Abstract
Objective
To identify whether intraventricular hemorrhage (IVH) and cerebellar hemorrhage (CH) have common or divergent risk factors.
Study Design
This is a retrospective cross-sectional cohort of infants including all infants born <30 weeks from 2007–16. Comprehensive perinatal, and clinical factors were extracted from the medical record. Outborn infants, infants with major congenital anomaly, those transferred prior to discharge, and those with mixed or no brain injury were excluded. The remaining infants were divided into two groups: IVH only and cerebellar hemorrhage only.
Continuous variables were evaluated with the Wilcoxon-Mann-Whitney Test, categorical variables were evaluated with Fisher’s Exact Test. Multinomial logistic regression was used to identify factors which predispose infants towards injury type more than another, holding other factors held constant.
Results
127 infants were included (CH n=27, IVH n=100). Compared to those with IVH, infants with CH were of lower EGA (p=0.03), lower birth weight (p=0.01), more often of multiple gestation (p=0.03), more frequently born emergently (p=0.03), had a greater number of ventilator days (p=0.03), received postnatal steroids more often (p=0.02), had a greater incidence of hemodynamically significant PDA, and less frequently had pulmonary hemorrhage (p=0.04)
In multinomial regression analysis, three factors were identified which favored cerebellar hemorrhage over IVH: multiple gestation (RR 4.70, 95% CI 1.56–14.21, p<0.01), chorioamnionitis (RR 3.18, 95% CI 1.13–8.92, p=0.03), and emergent delivery (RR 4.14, 95% CI 1.48–11.55, p<0.01). Only advancing gestational favored IVH over cerebellar hemorrhage (RR 0.74, 95% CI 0.65–0.85, p<0.01).
Conclusions
IVH and CH have unique risk factors. These results highlight the need to tailor neuroimaging surveillance to specific patient risk factors.
Introduction
Located near the lateral ventricle, the germinal matrix is a highly vascular region in the brain which plays an important role in neuronal migration.1 Preterm birth interrupts the normal developmental trajectory of the germinal matrix and places great hemodynamic stress on this fragile vascular bed. During a premature infant’s first week of life, a disruption of blood flow regulation in the germinal matrix may lead to hemorrhage into the intraventricular space1, termed intraventricular hemorrhage (IVH). This condition affects approximately 40% of all infants with birthweights less than 1000g.2–4 The consequences of this hemorrhage can be severe; IVH is associated with a 40% increased risk of moderate to severe neurodevelopmental impairment5 including cognitive delay and cerebral palsy.1,6
Cerebellar hemorrhage (CH) is another form of acute brain injury in the preterm infant with an incidence of 5–25% in infants born before 32 weeks gestation.1 However, unlike IVH, which is readily recognized on standard cranial ultrasound imaging through the anterior fontanelle, cerebellar hemorrhage can only be seen when the “mastoid window” is used, obtained by aiming the ultrasound beam through the junction of the squamosal, lambdoidal and occipital sutures.7,8 CH has been linked to hemodynamic disruption included impaired vascular autoregulation, hypotension requiring inotropic support9, and a patent ductus arteriosus.1
Despite apparent temporal and mechanistic overlap, the link between IVH and CH has not been fully explored, and it remains unclear whether the two different forms of brain injury have common or divergent risk factors.10,11 In this cross-sectional cohort study, we compare a comprehensive set of risk factors in two groups of infants: those with IVH only and those with CH only, in order to define differences in risk factors.
Methods
Patient selection
The study cohort was derived from all infants delivered at Barnes-Jewish Hospital and admitted to the NICU at St. Louis Children’s Hospital between 1 January 2007 and 31 December 2016. Inclusion criteria included preterm birth (< 30 weeks EGA) and absence of major congenital anomalies. Exclusion criteria included presence of mixed hemorrhage (IVH and CH) or no hemorrhage, transfer to other hospital before discharge, or birth at outside hospital due to incomplete data (e.g. cord blood gas analysis and placental pathology not done) and confounding factors of heterogeneous skill levels of resuscitating providers and the unknown effects of transportation. The remaining infants were subdivided into two groups based on type of intracranial hemorrhage (IVH only and CH only).
Data collection
A comprehensive set of characteristics were abstracted from the electronic medical record for all infants identified and encompassed demographic, perinatal, and hospital course factors.
Demographic factors included gestational age, birth weight, sex, multiple gestation, growth restriction, race, and mortality during initial hospitalization. Perinatal factors included antenatal steroid and/or magnesium sulfate exposure, pre-eclampsia, and prolonged, preterm rupture of membranes (defined as rupture > 18h). Delivery factors included presence of chorioamnionitis (defined by histopathology), delivery mode, need for emergent delivery, Apgar scores at 1 and 5 minutes, arterial cord blood pH and base excess, presence of abruption, CPR at delivery (defined as chest compressions and/or epinephrine administration), and temperature at NICU admission.
Detailed information about the NICU hospital course was collected and organized by affected organ system. Infection problems included culture-positive and –negative sepsis. Pulmonary problems included pneumothorax, pulmonary hemorrhage, number of ventilator days, need for high-frequency oscillatory ventilation in the first five days, blood gas pH and base excess at 5 days, bronchopulmonary dysplasia (BPD, defined as need for supplemental oxygen after 36 weeks post-menstrual age), and steroid treatment for severe BPD (hydrocortisone or dexamethasone). Cardiovascular problems included patent-ductus arteriosus requiring medical or surgical treatment, hypotension requiring treatment with inotropic medication, and stress-dose hydrocortisone for treatment of refractory hypotension. Surgical problems included spontaneous intestinal perforation (SIP), necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP) requiring surgical treatment.
Although initial case selection was automated, manual review of all selected charts was performed by two authors (ZAV, MH) to ensure accuracy and completeness of abstracted data.
Statistical approach
To examine differences between infants with IVH and infants with CH, univariate comparison of the two groups was made using the Wilcoxon-Mann-Whitney test for continuous variables and Fisher’s Exact test for categorical variables. Multinomial logistic regression was then used to identify factors which predispose infants towards one injury type more than another, other factors held constant. The choice of variables in the regression model was driven by those factors with a p value < 0.10 in the univariate analysis, with the goal of the greatest explanatory power while minimizing over-fitting (done by minimizing Akaike’s Information Criterion during model construction). The year of diagnosis was forced into the final model to account for practice variation over time. Results were considered significant where p < 0.05. Statistical analysis was conducted using R version 3.3.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Study procedures were reviewed and approved by the Human Subjects Research Protection Office at Washington University.
Results
Cohort selection
A total of 493 infants who were born before 30 completed weeks of gestation were admitted to the NICU at St. Louis Children’s Hospital during the study period. A total of 142 infants were excluded with the primary screen (transfer before discharge n=8; major congenital anomaly n=4; outborn infants n=130). An additional 224 infants were excluded with the secondary screen (mixed hemorrhage type n=42, no hemorrhage n=182). Of the remaining 127 infants, 27 had cerebellar hemorrhage and 100 had intraventricular hemorrhage. A flow diagram of patient selection is shown in Figure 1.
Figure 1.
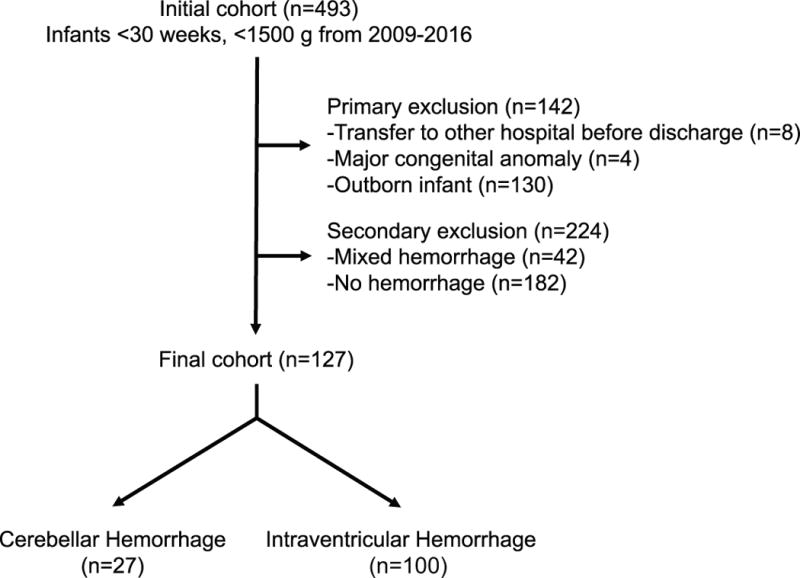
Flow diagram of patient selection including both primary and secondary screens and the composition of the final study cohort.
Demographic, perinatal, and delivery characteristics
Infants with CH were of younger gestational age at birth (25.1 vs. 26.1 weeks, p=0.03), lower birth weight (778 vs. 909 g, p=0.01), were more likely to be of multiple gestation (37 vs. 16%, p=0.03), and delivered emergently more often (59 vs. 35%, p=0.03). A complete overview of all variables is shown in Table 1.
Table 1.
Demographic, perinatal, and delivery characteristics
| CH (n=27) | IVH (n=100) | P value | |
|---|---|---|---|
| Demographic Factors | |||
| EGA, mean (SD), weeks | 25.1 (1.5) | 26.1 (1.9) | 0.03* |
| Birth weight, mean (SD), g | 788 (206) | 909 (257) | 0.01* |
| Male sex, n(%) | 13 (48) | 58 (58) | 0.39 |
| Multiple gestation, n (%) | 10 (37) | 16 (16) | 0.03* |
| IUGR, n (%) | 2 (7) | 8 (8) | 1.00 |
| Race/ethnicity | |||
| White, n (%) | 14 (52) | 47 (47) | 0.38 |
| Black, n (%) | 13 (48) | 51 (51) | |
| Asian, n (%) | 0 (0) | 2 (2) | |
| Died during initial hospitalization, n (%) | 3 (11) | 18 (18) | 0.56 |
| Cause of death | |||
| Acute decompensation, n (%) | 0 (0) | 3 (17) | 0.29 |
| Hepatic hemorrhage, n(%) | 1 (33) | 0 (0) | |
| NEC totalis, n (%) | 0 (0) | 4 (22) | |
| Pulmonary hemorrhage, n (%) | 0 (0) | 3 (17) | |
| Renal failure, n (%) | 0 (0) | 3 (17) | |
| Selective withdrawal of care, n (%) | 2 (67) | 5 (27) | |
| Perinatal factors | |||
| Received antenatal steroids | |||
| Any steroids, n (%) | 25 (93) | 82 (82) | 0.24 |
| Complete steroid course, n (%) | 12 (44) | 49 (49) | 0.83 |
| Antenatal magnesium sulfate, n (%) | 16 (59) | 65 (65) | 0.65 |
| Pre-eclampsia, n (%) | 6 (22) | 22 (22) | 1.00 |
| PPROM, n (%) | 3 (11) | 19 (19) | 0.41 |
| Delivery Factors | |||
| Chorioamnionitis, n (%) | 12 (44) | 26 (26) | 0.09 |
| Vaginal delivery, n (%) | 7 (26) | 36 (36) | 0.37 |
| Emergent delivery, n (%) | 16 (59) | 35 (35) | 0.03* |
| 1-min Apgar, median (range) | 4 (1–9) | 3 (0–9) | 0.43 |
| 5-min Apgar, median (range) | 6 (0–9) | 6 (0–9) | 0.50 |
| Cord blood pH, mean (SD) | 7.33 (0.08) | 7.29 (0.10) | 0.13 |
| Cord blood base excess, mean (SD) | −2.62 (1.96) | −2.58 (3.92) | 0.97 |
| Abruption, n (%) | 1 (4) | 11 (11) | 0.46 |
| CPR at delivery, n (%) | 3 (11) | 20 (20) | 0.40 |
| Admission temperature < 36C, n (%) | 4 (15) | 14 (14) | 1.00 |
Footnote:
denotes significance at p<0.05. Continuous variables evaluated with the Wilcoxon-Mann-Whitney Test, categorical variables evaluated with Fisher’s Exact Test. Abbreviations: EGA=estimated gestational age, IUGR=intrauterine growth restriction, NEC=necrotizing enterocolitis, PPROM=prolonged, preterm rupture of membranes.
Clinical characteristics
Infants with CH were less likely to have pulmonary hemorrhage (0 vs. 14%, p=0.04), had a greater median number of ventilator days (31 vs. 6, p=0.01), were more likely to receive postnatal steroid treatment for severe BPD (hydrocortisone 44 vs 22%, p=0.03; dexamethasone 26 vs. 8%, p=0.02), and more likely to received medical or surgical treatment of a patent ductus arteriosus (59 vs. 35%, p=0.01). See Table 2 for a comparison of all clinical factors.
Table 2.
Clinical characteristics
| Cerebellar Hemorrhage (n=27) | IVH (n=100) | P value | |
|---|---|---|---|
| Infection Problems | |||
| Culture-negative sepsis, n (%) | 12 (44) | 33 (33) | 0.36 |
| Culture-positive sepsis, n (%) | 9 (33) | 25 (35) | 0.46 |
| Pulmonary Problems | |||
| Pneumothorax, n (%) | 1 (4) | 11 (11) | 0.46 |
| Pulmonary hemorrhage, n (%) | 0 (0) | 14 (14) | 0.04* |
| Ventilator days, median (range) | 31 (0–137) | 6 (0–114) | 0.01* |
| HFOV ≤ 5 days of life, n (%) | 16 (59) | 37 (37) | 0.05 |
| Blood gas pH at 5 days of life, mean (SD) | 7.27 (0.09) | 7.27 (0.08) | 0.76 |
| Blood gas base excess at 5 days of life, mean (SD) | −5.6 (3.5) | −4.4 (8.4) | 0.66 |
| BPD, n (%) | 17 (62) | 45 (45) | 0.16 |
| Lung-dose hydrocortisone, n (%) | 12 (44) | 22 (22) | 0.03* |
| Lung-dose dexamethasone, n (%) | 7 (26) | 8 (8) | 0.02* |
| Cardiovascular Problems | |||
| PDA treatment | |||
| No treatment, n (%) | 11 (41) | 65 (65) | 0.01* |
| Medical treatment only, n (%) | 12 (44) | 20 (20) | |
| Surgical treatment only, n (%) | 0 (0) | 8 (8) | |
| Medical and surgical treatment, n (%) | 4 (15) | 7 (7) | |
| Inotropic medication during hospitalization, n (%) | 18 (67) | 52 (52) | 0.19 |
| Stress-dose hydrocortisone, n (%) | 9 (33) | 23 (23) | 0.31 |
| Surgical Problems | |||
| Spontaneous intestinal perforation, n (%) | 5 (19) | 15 (15) | 0.77 |
| Necrotizing enterocolitis, n (%) | 5 (19) | 17 (17) | 0.78 |
| ROP requiring surgery, n (%) | 8 (30) | 15 (15) | 0.16 |
Footnote:
denotes significance at p<0.05. Continuous variables evaluated with the Wilcoxon-Mann-Whitney Test, categorical variables evaluated with Fisher’s Exact Test. Abbreviations: HFOV=high-frequency oscillatory ventilation, BPD=bronchopulmonary dysplasia, PDA=patent ductus arteriosus, ROP=retinopathy of prematurity.
Regression model
Utilizing variables which were statistically different at a level of p < 0.10 as a basis, a multinomial logistic regression model was constructed. Among preterm infants with brain injury, three factors were identified which favored cerebellar hemorrhage over IVH: multiple gestation (RR 4.70, 95% CI 1.56–14.21, p<0.01), chorioamnionitis (RR 3.18, 95% CI 1.13–8.92, p=0.03), and emergent delivery (RR 4.14, 95% CI 1.48–11.55, p<0.01). Only advancing gestational favored IVH over cerebellar hemorrhage (RR 0.74, 95% CI 0.65–0.85, p<0.01). Birth weight, antenatal steroid administration, need for inotropic support, hemodynamically significant PDA, hydrocortisone treatment for severe BPD, and year of diagnosis were not differentiating factors between the two forms of injury. A complete overview of the model is shown in Table 3.
Table 3.
Multinomial logistic regression
| Parameter | RR for CH compared to IVH (95% CI) | Z statistic | P value |
|---|---|---|---|
| EGA | 0.74 (0.65–0.85) | −4.26 | <0.01* |
| Birth weight | 1.00 (0.99–1.01) | 0.10 | 0.92 |
| Multiple gestation | 4.70 (1.56–14.21) | 2.74 | <0.01* |
| Complete antenatal steroids | 1.19 (0.44–3.18) | 0.34 | 0.73 |
| Chorioamnionitis | 3.18 (1.13–8.92) | 2.19 | 0.03* |
| Emergent delivery | 4.14 (1.48–11.55) | 2.71 | <0.01* |
| Need for inotropic support | 0.67 (0.21–2.16) | −0.67 | 0.50 |
| Hemodynamically significant PDA | 1.52 (0.52–4.46) | 1.52 | 0.44 |
| Hydrocortisone for treatment of severe BPD | 1.40 (0.44–4.50) | 1.40 | 0.57 |
| Year of diagnosis | 1.00 (0.99–1.01) | 0.79 | 0.42 |
Footnote: Final model AIC = 53.65
Discussion
In this study our goal was to identify common and divergent factors associated with the IVH and CH injury “phenotypes” in a group of preterm infants. In particular, our goal was to define unique risk factors which predispose infants towards cerebellar hemorrhage in comparison to those with intraventricular hemorrhage. In univariate analysis, infants with cerebellar hemorrhage were more premature, lower birth weight, more often of multiple gestation, were born under emergent circumstances more frequently, had more severe lung disease, and more frequently had a hemodynamically significant PDA which required treatment. When these factors are entered into a multinomial regression model, holding other factors constant, a more clear picture of the cerebellar hemorrhage phenotype became clear, defined by multiple gestation, chorioamnionitis and emergent delivery. In contrast, as infants advanced in gestational age the likelihood of CH dropped, while the risk of IVH increased.
Although not surprising, these data confirm that infants with intracranial hemorrhage of any type are amongst the sickest infants in the NICU and experience many of the most severe complications. However, these results suggest that there are subtle, but important distinguishing characteristics between the two injury types which should alter surveillance practices. These divergent risk factors also point a potential different time scale for the evolution of each injury phenotype—IVH is more often associated with acute, hemodynamically compromising events (pulmonary hemorrhage) while CH is more frequently associated with sub-acute events marked by extreme prematurity, inflammation (chorioamnionitis), prolonged hypoxia (severe lung disease), and hypotension (patent ductus arteriosus).
While some risk factors are divergent, it is important to point out that several key factors, long associated with IVH, have equal association with cerebellar hemorrhage, suggesting that both forms of brain injury share common pathways. Two major IVH risk factors, hypotension requiring inotropic support12–14 and patent ductus arteriosus15,16, are equally important in the development of CH. Likewise, antenatal steroids, which have a well-described association with a reduction in IVH rates17,18, do not exert a disease-specific effect in this analysis.
One particular factor which merits individual discussion is the use of antenatal magnesium sulfate. Gano et al. reported a reduction in CH in association with magnesium sulfate administration19, but infants were not stratified by injury type. Although antenatal magnesium sulfate administration has become widespread in obstetrical practice after the publication of three randomized trials20–22 which showed a reduction in mortality and cerebral palsy, the exact mechanism by which it affords neuroprotection is still not understood, and at least one study22 has refuted this finding. One potential mechanism for neuroprotection is the anti-inflammatory/anti-oxidant properties of magnesium23, however the results of this study suggest that whatever effects magnesium may have are not sufficient to overcome the inflammatory component of chorioamnionitis.
The role that poor temperature control plays in the development of intracranial hemorrhage is more clear, with repeated demonstration of an association24,25. This knowledge has led to the development of so-called “Golden Hour” protocols26,27, which place great emphasis on the maintenance of normothermia for neuroprotection. In the present study, the rates of hypothermia (temperature < 36º C) on admission were roughly equivalent in the CH and IVH groups (14–15%). This is roughly double the rate in the no injury group excluded earlier in the study (approximately 8%), again reinforcing the link to injury, although without specificity for injury type. However, there is difficulty extrapolating the meaning of this data, as reported rates of hypothermia on admission are widely variable, ranging between 11%28 and 56%29 in normative populations. Regardless, there is no doubt that close attention to thermoregulation remains a crucial component of a comprehensive neuroprotective strategy in the delivery room and in the hours after admission.
This study has several important limitations. First, the retrospective nature of the study introduces the specter of missing or incomplete data, although every attempt was made to completely and accurately extract data from the electronic medical record. Second, the results of this study represent associations between perinatal/clinical factors and intracranial hemorrhage and should not be misconstrued as causative. Third, while the longitudinal, cross-sectional design affords a large and diverse sample, it may also be a source of bias if the incidence of each form of hemorrhage varies over time or if there are variations in practice. However, as indicated by Figure 2, there is no obvious pattern in incidence of any of the injury, with waxing and waning rates. Furthermore, the regression model was corrected for year of diagnosis.
Figure 2.
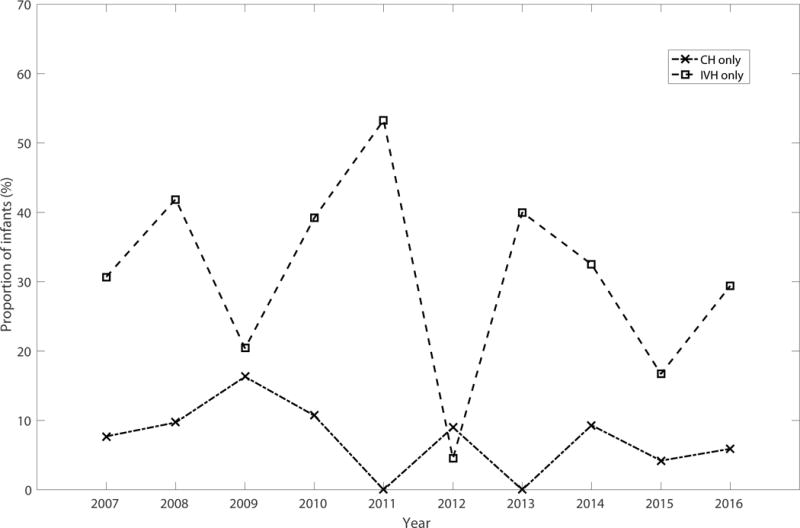
Incidence of CH only (dashed line with X markers) and IVH only (dashed line with square markers are shown over the course of the study period.
The results of this study underscore the routine use of the mastoid window to image the posterior fossa for detection of cerebellar hemorrhage. With an estimated incidence of 10–20%1 (20% in this cohort), it is a common lesion in preterm infants born before 30 weeks gestation and has significant associations with adverse neurodevelopmental outcomes.11 The data presented here suggest that surveillance for IVH should be focused on those with acute hemodynamically compromising events, while infants with protracted and respiratory disease, chorioamnionitis, or a hemodynamically significant PDA should be more closely monitored for CH. Additionally, these findings offer insight into risk factors for brain injury, permitting more accurate targeting of MRI or ultrasound imaging at term-equivalent age to those infants at greatest risk. Future studies should include daily cranial ultrasounds to more precisely obtain the timing of the hemorrhage.
Acknowledgments
The authors wish to acknowledge the assistance of Amy Distler, RN in the identification of infants with cerebellar hemorrhage.
Funding source: This work was supported by the following grants:
Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450)
The Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448)
Washington University in St. Louis Center for Biomedical Informatics, Clinical Investigation Data Exploration Repository (NIH/NCATS UL1 TR000448)
Footnotes
Conflict of Interest Statement:
None of the authors have competing financial interests in relation to this work.
References
- 1.Volpe JJ. Neurology of the newborn. 5th. Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 4.Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2009;22:491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- 5.Mukerji A, Shah V, Shah PS. Periventricular/Intraventricular Hemorrhage and Neurodevelopmental Outcomes: A Meta-analysis. Pediatrics. 2015;136:1132–1143. doi: 10.1542/peds.2015-0944. [DOI] [PubMed] [Google Scholar]
- 6.Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103:273–277. doi: 10.1016/s0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 7.Cramer BC, Jequier S, O’Gorman AM. Sonography of the neonatal craniocervical junction. AJR Am J Roentgenol. 1986;147:133–139. doi: 10.2214/ajr.147.1.133. [DOI] [PubMed] [Google Scholar]
- 8.Di Salvo DN. A new view of the neonatal brain: clinical utility of supplemental neurologic US imaging windows. Radiogr Rev Publ Radiol Soc N Am Inc. 2001;21:943–955. doi: 10.1148/radiographics.21.4.g01jl14943. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal A, El-Naggar W, Glanc P, Asztalos E. Risk factors and ultrasonographic profile of posterior fossa haemorrhages in preterm infants. J Paediatr Child Health. 2009;45:215–218. doi: 10.1111/j.1440-1754.2008.01456.x. [DOI] [PubMed] [Google Scholar]
- 10.Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. 2005;116:717–724. doi: 10.1542/peds.2005-0556. [DOI] [PubMed] [Google Scholar]
- 11.Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 12.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 13.Vesoulis ZA, Ters NE, Foster A, Trivedi SB, Liao SM, Mathur AM. Response to dopamine in prematurity: a biomarker for brain injury? J Perinatol Off J Calif Perinat Assoc. 2016;36:453–458. doi: 10.1038/jp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Noori S, McCoy M, Anderson MP, Ramji F, Seri I. Changes in Cardiac Function and Cerebral Blood Flow in Relation to Peri/Intraventricular Hemorrhage in Extremely Preterm Infants. J Pediatr. 2014;164:264–270.e3. doi: 10.1016/j.jpeds.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Sellmer A, Bjerre JV, Schmidt MR, McNamara PJ, Hjortdal VE, Høst B, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–510. doi: 10.1136/archdischild-2013-303816. [DOI] [PubMed] [Google Scholar]
- 17.Liebowitz M, Clyman RI. Antenatal Betamethasone: A Prolonged Time Interval from Administration to Delivery Is Associated with an Increased Incidence of Severe Intraventricular Hemorrhage in Infants Born before 28 Weeks Gestation. J Pediatr. 2016;177:114–120.e1. doi: 10.1016/j.jpeds.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW. Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants. Am J Obstet Gynecol. 1995;172:795–800. doi: 10.1016/0002-9378(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 19.Gano D, Ho M-L, Partridge JC, Glass HC, Xu D, Barkovich AJ, et al. Antenatal Exposure to Magnesium Sulfate Is Associated with Reduced Cerebellar Hemorrhage in Preterm Newborns. J Pediatr. 2016;178:68–74. doi: 10.1016/j.jpeds.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowther CA, Hiller JE, Doyle LW, Haslam RR, Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 21.Marret S, Marpeau L, Zupan-Simunek V, Eurin D, Lévêque C, Hellot M-F, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG Int J Obstet Gynaecol. 2007;114:310–318. doi: 10.1111/j.1471-0528.2006.01162.x. [DOI] [PubMed] [Google Scholar]
- 22.Rouse DJ, Hirtz DG, Thom E, Varner MW, Spong CY, Mercer BM, et al. A Randomized, Controlled Trial of Magnesium Sulfate for the Prevention of Cerebral Palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto J, Romani AM, Valentin-Torres AM, Luciano AA, Ramirez Kitchen CM, Funderburg N, et al. Magnesium Decreases Inflammatory Cytokine Production: A Novel Innate Immunomodulatory Mechanism. J Immunol. 2012;188:6338–6346. doi: 10.4049/jimmunol.1101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levene MI, Fawer CL, Lamont RF. Risk factors in the development of intraventricular haemorrhage in the preterm neonate. Arch Dis Child. 1982;57:410. doi: 10.1136/adc.57.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SS, Lee HC, Gould JB. Hypothermia in very low birth weight infants: distribution, risk factors and outcomes. J Perinatol. 2011;31:S49–S56. doi: 10.1038/jp.2010.177. [DOI] [PubMed] [Google Scholar]
- 26.Bissinger RL, Annibale DJ. Thermoregulation in Very Low-Birth-Weight Infants During the Golden Hour: Results and Implications. Adv Neonatal Care. 2010;10:230–238. doi: 10.1097/ANC.0b013e3181f0ae63. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds RD, Pilcher J, Ring A, Johnson R, McKinley P. The Golden Hour: Care of the LBW Infant During the First Hour of Life One Unit’s Experience. Neonatal Netw J Neonatal Nurs. 2009;28:211–219. doi: 10.1891/0730-0832.28.4.211. [DOI] [PubMed] [Google Scholar]
- 28.Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, et al. Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks’ gestation. JAMA Pediatr. 2015;169:e150277. doi: 10.1001/jamapediatrics.2015.0277. [DOI] [PubMed] [Google Scholar]
- 29.Laptook AR, Salhab W, Bhaskar B, Neonatal Research Network Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics. 2007;119:e643–649. doi: 10.1542/peds.2006-0943. [DOI] [PubMed] [Google Scholar]


