Introduction
Mitogen-activated protein kinase (MAPK) signaling in the liver occurs in response to physical and chemical stress, including alterations in nutrients, growth factors, cytokines, extracellular matrix, DNA damage, drugs and toxins. This signaling pathway plays a role in liver injury and diseases such as drug induced hepatotoxicity, viral hepatitis, infection and inflammation, NAFLD, NASH, ALD, ischemia/reperfusion, fibrosis, regeneration, and carcinogenesis (1–4). In mammals, three major groups of MAPK have been identified. Each of these groups of MAPK is activated by a protein kinase cascade. MAPK signaling cascades consist of at least three components, or tiers: MAPK kinase (MAP3K), MAPK kinase (MAP2K), and MAPK. The groups are named according to their executing downstream MAPK, such as the extracellular signal-regulated kinase (ERK), the p38 kinase, and the c-Jun N-terminal kinase (JNK) families. In the liver, JNK is a dominant effector MAPK which catalyzes the phosphorylation of numerous substrate proteins including nuclear AP1 transcription factors (c-Jun, etc) as well as protein kinases and phosphatases, scaffold proteins, and other functional proteins (4, 5). JNK activation and substrate phosphorylation has two major direct consequences: regulation of gene expression through AP1 transcription factors and direct activation or inhibition of protein targets (Fig. 1). The liver expresses both JNK1 and JNK2. In this review we will focus on recent studies from many different laboratories in the past 5 years which have improved our understanding of the role of JNK signaling pathway in the pathogenesis of liver diseases and promise to lead to exciting therapeutic applications. Because of the broad nature of this subject, we have selected specific areas which illustrate the important recent conceptual advances.
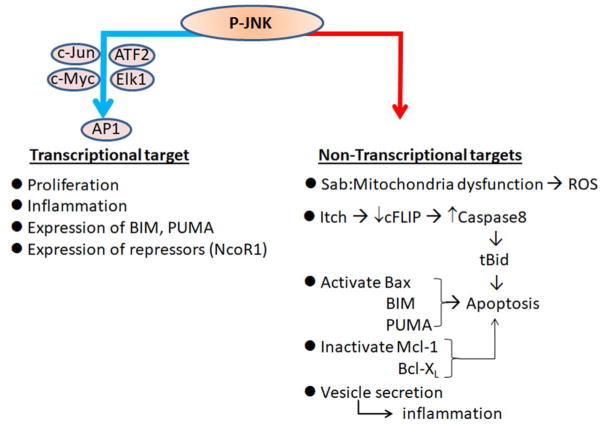
Fig. 1. Classification of substrates of activated JNK (P-JNK).
Transcriptional targets are identified by AP1 binding sequence with JNK phospho-activated substrates such as c-Jun, c-Myc, ATF2, Elk1. Non-transcriptional targets are identified by kinase-interacting-motif (KIM) and serine/threonine-proline (S/TP) phosphorylation sites on substrates. Sab is a substrate as well as scaffold protein of activated JNK on mitochondria. Other P-JNK phosphorylated substrates are E3 ubiquitin ligase (Itch), mitochondrial apoptosis inducers and inhibitors among the Bcl family proteins such as Bax, Bim, PUMA, Mcl1, Bcl-XL. JNK activation is required for vesicle trafficking to export proteins such as CXCL10 from hepatocytes. Not described in this review, other JNK phospho-regulated targets include p53 in cell cycle regulation and cell death; Mfn2 in mitochondrial fusion; autophagy regulation.
The JNK signaling pathway in the liver: recent advances
JNK regulates an intramitochondrial signal transduction pathway
Activated JNK (P-JNK) translocates to mitochondria and binds to the JNK target protein Sab (Sh3bp5) in hepatic mitochondria in acute liver injury models where massive JNK activation has been observed (6). Sab is an outer membrane scaffold protein with one hydrophobic transmembrane domain which separates the N-terminal SH3 domain facing the intermembrane space and the C-terminal JNK kinase interacting motifs (KIM) facing the cytoplasm (7). The interaction of P-JNK and Sab was first recognized in 2002 (8) and subsequently confirmed by others (6, 9, 10). In parallel with studies in the liver (6, 7), interaction of JNK and Sab was observed in Hela cells and inhibited by Tat-Sab inhibitory peptide (KIM1 20 amino acid peptide linked to membrane permeant Tat peptide) which competitively interferes with JNK interaction with the KIM domain on the Sab platform, blocking the JNK induced inhibition of respiration and increased production of ROS (9). Protective role of Tat-Sab inhibitory peptide was studied in heart and brain injury models (11, 12). Further studies using primary mouse hepatocytes and isolated liver mitochondria confirmed the mitochondrial production of ROS as a consequence of the JNK interaction with Sab and the prevention of ROS production by knockdown of Sab or by Sab blocking peptide (13).
JNK is not taken up by mitochondria. Therefore, a key question was how does the interaction of JNK with an outer membrane platform on the surface of the mitochondria exert effects inside the mitochondria. Recently, a JNK/Sab mediated intramitochondrial signaling pathway was elucidated (7). Briefly, P-JNK binding to Sab initiates intramitochondrial release of tyrosine phosphatase Shp1 from Sab leading to dephosphorylation of activated-Src (P-419-Src), which occurs on and requires the platform, DOK4, located on the mitochondrial inner membrane. P-Src is required to maintain electron transport. Decreased P-Src inhibits mitochondrial respiration and enhances ROS production. Using mitochondria after in-vivo knockdown of Shp1 or DOK-4, P-JNK mediated inhibition of mitochondrial respiration was abrogated. After knockdown of DOK4, cell death in vitro or in vivo was inhibited in several acute injury models (7). Of note P-MKK4 translocates to mitochondria along with JNK only when Sab is expressed (6, 14). The requirement of ATP for P-JNK to inhibit mitochondrial respiration (7, 13) supports earlier evidence that Sab is a JNK substrate. Interestingly, P-JNK1 or P-JNK2 with ATP equally inhibits mitochondrial respiration and only when Sab is expressed in mitochondria (7). Further studies are required to determine the JNK phosphorylation site(s) on Sab, and whether MAP3K is also associated with the Sab signalosome. However, these recent findings support the overall concept that Sab functions in an analogous fashion to a plasma membrane receptor which is activated by binding of its ligand and its phosphorylation leading to a transmembrane signal transduction pathway.
The significance of sustained JNK activation loop
In the context of receptor signaling, such as TNFR1, JNK activation is transient due to dampening by the concomitant activation of NF-κB responsive survival genes. However, sustained JNK activation directly and positively correlates with liver injury (cell death) and liver metabolic stress/dysfunction (2, 3). A feedforward self-sustaining signaling pathway has been elucidated in liver models which we refer to as the JNK amplification loop (P-JNK → Sab → Intramitochondrial pathway → ↑ ROS → ↑ P-ASK1 → ↑ P-MKK4 → ↑ P-JNK) (6, 7, 14). In normal liver, JNK is minimally or transiently activated and is not detectably associated with mitochondria. P-JNK translocates to mitochondria and binds with mitochondrial outer membrane protein Sab (Sh3bp5) (6, 7, 10). Depletion of Sab or the presence of inhibitory Sab-peptide completely prevents/blocks translocation of P-JNK to mitochondria (6, 7, 9). As a consequence of the binding of P-JNK to Sab, the above described intramitochondrial signaling pathway leads to inhibition of mitochondrial respiration and electron transport, leading to ROS (presumably H2O2) release from mitochondria and activation of MAP3K/MAP2K pathway sustaining JNK activation. Increased ROS also feeds back to inhibit JNK phosphatase such as MKP1 (DUSP1), which contributes to the sustained level of JNK activation (15). Sustained JNK activation then mediates phosphorylation of transcription factors or directly regulates cell death, eg. phosphorylation of Bcl family. Interruption of JNK activation at multiple sites in the loop can dampen the sustained activation of JNK and protect against cell death or metabolic consequences. The initiation of the MAPK cascade can occur through cell membrane receptor or intracellular inflammasomes (via TAK1, MLK2/3, ASK1) or through organelle stress, such as mitochondria, ER, or nuclear DNA. However, for the activation to be sustained usually requires the participation of ASK1. ASK1 is normally held in an inactivated state bound to various inhibitors such as thioredoxin or GSH S-transferases, which are redox sensitive so that ROS can release ASK1 from inhibition (3, 16, 17). In addition, cFLIP and CREG have been shown to interfere with the dimerization of ASK1, preventing activation (18, 19). Therefore, conditions in which the expression of cFLIP or CREG is inhibited can lead to enhanced ASK1 activation. The importance of ASK1 in cell death has been confirmed by knockout and specific inhibitors (20, 21). Other approaches which confirm the importance of the JNK-mitochondrial-ROS activation loop include specific inhibition of interaction of P-JNK with Sab using peptides (9, 11–13), small molecules (22), or knockdown and knockout of Sab (6,7), as well as the direct suppression of mitochondrial ROS with antioxidant such as BHA (15) and NAC (18,23), the increased expression of SOD2 (24), mitochondrial targeted SOD mimetics (25, 26), and Keap1 deletion to enhance Nrf2 regulated antioxidant defense (27). Importantly, inhibition of the feedforward JNK activation loop spares the transient JNK signal transduction to nucleus and gene regulation, thereby not interfering with the prosurvival functions of the MAPK and JNK signaling pathways. Figure 2 summarizes the role of the feedforward loop and the integration of mitochondria and ER in sustained JNK activation.
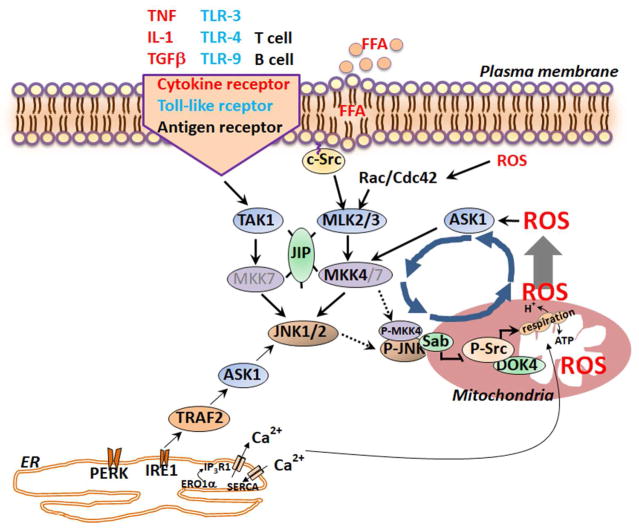
Fig. 2. Sustained JNK activation loop.
MAP3K such as TAK1, ASK1 and MLK2/3 can be initially activated by cytokine receptors, saturated fatty acids, ER stress and mitochondrial ROS release. MAP3K the activate MAP2K kinases, such as MKK4/7, which activate JNK1/2. P-JNK then phosphorylates Sab on the outer mitochondrial membrane which leads to the release and activation of Shp1, a phosphatase which then inactivates phospho-Src on the inner membrane platform, DOK-4. Inactivation of Src then impairs electron transport and leads to increased mitochondrial O2− production and H2O2 is released from mitochondria. ROS mediates activation of MAP3K (ASK1 and MLK/2/3) which continue to activate JNK. This activation leads to sustained JNK activation which promotes metabolic dysregulation and cell death through the various targets in Fig. 1.
Role of JNK in liver disease models: recent advances
Acute liver Injury models: role of JNK in cell death
Death of hepatocyte mainly occurs by apoptosis or necrosis. Hepatocytes express death receptors including TNFR, FAS and TRAIL. Death receptor mediated acute liver injury is mediated by inflammatory cell membrane bound or secreted death receptor ligands in infections/sepsis, viral hepatitis, acute alcoholic hepatitis, NASH. LPS from gut microbiome or infection activates T cells, NK-T cells, macrophages/Kupffer cell to express soluble or membrane bound cytokines (TNFα, FASL, IL-1, IFNγ). Cytokines activate death receptor signaling in hepatocytes. TNFα/GalN and Fas agonist monoclonal antibody (Jo2) induce apoptosis of hepatocytes and are widely used models to study the death receptor mediated acute liver injury. Sustained JNK activation precedes apoptotic hepatic injury in both models. In the FasL induced model, TRAIL has been shown to amplify Fas mediated cell death through activation of JNK (2, 3).
In addition to death receptor initiated JNK activation in hepatocytes, intrinsic cellular stress induced by acetaminophen (APAP), fatty acids, and bile acids leads to the sustained-JNK activation loop (28–30). This loop has been extensively studied in the APAP model. A fraction of an APAP dose is converted to an electrophilic reactive metabolite, NAPQI, mainly mediated by Cyp2e1. When sufficient NAPQI is produced, GSH in cytoplasm and mitochondria is depleted. After marked GSH depletion, NAPQI covalently binds to protein-thiols throughout the cells, but binding in mitochondria is critical. The depleted mitochondria GSH level and covalent binding cause moderate mitochondrial dysfunction and ROS release, activating the MAPK cascade (28). It is possible that covalent binding induced ER stress may also contribute to the initial MAPK activation (31). The magnitude and duration of JNK activation is a major determinant of acute injury from APAP. The extent of APAP induced JNK activation and translocation is dose related and correlates well with subsequent liver necrosis: minimal JNK activation but no translocation and no injury at 100mg/kg nontoxic dose, whereas JNK activation and translocation to mitochondria increase with APAP dose from 150 to 300mg/kg and this dose relationship is inversely related to Src activity in mitochondria (7). Previous studies showed that female mice are resistant to APAP induced liver injury compared to age matched male mice. We found that Sab protein expression was markedly lower in female than in male liver mitochondria which may contribute to less liver injury in female mice (32), suggesting the level of expression of Sab is an important determinant of the level of sustained JNK activation loop. Intriguingly, in Sab knockdown or knockout mice, mitochondrial translocation of P-JNK is completely inhibited irrespective of various isoforms of JNK (6, 7). Therefore, in acute APAP liver injury mitochondrial Sab is pivotal in leading to sustained JNK activation loop. In the APAP model, the sustained JNK activation loop does not cause apoptosis as caspases are inactivated. In this special case, the marked amplification of mitochondrial ROS due to the JNK activation loop overwhelms mitochondria leading to MPT mediated necrosis. Figure 3 provides a comparison of the role of signaling pathways in APAP necrosis and TNF/GalN apoptosis.
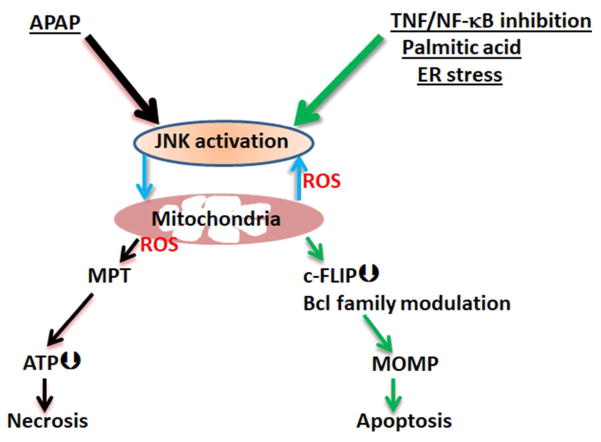
Fig. 3. Comparison of the mechanisms of sustained JNK activation in APAP induced hepatic necrosis and hepatocyte apoptosis in response to TNF, palmitic acid or ER stress.
(A) A fraction of APAP is metabolized to NAPQI by CYP2E1 which impairs mitochondrial function and causes initial ROS release leading to early activation of MLK3 and later activation of ASK1. At the same time NAPQI in cytoplasm and mitochondria deplete GSH (to 10% of basal), and the magnitude of initial activated JNK (P-JNK) reaches to the threshold to bind to available substrates including Sab on the mitochondrial outer membrane. The P-JNK and Sab interaction event leads to decreased mitochondrial respiration and increased release of ROS which then oxidizes thioredoxin, a redox sensitive ASK1 binding/inactivating protein. Oxidation of thioredoxin releases ASK1 and allows ASK1 dimerization and self-activation. Then ASK1/MKK4/JNK/Sab/ROS feedforward loop continues, leading to exposure of already damaged mitochondria to sufficient oxidant stress to cause the MPT with consequent ATP depletion, release of proteins from mitochondrial matrix and oncotic necrosis ensues. In the APAP model, ATP depletion and a high level of ROS incapacitate caspase activity, blocking apoptosis. (B) Sustained JNK activation also plays an important role in apoptosis by its known actions on c-FLIP degradation by activating itch, allowing caspase-8 to cleave Bid to t-Bid, as well as activation of Bax and Bim, and inactivation of Bcl-XL and Mcl-1, thereby promoting mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release followed by caspase dependent apoptosis.
Despite the many independent studies (6, 7, 14, 20–23, 26–28, 33, 34) demonstrating the importance of MAPK and JNK signaling in APAP toxicity, one study reported the apparent opposite, namely embryonic liver specific JNK1 and 2 double knockout (JNK2−/− global knockout crossed with JNK1f/f mice and liver specific Cre transgenic mice) increased the severity of APAP toxicity (35). However, the sensitization to APAP toxicity seemed to be due to enhanced mitochondrial oxidative stress under basal conditions, suggesting a developmental impairment in antioxidant defense, the nature of which is presently unknown but potentially related to long-term suppression of AP-1 (36, 37). Study of APAP in adult inducible double JNK1 and 2 floxed knockout mice is needed for further clarification.
When the magnitude of JNK activation reaches a threshold to translocate to mitochondria and interact with mitochondrial outer membrane protein Sab (6, 7), amplification of oxidant stress in the mitochondria occurs (7, 24). Thus, in PMH chemical ER stressor (tunicamycin, brefeldin A) (13) or saturated free fatty acids (palmitic acid)(29, 38) activate JNK and activate the sustained-JNK activation loop. In these models, membrane permeable Sab peptide blocks the binding of P-JNK and prevents sustained JNK activation and inhibits apoptotic cell death (13, 38). Thus, Sab is a common binding target of isoforms of P-JNK for both apoptosis and necrosis signal transduction pathways in many liver injury scenarios. However, there are exceptions: for example, hepatocellular JNK/Sab does not appear to play a role in furosemide (39) or CCL4 induced acute liver injury (23). Other forms of cell death have been identified, such as necroptosis, pyroptosis, and feroptosis, but knowledge concerning their contribution to liver injury or the role of JNK is either non-existent or controversial.
NAFLD/NASH and Metabolic disease models
The role of MAPK signaling pathway in the development of NAFLD, NASH, fibrosis, obesity and insulin resistance has been extensively discussed (40–43). Prior research using global knockouts in these models has lead to conflicting and inconclusive results. Recently, however, cell type specific Cre mediated JNK1 and/or JNK2 conditional knockout mice have become available to study the role of JNK in different liver cell types has improved our understanding. Still there remain concerns about potential confounding effects of embryonic conditional knockouts with respect to compensatory adaptive responses which may be obviated to some extent by use of inducible cell type specific knockouts in adult mice.
Role of Hepatic JNK in fatty liver disease and metabolic syndrome
A number of prior studies have linked sustained JNK activation to ER stress and insulin resistance in the liver. More recently, the role of JNK activation in hepatocytes has been linked to PPARα-FGF21 hepatokine axis (44). FGF21 regulates adipose tissue metabolism in part by reducing PPARγ inhibitory sumoylation and increasing PGC1α expression (45). Thus, hepatic deletion of JNK1+2 mice decreased adipocyte hypertrophy and macrophage infiltration of adipose tissue induced by HFD (44) (Fig. 4). Sustained hepatic JNK activity in HFD fed mice potently repressed the nuclear hormone receptor peroxisome proliferator-activated receptor-α (PPARα) responsive genes through increased expression of PPARα-corepressor NcoR1. Therefore, hepatic P-JNK caused decreased expression of PPARα target genes that increase fatty acid β-oxidation, ketogenesis, and FGF21 expression and promoted the development of insulin resistance. FGF21 is produced preferentially in the liver as a hepatokine which possesses potent regulatory effects on adipose tissue, liver and brain such as glucose and lipid metabolism, insulin sensitivity and adaptive starvation response in animal models (45–47). In addition, FGF21 regulates Nrf2 expression and enhances antioxidant defense which could dampen the JNK activation loop (48). Hepatocyte deletion of JNK2, but not JNK1, alone only slightly increased hepatocyte expression of Fgf21 mRNA and decreased body weight gain induced by HFD. Combined JNK1+2 hepatocyte specific deletion markedly increased hepatic expression of Fgf21 mRNA, circulating amounts of FGF21 in the blood, and was associated with reduced fatty liver and body weight gain by HFD, thus indicating the synergistic effects of JNK1 and 2 in hepatocyte (44). Though JNK2 seemed to have a greater role than JNK1 in this study, several other recent reports suggest that hepatocyte JNK1 plays the dominant role in mediating the hepatic effects of HFD models (18, 19). The relative contribution of JNK1, 2, or both remain an unresolved issue and the effects of knocking out one isoform on the expression and activation of the other complicates these types of studies. Conditional double knockout appears to provide the most interpretable results.
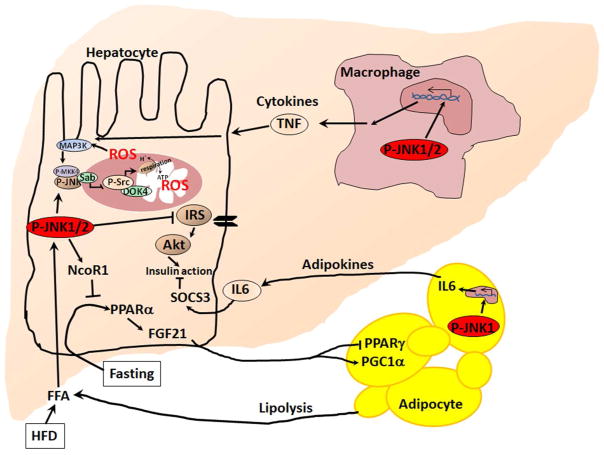
Fig. 4. High fat diet induced JNK activation in hepatocytes, Kupffer cells and adipocytes in the development of NAFLD and NASH.
JNK signaling in hepatocytes inhibits insulin signaling and causes insulin resistance. Sustained JNK1/2 activation upregulates PPARα-corepressor NcoR1 which in turn inhibits PPARα-responsive gene transcription. Both JNK1 and JNK2 participate in the regulation of NcoR1 in hepatocytes. PPARα regulates Fgf21 expression. Serum FGF21 activates adipocyte PGC1α and inhibits PPARγ and downstream target genes. P-JNK1 in adipocytes activates adipokines expression including IL-6 which targets hepatocytes to increase SOCS3. SOCS3 inhibits insulin signaling in hepatocytes and many other cells. JNK1/2 regulates M1 macrophage differentiation, tissue infiltration, and release of cytokines including TNF.
Role of extra-hepatic JNK in fatty liver disease and metabolic syndrome
JNK is activated in the adipose tissue, macrophage, and striated muscle of HFD-fed mice. This was associated with decreased whole-body insulin sensitivity (43) as a consequence of JNK inhibiting insulin-stimulated Akt activation (49). Interestingly insulin resistance and hepatic steatosis were suppressed by JNK1 deficiency in adipose tissue of Fabp4-Cre+ JNK1f/− mice. Lack of JNK1 in adipose tissue prevented the secretion of inflammatory cytokine IL-6 by adipose tissue in response to HFD feeding (49). IL-6 causes increased expression of hepatic SOCS3, a protein that induces hepatic insulin resistance by inhibiting insulin receptor and by targeting IRS degradation (49). However, the role of IL-6 in whole body insulin resistance has been debated because of tissue specific signaling differences of IL-6. Nevertheless, JNK1 activation in adipose tissue can cause insulin resistance in the liver (49), but JNK2 in adipose tissue may be dispensable. However, direct comparison of JNK1 deletion versus JNK2 deletion or JNK1+2 deletions in adipose tissue is required for further clarification.
Tissue infiltration by pro-inflammatory macrophages (M1) is a major contributor to inflammation and insulin resistance (50). In an earlier study, transfer of bone marrow (myeloid cells) from global JNK1−/− mice to irradiated WT mice fed with HFD improved the liver and peripheral tissue insulin sensitivity. In this model, most immune cells including Kupffer cells are replaced by transferred JNK1−/− myeloid cells (51). However, JNK1 deficiency in macrophages of Lyzs-Cre+ Jnk1f/f mice did not suppress HFD-induced hepatic insulin resistance, suggesting JNK 2 is the relevant isoform (49). In both models JNK1 depletion in macrophages did not suppress macrophage infiltration of the liver and adipose tissue. A recent study using JNK1+2 conditional deletion in macrophages (Lyzs-Cre+ Jnk1f/f Jnk2f/f) showed that JNK is required for polarization of pro-inflammatory macrophages to promote infiltration of tissue and suppression of insulin sensitivity in the liver, muscle and peripheral tissues in HFD-fed mice. However, HFD-induced obesity was not prevented by deleting JNK1+2 in macrophage (50).
Role of MAP3K upstream of JNK in metabolic syndrome
Two MAP3K, MLK3 and ASK1, have been implicated in diet induced regulation of hepatic JNK. Prior work has shown that global knockout of MLK3 prevents JNK activation in HFHC diet fed mice (52, 53). Despite a comparable increase in weight gain, hepatic steatosis by histological examination and hepatic triglyceride quantification were reduced in HFHC diet-fed Mlk3−/− mice compared with WT mice. The ubiquitously expressed MLK2 and MLK3 protein kinases have partially redundant functions. Therefore, in compound mutant global MLK2−/− and MLK3−/− mice JNK activation in liver was inhibited and the mice were protected against HFD-induced insulin resistance and obesity (54).
More recently, ASK1 has been the focus of attention. cFLIP directly targets ASK1 and interrupts its N-terminus-mediated dimerization, thereby blocking signaling involving ASK1 to JNK1. Activation of JNK in high fat diet feeding may activate the E3 ligase ITCH and then destabilize cFLIP by proteosomal degradation. Decreased cFLIP expression has been observed in human liver samples from NAFLD/NASH patients (18). Interestingly, liver specific ASK1 deletion protected against HFD induced insulin resistance, fatty liver and obesity (18). In addition, the activation of ASK1 is inhibited by CREG. CREG expression is decreased in NAFLD patients and liver specific CREG deletion increases ASK1-JNK axis in HFD mice and worsens fatty liver and insulin resistance (19). Of note, small molecule ASK1 inhibitor (GS-4997) reduces hepatic steatosis and fibrosis in diet induced NASH model (55).
Hepatocyte apoptosis plays a critical role in the progression of NASH to cirrhosis and HCC. JNK activation is crucial in the development of hepatocyte apoptosis accompanying NASH (29). MLK3 activates JNK during lipotoxic insults (53). JNK activation is critical in mediating saturated fatty acid induced apoptosis by modulating the activity and expression of Bcl family proteins (56). In addition, JNK inhibition results in retention of chemokine, CXCL10, in hepatocytes and decreases CXCL10 trafficking into the EVs, suggesting a regulatory role of hepatocyte JNK in liver inflammation (57). Although strong evidence has been provided for a key role for both MLK3 and ASK1 in lipotoxicity, fatty liver and insulin resistance, a key question has been the mechanism of their sustained activation of the MAPK cascade.
Role of Sustained-JNK activation loop in metabolic syndrome
Activating signals from Rac/Cdc42, Src, and PKC have been proposed for MLK3 activation based on cell studies (58, 59), but reports of in vivo confirmation are sparse. The effect of liver specific MLK2/3 deletion on the sustained JNK activation loop and the relative contribution of hepatic MLK2/3 versus ASK1 are needed. The involvement of JNK/mitochondria/ROS feedforward loop has been examined in the diet induced NAFLD/NASH and obesity by targeting Sab. HFHC diet was fed to Sabf/f and SabLPC-KO mice for 16 weeks and inducible deletion of hepatic Sab prevented sustained JNK activation, suppressed insulin resistance and consequently prevented NAFLD/NASH and obesity (60). Thus, the sustained activation of hepatic JNK in diet induced obesity models can be explained by the Sab dependent activation loop in which JNK induces mitochondrial dysfunction and ROS release in a Sab-dependent fashion and the ROS production enhances MAP3K. The relative contribution of ASK1 and MLK2/3 in this loop needs additional study. Ultimately, signaling through cytokine receptors and intrinsic stress (ER and mitochondria) converge on the interaction of JNK and Sab which continues to stimulate the JNK loop and the metabolic and apoptotic consequences of sustained JNK activation.
JNK in liver cancer models
Liver cancer has been studied mainly using two approaches: carcinogen (DEN) induced and genetic models (liver specific conditional KO of Mcl-1, TAK-1 or Hspd-1). Prior studies in DEN model have been performed using embryonic JNK1−/− or JNK2−/− or liver specific JNK1 and 2 double knockout (JNK2−/− global knockout crossed with JNK1f/f mice and liver specific Alb-Cre or liver+lymphocyte specific Mx1-Cre transgenic mice) mice to test whether JNK in hepatocyte or non-parenchymal cells is required for HCC development. Conflicting results on DEN induced hepatocyte apoptosis and HCC formation in embryonic JNK KO mice were discussed in prior review (2). Recently, mice with JNK1+2 conditional deletions in myeloid cells (Lyzs-Cre+ Jnk1f/f Jnk2f/f) such as Kupffer cells, macrophages and neutrophils were treated with DEN 25mg/kg single ip injection. Indeed, myeloid cell infiltration and cytokine expression, including IL-6, TNFα, IFNγ, IL-1b in the liver and blood, were significantly decreased by JNK1+2 deletion in myeloid cells. JNK deficiency in myeloid cells significantly decreased liver mass and tumor size, but tumor number was not different (61). Further evaluation of DEN induced liver tumor development in hepatic conditional deletion of JNK1, JNK2, or JNK1+2 mice is required to examine the role of JNK in DEN induce hepatocyte apoptosis and compensatory proliferation.
In most genetic models, HCC develops in response to liver cell death and compensatory replication proliferation. For example, more than 50% of mice with liver specific conditional deletion of Mcl-1 (Alb-Cre Mcl-1 f/f) develop spontaneous HCC within in 8 months (62). Similarly, liver specific TAK-1 deleted mice develop spontaneous apoptosis and HCC (63). In these models, HCC then develops in the absence of overt inflammation. Recently, the signaling pathway in hepatocytes mediating the progression of tumor development of Alb-Cre Mcl-1 f/f mice has been further elucidated. Cross breeding of liver Mcl-1 KO mice or TAK 1 KO mice with global TNFR1−/− mice prevented spontaneous apoptosis and decreased expression of inflammatory cytokines IL6, IL33, and IFNγ, indicating the involvement of TNFR1-dependent apoptosis and downstream signaling, TNFR-caspase-8-BID/tBID-Mcl-1 (64). The role of JNK in HCC development in the apoptosis mediated pathway is complicated by the promotion of apoptosis versus the potential role in DNA repair. JNK plays a pivotal role in apoptosis but also appears to regulate DNA repair through a novel platform consisting of non-enzymatic caspase-8 platform plus RIPK1 and cFLIP leading to γH2AX phosphorylation which activates its role in DNA repair. These apparent contradictory roles of JNK need to be further studied, particularly to understand the impact of abrogation of the activation loop during various stages of the progression from chronic apoptosis to HCC.
In hepatocyte specific Hspd1−/− mice lacking mitochondrial chaperone Hsp60, mitochondrial oxidative stress leads to sustained JNK activation in hepatocytes. P-JNK in hepatocytes increased the production and release of cytokines Ccl2, Ccl5, IL-1b which then activated Kupffer cells, leading to release of TNF. TNF then activated JNK dependent cholangiocyte proliferation and development of intrahepatic cholangiocarcinoma (65). These recent insights indicate that the complexity of the interplay of effects of JNK signaling in hepatocytes, cholangiocytes and non-parenchymal cell (eg. Kupffer cells) required for development and progression of various types of liver cancer which can be prevented by inhibiting sustained JNK mediated chronic apoptosis and proliferation-replication-associated DNA damage, as well as JNK dependent proliferative signals from parenchymal and non-parenchymal cells.
Therapeutic targets in the JNK activation loop
The magnitude and duration of JNK activation determine its physiological and pathological consequences. Many important physiological processes are regulated by transcription factors which are activated by JNK phosphorylation. These are largely mediated by receptor signaling and are typically transient, lasting minutes. However sustained high level JNK activation in pathological process lasts hours or more. Direct inhibition of JNK inhibition is generally considered an unfavorable target as it would interfere with the physiological aspects of JNK signaling. In contrast, the JNK activation loop mainly regulates metabolism and cell death. Therefore, the JNK/ROS feedforward loop could be a prime target for therapeutic intervention.
ASK1, a key MAP3K in the activation loop, is a very promising target. Recently, ASK1 inhibitors (GS-4997, GS-444217) have been examined in diet induced NASH and encouraging results have been obtained in preclinical and clinical studies. A phase 2 study has reported that the ASK1 inhibitor reverses liver fibrosis in humans with NASH (55). As noted above, ASK1 inhibitors or liver specific knockout exhibit striking protection against liver injury in mouse models of APAP and NAFLD/NASH (18, 20, 21). Detailed study of mechanism and target cells of ASK1 inhibitors need to be established. Since ROS activates ASK1, upregulation of antioxidant genes or anti-oxidant supplementation is another strategy for inhibiting the sustained JNK activation loop by dampening the release of ROS from mitochondria (24–27).
Recently, Antcin H, an herbal chemical, was discovered to selectively block the JNK binding to Sab and thereby inhibit the JNK/ROS feedforward loop by preventing JNK inhibition of mitochondrial respiration and release of ROS. Antcin H protected against APAP necrosis and TNF/GalN apoptosis (22). However, further detailed studies of the mechanism of binding inhibition and possible off-targets of Antcin H are required. Another way to block JNK binding to Sab is the use of membrane permeable (Tat) peptide corresponding to 20 amino acids of docking site on Sab (KIM 1 peptides). KIM1 peptide blocks the interaction of JNK and Sab and has shown efficacy in preventing cardiac and brain injury in ischemia/reperfusion (11, 12). Thus, in principle, approaches to blocking the interaction of JNK and Sab show promise. Alternatively, decreasing Sab expression is a potential strategy. Approaches to repressing Sab expression at the transcriptional or posttranscriptional level may be possible using antisense oligonucleotides to target hepatic Sab. Moreover, an estrogen-receptor-α agonist repressed Sab expression and protected against acute liver injury so that elucidation of the mechanism of estrogen receptor target genes involved in Sab repression may provide an approach to targeted therapy (32). Clearly, specifically targeting the individual steps in the JNK activation loop offers considerable promise as a therapeutic strategy in acute and chronic liver diseases in which JNK is a key mediator.
Conclusions
The role of the MAPK cascade is complex with effects on survival, proliferation, metabolism and cell death in cell type specific fashion. Among the MAPKs, JNK in hepatocytes has been shown to play a significant role in acute and chronic liver injury both in regulating cell death and metabolism in the liver. A key aspect of its effects is determined by the duration and level of sustained JNK activation which is influenced by mitochondrial Sab/ROS signaling loop. The level of activation of this signaling loop is critical in determining the pathophysiological consequences. Targeting the steps involved in the sustained JNK activation loop has therapeutic potential.
Acknowledgments
Financial Support
This research was supported by R01DK067215 (N.K.) and the USC Research Center for Liver Disease’s Cell Culture, Cell and Tissue Imaging, Histology and Metabolic/Analytical/Instrumentation Cores (P30DK048522) (N.K).
Nonstandard Abbreviations
- Akt
AKT serine/threonine kinase 1
- ALD
Alcoholic liver disease
- APAP
Acetaminophen
- ASK1
Apoptosis signal-regulating kinase 1
- ATF2
Activating transcription factor 2
- ATP
Adenosine triphosphate
- BHA
Butylated hydroxyanisole
- Bid
BH3 interacting domain death agonist
- Ccl2
C-C motif chemokine ligand 2
- CCl4
Carbon tetrachloride
- Ccl5
C-C motif chemokine ligand 5
- Cdc42
cell division cycle 42
- cFLIP
CASP8 and FADD like apoptosis regulator
- cFos
Fos proto-oncogene
- c-Jun
Jun proto-oncogene
- c-Myc
MYC proto-oncogene
- Con A
Concanavalin A
- Cre
Cre recombinase
- Creg
Cellular Repressor of E1A-stimulated Genes
- CXCL10
C-X-C motif chemokine ligand 10
- Cyp2e1
Cytochrome P450 2E1
- DEN
Diethylnitrosamine
- DOK-4
Docking protein 4
- DUSP
Dual specificity phosphatase
- Elk1
ETS-like gene 1
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- Ets1
ETS proto-oncogene 1
- EV
Exosome vesicle
- FABP4
fatty acid binding protein 4
- FAK
Focal adhesion kinase
- FAS
Fas cell surface death receptor
- FASL
Fas ligand
- FFA
Free fatty acid
- FGF21
Fibroblast growth factor 21
- GalN
N-acetyl-galactosamine
- γH2AX
gamma H2A histone family, member X
- GSH
Glutathione
- HFD
High fat diet
- HCC
Hepatocellular carcinoma
- HFHC
High fat high carbohydrate diet
- Hspd1
heat shock protein 1
- IFNγ
Interferon gamma
- IL-1
Interleukin 1
- IL-4
Interleukin 4
- IL-6
Interleukin 6
- IRS
insulin receptor substrate 1
- ITCH
Itchy E3 ubiquitin protein ligase
- JIP
JNK-interacting protein
- JNK
c-Jun N-terminal kinases
- JNK1
mitogen-activated protein kinase 8 (MAPK8)
- JNK2
mitogen-activated protein kinase 9 (MAPK9)
- KD
Knock down
- Keap1
kelch-like ECH-associated protein 1
- KIM
Kinase interaction motif
- KO
Knock out
- LPS
Lipopolysaccharide
- Lyzs
lysozyme 2
- MAPK
Mitogen-activated protein kinase
- MAP2K
Mitogen-activated protein kinase kinase
- MAP3K
Mitogen-activated protein kinase kinase kinase
- MKK4
MAPK kinase 4
- Mcl-1
myeloid cell leukemia sequence 1
- MKP
Mitogen-activated protein kinase phosphatase
- MLK
Mixed lineage kinase
- MPT
Mitochondrial permeability transition
- NAC
N-Acetyl Cysteine
- NAFLD
Non-alcoholic fatty liver disease
- NAPQI
N-acetyl-p-benzoquinone imine
- NASH
Non-alcoholic steatohepatitis
- NcoR1
Nuclear receptor corepressor 1
- NcoR2
Nuclear receptor corepressor 2
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor erythroid 2-related factor 2
- Nrip1
nuclear receptor interacting protein 1
- PGC1α
peroxisome proliferative activated receptor, gamma, coactivator 1 alpha
- P-JNK
Phospho-activated JNK
- PKC
Protein kinase C
- PPARα
peroxisome proliferator-activated receptor-α
- PPARγ
peroxisome proliferator-activated receptor-γ
- Rac
Rac family small GTPase 1
- ROS
Reactive oxygen species
- Sab (Sh3bp5)
SH3-domain binding protein 5
- SabLPC-KO
Sab gene deletion in liver parenchyma cell
- SAP1
Stress-associated protein 1
- SH3
SRC Homology 3 Domain
- Shp1
SH2 phosphatase 1
- shRNA
Small hairpin RNA
- SOCS3
Suppressor of cytokine signaling 3
- SOD2
Superoxide dismutase 2
- SP-1
Sp1 transcription factor
- Src
SRC proto-oncogene, non-receptor tyrosine kinase
- TAK1
TGF-beta activated kinase 1
- Tat
transactivator of transcription of human immunodeficiency virus
- TNF
Tumor necrosis factor
- TNFR
TNF receptor
- TRAIL
TNF-related apoptosis inducing ligand
Footnotes
Conflict of interest statement:
S.W, T.A.T, J.Z, C.O, R.W.M.M and N.K have declared that no conflict of interest exists.
References
Author names in bold designate shared co-first authorship
- 1.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci. 2013;34:243–253. doi: 10.1016/j.tips.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Win S, Than TA, Min RW, Aghajan M, Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016;63:1987–2003. doi: 10.1002/hep.28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiltshire C, Matsushita M, Tsukada S, Gillespie DA, May GH. A new c-Jun N-terminal kinase (JNK)-interacting protein, Sab (SH3BP5), associates with mitochondria. Biochem J. 2002;367:577–585. doi: 10.1042/BJ20020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem. 2011;286:16052–16062. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Rehman H, Krishnasamy Y, Schnellmann RG, Lemasters JJ, Zhong Z. Improvement of liver injury and survival by JNK2 and iNOS deficiency in liver transplants from cardiac death mice. J Hepatol. 2015;63:68–74. doi: 10.1016/j.jhep.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers JW, Pachori A, Howard S, Iqbal S, LoGrasso PV. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J Biol Chem. 2013;288:4000–4011. doi: 10.1074/jbc.M112.406777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers JW, Howard S, LoGrasso PV. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J Biol Chem. 2013;288:1079–1087. doi: 10.1074/jbc.M112.421354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Min R, Le K, Zhou S, Aghajan M, Than TA, et al. The role of MAP2kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017;8:e2903. doi: 10.1038/cddis.2017.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Weijman JF, Kumar A, Jamieson SA, King CM, Caradoc-Davies TT, Ledgerwood EC, et al. Structural basis of autoregulatory scaffolding by apoptosis signal-regulating kinase 1. Proc Natl Acad Sci U S A. 2017;114:E2096–E2105. doi: 10.1073/pnas.1620813114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, et al. Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:12749–12755. doi: 10.1074/jbc.M005561200. [DOI] [PubMed] [Google Scholar]
- 18.Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:439–449. doi: 10.1038/nm.4290. [DOI] [PubMed] [Google Scholar]
- 19.Zhang QY, Zhao LP, Tian XX, Yan CH, Li Y, Liu YX, et al. The novel intracellular protein CREG inhibits hepatic steatosis, obesity, and insulin resistance. Hepatology. 2017;66:834–854. doi: 10.1002/hep.29257. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, et al. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286:1–9. doi: 10.1016/j.taap.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Win S, Than TA, Yin S, Ye M, Hu H, et al. Antcin H Protects Against Acute Liver Injury Through Disruption of the Interaction of c-Jun-N-Terminal Kinase with Mitochondria. Antioxid Redox Signal. 2017;26:207–220. doi: 10.1089/ars.2016.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Wong GH, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 25.Kelso GF, Maroz A, Cocheme HM, Logan A, Prime TA, Peskin AV, et al. A mitochondria-targeted macrocyclic Mn(II) superoxide dismutase mimetic. Chem Biol. 2012;19:1237–1246. doi: 10.1016/j.chembiol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Du K, Farhood A, Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol. 2017;91:761–773. doi: 10.1007/s00204-016-1692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 28.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 30.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foufelle F, Fromenty B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect. 2016;4:e00211. doi: 10.1002/prp2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min R, Win S, Than TA, Kaplowitz N. Decreased Sab (SH3BP5) expression in female liver mitochondria accounts for resistance to acute liver injury in female mice. Hepatology. 2016;64:343A. [Google Scholar]
- 33.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246(1–2):8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cubero FJ, Zoubek ME, Hu W, Peng J, Zhao G, Nevzorova YA, et al. Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury. Gastroenterology. 2016;150:968–981. doi: 10.1053/j.gastro.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015;11:1769–1779. doi: 10.1517/17425255.2015.1071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H. Mechanisms of Acetaminophen Hepatotoxicity: Do We Need JNK for Cell Death? Gastroenterology. 2016;151:371–372. doi: 10.1053/j.gastro.2016.02.087. [DOI] [PubMed] [Google Scholar]
- 38.Win S, Than TA, Le BH, Garcia-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. 2015;62:1367–1374. doi: 10.1016/j.jhep.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGill MR, Du K, Xie Y, Bajt ML, Ding WX, Jaeschke H. The role of the c-Jun N-terminal kinases 1/2 and receptor-interacting protein kinase 3 in furosemide-induced liver injury. Xenobiotica. 2015;45:442–449. doi: 10.3109/00498254.2014.986250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35(9):490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 44.Vernia S, Cavanagh-Kyros J, Garcia-Haro L, Sabio G, Barrett T, Jung DY, et al. The PPARα-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 2014;20:512–525. doi: 10.1016/j.cmet.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye D, Wang Y, Li H, Jia W, Man K, Lo CM, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1α-mediated antioxidant capacity in mice. Hepatology. 2014;60:977–989. doi: 10.1002/hep.27060. [DOI] [PubMed] [Google Scholar]
- 49.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Gadang V, Kohli R, Myronovych A, Hui DY, Perez-Tilve D, Jaeschke A. MLK3 promotes metabolic dysfunction induced by saturated fatty acid-enriched diet. Am J Physiol Endocrinol Metab. 2013;305:E549–56. doi: 10.1152/ajpendo.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, et al. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014;34:427–437. doi: 10.1111/liv.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kant S, Barrett T, Vertii A, Noh YH, Jung DY, Kim JK, et al. Role of the mixed-lineage protein kinase pathway in the metabolic stress response to obesity. Cell Rep. 2013;4:681–688. doi: 10.1016/j.celrep.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budas G, Karnik S, Jonnson T, Shafizadeh T, Watkins S, Breckenridge D, et al. Reduction of liver steatosis and fibrosis with an ASK1 inhibtor in a murine model of NASH is accompanied by improvements in cholesterol, bile acid and lipid metabolism. Hepatology. 2016;64:S170. [Google Scholar]
- 56.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52:586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, et al. Saturated fatty acids induce c-Src. clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56:192–198. doi: 10.1016/j.jhep.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Win S, Than TA, Kaplowitz N. Hepatic mitochondrial Sab (SH3BP5) plays a pivotal role in sustained JNK activation and steatohepatitis in diet-induced NASH. Hepatology. 2016;64:128A. [Google Scholar]
- 61.Han MS, Barrett T, Brehm MA, Davis RJ. Inflammation Mediated by JNK in Myeloid Cells Promotes the Development of Hepatitis and Hepatocellular Carcinoma. Cell Rep. 2016;15:19–26. doi: 10.1016/j.celrep.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber A, Boger R, Vick B, Urbanik T, Haybaeck J, Zoller S, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 64.Boege Y, Malehmir M, Healy ME, Bettermann K, Lorentzen A, Vucur M, et al. A Dual Role of Caspase-8 in Triggering and Sensing Proliferation-Associated DNA Damage, a Key Determinant of Liver Cancer Development. Cancer Cell. 2017;32:342–359. doi: 10.1016/j.ccell.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan D, Huang S, Berger E, Liu L, Gross N, Heinzmann F, et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31:771–789. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]