Abstract
Background
This brief methodology report estimates the associations among diabetes, heart disease, and dementia, which may increase the difficulty of self-care, with functional disability trajectories jointly modeled with attrition over five years. National estimates are generated using sampling weights.
Design
Population-based complex survey design.
Setting
National Health and Aging Trends Study
Participants
Community-dwelling adults ≥65 years old (N=7,609).
Measurements
Annual in-person interviews included sociodemographic information, self-reported physician-diagnosed chronic conditions, six activities of daily living (ADL), and cognitive status. A joint model using group-based trajectory modeling estimated the number of ADL disabilities and attrition probability. Multinomial logistic regression with survey weights estimated the association among diabetes, heart disease, and dementia to resultant trajectories with the least disabled trajectory as reference.
Results
Three functional disability trajectories were identified: 26.9 million (76.3%) community-dwelling Medicare beneficiaries had no disability and a constant study attrition of 14.3%, 4.9 million (13.9%) had mild and increasing disability with 12% attrition in 2012 to 27.2% in 2015, and 3.4 million (9.7%) had severe and increasing disability with 25.4% attrition in 2012 to 35% in 2015. Persons with possible dementia, or possible dementia and diabetes, or possible dementia with both diabetes and heart disease, had significantly increased odds of being on the “mild disability” trajectory relative to “no disability.” Persons with probable dementia, representing over 1.5 million persons, regardless of concurrent conditions had significantly increased the odds of being on the “severe disability” trajectory relative to persons on the “no disability” trajectory.
Conclusions
Methods that generate national estimates, account for attrition and for multiple chronic conditions and cognitive status, may be useful for health policy-makers to provide care, support and services.
Keywords: Dementia, Diabetes, Heart disease, Functional Disability, Attrition, National Health and Aging Trends Study
Introduction
Functional independence is important for the majority of older patients who tend to prioritize remaining independent over living longer.1 However, functional disability, whether transient or persistent, is common in older adults.2 Estimating the national burden of disability among community-dwelling older adults may help policy makers plan and prepare for the needs of this population.
Although 75% of older adults have ≥2 chronic conditions, trajectory studies of function have typically focused on adults with individual health conditions3. Moreover, prior studies rarely accounted for attrition or provided nationally representative estimates. Although diabetes, heart disease, and dementia have been individually associated with functional decline4, their joint impact on functional disability is less clear. Furthermore, diabetes and heart disease are highly prevalent and require complicated self-care management, including diet control, blood glucose and/or blood pressure monitoring, and use of multiple medications. Optimal management of diabetes and heart disease requires competent executive function. As a result, persons with dementia may face greater challenges in self-managing diabetes and heart disease and other complicated self-care conditions. Previous research found that persons with dementia were difficult to recruit and retain in studies; therefore, accounting for missing data is imperative to avoid bias5,6.
In this brief methodology report, our aim was to quantify the associations between diabetes, heart disease, dementia, and their combinations with trajectories of functional disability accounting for attrition in a nationally-representative sample of American community-dwelling older adults.
Methods
Study sample
The National Health and Aging Trends Study (NHATS) is a nationally-representative sample of Medicare beneficiaries ≥65 years of age initiated in 2011 “designed to enhance understanding of trends and trajectories of late-life disability”7. The survey sampling weights allow for generation of national estimates (details were previously published7). The study protocol was approved by the Johns Hopkins University Institutional Review Board (IRB) and the Yale IRB (HIC# 1510016585). Written informed consent was obtained from all study participants or their proxy respondents.
In-person interviews, including cognitive and activities of daily living (ADL) assessments, were conducted by trained research staff in the homes of study participants living in the community7. Nursing home residents (n= 468, 5.7%) and residential care participants (n= 168, 2.0%) were excluded from the analysis because interviews were not done or incomplete7. The analytical sample was 7,609 community-dwelling participants, of whom 1,393 died (18.3%) and 2,511 (33.0%) dropped out over five years of annual follow-up.
Measures
NHATS Classifications of Possible and Probable Dementia
NHATS assessed cognition in three ways: 1) confirmation from the study participant or proxy of physician-diagnosed dementia or Alzheimer’s disease, 2) probable dementia classification score (score≤ 2/8 items) from the validated proxy-report, the Alzheimer’s Disease (AD)-8 Screening interview and 3) cognitive test battery8,9. Domains of the cognitive tests included orientation (scale: 0-8, cut-off ≤ 3), memory (scale: 0-20, cut-off ≤3) and executive function (scale: 0-5, cut-off ≤1)8. Cut-off scores were defined as 1.5 standard deviations (SD) below the mean to indicate cognitive impairment8. Probable dementia was defined as cut-off scores in two of the three domains and as possible dementia was defined as a cut-off score in one domain8. The NHATS definition of probable and possible dementia was previously validated against dementia cases (sensitivity: 85.7%, 95% CI: 69.7; 95.2)8.
Complicated self-care conditions
Participants or proxy respondents were asked if they ever had physician-diagnosed diabetes or heart disease, referred to as complicated self-care conditions.
Composite variable of diabetes, heart disease and cognitive status
We created a 12-level categorical variable based on all combinations of diabetes, heart disease and dementia (possible, probable or no dementia). This coding creates a parsimonious model. By using a composite variable, the model has more direct interpretation than two and three-way interactions.
Disability
The functional disability score was a sum of six ADLs (eating, dressing, bathing, toileting, transferring from bed, and getting around inside one's home) that the participants was unable to perform10.
Covariates
Baseline demographic characteristics were collected via interview and included: age (65-74, 75-84 and ≥85), sex, race (white versus other), living status (alone versus with someone), education (≥high school versus below high school), and having had a hospital stay in last 12 months (yes versus no). Baseline body mass index (BMI) was categorized as <25, 25-30, and ≥ 30 based on self-reported height and weight. A sum of physician-diagnosed, self-reported chronic conditions included: high blood pressure, arthritis, osteoporosis, lung disease, stroke, and cancer. A self-reported scale of overall health was used as an indicator for severity of illness. A total score of instrumental ADLs that participants were unable to do included: help with meals, laundry, light housework, groceries, taking medication, managing money and making telephone calls10.
Statistical analyses
Baseline characteristics of the cohort were compared across functional trajectories using survey adjusted chi-square tests and one-way analysis of variance11. A joint model using group-based trajectory modeling estimated the number of ADL disabilities and attrition probability (death or dropout) over five years using Stata version 12 (see supplementary section)12–14. Therefore, nonrandom participant attrition was accounted for across trajectories and within trajectories over time12. As a sensitivity analysis, we re-ran the trajectory modeling without accounting for attrition.
Subsequently, a multinomial logistic regression that incorporates complex survey designs (Proc Surveylogistic, SAS version 9.4), estimated the association of complicated self-care conditions and dementia to each functional disability trajectory with the least disabled trajectory as reference. Potential confounding was adjusted for by including baseline covariates: age, sex, race, BMI, living situation, overall health, education, the number of instrumental ADLs disabled and the number of other chronic conditions. Wave one analytic weights along with the appropriate cluster and strata variables were applied to all analyses to generate national estimates of older Medicare beneficiaries that account for differential probabilities of selection and adjust for potential bias related to unit non-response11.
Results
Study population characteristics
Baseline national estimates indicated that the majority (52.9%) of the people were 65-75 years old, had ~2 chronic conditions and 14.2% needed assistance with at least one ADL, 35.1% had either diabetes or heart disease and 10.9% and 10.0% had possible and probable dementia, respectively (Table 1).
Table 1.
National estimates based on characteristics of NHATS participants at baseline by functional disability trajectories
| All | No disability | Mild increasing disability | Severe increasing disability | p-valued | |
|---|---|---|---|---|---|
| Population-weighted estimates of trajectories | 35,305,630 | 26,941,212 | 4,922,933 | 3,441,485 | |
| Demographics | |||||
| Age (%) | <0.0001 | ||||
| 65-74 | 52.9 | 59.7 | 36.7 | 22.5 | |
| 75-84 | 33.7 | 32.2 | 39.3 | 38.0 | |
| 85+ | 13.4 | 8.1 | 24.0 | 39.5 | |
| Female (%) | 56.6 | 54.4 | 61.9 | 66.1 | <0.0001 |
| White, non-Hispanic (%) | 81.5 | 82.6 | 79.37 | 75.4 | <0.0001 |
| ≥ High school (%) | 78.2 | 81.4 | 71.2 | 63.1 | <0.0001 |
| Living with someone (%) | 70.2 | 70.8 | 69.0 | 67.6 | <.0001 |
| BMI | <0.0001 | ||||
| <25 normal | 34.4 | 32.7 | 37.1 | 44.6 | |
| 25 – 29.9 overweight | 37.7 | 39.6 | 32.0 | 31.1 | |
| ≥30 obese | 27.8 | 27.7 | 30.9 | 24.4 | |
| Cognition | |||||
| Dementia (%) | <0.0001 | ||||
| No cognitive impairment | 79.0 | 86.1 | 68.9 | 38.1 | |
| Possible dementia | 10.9 | 9.4 | 15.8 | 15.5 | |
| Probable dementia | 10.0 | 4.4 | 15.3 | 46.4 | |
| Used proxy for interview | 5.8 | 2.2 | 6.8 | 32.8 | <0.0001 |
| Health | |||||
| Diabetes present (%) | 23.9 | 21.4 | 29.7 | 34.7 | <0.0001 |
| Heart disease present (%) | 17.5 | 15.0 | 22.7 | 29.4 | <0.0001 |
| Complicated-self-care present (diabetes and/or heart disease (%) | 35.1 | 31.6 | 43.5 | 50.8 | <0.0001 |
| Total chronic conditions c mean, (SD) | 1.9 (0.02) | 1.8 (0.02) | 2.3 (0.04) | 2.5 (0.05) | <0.0001 |
| Self-rated health | <0.0001 | ||||
| Excellent | 14.8 | 17.6 | 6.6 | 4.6 | |
| Very good | 29.5 | 33.5 | 19.1 | 13.0 | |
| Good | 30.7 | 30.7 | 35.2 | 24.1 | |
| Fair | 18.4 | 14.9 | 27.5 | 32.3 | |
| Poor | 6.7 | 3.3 | 11.7 | 25.9 | |
| Hospital stays in last 12 months (%) | 21.0 | 16.5 | 29.3 | 45.0 | <0.0001 |
| Function | |||||
| Total ADLa (mean, SD) | 0.4 (0.01) | 0.025 (0.003) | 0.4 (0.02) | 2.9 (0.08) | <0.0001 |
| Help with at least one ADL (%) | 14.2 | 2.2 | 31.2 | 85.4 | <0.0001 |
| Total IADLb (mean, SD) | 0.8 (0.03) | 0.3 (0.02) | 1.2 (0.06) | 4.0 (0.02) | <0.0001 |
| Shopping assistance (%) | 17.7 | 7.1 | 32.4 | 77.9 | <0.0001 |
| Meals assistance (%) | 11.4 | 4.2 | 15.5 | 61.3 | <0.0001 |
| Medication assistance (%) | 10.0 | 4.1 | 13.3 | 52.2 | <0.0001 |
Note. There were 7,609 people included from the National Health and Aging Trends study, representing 35.3 million older adults.
Total activities of daily living score (ADL) consisted of: Eating, bathing, dressing, toileting, transferring from bed and getting around inside one’s home.
Instrumental activities of daily living (IADL) score consisted of a sum score of help with: meals, laundry, housework, groceries, money, medications and the telephone. With both scales higher scores mean increased dependency.
Sum of chronic conditions included: Blood pressure, Arthritis, Osteoporosis, Lung disease, Stroke and Cancer.
P-values represent second-order chi-square values for categorical variables against group membership and analysis of variance for continuous variables compared to group membership.
Joint trajectory of disability and attrition over five years
Among the models evaluated, a zero-inflated Poisson model with three trajectories was the best solution, with an average posterior probability of assignment (PPA) ≥0.88. After applying analytic weights to the trajectory analysis, national estimates were: 76.3% of US community-dwelling adults≥ 65 with no disability, 13.9% with mild increasing disability, and 9.7% with severe increasing disability (Figure 1A). Table 1 presents baseline characteristics per trajectory, which shows significant monotonic worsening of cognitive, health and functional measures from no disability to mild increasing disability and to severe increasing disability. For example, at baseline persons on the “severe disability” trajectory had the greatest prevalence of complicated-self-care condition (50.8%, p-value <0.0001), worst perceived overall health (25.9% poor, p-value <0.0001), greatest number of other chronic conditions (mean 2.5, p-value <0.0001), required assistance with groceries (77.9%, p-value<0.0001), making meals (61.3%, p-value<0.0001), taking medications (52.2%, p-value<0.0001); diet control and medications are part of self-care of chronic conditions (Table 1). Frequencies, percentages at the sample and national estimates for diabetes, heart disease, dementia and their combinations across trajectories are provided in Supplementary table 1.
Figure 1.
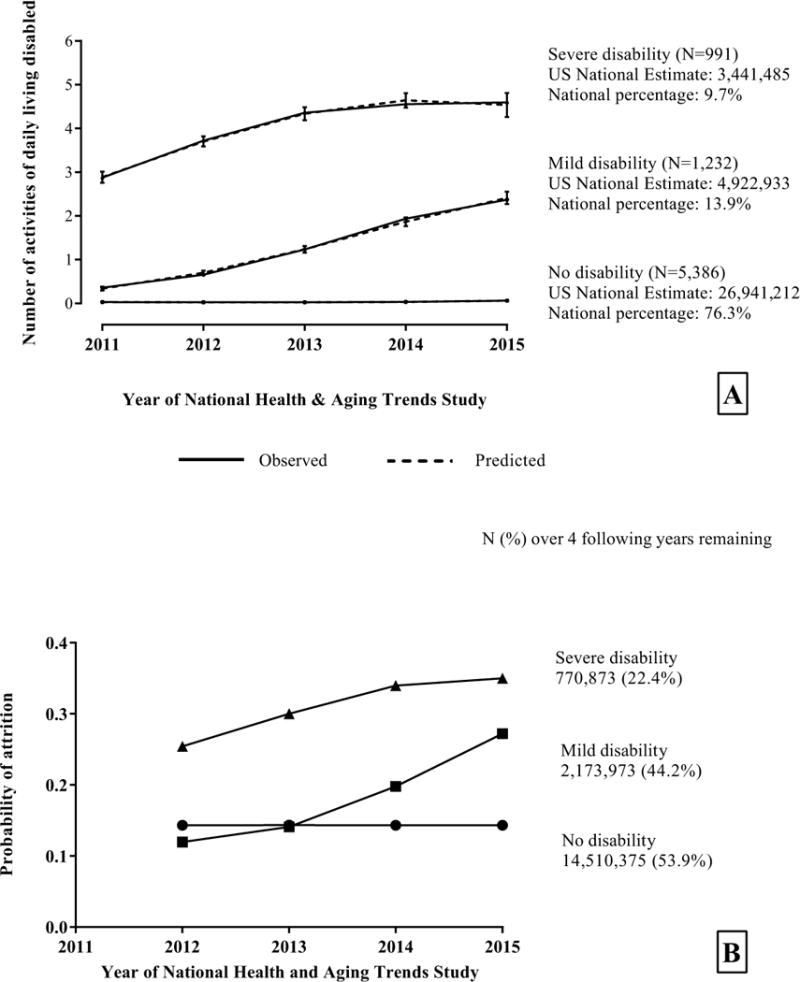
Panel A: Trajectories of functional disability over five years jointly modelled with attrition (panel B) using the National Health and Aging Trends Study with 2011 analytic weights to derive national estimates. Higher scores indicate increased functional disability. Panel B: Annual attrition probabilities and national estimates based on the joint modeling of functional disability (panel A) and attrition trajectories. Attrition was jointly modelled with the functional disability trajectories and 2011 analytic weights were applied to determine the number of participants remaining in each trajectory per year. The four-group model had the highest BIC (-20,771), and the incremental change from the three-group model was smaller than that from the two-group to the three-group models (2*Δ (BICij): 468 versus 3,478). We choose the two models with the highest BIC, i.e., the four- and three-group models, as contenders for further evaluation. The final three-group model was chosen over the four-group alternative after evaluating the model outputs and trajectory plots, comparing the average and minimal Posterior Probability of assignment (PPA) for each group, examining the distinctiveness and interpretability of the trajectories and group sizes.
National estimates of the probabilities of attrition per wave by trajectory are shown in Figure 1B. The “no disability” trajectory had the lowest attrition probabilities over time (14.3%), followed by the “mild disability” trajectory (12.0%-27.2%) and the “severe disability” trajectory had the highest probability of attrition (25.4%-35.0%).
Sensitivity analysis of the trajectory model without attrition over-represented the proportion of older adults on the mild increasing disability trajectory and under-represented the proportion of older adults on the no disability trajectory when compared to the joint model that included attrition. The posterior probability of assignment showed a better fit for the joint model with attrition (Supplemental Table 2).
Figure 2 presents multinomial logistic regression results. All covariates were significant except for race (p-value=0.44) and education (p-value=0.65).
Figure 2.
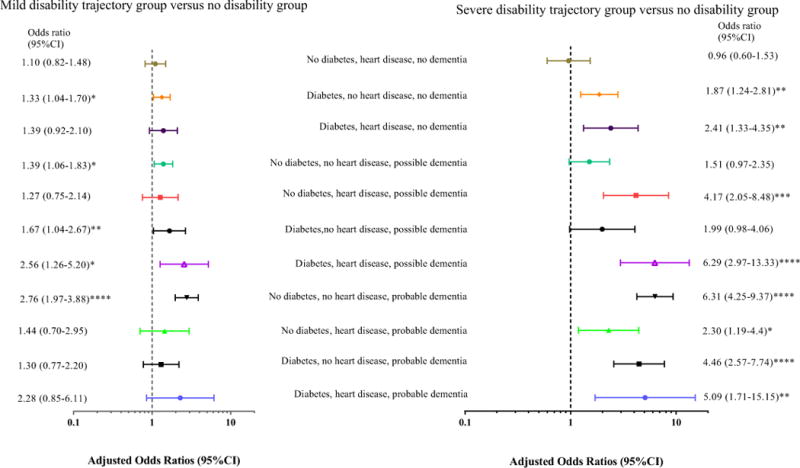
Log-scale forest plot of the adjusted odds ratios and their 95% confidence intervals of being on the “mild” or “severe” disability trajectories for combinations of diabetes, heart disease and dementia status. The reference group, which has the value of one on the x-axis, were persons on the no functional disability trajectory. *=0.05, **=0.01, ***<0.001, ****<0.0001. Multinomial logistic regression models adjusted for age (five year intervals from 65 years old), sex, race, living situation, education, the number of IADLS disabled, BMI, self-reported overall health and the number of chronic conditions. See Supplementary table 1 for a breakdown of original sample sizes and national estimates per combination.
Associations of diabetes, heart disease and dementia status with the “mild disability” trajectory
For cognitively intact persons, having diabetes in the absence of heart disease (hereafter referred to as diabetes alone) significantly increased the odds of being on the “mild disability” trajectory compared with the “no disability” trajectory (Figure 2). Persons with possible dementia alone, or possible dementia and diabetes, or possible dementia with both diabetes and heart disease, had significantly greater odds of being on the “mild disability” trajectory relative to “no disability.” Persons with probable dementia alone had significantly greater odds of being on the “mild disability” trajectory relative to persons on the “no disability” trajectory.
Of the estimated 4.9 million persons on the “mild disability” trajectory, given the prevalences of diabetes and possible dementia, persons with these were most frequent. Although people with diabetes and no dementia were significantly at risk for both “mild” and “severe” disability, in absolute terms national estimates for “mild disability” were double the “severe disability” trajectory (710,407 and 336,852 respectively, Supplementary table 1).
Associations of diabetes, heart disease and dementia status with “severe disability” trajectory
For cognitively intact persons, having diabetes alone or having both diabetes and heart disease significantly increased the odds of being on the “severe disability” trajectory relative to persons on the “no disability” trajectory (Figure 2). For persons with possible dementia, having heart disease, or both diabetes and heart disease significantly increased the odds of being on the “severe disability” trajectory relative to persons the “no disability”. Persons with probable dementia, regardless of concurrent complicated self-care conditions had significantly increased the odds of being on the “severe disability” trajectory relative to persons on the “no disability” trajectory.
Of an estimated 3.4 million persons on the “severe disability” trajectory, over 1.5 million had probable dementia (Supplemental table 1). Persons with possible dementia and heart disease (with or without diabetes) had national estimates that were higher on the “severe disability” trajectory than the “mild disability” trajectory (Supplementary table 1).
Discussion
In this longitudinal nationally-representative cohort of community-dwelling adults ≥65 years old, we found three distinct functional disability trajectories. Our results found attrition rates closely followed the shape of each trajectory with greater disability leading to increased attrition rates. For the trajectory without disability over time, there was a low, stable level of attrition. Following the “mild disability” trajectory, at baseline there was negligible disability, which increased concurrently with attrition. At the end of five years, the “mild disability” trajectory had similar attrition and disability estimates compared to the baseline estimates of “severe disability” trajectory. There were combinations of complicated self-care conditions and cognitive status that were only significantly associated with “mild disability” (possible dementia with or without diabetes) or only significantly associated with “severe disability” (diabetes and heart disease, heart disease and possible dementia, probable dementia with any combinations of diabetes or heart disease).
Previous trajectory research in the oldest of the old that jointly modelled ADLs and survival in China, similarly found three distinct trajectories, with survival probabilities decreasing with ADL difficulty15. A study estimating trajectories of physical functioning in adults 26-70 years old over 11 years found five trajectories using the Short-form 36 where 54% of the individuals had a “stable slightly limited course” of physical functioning indicating some disability16. Our results may differ from these studies because of different outcome scales, covariates, country differences, jointly modelling attrition, age of the sample population and the length of the study. Neither of the prior studies focused on cognitive impairment or comorbid conditions that may require complicated-self-care.
Previous trajectory research found persons who died from advanced dementia, had the highest levels of disability in the last year of life17. Moreover, Han et al18 found increasing burden of disability, hospitalization and institutionalization as community-dwelling older people aged, particularly for those with a declining cognitive trajectory. This agrees with our findings that persons with probable dementia alone or in combination with either or both complicated-self-care conditions had significantly increased odds of being on the “severe disability” trajectory and had the highest (45%) hospitalizations in the year before baseline.
Our study reinforces the need for health policies addressing functional disability among persons with multiple chronic conditions. It is important to examine the unique combination of dementia with other chronic conditions because dementia may make self-care more difficult. Improving diabetes and/or heart disease self-care may decrease preventable hospitalizations, and in turn disability, as hospitalization has been associated with ADL disability2,19. A meta-analysis found preventative home visits that included a multicomponent geriatric assessment and multiple follow-up home visits reduced functional decline and mortality in community-dwelling older adults20. Another review presented evidence that home visits using exercise therapy reduced disability in community-dwelling dementia participants; however, they did not report multimorbidity21.
The strengths of this study include ≥7600 representative community-dwelling adults ≥65 years old with analytic weights to provide national estimates. Only one article previously jointly modeled disability and attrition to prevent a healthy survivor bias further increasing the novelty of these methods15. Previous simulation studies showed that jointly modelling attrition and functional disability reduces bias and misrepresentation of group allocation compared to the group based trajectory model that does not take attrition into account12. Even the least disabled trajectory had 46.1% attrition after five years. Cognitive impairment, heart disease and diabetes are associated with an increased risk of disability, death and lost to follow-up5,22,23. Missing data is known to bias results; therefore, research must use methods to address missingness6. This study used a range of participant characteristics, annual visits over five years and a validated dementia method. No studies have examined the influence of complicated-self-care with dementia.
This study also has limitations. NHATS recorded physician-diagnosed self-reported chronic conditions and 5.8% used a proxy to complete the baseline interview, a potential source of imprecision on confidence intervals. Proxy responses may introduce bias as previous research found that although older adults remember common chronic conditions well, they have more difficulty reporting ADLs24. Moreover diabetes, the 6th leading cause of disability is commonly under-diagnosed because many individuals are asymptomatic and type 1 and 2 diabetes may differ on associations with disability25,26. Cognitive status and complicated-self-care conditions were set at baseline and not updated over the course of follow-up. Finally, as disease-specific severity measures were not available, we used a self-reported score of overall health as a surrogate measure for overall disease severity.
Future research should focus not just on the presence of chronic conditions and their associations with healthcare utilization, but should also investigate interventions to reduce the burden of functional disability. For example, informal caregivers, who may provide medical assistance, may attenuate the impact of dementia on functional disability in the presence of chronic conditions that require self-management, such as diabetes and heart disease.
Conclusion
This paper provides a robust method to estimate the national burden of disability at a population-level in community-dwelling older adults to help plan for the needs of the US population. The majority of US community-dwelling older adults live without disability; however, approximately a quarter are likely to have increasing functional disability over five-years. Of health policy concern is that 4.9 million and 3.4 million older Americans are estimated to follow the “mild” and “severe” disability trajectories. This paper shows that combinations of diabetes, heart disease and cognitive status have differing odds of functional disability and attrition. Adoption of methods that provide national estimates, account for attrition and quantify the effects of chronic conditions, will reduce bias in estimates health policy-makers utilize.
Supplementary Material
Supplementary Table 1. Frequencies, percentages at the sample and national estimates for diabetes, heart disease, dementia and their combinations across trajectory groups
Supplemental Table 2. Sensitivity analysis of the trajectory models that jointly modelled attrition and the trajectory model without attrition
Impact Statement for Brief Report.
We certify that this work is novel. The potential impact of this research to health policy includes the following. Using nationally-representative data of older adults, we demonstrate a method to calculate national estimates on the trajectories of functional disability that accounts for attrition. We also quantify the association between cognitive impairment, diabetes and heart disease with differing functional disability trajectories.
Acknowledgments
We thank Maureen Skehan at NHATS for her advice on the use of the analytic weights.
This work was supported by the National Institute on Aging [R01 AG047891-01A1, HGA, P50AG047270 and a P30 AG021342-14S1 HGA, and JMV, as well as a Brown Cox fellowship to JMV. JMV, HGA, LH, KL and JM receive support from the National Institute on Aging Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). KL receives support from the National Institute on Aging and the American Federation of Aging Research through the Paul Beeson Career Development Award (K23AG048359). JM receives support from the National Institute on Aging (K01 AG042450-01).
Financial Disclosure: This work was supported by the National Institute on Aging [R01 AG047891-01A1, HGA, P50AG047270 and a P30 AG021342-14S1 HGA, and JMV, as well as a Brown Cox fellowship to JMV. JMV, HGA, LH, KL and JM receive support from the National Institute on Aging Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). KL receives support from the National Institute on Aging and the American Federation of Aging Research through the Paul Beeson Career Development Award (K23AG048359). JM receives support from the National Institute on Aging (K01 AG042450-01).
Footnotes
Conflict of Interest: none.
Authors Contributions: All the authors participated in designing the study, interpreting the results, critically revising the manuscript, and approved the final version for submission
Sponsor’s Role: The National Institute on Aging had no role in the design or conduct of the study; collection, management, analysis, or interpretation of data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. New Engl J Med. 2002;346(14):1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 2.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson G. Making the case for ongoing care. Princeton, NJ: Feb, 2010. 2010. [Google Scholar]
- 4.Jutkowitz E, MacLehose RF, Gaugler JE, Dowd B, Kuntz KM, Kane RL. Risk Factors Associated With Cognitive, Functional, and Behavioral Trajectories of Newly Diagnosed Dementia Patients. J Gerontol A Biol Sci Med Sci. 2016 doi: 10.1093/gerona/glw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coley N, Gardette V, Toulza O, et al. Predictive factors of attrition in a cohort of Alzheimer disease patients. The REAL.FR study. Neuroepidemiology. 2008;31(2):69–79. doi: 10.1159/000144087. [DOI] [PubMed] [Google Scholar]
- 6.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009:338. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasper JD, Freedman VA. Findings from the 1st round of the National Health and Aging Trends Study (NHATS): introduction to a special issue. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 1):S1–7. doi: 10.1093/geronb/gbu125. [DOI] [PubMed] [Google Scholar]
- 8.Kasper JD, Freedman VA, Spillman BC. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Baltimore: Johns Hopkins University School of Public Health; 2013. (Technical Paper #5). [Google Scholar]
- 9.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 10.Wolff JL, Spillman BC, Freedman VA, Kasper JD. A National Profile of Family and Unpaid Caregivers Who Assist Older Adults With Health Care Activities. JAMA Intern Med. 2016;176(3):372–379. doi: 10.1001/jamainternmed.2015.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaquila J, Freedman VA, Spillman B, Kasper JD. National Health and Aging Trends Study Development of Round 1 Survey Weights. Baltimore: Johns Hopkins University School of Public Health; 2012. (NHATS Technical Paper #2). NHATS Technical Paper #2. [Google Scholar]
- 12.Haviland A, Jones B, Nagin D. Group-based Trajectory Modeling Extended to Account for Nonrandom Participant Attrition. Sociological Methods & Research. 2011;40(2):367–390. [Google Scholar]
- 13.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 14.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- 15.Zimmer Z, Martin LG, Nagin DS, Jones BL. Modeling disability trajectories and mortality of the oldest-old in China. Demography. 2012;49(1):291–314. doi: 10.1007/s13524-011-0075-7. [DOI] [PubMed] [Google Scholar]
- 16.Rooth V, van Oostrom SH, Deeg DJH, Verschuren WMM, Picavet HSJ. Common trajectories of physical functioning in the Doetinchem Cohort Study. Age Ageing. 2016;45(3):382–388. doi: 10.1093/ageing/afw018. [DOI] [PubMed] [Google Scholar]
- 17.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of Disability in the Last Year of Life. New Engl J Med. 2010;362(13):1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han L, Gill TM, Jones BL, Allore HG. Cognitive Aging Trajectories and Burdens of Disability, Hospitalization and Nursing Home Admission Among Community-living Older Persons. J Gerontol A Biol Sci Med Sci. 2016;71(6):766–771. doi: 10.1093/gerona/glv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill TM, Allore HG, Gahbauer EA, Han L. Establishing a Hierarchy for the Two Components of Restricted Activity. J Gerontol A Biol Sci Med Sci. 2015;70(7):892–898. doi: 10.1093/gerona/glu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287(8):1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 21.McLaren AN, LaMantia MA, Callahan CM. Systematic Review of Non-Pharmacologic Interventions to Delay Functional Decline in Community-Dwelling Patients with Dementia. Aging & mental health. 2013;17(6):655–666. doi: 10.1080/13607863.2013.781121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks J, Muriel A, Smith JP. Attrition and Health in Ageing Studies: Evidence from ELSAS and HRS. The RAND corporation; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thygesen LC, Johansen C, Keiding N, Giovannucci E, Gronbaek M. Effects of sample attrition in a longitudinal study of the association between alcohol intake and all-cause mortality. Addiction. 2008;103(7):1149–1159. doi: 10.1111/j.1360-0443.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- 24.Shardell M, Hicks GE. Statistical analysis with missing exposure data measured by proxy respondents: a misclassification problem within a missing-data problem. Stat Med. 2014;33(25):4437–4452. doi: 10.1002/sim.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Diabetes Federation. IDF Diabetes Atlas. Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 26.Global regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Frequencies, percentages at the sample and national estimates for diabetes, heart disease, dementia and their combinations across trajectory groups
Supplemental Table 2. Sensitivity analysis of the trajectory models that jointly modelled attrition and the trajectory model without attrition


