Abstract
Periostin is a matricellular protein involved in development, maintenance and regulation of tissues and organs via by binding to cell surface integrin receptors. Pathologically, periostin plays an important role in the process of wound healing: as a deficiency of the Postn gene delays wound closure and periostin is consistently upregulated in response to injury and skin diseases. However, the functional role of elevated periostin in the process of wound healing has not been tested. In this study, we generated Postn-transgenic mice under the control of the CAG promoter/enhancer to investigate the effects of constitutive overexpression of full length periostin during its pathophysiological roles. Transgenic mice showed significant overexpression of periostin in skin, lung, and heart, but no morphological changes were observed. However, when these transgenic mice were injured, periostin overexpression delayed the closure of excisional wounds. Expression of IL-1β and TNFα, pro-inflammatory cytokines important for wound healing, was significantly decreased in the transgenic mice, prior to delayed healing. Infiltration of neutrophils and macrophages, the main sources of IL-1β and TNFα, was also downregulated in the transgenic wound sites. From these data, we conclude that enforced expression of periostin delays wound closure due to reduced infiltration of neutrophils and macrophages followed by downregulation of IL-1β and TNFα-expression. This suggests that regulated spatiotemporal expression of periostin is important for efficient wound healing and that constitutive periostin overexpression interrupts the normal process of wound closure.
Keywords: periostin, wound healing, fibrosis, matricellular protein, overexpressing mouse
Introduction
Cutaneous wound healing is a well-organized and highly coordinated physiological function.1 Wound healing comprises three sequential and overlapping phases: inflammation, tissue formation, and tissue reorganization. Spatiotemporally regulated expression of key mediators is crucial for implementing the wound healing process. For example, transforming growth factor-β1 (TGF-β) is important for cutaneous wound healing by stimulating keratinocyte migration, granulation tissue formation, and myofibroblast differentiation.2, 3 However, constitutive overexpression of TGF-β1 in keratinocytes delays wound closure by prolonging re-epithelialization and generation of granulation tissue.4, 5 Thus, spatiotemporal regulation of mediator expression is critical during the process of wound healing.
Periostin is an ECM protein belonging to the fasciclin family, acting as a matricellular protein by binding integrins such as αvβ3 or αvβ5 on the cell surface, thereby transducing signals within the cells.6, 7 Periostin plays important roles in developing and maintaining several tissues and organs. Specifically, periostin is required for the normal development of cardiac valves and periodontal ligament 8 and in remodeling of the neonatal lung.9 Induction of periostin is also important in mediating pathological processes, such as fibrosis after myocardial infarction,10 pulmonary fibrosis,11 and skin fibrosis such as scleroderma.12 In normal adult skin, periostin is predominantly expressed in the basement membrane and peri-hair follicular region in the dermis, but during excisional wound healing the spatiotemporal expression of periostin is expanded and robustly upregulated. 13 Moreover, periostin deletion delays the wound repair process by reducing myofibroblast-mediated wound contraction,14 re-epithelialization of the epidermis,15 and dermal fibroblast activation.16 These findings have been based mostly on analyses using wild-type and Postn-deficient mice.17 However, the effects of constitutively and ubiquitously overexpressed periostin in vivo have not yet been examined in skin. Only analyses using mice overexpressing periostin ectopically in adult cardiomyocytes under the α-myosin heavy chain promoter have been reported.10, 18
In this study, for the first time, we generated periostin-overexpressing mice under the control of the ubiquitous CAG promoter (CAG-Postn Tg mice). We then analyzed resultant morphological changes in the overexpression within skin, heart and lung at steady state; and then subsequently the effects of cutaneous wound healing in the presence of constitutive periostin.
Materials and methods
Animal studies
Eight- to twelve-week-old male C57BL6/J mice were used (Japan SLC, Hamamatsu, Japan). All experiments were performed following the guidelines for care and use of experimental animals required by the Japanese Association for Laboratory Animals Science (1987) and were approved by the Saga University Animal Care and Use Committee (Saga, Japan).
Generation of CAG-Postn transgenic mice
Full-length cDNA (2.5 kb) of murine periostin (NM_015784) was cloned into the pCAGGS plasmid vector harboring the CAG promoter and a rabbit β-globin poly (A) tail. The linearized construct was microinjected into the pronuclei of one-cell embryos of BDF1×BDF1 mice. Founder transgenic mice were identified by PCR with the primers specific for periostin cDNA and rabbit β-globin poly (A). The PCR primers for the transgene detection were as follows: forward primer, 5′-AGAAGACGATCAAGAGAAGGCCGTAGC-3′; reverse primer, 5′-CCTGCACCTGAGGAGTGAAT-3. CAG-Postn transgenic mice were crossed with C57BL6/J mice for at least six generations. The littermates of transgenic mice were used as non-transgenic mice.
Murine wound repair model
Mouse skin excisional wounds (6 mm) were generated using methods slightly modified from our earlier report (8 or 10 mm).16 Wounds were photographed with a reference object to allow image analysis. Area measurement and carburation were performed using Image-J software. Wound tissues were collected at indicated time points after injury and used for quantitative RT-PCR, ELISA, or histological analysis.
Immunohistochemistry
Wounded skin tissues were fixed in 10% buffered formalin and embedded in paraffin. Histological analyses were performed on serial sections spanning the central portion of the wound and were stained with H&E. In some experiments, heart and lung tissues were stained with Masson’s trichrome. Mouse anti–α-SMA antibody (Ab) (Dako, Glostrup, Denmark), rabbit anti-neutrophil elastase Ab (Abcam, Cambridge, UK), amti-F4/80 Ab (Abcam), and anti-periostin Ab16 were used for immunohistochemical staining. These sections were incubated with polymer-HRP and then developed with the DAB Detection System (Dako). For quantitative analysis of infiltrated neutrophil elastase+ and F4/80+ cells in wounded tissues, the number of elastase+ and F4/80+ cells in a center and two border regions of granulation tissue and two regions beneath the fibrin clot was counted at the high power fields (HPF) (400×).
Measurement of periostin
Periostin in the serum or tissue homogenates was measured by a sandwich ELISA with originally developed anti-periostin Abs (clone no. SS19C and SS19D).19 Briefly, a Nunc MaxiSorp ELISA plate (Affymetrix Japan, Tokyo, Japan) was coated with 2 μg/mL mouse anti-periostin Ab (SS19D) and then blocked with 0.5% casein. Diluted serum or tissue homogenates were added to the wells and incubated for 2 hours. After washing with phosphate-buffered saline (PBS) containing 0.05% Tween20 (PBS-T), biotinylated mouse anti-periostin Ab (SS19C) was added to each well and incubated for 1.5 hours. After washing with PBS-T, the wells were incubated with streptavidin-conjugated poly-HRP (Stereospecific Detection Technologies, Baesweiler, Germany) for 30 minutes and then washed with PBS-T. The reaction was developed with 3,3′, 5,5′-tetramethylbenzidine substrate for 5 minutes and stopped by adding an equal volume of 2 M sulfuric acid. Absorbance at 450 nm was measured. The recombinant mouse periostin (R&D Systems, Minneapolis, MN) was used as a standard.
Measurement of IL-1β and TNFα protein
IL-1β and TNFα proteins in tissue homogenates were measured with specific ELISA kits (Thermo Fisher Scientific, Waltham, MA).
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously described.20 Briefly, total RNA was prepared using an RNAiso plus reagent (Takara Bio, Otsu, Japan), and reverse-transcribed with the ReverTra Ace (TOYOBO, Osaka, Japan). Quantitative analyses were performed on StepOnePlus Real-Time PCR System (Life Technologies Japan, Tokyo, Japan) using the Thunderbird SYBR qPCR Mix (TOYOBO). The primer sequences were shown in Table S1.
Statistical analyses
The data shown are the mean ± standard deviation (SD) or standard error (SE). The statistical analyses were performed with the one-sided, Mann-Whitney t-test or one-way analysis of variance with multiple comparisons. p values less than 0.05 were considered to indicate statistically significant differences.
Results
Generation of Postn-transgenic mice overexpressing periostin constitutively and systemically in skin and serum
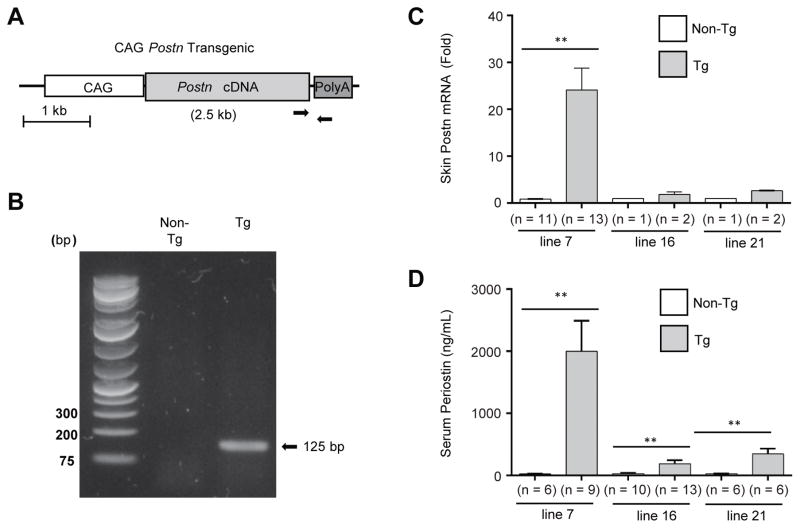
To test the in vivo functions of constitutively overexpressing periostin, we generated transgenic mice expressing full-length cDNA of mouse periostin controlled by the synthetic CAG promoter/enhancer that would drive ubiquitous mammalian expression of periostin (Figures 1A and B). We obtained three different lines of founder mice; lines 7, 16, and 21. As shown in Figure 1C, Postn mRNA expression in the skin was much higher in line 7 than in line 16 or in line 21. We further measured serum concentrations of periostin to examine the systemic periostin levels in these transgenic lines. Serum periostin markedly increased in line 7 compared to line 16 or line 21 (Figure 1D, line 7: 2001.2 ± 489.9 ng/mL, line 16: 187.6 ± 59.6 ng/mL, line 21: 347.7 ± 84.8 ng/mL). Since line 7 had higher periostin mRNA and protein expression than the other two lines, we used it for further experiments as Postn-overexpressing mice along with their age-matched littermates as non-transgenic controls.
Figure 1. Generation of Postn-overexpressing mice.
(A) Structure of the transgene. Transgene contains the ubiquitous CAG promoter and the full-length periostin cDNA followed by the rabbit β-globin polyadenylation signal. Arrows indicate the positions of forward and reverse primers used for transgene specific-PCR. (B) A representative PCR genotyping result from tails of Postn-overexpressing and non-transgenic mice. Skin sections and sera were collected from naïve Postn-overexpressing lines and non-transgenic littermates. (C) qRT-PCR analysis of periostin mRNA in transgenic lines 7, 16, and 21. (D) Quantitative analysis of periostin protein in serum within lines 7, 16, and 21. The data shown are the mean ± SE of three independent experiments. Statistical analysis was performed using a one-sided, Mann-Whitney t-test; **p < 0.01 versus non-transgenic.
No significant morphological changes in the skin or hematopoietic cells at steady state in Postn-overexpressing mice
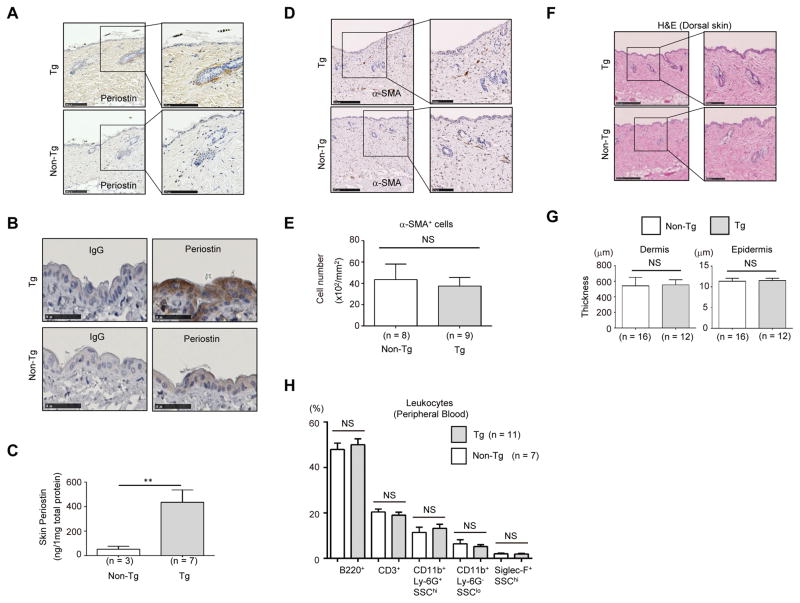
We examined the expression pattern of periostin in the skin of Postn-overexpressing mice by immunohistochemical staining. Periostin was weakly expressed in the dermis and in the peri-hair follicles in the dorsal skin of non-transgenic mice. Compared to non-transgenic mice, periostin expression substantially increased, particularly in the dermis and in the peri-hair follicles in Postn-overexpressing mice (Figure 2A). Although it is conceivable that CAG promoter can provide strong expression in all cell types, immunoreactivity of the epidermis to periostin Ab was lower than that of the dermis. Increased ectopic periostin expression in the epidermis of Postn-overexpressing mice was detected via longer exposure to immunostaining (Figure 2B). Consistent with these observations, protein concentrations of periostin markedly increased in the skin homogenates of Postn-overexpressing mice (Figure 2C). A previous study had suggested that periostin played a key role in the differentiation of dermal fibroblasts into myofibroblasts.14 However, the number and intensity of dermal alpha-smooth muscle actin (α-SMA+) cells did not increase in Postn-overexpressing mice (Figure 2D and E), and the skin sections did not show any specific morphological change (Figure 2F). There were no significant differences in the thickness of epidermis or dermis between Postn-overexpressing mice and non-transgenic mice (11.5 ± 0.5 μm and 554.6 ± 65.8 μm, Postn-overexpressing mice versus 11.3 ± 0.7 μm and 543.5 ± 108.2 μm, non-transgenic mice) (Figure 2G). Moreover, the ratio of peripheral blood T cells (CD3+/B220− cells), B cells (CD3−/B220+ cells), neutrophils (CD11bhi/Ly-6G+/SSCint cells), monocytes (CD11bhi/Ly-6G−/SSClo cells), and eosinophils (CD11b+/Siglec-Fhi/SSChi cells) did not change, even in the presence of excess circulating periostin levels (Figure 2H). These data suggest that constitutive overexpression of periostin does not cause any particular morphologic change in skin or in the proportions of hematopoietic cells, and that the mere presence of elevated periostin in uninjured skin within both normal and ectopic locations is not pathological.
Figure 2. Histological analysis of the uninjured dorsal skin of Postn-overexpressing mice.
(A, B) Immunostaining of periostin in the dorsal skin of Postn-overexpressing (Tg) mice (upper) and non-transgenic (Tg) mice (lower). In panel B, immunostaining with anti-periostin Ab or control IgG with long exposure is depicted. Scale bars: 250 μm (A, left panels), 100 μm (A, right panels), 50 μm (B). (C) Periostin levels in dorsal skin from uninjured Tg and non-Tg mice. Protein values were calculated based on 1 mg of the homogenate protein. (D) Immunostaining of α-SMA in the dermis of Postn-overexpressing mice and non-transgenic mice. Scale bars: 250 μm (left panels), 100 μm (right panels). (E) Quantitative data of the number of α-SMA+ cells with statistical analysis. (F) H&E staining of dorsal skin from uninjured Tg (upper) and non-Tg mice (lower). Low magnification (left panel) and high magnification (right panel) images are depicted. Scale bars: 250 μm (left panels), 100 μm (right panels). (G) Quantitative data of dermis and epidermis thickness. (H) Proportions of B220+ cells (B cells), CD3+ cells (T cells), CD11b+Ly-6G+SSChigh cells (neutrophils), CD11b+Ly-6G−SSClow cells (monocytes), and Siglec-F+SSChigh cells (eosinophils) in peripheral blood leukocytes of Postn-overexpressing mice and non-transgenic mice are depicted. The data shown are the mean ± SE (C) or SD (E, G, and H). Statistical analysis was performed using a one-sided, Mann-Whitney t-test; **p < 0.01 versus non-transgenic. NS; not significant
No overt cardiac or pulmonary fibrosis at steady state in Postn-overexpressing mice
Since periostin upregulation is involved in the pathogenesis of cardiac and pulmonary fibrosis as well as in cutaneous fibrosis,10–12 we examined whether overexpression of periostin spontaneously promotes fibrosis in lung or heart tissues. Postn-overexpressing mice exhibited increased periostin expression in heart and lung tissues (Figure S1A). Immunohistochemical staining showed that periostin deposition was elevated in the endocardium and subepithelial spaces around bronchioles (Figure S1B). However, fibrotic areas detected via Masson’s trichrome staining did not increase in Postn-overexpressing mice (Figure S1C). These results suggest that enforced expression of periostin does not induce overt fibrosis of heart or lung tissues at steady state. This lack of response is in agreement with prior studies that ectopically drove increased periostin levels in adult mouse hearts.10, 18
Induction of periostin protein expression by skin injury during wound healing
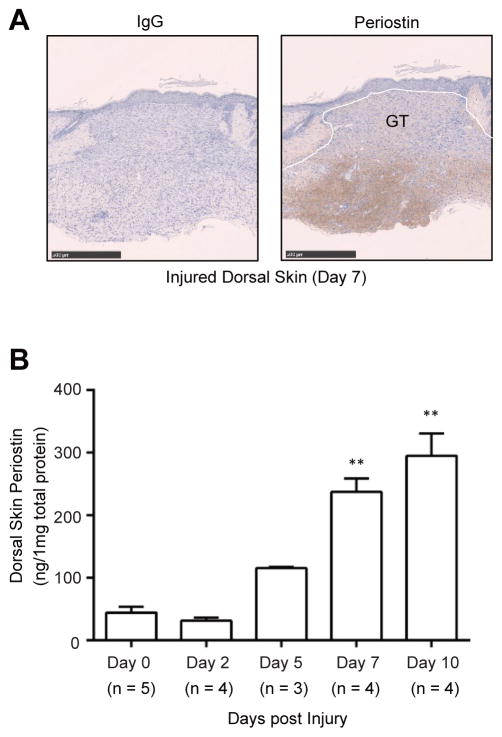
We and others have already reported that excisional wounds induce spatiotemporal upregulation of periostin in skin of mice.14–16 However, the changes in expression of periostin protein were not quantitatively measured in these prior analyses. Thus, we next examined time-dependent expression of endogenous periostin protein during wound healing. Full-thickness excisional wounds were created on the backs of mice. Periostin protein was substantially expressed beneath the granulation tissue of C57BL/6 mice at day 7 post-wounding in accordance with previous reports by us and others15, 16 (Figure 3A). Furthermore, we analyzed quantitative differences in periostin expression levels in unwounded (day 0) and wounded (days 2, 5, 7, and 10) skin homogenates using an ELISA system (Figure 3B). There was no significant increase in the levels of periostin protein at day 2 post-wounding (day 0: 44.5 ± 5.1 ng/mg protein, day 2: 31.5 ± 4.5 ng/mg protein). However, the amounts of periostin protein in wounded skin homogenates gradually and progressively increased (day 5: 115.4 ± 3.2 ng/mg protein, day 7: 237.6 ± 14.4 ng/mg protein, day 10: 295.3 ± 29.5 ng/mg protein). These results suggest that skin injury-inducing expression of periostin protein is spatiotemporally regulated during normal wound repair.
Figure 3. Spatiotemporal periostin expression in the dorsal skin following wounding.
(A) Immunostaining of periostin in the wounded dorsal skin (Day 7). GT: granulation tissue. (Scale bar = 500 μm) (B) Periostin protein in wounded skin homogenates during wound healing. Protein values were calculated based on 1 mg of the homogenate protein. The data shown are the mean ± SE of two independent experiments. Statistical analysis was performed using a one-sided, Mann-Whitney t-test; **p < 0.01 versus Day 0.
Delay of wound healing in Postn-overexpressing mice
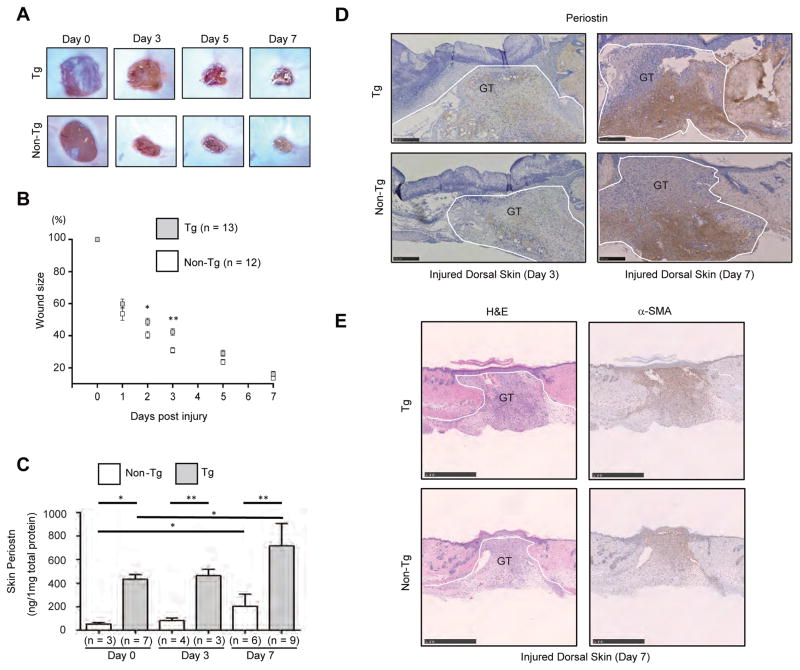
To examine the effects of constitutive overexpression of periostin on wound healing, we generated full-thickness excisional wounds on the dorsal skins of Postn-overexpressing mice and of sex- and age-matched non-transgenic mice. Wound closure was significantly delayed in Postn-overexpressing mice, especially in the early phase of the wound repair process (Figure 4A). At days 2 and 3 after injury, in non-transgenic mice, wound sizes were reduced from their initial wound areas to 53.7 ± 4.6% and 40.7 ± 2.6%, respectively. In Postn-overexpressing mice, wound size was significantly larger than that of non-transgenic mice (60 ± 3.3% at day 2, p <0.05 and 48.6 ± 2.5% at day 3, p <0.01) (Figure 4B). However, by day 7, the wound size in Postn-overexpressing mice reached a level similar to that of non-transgenic mice (16.1 ± 1.6%, Postn-overexpressing mice versus 13.5 ± 1.4%, non-transgenic mice). No significant difference in granulation tissue sizes between Postn-overexpressing and non-transgenic mice was observed at day 7 after injury (2.1 ± 0.35 mm2, Postn-overexpressing mice versus 2.0 ± 0.2 mm2, non-transgenic mice) (Figure S2). These results demonstrate that constitutive overexpression of periostin delays the closure of wounds in mouse skin.
Figure 4. Delayed wound healing in Postn-overexpressing mice.
(A, B) Successive photographs (A) or size (B) of the wounds at the indicated days post injury in the dorsal skin of Postn-overexpressing (Tg) mice and non-transgenic (Tg) mice. Statistical analysis was performed using Mann-Whitney t-test and mean ± SE is shown; *p < 0.05, **p < 0.01 versus non-transgenic mice. (C, D) Periostin protein in skin homogenates (C) or histology of the wounded dorsal skin of Postn-overexpressing (Tg) mice and non-transgenic (Tg) mice (D) (day 3 and 7). GT: granulation tissue. The data shown are the mean ± SE. Statistical analysis was performed using one-way analysis of variance with multiple comparisons; *p < 0.05, **p < 0.01. (E) The skin sections were stained with H&E or anti–α-SMA Ab. (Scale bar = 1000 μm).
To examine whether skin injury induced periostin protein levels are altered in Postn-overexpressing mice, we evaluated the amounts of periostin using the skin homogenates. Slight but not significant increase in periostin protein was similarly induced in both Postn-overexpressing and non-transgenic mice at day 3 post-wounding. At 7 day after injury, periostin protein significantly increased in both (717.2 ± 63.6 ng/mg protein, Postn-overexpressing mice versus 204.4 ± 42.1 ng/mg protein, non-transgenic mice) (Figure 4C). As the increases in periostin were comparable in the two groups of mice, this suggests that expression of periostin protein triggered by the wound is not affected in Postn-overexpressing mice. As shown in Figure 4D, however, distribution of periostin at 3 day after injury differed between Postn-overexpressing mice and non-transgenic mice. Periostin was present throughout maturating granulation tissue in Postn-overexpressing mice but not in non-transgenic mice. Infiltrated leukocytes in wounds failed to react with periostin Ab even in Postn-overexpressing mice. At day 7 post-wounding, periostin distribution in the granulation tissue was comparable in both groups of mice and wound-inducible pattern of α-SMA expression was also similar in both groups of mice (Figure 4E). Moreover, circulating periostin in serum did not change in either wounded Postn-overexpressing or non-transgenic mice (Figure S3).
These observations suggest that constitutive overexpressed periostin delays wound repair without abolishing expression of tissue-restricted periostin induced by the injury, excluding the possibility that the delayed wound healing is due to transgenic abrogation of wound-induced periostin expression.
Attenuation of TNFα and IL-1β expression induced by skin injury in Postn-overexpressing mice
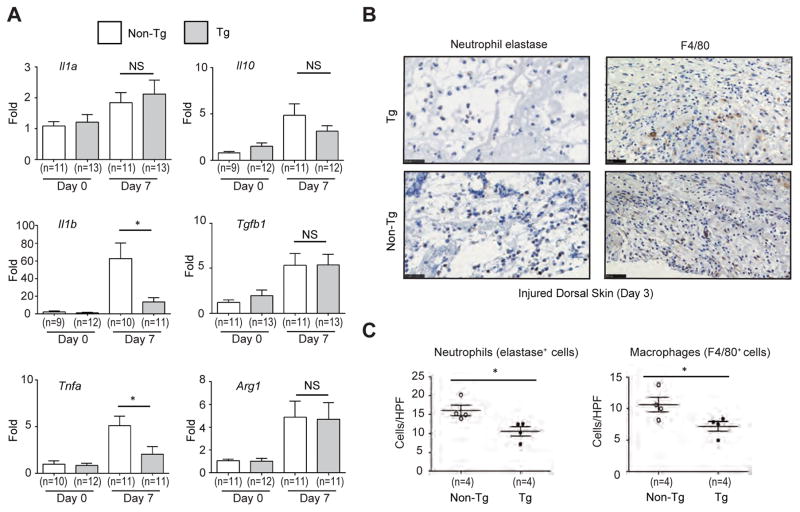
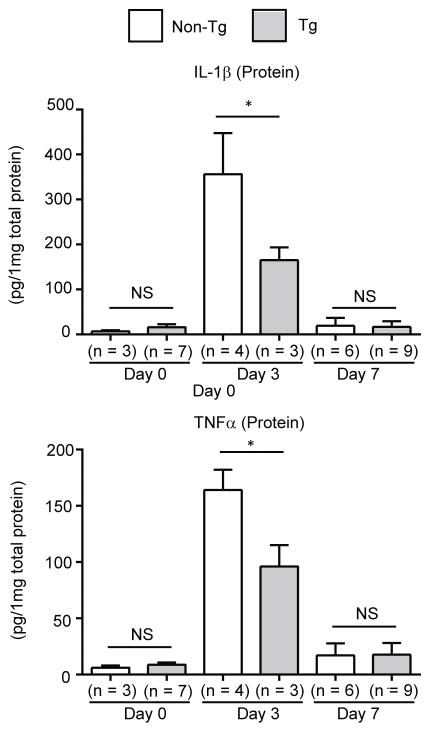
It is well known that balanced expression of pro- and anti-inflammatory cytokines is important for the regulation of wound repair.21 Thus, we analyzed the expression of pro- (Il1a, Il1b, and Tnfa) and anti- (Il10, Tgfb1, and Arg1) inflammatory cytokines. After cutaneous wounding, expression of most of these cytokines increased in both groups of mice. However, expression of Il1b and Tnfa in the wounded tissue of Postn-overexpressing mice was significantly lower than in non-transgenic control littermates (Figure 5A). This result raises the possibility that recruitment of inflammatory cells which express Il1b and Tnfa into the wounds is reduced in Postn-overexpressing mice. Since macrophages and neutrophils are main sources of IL-1β and TNFα during skin wound healing,22–24 we assessed the numbers of F4/80+ cells (macrophages) and neutrophil elastase+ cells (neutrophils) which are present in the wounds at the earlier time point (day 3). Both macrophages defined as F4/80+ cells and neutrophils defined as elastase+ cells significantly decreased in maturating granulation tissue in Postn-overexpressing mice compared to non-transgenic mice (Figure 5B and C). At the same time point, production of IL-1β and TNFα protein was reduced in Postn-overexpressing mice (Figure 6). These results demonstrated that, in Postn-overexpressing mice, recruitment of inflammatory cells such as macrophages and neutrophils was inhibited and consequently the balance between pro- and anti-inflammatory cytokines during wound repair are dysregulated, which would lead to the observed delayed wound closure phenotype.
Figure 5. Downregulated expression of Il1b and Tnfa in wounded skin of Postn-overexpressing mice.
(A) Expression of pro- (Il1a, Il1b, and Tnfa) and anti-inflammatory genes (Il10, Tgfb1, and Arg1) in wounded dorsal skin of Postn-overexpressing and non-transgenic mice on days 0 and 7 after wounding. (B) Immunostaining of neutrophil elastase and F4/80 in wounded dorsal skin of Postn-overexpressing mice and non-transgenic mice (day 3). Scale bars: 25 μm (left panels), 50 μm (right panels). (C) Quantitative data of the number of neutrophil elastase+ and F4/80+ cells in granulation tissues. The data shown are the mean ± SE. Statistical analysis was performed using a one-sided, Mann-Whitney t-test; *p < 0.05 versus non-transgenic mice. NS; not significant
Figure 6. Expression of IL-1β and TNFα is reduced in wounded skin of Postn-overexpressing mice.
Expression of IL-1β and TNFα proteins in wounded dorsal skin of Postn-overexpressing and non-transgenic mice on days 0, 3, and 7 after wounding. Protein values were calculated based on 1 mg of the homogenate protein. The data shown are the mean ± SD. Statistical analysis was performed using a one-sided, Mann-Whitney t-test; *p < 0.05 versus non-transgenic mice. NS; not significant
Discussion
Periostin exerts various actions in vivo as both an ECM protein and a matricellular protein.6 The analyses of periostin-deficient mice have contributed a great deal to elucidating the pathophysiological requirement of periostin; as Postn-deficient mice show impaired neonatal valvulogenesis in heart and development of pulmonary remodeling.8, 9 In addition to the normal physiological roles of periostin, applying Postn-deficient mice to model various diseases has revealed that upregulated periostin plays important roles in fibrosis after myocardial infarction,10 pulmonary fibrosis,11 and skin fibrosis mimicking scleroderma.12 Moreover, using Postn-deficient model mice in the study of cutaneous wound repair has shown that genetic deficiency of periostin delays wound closure, highlighting its significance for wound healing in skin.14–16 However, thus far, no Postn-transgenic mice in which periostin is ubiquitously overexpressed have been established. Only transgenic mice overexpressing periostin ectopically in adult cardiomyocytes has been reported.10, 18 In this study, for the first time, we generated Postn-overexpressing mice under the control of the ubiquitous CAG promoter. In these transgenic mice, periostin expression was constitutively increased in various tissues such as skin, heart, lung, and blood although the CAG promoter-derived protein expression levels differed among tissues or cell types. The differences in enforced periostin deposition may be explained by a recent study demonstrating that CpG methylation of CAG promoter in each tissue results in varied expression of transgenes25, that different tissues and cell type express different levels and/or combinations of its integrin receptor pairs, or that periostin deposition is only permissible in certain tissues or cell types. Moreover, we applied these Postn-overexpressing mice to the model of skin wound healing. The findings in this study contribute to elucidating the pathophysiological roles of periostin induction in vivo.
In this study, we demonstrated that Postn-overexpressing mice show no morphological change in skin and no overt cardiac or pulmonary fibrosis (Figures 2 and S1). It has been reported that neither heart size nor the number of cardiomyocytes change in transgenic mice with ectopic periostin in the heart.10, 18 Although we have not examined cardiac function in our transgenic mice, the present findings can be expected to be compatible with the previous ones. Although in vitro experiments have shown that periostin alone can induce differentiation of quiescent fibroblasts into α-SMA+ myofibroblasts,14 proliferation of dermal fibroblasts and keratinocytes,16, 26 and collagen I expression in lung mesenchymal cells,27 these changes did not occur in vivo in our mice (Figures 2 and S1). We and others have already shown that cross-talk of periostin and various cytokines such as IL-1α, TNFα, or TGF-β is important to transduce their signals in skin and lung.11, 12, 26 These results suggest that periostin cooperates with various cytokines rather than mediating pathogenesis by itself. This may explain why there is a discrepancy in the results based on Postn-deficient mice in which the cross-talk of periostin and other mediators is impaired and in the mice in which endogenous periostin alone is overexpressed.
We and others have previously demonstrated that genetic deficiency of periostin delays wound closure in mice, demonstrating that periostin is an important mediator in the process of wound healing.14–16 In contrast, in this study, we showed that constitutive overexpression of periostin also delays wound closure, particularly at the early phases (days 2 and 3). Such a paradoxical result has been observed also in the case of matricellular thrombospondin-1 (TSP-1) and TGF-β1; wound healing is delayed in Tgfb1−/−/Scid−/− mice compared to Scid−/− mice.28 Moreover, deficiency of TSP-1 delays wound closure accompanied with decreases of macrophage infiltration and TGF-β1 expression.29 In contrast, constitutive expression of either TGF-β1 or TSP-1 causes a delay of wound closure as well.4, 5, 30 The exact mechanism of how constitutive overexpression of periostin, an important mediator in wound healing, delays wound closure has previously not been well understood. Upregulation and expression of these mediators is usually under tight spatiotemporal regulation. Taking these data into consideration, we assume that these delays can be attributed to spatiotemporal dysregulation of periostin expression. The amount of net increase in periostin protein by the wound was not suppressed in Postn-transgenic mice, suggesting that such transgenic periostin expression can interfere with wound healing without reducing the wound-induced expression of periostin (Figure 4C).
In this study, we investigated how constitutive overexpression of periostin impairs wound healing, finding that expression of pro-inflammatory cytokines, IL-1β and TNFα, is downregulated in the wound sites (Figures 5A and 6). It is well known that IL-1β and TNFα play important roles in wound repair by activating macrophages and neutrophils, followed by upregulating the production of growth factors,22, 31 inducing migration and proliferation of keratinocytes via matrix metalloproteinases,32 and activating fibroblasts followed by induction of FGF7 important for re-epithelialization.33, 34 Genetic deficiency of TNFα enhances granulation tissue formation by up-regulating the TGF-β signals and delays re-epithelialization.35 Moreover, deficiency of IL-1 receptor, the common receptor for both IL-1α and IL-1β, impairs oral, but not cutaneous, wound repair by supporting inflammation.36 Taking these findings together, we assume that down-regulation of IL-1β and TNFα expression is one of the underlying mechanisms for how wound healing is impaired in Postn-overexpressing mice. M1-like macrophages and neutrophils are the principal sources of IL-1β and TNFα during wound repair.22–24 It was recently reported that treating mouse intraperitoneal macrophages with high doses of recombinant periostin changes the characteristics of these macrophages, decreasing expression of M1 markers including IL-1β.37 We found that constitutive overexpression of periostin repressed infiltration of macrophages and neutrophils at early wound sites (Figure 5C). This suggests that infiltration of neutrophils and macrophages followed by downregulated expression of IL-1β and TNFα would be one mechanism of how enforced expression of periostin delays the wound closure. Alternatively, spatiotemporal dysregulation of periostin expression (including non-physiological ectopic expression in the epidermis, lung, heart or blood) may disturb infiltration and accumulation of macrophages, neutrophils, or fibroblasts into the wound sites; as it has been shown that periostin is important for production of chemokines for the recruitment of inflammatory cells and the migration of fibroblasts.11, 26 It is still unclear whether enforced expression of periostin in cells that would not express Postn—macrophages, neutrophils, and fibroblasts—affects their abilities of cytokine production or migration independently of extracellular excess periostin. Further analysis would be required to clarify these important issues.
We previously demonstrated that IL-4 and IL-13, signature type 2 cytokines, can induce periostin and that periostin is highly expressed in the dermis of hapten-induced model mice with allergic skin inflammation or in atopic dermatitis (AD) patients, in parallel with the clinical severity downstream of these cytokines.38 In contrast, it is known that in patients with allergic skin inflammation to haptens, wound healing is delayed.39 Taken together with the present findings, high expression of ubiquitous periostin may contribute to impaired wound repair in patients with allergic skin inflammation. Consistent with this notion, it has been recently demonstrated that excisional wound closure is significantly delayed in mice overexpressing IL-4 or the active form of STAT6, a transcription factor critical for the IL-4/IL-13 signals that manifest AD-like skin disorders.40, 41 These results support the intriguing possibility that periostin mis-expression may be involved in the impaired wound repair seen in AD patients.
Supplementary Material
Acknowledgments
We thank Dr. Dovie R. Wylie for the critical review of this manuscript. We also thank Kazuyo Yoshida, Maki Watanabe, Miho Miyake, Yukiko Tokuda, Yoko Esaki and Saki Nishioka for technical assistance. We thank NPO Biotechnology Research and Development for generating transgenic animals.
Source of funding: This work was supported in part by Grants-in-Aid for Scientific Research from the Saga Medical School (to KI); and in part, by JSPS KAKENHI Grant Number #JP25293224, #JP16H05343 (to KI); and by the National Institutes of Health (to SJC).
Abbreviations
- α-SMA
alpha-smooth muscle actin
- Ab
antibody
- AD
atopic dermatitis
- DAB
3,3′-diaminobenzidine tetrahydrochloride
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- HPF
phosphate buffered saline
- PBS
high power fields
- SD
standard deviation
- SE
standard error
- STAT6
signal transducer and activator of transcription 6
- Tg
transgenic
- TGF-β1
transforming growth factor-β1
- TNFα
tumor necrosis factor-α
- TSP
thrombospondin
Footnotes
Conflicts of interest: The authors declare no competing interests.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Eng J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gailit J, Welch MP, Clark RA. TGF-β1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103(2):221–7. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- 4.Chan T, Ghahary A, Demare J, Yang L, Iwashina T, Scott PG, et al. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-β1 transgenic mouse. Wound Repair Regen. 2002;10(3):177–87. doi: 10.1046/j.1524-475x.2002.11101.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Chan T, Demare J, Iwashina T, Ghahary A, Scott PG, et al. Healing of burn wounds in transgenic mice overexpressing transforming growth factor-β1 in the epidermis. Am J Pathol. 2001;159(6):2147–57. doi: 10.1016/s0002-9440(10)63066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71(7):1279–88. doi: 10.1007/s00018-013-1494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto K. Periostin in allergic inflammation. Allergol Int. 2014;63(2):143–51. doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]
- 8.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102(7):752–60. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozyk PD, Bentley JK, Popova AP, Anyanwu AC, Linn MD, Goldsmith AM, et al. Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication. PLoS One. 2012;7(2):e31336. doi: 10.1371/journal.pone.0031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101(3):313–21. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46(5):677–86. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Serada S, Fujimoto M, Terao M, Kotobuki Y, Kitaba S, et al. Periostin facilitates skin sclerosis via PI3K/Akt dependent mechanism in a mouse model of scleroderma. PLoS One. 2012;7(7):e41994. doi: 10.1371/journal.pone.0041994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal. 2009;3(2):125–33. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, et al. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci. 2012;125(1):121–32. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, et al. Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One. 2011;6(4):e18410. doi: 10.1371/journal.pone.0018410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21(5):331–6. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 17.Izuhara K, Nunomura S, Nanri Y, Ogawa M, Ono J, Mitamura Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74(23):4293–4303. doi: 10.1007/s00018-017-2648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104(1):e1–7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama A, Kanno K, Nishimichi N, Ohta S, Ono J, Conway SJ, et al. Periostin promotes hepatic fibrosis in mice by modulating hepatic stellate cell activation via αv integrin interaction. J Gastroenterol. 2016;51(12):1161–74. doi: 10.1007/s00535-016-1206-0. [DOI] [PubMed] [Google Scholar]
- 20.Arima K, Ohta S, Takagi A, Shiraishi H, Masuoka M, Ontsuka K, et al. Periostin contributes to epidermal hyperplasia in psoriasis common to atopic dermatitis. Allergol Int. 2015;64(1):41–8. doi: 10.1016/j.alit.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haertel E, Werner S, Schäfer M. Transcriptional regulation of wound inflammation. Semin Immunol. 2014;26(4):321–8. doi: 10.1016/j.smim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Feiken E, Rømer J, Eriksen J, Lund LR. Neutrophils express tumor necrosis factor-α during mouse skin wound healing. J Invest Dermatol. 1995;105(1):120–3. doi: 10.1111/1523-1747.ep12313429. [DOI] [PubMed] [Google Scholar]
- 23.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. doi: 10.1016/j.semcdb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine. 2015;71(2):409–12. doi: 10.1016/j.cyto.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Zhang T, Zhang QK, Jiang Y, Xu DG, Zhang M, et al. Unstable expression of transgene is associated with the methylation of CAG promoter in the offspring from the same litter of homozygous transgenic mice. Mol Biol Rep. 2014;41(8):5177–86. doi: 10.1007/s11033-014-3385-1. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 loop. J Invest Dermatol. 2014;134(5):1295–304. doi: 10.1038/jid.2013.500. [DOI] [PubMed] [Google Scholar]
- 27.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, et al. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1046–56. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-β1 knockout mice. J Invest Dermatol. 2000;115(1):3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 29.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831–9. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19(13):3272–82. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Saeki K, Matsunobu T, Okuno T, Koga T, Sugimoto Y, et al. 12-hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J Exp Med. 2014;211(6):1063–78. doi: 10.1084/jem.20132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang A, Gilchrest BA. Regulation of keratinocyte growth factor gene expression in human skin fibroblasts. J Dermatol Sci. 1996;11(1):41–50. doi: 10.1016/0923-1811(95)00418-1. [DOI] [PubMed] [Google Scholar]
- 34.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 35.Shinozaki M, Okada Y, Kitano A, Ikeda K, Saika S. Impaired cutaneous wound healing with excess granulation tissue formation in TNFα-null mice. Arch Dermatol Res. 2009;301(7):531–7. doi: 10.1007/s00403-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 36.Graves DT, Nooh N, Gillen T, Davey M, Patel S, Cottrell D, et al. IL-1 plays a critical role in oral, but not dermal, wound healing. J Immunol. 2001;167(9):5316–20. doi: 10.4049/jimmunol.167.9.5316. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17(2):170–82. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122(7):2590–600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lhotka CG, Szekeres T, Fritzer-Szekeres M, Schwarz G, Steffan I, Maschke M, et al. Are allergic reactions to skin clips associated with delayed wound healing? Am J Surg. 1998;176(4):320–23. doi: 10.1016/s0002-9610(98)00197-4. [DOI] [PubMed] [Google Scholar]
- 40.Serezani AP, Bozdogan G, Sehra S, Walsh D, Krishnamurthy P, Sierra Potchanant EA, et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J Allergy Clin Immunol. 2017;139(1):142–51. e5. doi: 10.1016/j.jaci.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Bao L, Chan LS, DiPietro LA, Chen L. Aberrant wound healing in an epidermal interleukin-4 transgenic mouse model of atopic dermatitis. PLoS One. 2016;11(1):e0146451. doi: 10.1371/journal.pone.0146451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.