weight by ultrasound within two weeks of delivery in the detection of fetal macrosomia. Aust N Z J Obstet Gynaecol. 2014;54(5):441–444. doi: 10.1111/ajo.12214. [DOI] [PubMed] [Google Scholar]
- 20.Valent AM, Newman T, Kritzer S, Magner K, Warshak CR. Accuracy of Sonographically Estimated Fetal Weight Near Delivery in Pregnancies Complicated With Diabetes Mellitus. J Ultrasound Med. 2017;36(3):593–599. doi: 10.7863/ultra.15.12021. [DOI] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 173: Fetal Macrosomia. Obstet Gynecol. 2016;128(5):e195–e209. doi: 10.1097/AOG.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 22.Sekar R, Khatun M, Barrett HL, Duncombe G. A prospective pilot study in assessing the accuracy of ultrasound estimated fetal weight prior to delivery. Aust N Z J Obstet Gynaecol. 2016;56(1):49–53. doi: 10.1111/ajo.12391. [DOI] [PubMed] [Google Scholar]
- 23.Fadigas C, Saiid Y, Gonzalez R, Poon LC, Nicolaides KH. Prediction of small-for-gestational-age neonates: screening by fetal biometry at 35-37 weeks. Ultrasound Obstet Gynecol. 2015;45(5):559–565. doi: 10.1002/uog.14816. [DOI] [PubMed] [Google Scholar]
- 24.Sovio U, White IR, Dacey A, Pasupathy D, Smith GC. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097. doi: 10.1016/S0140-6736(15)00131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kintiraki E, Goulis DG, Mameletzi S, et al. Large- and small-for-gestational-age neonates born by women with gestational diabetes mellitus diagnosed by the new IADPSG criteria: a case-control study of 289 patients and 1 108 controls. Exp Clin Endocrinol Diabetes. 2013;121(5):262–265. doi: 10.1055/s-0033-1334907. [DOI] [PubMed] [Google Scholar]
- 26.Kurnit KC, Overcash RT, Ramos GA, LaCoursiere DY. The impact of inadequate gestational weight gain in obese diabetic women. J Perinatol. 2016;36(2):86–89. doi: 10.1038/jp.2015.155. [DOI] [PubMed] [Google Scholar]
- 27.Leguizamon G, Trigubo D, Pereira JI, Vera MF, Fernandez JA. Vascular complications in the diabetic pregnancy. Curr Diab Rep. 2015;15(4):22. doi: 10.1007/s11892-015-0586-5. [DOI] [PubMed] [Google Scholar]
- 28.Mendez-Figueroa H, Truong VT, Pedroza C, Chauhan SP. Morbidity and Mortality in Small-for-Gestational-Age Infants: A Secondary Analysis of Nine MFMU Network Studies. Am J Perinatol. 2017;34(4):323–332. doi: 10.1055/s-0036-1586502. [DOI] [PubMed] [Google Scholar]


