Abstract
While the peripheral auditory system of fishes has been well studied, less is known about how the fish’s brain and central auditory system process complex social acoustic signals. The plainfin midshipman fish, Porichthys notatus, has become a good species for investigating the neural basis of acoustic communication because the production and reception of acoustic signals is paramount for this species’ reproductive success. Nesting males produce long duration advertisement calls that females detect and localize amongst the noise in the intertidal zone to successfully find mates and spawn. How female midshipman are able to discriminate male advertisement calls from environmental noise and other acoustic stimuli is unknown. Using the immediate early gene product cFos as a marker for neural activity, we quantified neural activation of the ascending auditory pathway in female midshipman exposed to conspecific advertisement calls, heterospecific white seabass calls or ambient environment noise. We hypothesized that auditory hindbrain nuclei would be activated by general acoustic stimuli (ambient noise and other biotic acoustic stimuli) whereas auditory neurons in the midbrain and forebrain would be selectively activated by conspecific advertisement calls. We show that neural activation in two regions of the auditory hindbrain, the rostral intermediate division of the descending octaval nucleus and the ventral division of the secondary octaval nucleus, did not differ via cFos immunoreactive (cFos-ir) activity when exposed to different acoustic stimuli. In contrast, female midshipman exposed to conspecific advertisement calls showed greater cFos-ir in the nucleus centralis of the midbrain torus semicircularis compared to fish exposed only to ambient noise. No difference in cFos-ir was observed in the torus semicircularis of animals exposed to conspecific versus heterospecific calls. However, cFos-ir was greater in two forebrain structures that receive auditory input, the central posterior nucleus of the thalamus and the anterior tuberal hypothalamus, when exposed to conspecific calls versus exposure to either ambient noise or heterospecific calls. Our results suggest that higher order neurons in the female midshipman midbrain torus semicircularis, thalamic central posterior nucleus and hypothalamic anterior tuberal nucleus may be necessary for the discrimination of complex, specie-specific social acoustic signals. Furthermore, neurons in the central posterior and anterior tuberal nuclei are differentially activated by exposure to conspecific vs. other acoustic stimuli.
Keywords: Acoustic communication, animal communication, auditory pathways, fish, teleost
Introduction
Acoustic communication is a fundamental component of social behavior across vertebrate taxa. Socially relevant acoustic signals can vary greatly across context-specific behaviors such as during aggression, affiliation and reproduction. The discrimination of biologically relevant acoustic signals from background environmental noise is paramount for appropriate behavioral decision-making. Among vertebrates, gnathostome fishes represent perhaps the most ancestral design of the vertebrate auditory receiver system and it is thought that vocal-acoustic communication evolved first in bony fishes (Bass and McKibben 2003, Bass et al. 2008). Understanding the underlying neural circuitry responsible for the discrimination of behaviorally relevant acoustic signals can potentially provide important insights into the co-evolution of central auditory and vocal-acoustic communication systems that may be conserved across vertebrate taxa.
The production and reception of social acoustic signals is necessary for successful reproduction in the plainfin midshipman fish, Porichthys notatus (Bass and McKibben 2003, Bass and Ladich 2008). Furthermore, the central auditory and vocal motor pathways of this species have been extensively studied in terms of their connections and neurochemistry, thus making the plainfin midshipman a valuable system for studying vocal-acoustic communication. Plainfin midshipman are a nocturnal marine teleost fish found on the west coast of North America that make seasonal migrations from deep off shore sites (>100 m) into the shallow intertidal zone to breed. Type I males excavate nests under rocky shelters from which they contract their sonic swim bladder muscles to produce long duration multiharmonic advertisement calls to attract reproductively receptive females for spawning (Brantley and Bass 1994, Bass and Ladich 2008). Females must be able to detect, discriminate and localize calling type I males amongst the background noise of other soniferous fishes, invertebrates and abiotic factors to successfully locate courting males. The auditory encoding of both conspecific and heterospecific vocalizations likely requires neural mechanisms for signal recognition and discrimination at the level of the midbrain torus semicircularis (TS) and/or higher nuclei in the midshipman auditory pathway, as shown in anurans (Hoke et al. 2004, Hoke et al. 2010), however currently it is unknown how fish discriminate social acoustic signals.
All teleost fishes are thought to be able to detect the particle motion component of underwater sound using their otolithic end organs, which act as biological accelerometers to sense the direct movement of underwater particles relative to the fish. The saccule is thought to be the primary hearing organ in the midshipman as in most other fishes and it is where sound is first transduced and processed before auditory information is sent to hindbrain nuclei in the ascending auditory pathway. Studies in the closely related oyster toadfish, Opsanus tau, have shown that neurons in the auditory hindbrain are broadly tuned (Edds-Walton and Fay 2008, Edds-Walton 2016) and that tuning sharpens along the ascending auditory pathway into the midbrain TS (Edds-Walton and Fay 2003, Edds-Walton and Fay 2005). Studies in goldfish and anurans, have revealed that the auditory thalamus is likely involved in the discrimination of complex social acoustic signals and is selectively responsive to ecologically-relevant signals (Fuzessey and Feng 1983, Hall and Feng 1987, Mudry and Capranica 1987, Lu and Fay 1995).
The purpose of this study was to characterize neural circuits necessary for the discrimination of complex acoustic signals including conspecific vocalizations. We hypothesized that auditory neurons within major nuclei of the midshipman auditory pathway would be differentially activated by the exposure to ambient noise, conspecific and heterospecific acoustic stimuli. Specifically, we predicted that reproductive females exposed to conspecific advertisement calls would show greater activity of cFos, an immediate early gene product used as a marker for neural activiation, in midbrain and forebrain auditory nuclei compared to fish exposed to heterospecific vocalizations and ambient noise. We analyzed five nuclei within the central auditory system, specifically chosen based on their consistent activation during preliminary playback experiments in females and earlier studies in males (RM and PMF, personal observations, Petersen et al., 2013) and their known neurochemical input and multi-sensory connectivity (Bass et al. 2000, Goodson and Bass, 2002, Forlano et al. 2014, Forlano et al. 2015a). Our results support the hypothesis that higher order auditory nuclei are selectively activated by conspecific vocal signals compared to ambient noise in the midbrain TS, and to both ambient noise and heterospecific signals in the thalamic CP and hypothalamic AT in female midshipman fish.
Methods
Fish collection and housing
The 39 female plainfin midshipman used in this study were collected by hand during the morning low tides in the intertidal zone at Seal Rock near Brinnon, WA. Fish were housed in aerated 5 gallon buckets with fresh intertidal seawater changed every 2–3 hours until experimentation. After dark, fish were transferred to individual buckets with fresh seawater to acclimate for at least 30 minutes prior to testing.
Experimental setup
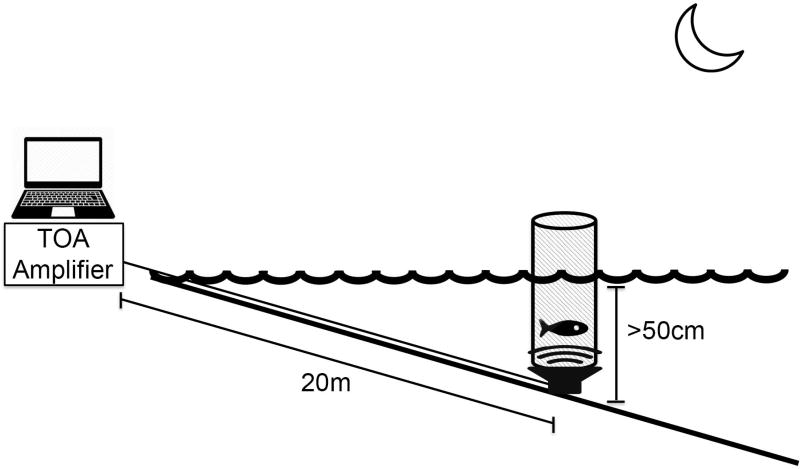
An experimental playback arena was setup in the intertidal zone at Seal Rock (Figure 1). At low tide, a UW-30 underwater speaker (Telex Communications, Burnsville, MN, USA) was buried in the substrate ~20 meters from the high tide line and four 1.5 meter rebar were staked into the ground around the speaker for cage support. A removable cage (diameter 40cm, height 120cm) was positioned and secured directly above the speaker. The speaker was powered by an audio amplifier (TOA BG-1120), which broadcast audio sound files from a laptop computer. Prior to testing, a field hydrophone (High Tech Inc. HTI-96, Long Beach, MS, USA) and recorder (Zoom H2, Hauppauge, NY, USA) were used to measure ambient noise levels of the testing arena in the natural acoustic environment and calibrate the playback sound levels such that average peak-to-peak amplitude of the acoustic stimuli was adjusted to 130db re: 1µPa at the outer edge of the testing arena. Experiments were then commenced at night after dark when the underwater speaker was submerged 50 cm or greater by the rising or falling tide, and then one female midshipman fish was gently placed into the arena for testing. Water depth at the testing arena was measured at the start and conclusion of each auditory playback experiment, as was water temperature.
Figure 1.
A schematic representation of the playback paradigm used during auditory exposure. An underwater speaker was buried in the substrate ~20 meters from the shoreline, where it was powered by a TOA amplifier which received acoustic playback files from a laptop computer. Above the speaker, a mesh cage (diameter = 40cm) was suspended in place where the fish was allowed to swim freely during exposure to auditory playback. Experiments were conducted each night after sunset when the cage was covered at least 50cm by the tide. The sound pressure level of the acoustic stimuli was calibrated to 130db re: 1µPa at the perimeter of the cage.
Acoustic Stimuli and Playback Procedures
Female midshipman fish were exposed to one of three acoustic stimuli: conspecific advertisement calls, heterospecific calls of white seabass, Atractoscion nobilis, and ambient environmental noise. The playback of conspecific advertisement calls consisted of a 30 minute looped audio file containing acoustic recordings from 7 male midshipman advertisement calls or “hums” (Brantley and Bass 1994) previously recorded in situ from calling type I male midshipman nests at Seal Rock. The audio files were equalized to the same maximum peak-to-peak sound level in MatLab to account for any differences in amplitude between individual male callers (Figure 2A). Previous work by Brantley and Bass (1994) showed that the fundamental frequency of male advertisement calls increases with temperature and that female preference is tightly coupled to match the appropriate fundamental frequency across temperatures (McKibben and Bass 1998). To account for the daily fluctuations in water temperature, we linearly shifted the fundamental frequency (along with the harmonics) of the advertisement call stimuli in Matlab to compensate for temperature differences at time of playback. The heterospecific call was a 30 minute looped audio file containing recordings from white seabass (Atractoscion nobilis) (Figure 2B). The white seabass is a soniferous fish found on the west coast of the United States and is known to be sympatric with the plainfin midshipman (Aalbers and Drawbridge 2008). There is no known predator-prey interaction between these species and theoretically the sounds of the white seabass should represent a familiar and innocuous biotic background sound to plainfin midshipman. The control condition consisted only of the background environment noise present in the intertidal zone during the experiment without the experimental playback of conspecific or heterospecific calls.
Figure 2.
Representative spectrograms of the sound stimuli used during auditory playback. The advertisement call of the plainfin midshipman (A) is long in duration and primarily sinusoidal while the white seabass calls (B) are short duration pulses produced at random intervals and the ambient noise (C) condition lacks any prominent components.
After 30 minutes of continuous exposure to one of the three acoustic stimuli, the subjects were removed from the testing cage and were then kept individually in a 5 gallon bucket filled with water from the intertidal for an additional 120 minutes before being sacrificed. The 120 minute post-treatment time before sacrifice was chosen to allow adequate cFos synthesis after sound exposure based on the work by Petersen et al. (2013) and Forlano et al. (2017). Fish were first deeply anesthetized in a 0.025% aminobenzoate bath after which they were weighed and measured for standard length (SL) before being transcardially perfused with ice cold teleost ringers followed by 4% paraformaldehyde in 0.1 M Phosphate buffer (PB; pH 7.2). Brains were harvested and post-fixed for 1 hr before being rinsed 3× in 0.1M PB and stored at 4°C until cryo-sectioned. Brains were transferred to 30% sucrose in a 0.1M PB solution for 24–48 hrs before being sectioned at 25 µm. Every other section was analyzed in the current study. In addition, ovaries were dissected and weighed and gonadosomatic index was calculated [(gonad mass/body mass − gonad mass) × 100].
Immunohistochemistry
Slides were brought to room temperature and then the perimeter of each slide was traced with a hydrophobic pen and soaked 3× for 10 minutes in phosphate buffered saline (PBS; pH 7.2), followed by a 1 hour soak in a blocking solution made of PBS with 0.3% Triton X-100 and 10% normal donkey serum (PBS-DS). Following the blocking procedure, slides were incubated for 16–17 hours at room temperature in PBS-DS containing rabbit anti-cFos (1:2000, Santa Cruz Biotechnology lot# C2510) and mouse anti-Hu, a specific marker for neuronal somata (1:2000, Molecular Probes). After incubation, slides were briefly dipped (to remove the majority of the antibody solution) and then rinsed in PBS + 0.5% normal donkey serum 5× for 10 minutes. The slides were then incubated for 2 hours at room temperature with PBS-DS containing donkey anti-rabbit conjugated to Alexa Fluor 568 (1:200, Life Technologies) for cFos and donkey anti-mouse conjugated to Alexa Fluor 488 (1:200, Life Technologies) for Hu. Finally, slides were dipped and rinsed 4× for 10 minutes in PBS before being cover slipped using ProLong Gold with DAPI and then allowed to cure for 48–72 hours in a dark room. Once dry, the slides were sealed with nail polished and stored at 4°C.
Image Acquisition
Micrographs were obtained on an Olympus BX61 epifluorescence compound microscope using MetaMorph imaging and processing software. All auditory nuclei were identified at low magnification using the GFP and DAPI filters to visualize neurons and their nuclei before being photomicrographed using a 20× objective. Exposure times and light levels were held constant for each channel across all conditions. Each micrograph was imaged consecutively starting with Texas Red, followed by GFP and DAPI filter sets, respectively. Images were taken in z-series by setting a top and bottom focal plane in the GFP channel with a stack thickness of 1 µm. The stacked photomicrographs were combined into a single projection image using the Z projection maximum intensity feature in ImageJ. cFos immunoreactive (cFos-ir) neurons were quantified manually using a custom ImageJ macro. Individual background thresholds were determined for each image and cFos-ir cells were confirmed by comparing across the neuron specific anti-Hu and nucleus specific DAPI channels. The average number of cFos-ir neurons per section per nucleus was recorded for each animal across the sound exposure groups. All cFos-ir quantification was done via experimenters who were blind to the exposure conditions.
Central Acoustic Circuitry
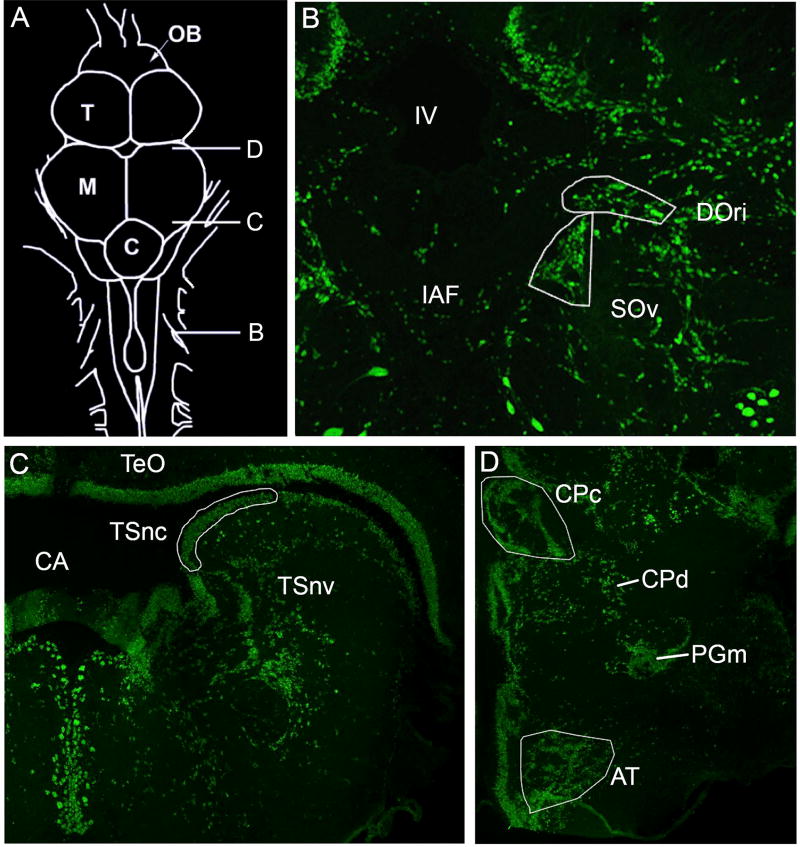
The ascending auditory pathway has been well characterized in midshipman fish (see: Bass et al. 2000). The nuclei and respective subdivisions described here represent a subset of the central acoustic pathway, which were analyzed in the present study. The rostral intermediate division of the descending octaval nucleus (DOri) and ventral division of the secondary octaval nucleus (SOv) (Figure 3B), the two auditory hindbrain nuclei analyzed, receive direct and indirect connections from the saccule, respectively. While there are several unique subdivisions of DO that receive saccular projections (Bass et al. 2000, Sisneros et al. 2002) previous preliminary studies have revealed greatest and most consistent cFos activation within DOri (RM and PMF, personal observations). The caudal extent of DOri was determined by its appearance at the level of the octavolateralis efferent nucleus and sampled rostrally until its disappearance. SOv lies just medial and ventral to DOri throughout most of its extent (slight variations are present due to the angle at which brains were mounted and sectioned). While SO also has a dorsal division, previous preliminary examination revealed less activation in response to auditory stimuli (RM and PMF, personal observation). All landmarks were determined by previously published neuroanatomical and physiological studies and the sampling techniques replicated from Petersen et al. (2013). Serial sections of DOri and SOv were sampled unilaterally on the right side throughout their entire extent. No left/right differences in hemispheric brain activation were predicted because fish were allowed to move freely above the underwater playback speaker. On average 8.67 (±2.2 SD) and 7.81 (±1.7 SD) sections were quantified per subject for DOri and SOv, respectively.
Figure 3.
Auditory neuroanatomy. Transverse sections with anti-Hu (green) stain showing neuronal cell bodies. Traced areas in white represent auditory nuclei in which cFos-ir neurons were quantified. A) Dorsal view drawing of the midshipman brain with the relative positions of B–D indicated B) The hindbrain auditory nuclei, rostral intermediate division (DOri) and ventral division of the secondary octaval nucleus (SOv) are shown. C) The nucleus centralis of the midbrain torus semicircularis (TSnc). D) The compact division of the central posterior nucleus in the auditory thalamus (CPc) and the anterior tuberal nucleus of the hypothalamus (AT). Abbreviations: Cerebellum (C); Cerebral aqueduct (CA); Diffuse division of the central posterior nucleus (CPd); Internal arcuate fiber tract (IAF); Fourth ventricle (IV); Midbrain (M); Olfactory Bulb (OB); Medial nucleus preglomerulosus (PGm); Telencephalon (T); Optic tectum (TeO); Torus semicircularis nucleus ventrolateralis (TSnv).
The nucleus centralis of the torus semicircularis (TSnc) was the auditory midbrain region imaged for cFos-ir activity (Figure 3C). Landmark and image acquisition for TSnc was held constant as with Petersen et al. (2013) and we sampled every fourth section with two adjacent images to encompass the entire nucleus. Similar to the hindbrain photomicrographs, TSnc images were taken only on the right side of the brain for all treatment groups. On average, 6.32 (± 1.3 SD) sections were analyzed in TSnc per animal. The TSnc sends projections to the central posterior nucleus of the thalamus (CP) in the auditory forebrain (Figure 3D). The compact division of CP (CPc) is defined by a wing-shaped nucleus adjacent to the midline. While there is a diffuse region of CP (CPd) just lateral and ventral to CPc, only CPc was imaged for analysis due to the lack of clear boundaries in CPd. Photomicrographs of CPc were taken serially on the right side. In CP, 5.1 (±0.9 SD) sections were analyzed on average per animal. Finally, TS also sends projections to the anterior tuberal nucleus of the ventral hypothalamus (AT). Photomicrographs of AT were also taken serially from the right side of the brain. Within AT, cFos-ir activity was quantified in 3.05 (± 0.6 SD) sections on average.
Statistics
Numbers of cFos-ir cells were averaged within each auditory nucleus and compared between playback condition groups using a one-way ANOVA with an alpha set at 0.05. Post-hoc Tukey tests were used to make pair-wise comparisons across groups for each nucleus. Correlations between water temperature and cFos-ir activity were conducted and the Benjamini-Hochberg procedure was used to correct for multiple comparisons with a false discovery rate of 0.25 (Butler and Maruska 2016, Forlano et al. 2017). All statistics were analyzed using IBM SPSS Statistics 19 and GraphPad Prism 5 software.
RESULTS
Of the 39 female midshipman used in this study, 15 animals were used in each of the ambient noise and conspecific playback conditions while nine animals were used in the heterospecific playback condition. Fish exposed to ambient noise had a standard length (SL) of 16.54 ± 1.57 cm (mean ± SD), body mass (BM) of 59.48 ± 17.61 g and a gonadosomatic index (GSI) of 22.07 ± 10.54. Fish exposed to conspecific advertisement calls had a SL of 16.43 ± 1.45 cm, BM of 61.95 ± 15.28 g and GSI of 26.53 ± 8.26. Fish exposed to heterospecific vocalizations had a SL of 16.50 ± 0.96 cm, BM of 60.57 ± 14.14 g and GSI of 23.77 ± 5.24. There were no differences between groups for any of the morphometric data analyzed (p>0.38 for all cases).
Brain activation of auditory nuclei was examined at the level of the hindbrain (DOri and SOv), midbrain (TS) and diencephalic forebrain (CP and AT) using cFos as a proxy for neural activity. Fluctuations in water temperature during the experiments on different nights ranged from 12–16°C with an average of 14.52 °C (± 1.3 SD). There was no correlation between water temperature at the testing site and cFos-ir activation (p>0.05 for all cases).
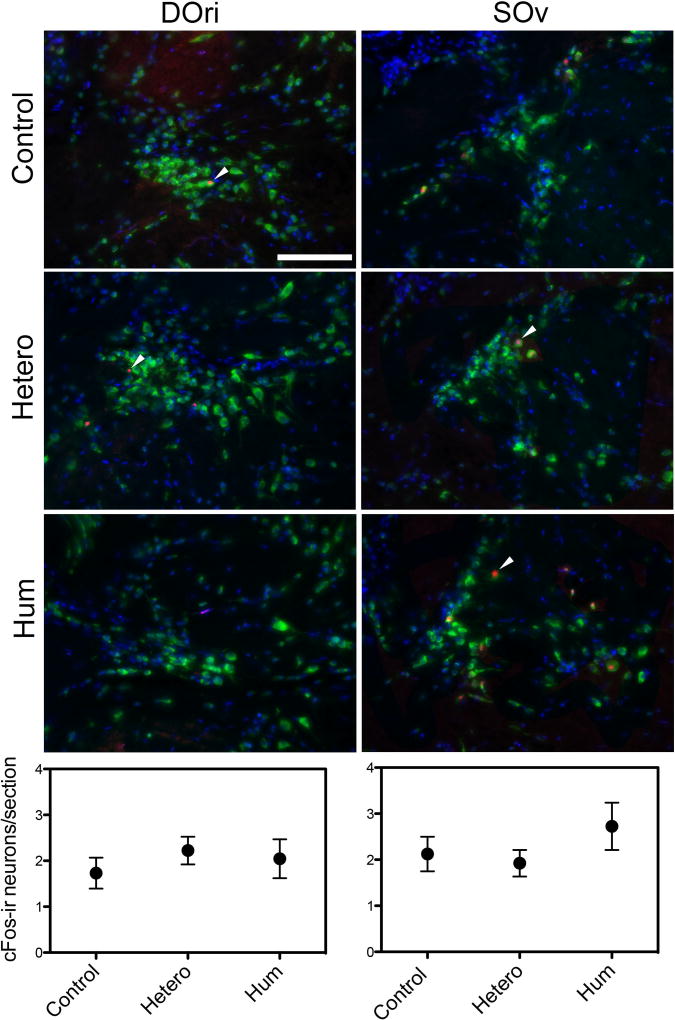
Neural activation of the hindbrain auditory nuclei did not vary with exposure to the tested acoustic stimuli. The average number of cFos-ir cells in the hindbrain DOri did not vary with acoustic playback stimuli: conspecific advertisement calls (mean = 2.05 ± 1.64 SD), heterospecific vocalizations (mean = 2.22 ± 0.85 SD) and ambient noise (mean = 1.73 ± 1.26 SD), (one-way ANOVA, F (2, 34) = 0.37, p=0.69) (Figure 4). There was also no difference in average cFos-ir neurons between groups for SOv: conspecific (mean = 2.73 ± 1.99 SD), heterospecific (mean = 1.92 ± 0.82 SD) vocalizations and ambient noise (mean = 2.12 ± 1.40 SD) (one-way ANOVA, F (2, 34) = 0.84, p=0.44; Figure 4). There were no differences in the number of sections analyzed within each nucleus between any of the experimental groups (p>0.05).
Figure 4.
Representative photomicrographs of the rostral intermediate division of the descending octaval nucleus (DOri) and in the ventral division of the secondary octaval nucleus (SOv). cFos-ir cells (red, arrowheads) were quantified as a marker for neuronal activity. The neuron specific stain anti-Hu (green) and the counterstain DAPI (blue) were used to confirm that the cFos-ir signal was neuron specific. Fish were randomly assigned to exposure of ambient noise (control), white seabass calls (hetero) or conspecific male advertisement calls (hum). Graphs show the mean number of cFos-ir neurons per section across the experimental conditions. Scale bar = 100µm.
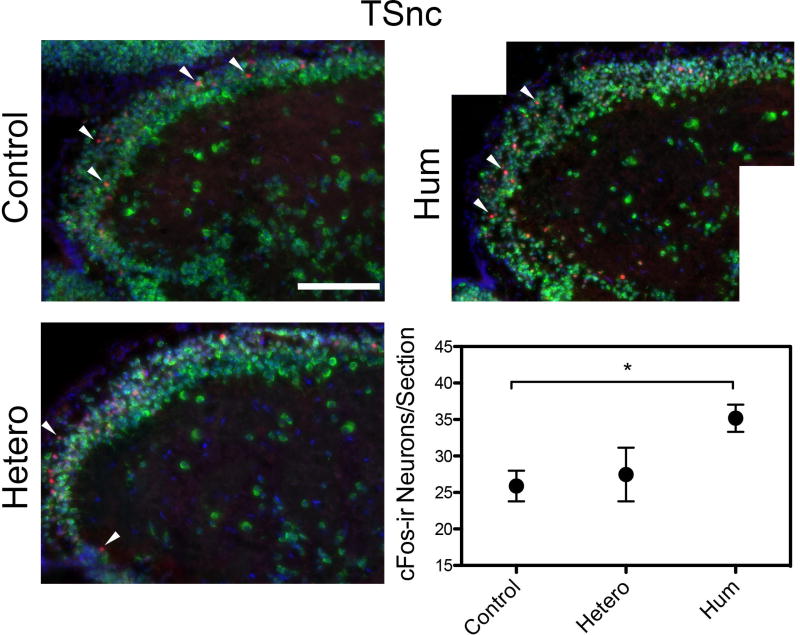
Midbrain neurons were differentially activated by exposure to conspecific advertisement calls versus ambient noise. Specifically, in the midbrain TSnc, there was a significant difference in average cFos number across groups and pairwise comparisons revealed that there were more cFos-ir neurons (p<0.05) with conspecific signal exposure (mean = 35.18 ± 7.21 SD) compared to the ambient noise exposure (mean = 25.89 ± 8.14 SD) (one way ANOVA, post hoc Tukey Test, F (2,34) = 4.88, p=0.013); however, no differences were found between the ambient noise and heterospecific signal exposure groups (p=0.90; mean = 27.47 ± 11.02 SD) or between the conspecific and heterospecific exposure groups (p=0.10) (Figure 5).
Figure 5.
Representative photomicrographs of the periventricular nucleus centralis of the torus semicircularis (TSnc) in the midbrain. Images were taken at 20× and are representative of the three experimental exposure conditions: ambient noise (control), white seabass calls (hetero) and conspecific advertisement calls (hum). Anti-Hu (green) and DAPI (blue) were used to visualize all neurons while only cFos-ir neurons (red, arrowheads) were quantified. Data are represented in the graph as the mean number of cFos-ir neurons per section in each of the experimental groups. *p<0.05, scale bar = 100µm.
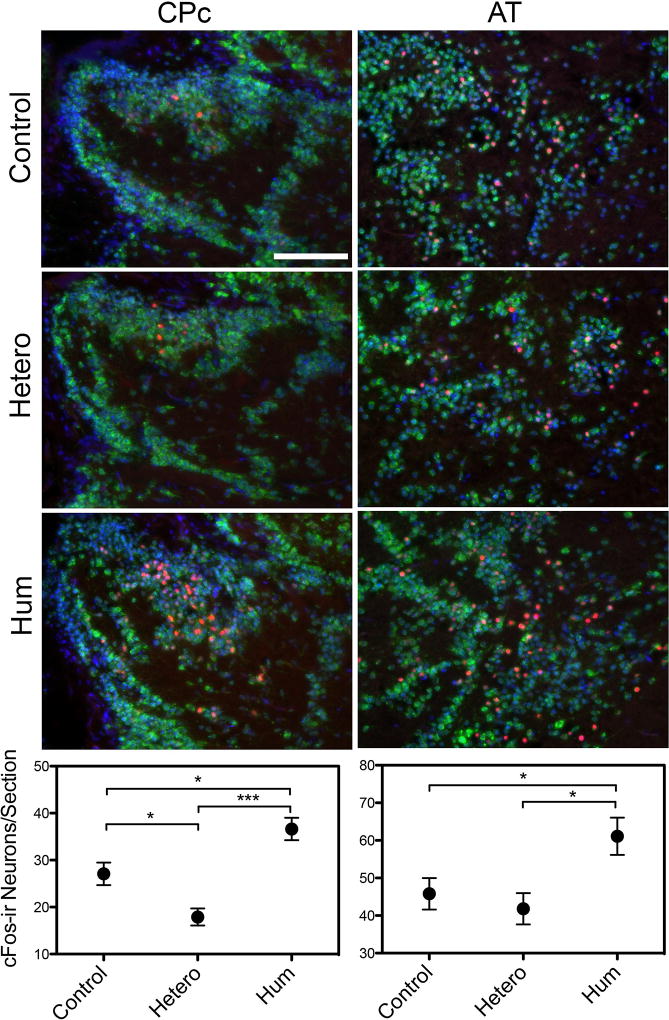
Forebrain auditory nuclei were differentially activated by exposure to the auditory stimuli presented. There were significant differences in the average number of cFos-ir neurons between playback conditions within CPc and pairwise comparisons revealed there were significantly more cFos-ir neurons in the conspecific signal exposure group (mean = 36.63 ± 8.90 SD) group compared to both the ambient noise (mean = 27.08 ± 9.29 SD, p<0.05) and heterospecific signal (mean = 17.89 ± 5.47 SD, p<0.001) exposure groups and the ambient noise group had significantly more cFos-ir neurons (p<0.05) than the heterospecific signal exposure group (one way ANOVA, post hoc Tukey Test, F (2,35) = 13.90, p<0.001) (Figure 6). In AT, there was a significant difference in average cFos-ir neurons between playback conditions and pairwise comparisons revealed that females exposed to the conspecific advertisement call (mean = 61.09 ± 19.23 SD) had a significantly higher average number of cFos-ir neurons compared to both ambient noise (mean = 45.80 ± 16.21 SD, p<0.05) and heterospecific signal exposure (mean = 41.80 ± 12.50 SD, p<0.05) groups (one way ANOVA, post hoc Tukey Test, F (2,36) = 4.80, p=0.014). There was no difference between the average number of cFos-ir cells between the ambient noise and heterospecific signal exposure groups (p=0.84) (Figure 6).
Figure 6.
Representative photomicrographs of the forebrain central posterior nucleus of the thalamus (CP) and the anterior tuberal nucleus of the ventral hypothalamus (AT). Neurons are identified by the presence of both the neuron specific anti-Hu (green) and the nuclear specific counterstain DAPI (blue) with red indicating cFos-ir activation. Fish were assigned one of three experimental exposure conditions: ambient noise (control), white seabass calls (hetero) or conspecific advertisement calls (hum). Graphical data are presented as the mean number of cFos-ir neurons per section for each of the experimental conditions. *p<0.05, ***p<0.001, scale bar = 100µm.
DISCUSSION
The goal of this study was to identify neural circuits involved in the recognition and discrimination of conspecific social acoustic signals in female midshipman. Using the immediate early gene (IEG) protein product cFos as a marker or proxy for neural activation, we mapped cFos activity of auditory neurons in specific components of the ascending auditory pathway of females held in a semi-naturalistic playback arena while exposed to either conspecific or heterospecific vocalizations or ambient noise. The use of IEG responses, including cFos, has become a powerful tool for mapping neuronal activation patterns as they can be used to assess the entire brain and the IEG response begins within minutes (Luckman et al. 1994, Clayton 2000, Kovacs 2008). Consistent with our hypothesis that exposure to complex acoustic signals would yield differential neural activation across auditory nuclei, our results revealed greater activation in response to conspecific vocalizations compared with ambient noise at the level of the midbrain and greater activation in response to conspecific vocalizations compared with both heterospecific calls and ambient noise in the forebrain. Our results suggest higher order processing is likely necessary for the processing and discrimination of complex social signals in teleosts, consistent with studies in tetrapods (Syka et al. 1997, Klug et al. 2002, Suta et al. 2003, Hoke et al. 2004).
cFos-ir Response in the Hindbrain
The rostral intermediate division of the descending octaval nucleus (DOri) and the ventral division of the secondary octaval nucleus (SOv) are auditory areas in the hindbrain that receive direct and indirect innervation from the auditory end organs via the VIIIth nerve, respectively (Bass et al. 2000, Sisneros et al. 2002). Neuroanatomical evidence from Bass et al. (2000) suggests a connection between DOri and SOV, and that a DOri-SOv complex is the major hindbrain site for auditory and vocal integration. Neurons within DOri and SOv revealed similar activation levels across all three experimental conditions. While the average number of cFos-ir neurons in DOri and SOv were relatively low, the percentage of neurons activated may be similar to other higher order brain areas analyzed given the intrinsically low number of neurons within these small nuclei. These results are consistent with previous findings in the oyster toadfish, Opsanus tau, which revealed that auditory hindbrain neurons in DO are typically “silent” or have low spontaneous activity (1–10 spikes/sec) (Edds-Walton and Fay 2008). Furthermore, hindbrain neurons in O. tau are broadly tuned and respond to a wide array of acoustic stimuli, including general acoustic stimuli and vocal signals alike (Edds-Walton 2016) which are consistent with results from the clawed frog, Xenopus laevis, where first-order hindbrain auditory nuclei have been shown to respond broadly to auditory stimuli (Elliott et al. 2007) which may help to explain the similarities in cFos-ir activation across our experimental conditions. It is also important to note that reduced cFos activity in DOri and SOv does not exclude the possibility of higher activation of these circuits than what is revealed with cFos-ir, which may be due to sub-threshold neuronal activity levels for cFos induction or activation of inhibitory inputs on auditory neurons in this hindbrain circuit. Other activity measures including the use of different IEGs (i.e. egr-1 or arc) or electrophysiology may also yield different results (Kawashima et al. 2014).
Midshipman fish receive auditory input from their main end organ of hearing, the saccule, which has been shown to undergo seasonal changes in morphology and auditory sensitivity related to reproductive state and circulating levels of steroid hormones (Sisneros and Bass 2003, Sisneros et al. 2004, Sisneros 2009, Rohmann and Bass 2011, Coffin et al. 2012, Rohmann et al. 2013, Forlano et al. 2015b, Forlano et al. 2016). Specifically, reproductive females, like those used in this study, have increased hearing sensitivity across their entire frequency range to better detect and localize advertising male midshipman fish. Recent studies have also revealed that females have elongated horn-like structures on the rostral ends of their swim bladders, which come into close proximity to the saccule and lagena, which are thought to increase sensitivity to sound pressure and high frequency signals (Mohr et al. 2017). Together these adaptations in female midshipman may lead to heightened auditory sensitivity across all frequencies, especially during the reproductive season. Abiotic noise factors along with biotic sounds from other species can cause the intertidal zone to be a very loud and noisy environment (up to 120dB re: 1µPa, personal observation) and such environmental noise levels are well within the hearing range of midshipman fish and are likely a constant source of stimulation to the midshipman auditory system.
Furthermore, DOri is also an important part of the vocal-acoustic circuitry in male midshipman fish, as it has direct descending connections to the prepacemaker nucleus of the vocal pattern generator (Bass et al. 1994, Bass et al. 2000, Bass and Ladich 2008). Petersen et al. (2013) found greater cFos-ir activity in DOri in male midshipman fish in response to conspecific playback versus ambient noise, while our results in females revealed no differences across auditory exposure conditions. Female midshipman fish are incapable of producing the long duration, reproductive advertisement call and the sex-specific differences in cFos-ir activity may be related to mate call production.
cFos-ir Response in the midbrain and forebrain
The TSnc receives direct innervation from the auditory hindbrain and is the primary auditory center within the midbrain of midshipman and other bony fishes (McCormick 1999, Bass et al. 2000, Bass et al., 2005). Our results revealed greater cFos-ir activation within TSnc in response to conspecific vocalizations compared to ambient noise but no difference when compared to the heterospecific playback condition. The TS is the homologue to the mammalian inferior colliculus, an area that has been implicated in selectivity to species-specific auditory signals (Feng and Lin 1991, Crawford 1993, Crawford 1997, Syka et al. 1997, Klug et al. 2002, Suta et al. 2003, Hoke et al. 2004). In mammals, subpopulations of auditory neurons within the inferior colliculus have been theorized to be selectively responsive to a wide array of biologically relevant acoustic stimuli including sounds made by both conspecifics and heterospecifics (Casseday and Covey 1996). Our findings are consistent with the hypotheses of Casseday and Covey (1996) along with results of Petersen et al. (2013), which showed greater cFos-ir activation in male midshipman TSnc in response to conspecific advertisement calls compared to ambient noise in an outdoor laboratory tank. Similarly, our findings also parallel the egr-1 activity quantified in response to auditory playback of conspecific vocalizations in the Tungara frog that showed greater activation to variations of conspecific advertisement calls (whines, chucks and whine-chucks) in TS compared to silence, but also greater activation to conspecific versus heterospecific vocalizations which were not observed until the level of the forebrain in midshipman (Hoke et al. 2004). However, unlike the anuran TS which has several distinct toral subdivisions with unique cytoarchitecture and connectivity related to processing of auditory information, no such subdivisions of the TSnc have been elucidated in the plainfin midshipman (Potter 1965, Wilczynski 1981, Bass et al. 2000, Endepols and Walkowiak 2001, Hoke et al. 2004).
The central posterior nucleus in the auditory thalamus (CP) is a major area of higher order processing of auditory information in midshipman and other teleosts (Bass et al., 2000, McCormick, 2011) and receives projections from TS) (Bass et al. 2000, Goodson and Bass 2002, McCormick 1999). While there are other ascending targets of TSnc, CP has been well characterized anatomically, physiologically and neurochemically (Bass et al., 2000; Goodson and Bass, 2002; Lu and Fay 1995, Forlano et al. 2015a). Our results from the compact division of CP (CPc), a CP region more easily discerned than its diffuse division, revealed greatest cFos-ir activation in response to conspecific vocalizations compared to both the ambient noise and heterospecific playback conditions. Interestingly, there was less cFos activity in the heterospecific condition compared to the ambient noise condition. Previous single unit physiological studies in goldfish, Carassius auratus, have suggested that CP may be involved in the processing of complex, wide bandwidth ecologically-relevant stimuli (Lu and Fay 1995) and along with TS may be a source for the discrimination of conspecific acoustic signals. Earlier work in anurans has also shown characteristics of species-specific selectivity of neurons in the auditory thalamus (Fuzessey and Feng 1983, Hall and Feng 1987, Mudry and Capranica 1987). However, as has been suggested more recently in both teleost fish and anurans, it is likely that CP and other forebrain areas are not solely auditory regions but highly integrative, receiving multisensory (auditory and visual) input with implications for sensorimotor responses (Northcutt 2006, Hoke et al. 2007, Wilczynski and Endepols 2007). Results from Petersen et al. (2013) showed that male midshipman exhibited the greatest differential cFos activity in conspecific vocalizations vs. ambient sounds in CP compared to TSnc, DOri and SOv.. While the quantitative response properties in CP are similar in both sexes to conspecific advertisement calls, it is possible that the sensorimotor and physiological responses vary greatly between the sexes as they relate to aggression and reproduction. Reproductive female midshipman are shown to exhibit increased density of tyrosine hydroxylase immunoreactive (TH-ir) fibers in CP which likely originates from projections of the dopaminergic periventricular posterior tuberculum (TPp) (Forlano et al. 2014, Forlano et al. 2015a, Forlano et al. 2016). TPp also sends TH-ir projections to the saccule, which could act to modulate sensitivity of the inner ear in response to conspecific vocalizations during the breeding season (Forlano et al. 2014, Forlano et al. 2015a, Forlano and Sisneros 2016, Perelmuter and Forlano 2017). Catecholamines (CA), including dopamine, are well known neuromodulators that can affect attention, motivation and arousal and enhance the valence of conspecific vocal signals (Berridge 2008, Hurley et al 2004, Riters 2012, Caras 2013). Future physiological, behavioral and pharmacological studies will be needed to further understand the role of CP In the processing of auditory information and how catecholamines may alter central processing and sensorimotor responses to the male advertisement call (see Forlano et al. 2017).
The CP shares reciprocal connections with the anterior tuberal nucleus (AT) of the hypothalamus, which also receives ascending projections from TSnc (McCormick 1999, Bass et al. 2000, Goodson and Bass 2002). Other dienchephalic connections with CP are present including the lateral preglomerular nucleus, however there is more known about the role of AT as it relates to vocal-acoustic circuitry than other forebrain regions (Goodson 2005, Petersen et al. 2013, Forlano et al. 2014). Similar to CP, our results revealed greater cFos-ir activation in AT in response to conspecific compared with both ambient noise and heterospecific acoustic playbacks. Our results parallel that of Petersen et al. (2013) in which they showed higher levels of cFos in AT in response to conspecific vocalizations compared to ambient noise. AT is not only a part of the ascending auditory system but also part of the descending vocal-motor circuitry (Bass et al. 2000, Goodson and Bass 2000a–b, Goodson and Bass 2002) and the social behavior network (SBN) (Newman 1999, Goodson 2005). The SBN is a group of reciprocally connected nuclei within the midbrain and basal forebrain that are involved in the processing, assessment and action of various social behaviors (Newman 1999, Goodson 2005, Goodson and Kabelik 2009). The AT is thought to be homologous, in part, to the ventral medial hypothalamus (VMH) in mammals and birds (Newman 1999, Goodson 2005, O’Connell and Hofmann 2011). Lesion and stimulation studies have revealed that VMH is an important area involved in sexual behavior and female receptivity in rodents and birds (Malsbury et al. 1977, Mathews and Edwards 1977, Pfaff and Sakuma 1979, Meddle et al. 1999, O’Connell and Hofmann 2011, Pawlisch et al. 2012). Future studies will be needed to further understand the role of AT and other forebrain acoustic and multi-sensory brain areas in social behavior and central auditory processing in fishes.
Acknowledgments
We would like to thank Scott Aalbers for providing us with the white seabass audio recordings. We also wish to thank Zack Ghahramani, Jon Perelmuter, Spencer Kim, Miky Timothy and the rest of the Forlano lab for assistance with immunohistochemistry procedures. We thank Miky Timothy for custom macro development in ImageJ for image analyses. We thank Nick Lozier and Ruiyu Zeng for help with the cFos analysis, Brooke Vetter for helping create the spectrograms and Melissa Reilly for editing help. This work was supported by the National Science Foundation (NSF) IOS-1456700 (to J.A.S.) and IOS-1456743 (to P.M.F.) and National Institutes of Health SC2DA034996 (to P.M.F.).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Aalbers SA, Drawbridge MA. White seabass spawning behavior and sound production. Trans Am Fish Soc. 2008;137:542–550. [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bass AH, Rose GJ, Pritz MB. Auditory midbrain of fish, amphibians and reptiles: Models systems for understanding auditory function. In: Winer JA, Schreiner CE, editors. The inferior colliculus. New York: Springer; 2005. pp. 459–492. [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Ladich F. Vocal-acoustic communication: From neurons to behavior. In: Webb JF, Fay RR, Popper AN, editors. Fish Bioacoustics. New York, NY: Springer; 2008. pp. 253–278. [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative spawning tactics and acoustic signals in the plainfin midshipman fish, Porichthys notatus Girard (teleosti, batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Butler JM, Maruska KP. The mechanosensory lateral line system mediates activation of socially relevant brain regions during territorial interactions. Front Behav Neurosci. 2016;10:93. doi: 10.3389/fnbeh.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML. Estrogenic modulation of auditory processing: A vertebrate comparison. Front Neuroendocrinol. 2013;34:285–299. doi: 10.1016/j.yfrne.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Covey E. A neuroethological theory of the operation of the inferior colliculus. Brain Behav Evol. 1996;47:323–336. doi: 10.1159/000113249. [DOI] [PubMed] [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Crawford JD. Central auditory neurophysiology of a sound-producing fish: The mesencephalon of Pollimyrus isidori (Mormyridae) J Comp Physiol A. 1993;172:139–152. doi: 10.1007/BF00189392. [DOI] [PubMed] [Google Scholar]
- Crawford JD. Feature-detecting auditory neurons in the brain of a sound producing fish. J Comp Physiol A. 1997;180:439–450. doi: 10.1007/s003590050061. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Mohr RA, Sisneros JA. Saccular-specific hair cell addition correlates with reproductive-state dependent changes in the auditory saccular sensitivity of a vocal fish. J Neurosci. 2012;32:1366–1376. doi: 10.1523/JNEUROSCI.4928-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR. Directional selectivity and frequency tuning of midbrain cells in the oyster toadfish, Opsanus tau. J Comp Physiol A. 2003;189:527–543. doi: 10.1007/s00359-003-0428-9. [DOI] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR. Sharpening of directional responses along the auditory pathway of the oyster toadfish, Opsanus tau. J Comp Physiol A. 2005;19:1079–1086. doi: 10.1007/s00359-005-0051-z. [DOI] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR. Directional and frequency response characteristics in the descending octaval nucleus of the toadfish (Opsanus tau) J Comp Physiol A. 2008;194:1013–1029. doi: 10.1007/s00359-008-0373-8. [DOI] [PubMed] [Google Scholar]
- Edds-Walton PL. What the Toadfish Ear Tells the Toadfish Brain About Sound. In: Sisneros JA, editor. Fish Hearing and Bioacoustics: An anthology in honor of Arthur N. Popper and Richard R. Fay. Advances in Experimental Medicine and Biology. Springer Science + Business Media; New York: 2016. pp. 197–226. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J Comp Physiol A. 2007;193:1243–1257. doi: 10.1007/s00359-007-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endepols H, Walkowiak W. Integration of ascending and descending inputs in the auditory midbrain of anurans. J Comp Physiol [A] 2001;186:1119–1133. doi: 10.1007/s003590000159. [DOI] [PubMed] [Google Scholar]
- Feng AS, Lin W. Differential innervation patterns of three divisions of frog auditory midbrain (torus semicircularis) J Comp Neurol. 1991;306:613–630. doi: 10.1002/cne.903060407. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Kim SD, Krzyminska Z, Sisneros JA. Catecholaminergic connectivity to the inner ear, central auditory and vocal motor circuitry in the plainfin midshipman fish, Porichthys notatus. J Comp Neurol. 2014;522:2887–2927. doi: 10.1002/cne.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Ghahramani ZN, Monestime C, Kurochkin P, Chernenko A, Milkis D. Catecholaminergic innervation of central and peripheral auditory circuitry varies with reproductive state in female midshipman fish, Porichthys notatus. PLoS ONE. 2015a;10(4):e0121914. doi: 10.1371/journal.pone.0121914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Sisneros JA, Rohmann KN, Bass AH. Neuroendocrine control of seasonal plasticity in the auditory and vocal systems of fish. Front Neuroendocrinol. 2015b;37:129–145. doi: 10.1016/j.yfrne.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Maruska KM, Sisneros JA, Bass AH. Hormone-dependent plasticity of auditory systems in fishes. In: Bass AH, Sisneros JA, Fay RR, Popper AN, editors. Hearing and Hormones. Springer Handbook of Auditory Research. Vol. 57. Springer Science+Business Media, LLC; New York: 2016. [Google Scholar]
- Forlano PM, Licorish RR, Ghahramani ZN, Timothy M, Ferrari M, Palmer WC, Sisneros JA. Attention and motivated response to simulated male advertisement call activates forebrain dopaminergic and social decision-making network nuclei in female midshipman fish. Integr Comp Biol. 2017;57:820–834. doi: 10.1093/icb/icx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery ZM, Feng AS. Mating call selectivity in the thalamus and midbrain of the leopard frog (Rana p. pipiens): single and multiunit responses. J Comp Physiol. 1983;150:333–344. [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Rhythmic midbrain-evoked vocalization is inhibited by vasoactive intestinal polypeptide in the teleost Porichthys notatus. Brain Res. 2000b;865:107–111. doi: 10.1016/s0006-8993(00)02232-0. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009;30:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC, Feng AS. Evidence for parallel processing in the frog’s auditory thalamus. J Comp Neurol. 1987;258:407–419. doi: 10.1002/cne.902580309. [DOI] [PubMed] [Google Scholar]
- Hoke KL, Burmeister SS, Fernald RD, Stanley Rand A, Ryan MJ, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J Neurosci. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke KL, Ryan MJ, Wilczynski W. Sexually dimorphic sensory gating drives behavioral differences in tungara frogs. J Exp Biol. 2010;213:3463–3472. doi: 10.1242/jeb.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding within mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: Activity-dependent promoters have come of age. Front Neural Circuits. 2014;8:1–9. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyong. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Lu Z, Fay RR. Acoustic response properties of single neurons in the central posterior nucleus of the thalamus of the goldfish, Carassius auratus. J Comp Physiol A. 1995;176:747–760. doi: 10.1007/BF00192623. [DOI] [PubMed] [Google Scholar]
- Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsbury CW, Kow LM, Pfaff DW. Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol Behav. 1977;19:223–237. doi: 10.1016/0031-9384(77)90331-6. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol Behav. 1977;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- McCormick CA. Anatomy of the central auditory pathways of fish and amphibians. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York, NY: Springer; 1999. pp. 155–217. [Google Scholar]
- McCormick CA. Auditory/lateral line CNS: Anatomy. In: Farrell AP, editor. Encyclopedia of fish physiology: From genome to environment. San Diego (CA): Academic Press; 2011. pp. 283–291. [Google Scholar]
- McKibben JR, Bass AH. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J Acoust Soc Am. 1998;104:3520–3533. doi: 10.1121/1.423938. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Foidart A, Wingfield JC, Ramenofskyand M, Balthazart J. Effects of sexual interactions with a male on fos-like immunoreactivity in the female quail brain. J Neuroendocrinol. 1999;11:771–784. doi: 10.1046/j.1365-2826.1999.00384.x. [DOI] [PubMed] [Google Scholar]
- Mohr RA, Whitchurch EA, Anderson RD, Forlano PM, Fay RR, Ketten DR, Cox TC, Sisneros JA. Intra- and Intersexual swim bladder dimorphisms in the plainfin midshipman fish (Porichthys notatus): Implications of swim bladder proximity to the inner ear for sound pressure detection. J Morph. 2017 doi: 10.1002/jmor.20724. [DOI] [PubMed] [Google Scholar]
- Mudry KM, Capranica RR. Correlation between auditory evoked responses in the thalamus and species-specific call characteristics. I. Rana catesbeiana (Anura: Ranidae) J Comp Physiol A. 1987;160:477–489. doi: 10.1007/BF00615081. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Pawlisch BA, Kelm-Nelson CA, Stevenson SA, Riters LV. Behavioral indices of breeding readiness in female European starlings correlate with immunolabeling for catecholamine markers in brain areas involved in sexual motivation. Gen Comp Endocrinol. 2012;179:359–368. doi: 10.1016/j.ygcen.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Petersen CL, Timothy M, Kim DS, Bhandiwad AA, Mohr RA, Sisneros JA, Forlano PM. Exposure to advertisement calls of reproductive competitors activates vocal-acoustic and catecholaminergic neurons in the plainfin midshipman fish, Porichthys notatus. PLoS ONE. 2013:e70474. doi: 10.1371/journal.pone.0070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Potter HD. Mesencephalic auditory region of the bullfrog. J Neurophysiol. 1965;28:1132–1154. doi: 10.1152/jn.1965.28.6.1132. [DOI] [PubMed] [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J Exp Biol. 2011;214:1931–1942. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Fergus D, Bass AH. Plasticity in ion channel expression underlies variation in hearing during reproductive cycles. Curr Biol. 2013;23:678–683. doi: 10.1016/j.cub.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Marchaterre MA, Bass AH. Otolithic endorgan projections of the inner ear in a vocal fish. Bioacoustics. 2002;12:137–139. [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J Neurophysiol. 2009;102:1121–1131. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- Suta D, Kvasnak E, Popelar J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J Neurophysiol. 2003;90:3794–3808. doi: 10.1152/jn.01175.2002. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelář J, Kvašñák E, Šuta J, Jilek M. Processing of Species-Specific Vocalizations in the Inferior Colliculus and Medial Geniculate Body of the Guinea Pig. In: Syka J, editor. Acoustical Signal Processing in the Central Auditory System. Springer; Boston, MA: 1997. pp. 431–441. [Google Scholar]
- Wilczynski W. Afferents to midbrain auditory center in the bullfrog Rana catesbeiana. J Comp Neurol. 1981;198:421–433. doi: 10.1002/cne.901980304. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Endepols H. Central auditory pathways in anuran amphibians: The anatomical basis of hearing and sound communication. In: Popper AN, Feng AS, Narins PN, editors. Hearing and Sound Communication in Amphibians. Berlin, Germany: Springer; 2007. pp. 221–249. [Google Scholar]