Abstract
Osteoarthritis (OA) is a disease of the synovial joint marked by chronic, low-grade inflammation leading to cartilage destruction. Regenerative medicine strategies for mitigating OA progression and/or promoting cartilage regeneration must be assessed using models that mimic the hallmarks of OA. More specifically, these models should maintain synovial macrophage phenotype in their native micro-environment. Herein, an in vitro coculture model of patient-matched human OA cartilage and synovium was assessed for viability, macrophage phenotype, and progressive cartilage destruction in the presence of an inflammatory milieu. Additionally, the influence of synovial macrophages and their polarization within the model was defined using depletion studies. Finally, the model was used to compare the ability of human amniotic stem cells (hAMSCs) and human adipose stem cells (hADSCs) to mitigate OA progression. OA cocultures demonstrated progressive and significant reductions in chondrocyte viability and cartilage glycosaminoglycan content within a proinflammatory environment. Selective depletion of synovial macrophages resulted in significant decreases in M1:M2 percentage ratio yielding significant reductions in concentrations of interleukin-1 beta, matrix metalloproteinase-13 and attenuation of cartilage damage. Finally, hAMSCs were found to be more chondroprotective versus hADSCs as indicated by significantly improved OA chondrocyte viability (89.8 ± 2.4% vs. 58.4 ± 2.4%) and cartilage glycosaminoglycan content (499.0 ± 101.9 μg/mg dry weight vs. 155.0 ± 26.3 μg/mg dry weight) and were more effective at shifting OA synovial macrophage M1:M2 ratio (1.3:1 vs. 5:1), respectively. Taken together, the coculture model mimics salient features of OA, including macrophage-mediated cartilage destruction that was effectively abrogated by hAMSCs but not hADSCs.
Keywords: amniotic stem cells, coculture, macrophages, osteoarthritis, regeneration, synovium
1 | INTRODUCTION
It is estimated that more than 30.8 million Americans and 40 million Europeans suffer from osteoarthritis (OA; Cisternas et al., 2016). Commonly affecting the synovial joints of the knee and hip, this disease often necessitates total joint arthroplasty and imparts significant socio-economic burden (Yelin, Weinstein, & King, 2016). OA is a slowly progressive, self-perpetuating disease of the synovial joint mediated by chronic, low-grade inflammation that prominently affects the articular cartilage as well as the synovium, ligaments, and subchondral bone (Liu-Bryan & Terkeltaub, 2015). Events that initiate joint tissue damage can include infection, mechanical overload, overuse, trauma, and/or genetic predisposition (Wojdasiewicz, Poniatowski, & Szukiewicz, 2014). Subsequently, damaged cartilage extracellular matrix (ECM) fragments bind to chondrocytes, synovial fibroblasts, and macrophages stimulating enhanced production of proinflammatory cytokines and proteases resulting in a vicious feed-forward cycle of joint tissue destruction and inflammation (Goldring & Marcu, 2009). Although our understanding of the many factors that mediate OA is improving, continued study is warranted.
A more recent area of interest is defining the role of synovial macrophages in OA progression (Bondeson, Blom, Wainwright, Hughes, & Caterson, 2010; Goldring & Marcu, 2009). Macrophages in general can be classified by their effector phenotype as existing along a dynamically shifting continuum spanning from “proinflammatory” (M1) to “proregenerative” (M2; Murray et al., 2014). Effector functions of these cells are carried out primarily via the production of a complex milieu of soluble mediators that influence local cells. Recent studies have sought to more clearly define the role of these cells by incorporating macrophages within in vitro cocultures to study their crosstalk with chondrocytes and to define the influence of macrophages on OA progression (Bauer et al., 2016; Samavedi, Diaz-Rodriguez, Erndt-Marino, & Hahn, 2017). These studies generally demonstrate that macrophage-mediated inflammation results in the dysfunction of chondrocytes at the transcriptional level. However, characterization of their effects at the joint tissue-level is limited. Several other limitations and gaps in knowledge also exist. For example, previous studies fail to incorporate macrophages within the natural three-dimensional tissue environment of the synovium. This is critical as macrophage phenotype and effector function has been shown to be largely influenced by the local tissue micro-environment (Okabe & Medzhitov, 2014). Additionally, in vitro OA models commonly incorporate M1 macrophages; however, to date, no one has quantitatively evaluated the number of M1 and M2 macrophages in human OA synovium nor have they incorporated both phenotypes within in vitro models. Taken together, addressing these limitations will result in the development of a model system that will more accurately represent the pathogenesis of OA and can ultimately be used to study the specific role of macrophages in OA while serving as a platform to evaluate regenerative medicine strategies targeting advanced OA.
Recent evidence has shown that stem cells have the ability to modulate inflammation in part by shifting macrophages towards an M2 phenotype (Abumaree et al., 2013); however, reports evaluating the ability of stem cells to shift human synovial macrophage phenotype in the context of the OA micro-environment do not exist. Additionally, although amniotic mesenchymal stem cells (hAMSCs) have demonstrated promise as an alternative stem cell source for orthopaedic regenerative medicine applications (Keeley, Topoluk, & Mercuri, 2014), few have evaluated their efficacy in OA (Raines et al., 2017), and moreover, none have compared their efficacy with hADSCs.
Thus, the primary goal of the studies herein was to establish an in vitro human tissue explant coculture model of OA consisting of patient-matched cartilage and macrophage-containing synovial biopsies in order to (a) demonstrate the model’s ability to recapitulate the hallmarks of OA including progressive tissue-level destruction in the presence of a complex inflammatory milieu, (b) define synovial macrophage phenotype and assess their direct contribution to OA progression, and (c) evaluate and compare the efficacy of hAMSCs and hADSCs to mitigate OA progression following direct- and indirect-contact coculture with OA cartilage.
2 | MATERIALS AND METHODS
2.1 | Human amniotic mesenchymal stem cell isolation and adipose mesenchymal stem cell procurement
Human placentas were obtained from consenting patients immediately following delivery via elective caesarean sections of full-term babies under an Institutional Review Board approved protocol (Greenville Health System: PRO00031185). hAMSCs were isolated from placentas per methods adapted from Barbati et al. Briefly, amnion was rinsed in sterile saline prior to two sequential digestions in 0.25% trypsin for 30 min at 37 °C with agitation (150 revolutions per minute) to completely liberate only the amniotic epithelial cells followed by digestion in two sequential changes of collagenase [2 mg/mL collagenase (249 U/mg)] for 30 min at 37 °C with agitation to subsequently liberate only the amniotic mesenchymal stem cells (hAMSCs). All digests were filtered through a 100-μm cell strainer prior to cell counting. hAMSCs were subsequently frozen in liquid nitrogen prior to thawing, pooling, and expansion to Passage 2 prior to experimentation. hADSCs were purchased from Invitrogen and expanded to Passage 2 per manufacturer’s instructions prior to experimentation.
2.2 | Human cartilage and synovium tissue collection and preparation
Human cartilage and synovium being discarded as medical waste were obtained from 15 consenting patients with OA (Kellgren-Lawrence Grade 4) immediately following total knee arthroplasty under an Institutional Review Board approved protocol (Greenville Health System: PRO00031185). Explants were transferred to the lab in sterile cell culture media consisting of Dulbecco’s modified eagle’s medium supplemented with 1× insulin transferrin selenium, 2% fetal bovine serum, 50-nM Ascorbate-2-phosphate, and 1% antibiotic/antimitotic. Tissue biopsies (Figure 1a,b) were obtained with 6-mm sterile punches. Cartilage biopsy depth was ~2–3 mm. Synovium biopsies were cleaned of extraneous fat prior to commencement of coculture. One biopsy of patient-matched OA cartilage and synovium were acclimated and cocultured in standard culture conditions (5% CO2; 37 °C) using indirect contact for 3 days. Following acclimation, cell culture media and biopsies were collected for “Day 0” samples.
FIGURE 1.
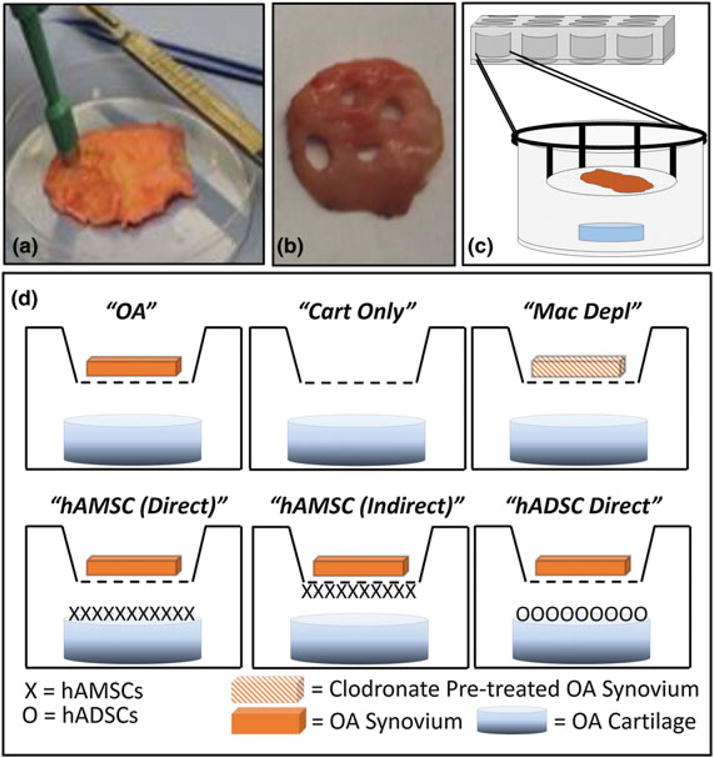
Human osteoarthritic cartilage and synovium explant coculture sample harvest and study group layout. Patient-matched 6-mm-diameter biopsies of (a) articular cartilage from the distal femoral condyle and (b) synovial membrane of patients undergoing total knee arthroplasty were (c) cocultured together using indirect contact in trans-well plates; cartilage was placed in the wells and synovial tissue was placed above the cartilage in porous trans-well inserts. Study groups for analysis included (d) cocultures of cartilage and synovium biopsies together [osteoarthritis (OA); n = 15], cartilage biopsies only (cart only; n = 5), synovium biopsies only (syn only = 5—not shown), cocultures containing cartilage and macrophage depleted synovium (Mac Depl; n = 5), cocultures treated with human amniotic stem cells (hAMSCs) or human adipose stem cells (hADSC) seeded directly on top of the OA cartilage (hAMSC and hADSC direct, n = 5, respectively) or by seeding hAMSCs on the underside of the trans-well inserts (hAMSC indirect) [Colour figure can be viewed at wileyonlinelibrary.com]
2.3 | Human OA explant coculture and stem cell treatments
Following acclimation, cartilage biopsies were placed in the bottom of 12-well trans-well plates (1 biopsy per well) and submerged in 1.5 ml of fresh culture media. A sterile rubber gasket was placed around the well prior to placement of porous (0.3 μm) well inserts containing patient-matched synovium biopsies (1 biopsy per well) submerged in 0.5 ml of fresh culture media (Figure 1c). Media was changed every 3 days for the duration of the studies (15 days). Additional culture control groups included cartilage (n = 5) and synovium (n = 5) only cultures (Figure 1d). For stem cell treated groups (n = 5 per study group per time-point), 1 × 105 hAMSCs or hADSCs (Passage 2) were seeded dropwise onto OA cartilage at Day 0 for the “direct” contact group (Figure 1d). For the “indirect” contact group, 1 × 105 hAMSCs were seeded drop-wise onto the underside of porous trans-well inserts and allowed to attached for 2 hr prior to placement of synovium biopsies in the overlying well and introduction into coculture with cartilage biopsies (Figure 1d). After 15 days of coculture, each cartilage biopsy was divided by segmenting the circular cross-section into three pieces, a left and right hemisphere of equal size interposed by a thin rectangular section. Samples were prepared for analysis as described below.
2.4 | Depletion of OA synovial macrophages
Synovial biopsies (n = 5) were placed in the wells of a 12-well plate and submerged in 1.5-ml medium. To deplete macrophages each synovial biopsy was treated with 0.2-mL Clophosome®-A (liposome encapsulated clodronate) for 24 hr. Biopsies were subsequently washed 3× in medium prior to coculture initiation with patient-matched OA cartilage (Figure 1d) as previously described.
2.5 | OA chondrocyte and synoviocyte viability
Live/Dead staining was completed on cartilage and synovium per manufacturer’s instructions immediately at the study time-points. Briefly, cartilage and synovium sections obtained from a thin centralized rectangular region of the biopsies were incubated in a working solution of 2-μM calcein antimitotic and 4-μM Ethd-1 at room temperature for 45 min. Tissues were placed on a microscope slide prior to fluorescent imaging. Positive controls for cell death included cartilage and synovium biopsy samples treated with 100% ethanol.
2.6 | OA cartilage glycosaminoglycan content and collagen leaching
The right hemisphere section of OA cartilage was frozen overnight at −80 °C prior to lyophilization, recording of dry mass and digestion overnight in 125 μg/ml papain in PBE buffer (pH 7.5) at 65 °C. Tissue digests were assessed for glycosaminoglycan (GAG) content via dimethylmethylene blue (DMMB) assay. Briefly, 200 μl of DMMB reagent (46-μg DMMB, 40-mM Glycine, 40-mM NaCl, pH 3) was added to 50 μl of digested sample in a 96-well plate. Absorbance was read at 525 nm, and GAG content was determined from a standard curve developed from known concentrations of chondroitin- 6-sulfate. Values were normalized to sample dry weights.
Culture media was assessed for hydroxyproline, a measure of collagen content per manufacturer’s instructions (Sigma). Briefly, media samples (100 μl) were hydrolysed with an equal volume of 12-N hydrochloric acid at 120 °C for 3 hr prior to transfer to a 96-well plate. Wells were evaporated to dryness at 60 °C. Equal amounts of chloramine T/oxidation buffer mixture and diluted p-dimethylaminobenzaldehyde reagent were added followed by 5-min room temperature and 90-min 60 °C incubations, respectively. Standards were created using a 1 mg/ml stock solution of hydroxyproline standard solution. Sample absorbance was read at 560 nm. Values were normalized to sample dry weights.
2.7 | Immunohistochemistry for OA synovial macrophage phenotype
Immunohistochemistry (IHC) on rehydrated paraffin embedded synovium thin sections (5 μm) was performed for detection of synovial macrophages. Briefly, antigen retrieval was accomplished via 10-mM citric acid incubation at 90 °C for 20 min. Slides were rinsed twice in tris buffered saline for 5 min, permeabilized in 0.025% Triton X-100; non-specific binding and endogenous peroxidases were blocked with normal serum and a solution of 0.3% hydrogen peroxide in 0.3% normal serum, respectively. A rabbit polyclonal antibody towards human C-C chemokine receptor Type 7 (CCR7-AbCam; 0.5 μg/ml dilution) or human mannose receptor C type 1 (MRC-AbCam; 1 μg/mL dilution) was incubated for 1 hr at room temperature prior to thorough rinsing and incubation at room temperature for 30 min with a secondary biotinylated antibody and avidin biotin complex according to manufacturer’s instructions (Vectastain®ABC Elite Kit Rabbit IgG—Vector Labs). A 3,3′-diaminobenzidine substrate kit (Vector Labs: SK4100) was used to visualize positive staining prior to counterstaining with a dilute hematoxylin solution for 30 s. Negative controls did not receive primary antibody. Representative images were captured using a Zeiss Axiovert. A1 microscope with Axiovision software (Release 4.9.1 SP08-2013) at 200× total magnification from three slides for each specimen (n = 5 per study group per time-point) for both antibodies used. The average number of positively stained macrophages was calculated from these images for each coculture group.
2.8 | Histopathological assessment of OA cartilage
The left hemisphere section of OA cartilage biopsies was secured within a tissue processing cassette, fixed in 10% phosphate buffered formalin overnight at room temperature prior to undergoing standard tissue processing, decalcification, paraffin embedding, and sectioning at 5-μm thickness. Sections were stained with safranin-O counter-stained with fast green for visualization of proteoglycan rich cartilage matrix. Briefly, rehydrated sections were differentiated in 1% acid alcohol for 2 s prior to room temperature incubation in 0.02% fast green for 2.5 min. After 30 s incubation in 1% acetic acid, sections were stained with 1% safranin-O for 15 min. Three images were taken spanning the surface length of the sample for each specimen analysed (n = 5 per study group per time-point). An observer blinded to the coculture study group and time-point completed the Osteoarthritis Research Society International (OARSI) histopathological assessment on each image according to the direction of Pritzker et al. (2006). These numerical results were averaged to obtain the final OARSI score for each sample.
2.9 | Cytokine analysis on OA co-culture media
Enzyme linked immunosorbent assay was performed according to manufacturer instructions for a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 (Raybiotech: ELH-ADAMTS4-1) and matrix metalloproteinase (MMP)-13 (Raybiotech: ELH-MMP13-1). Briefly, 96-well plates were incubated overnight at 4 °C with standards and coculture media samples (n = 3 per study group per time-point). After several washes, wells were incubated with biotinylated antibodies for either ADAMTS-4 or MMP-13 for 1 hr, followed by incubation in horseradish peroxidase conjugate-conjugated streptavidin for 45 min. Enzymatic reactions were allowed to develop, and the absorbance of each plate was read at 450 nm.
A human cytokine arrays (RayBiotech: QAH-CYT-1-4) was performed per manufacturer instructions on coculture media to detect proinflammatory and anti-inflammatory cytokines and chemokines. Briefly, media samples (n = 5 per study group per time-point) were incubated in sample diluent for 30 min on glass slides prior to washing and subsequent incubation in detection antibody for 1 hr. Slides were then washed and incubated in a Cy3 equivalent dye-conjugated streptavidin for 1 hr at room temperature. Analysis was performed by the manufacturer using Q-Analyzer software to quantitatively evaluate cytokine/chemokine concentrations.
2.10 | Statistical analysis
Results are represented as a mean ± standard error of the mean. All statistical analyses were performed in GraphPad Prism 7 software. Cytokine array data and hAMSC direct versus indirect comparisons were performed using two-tailed Student’s t-tests of equal variance. All other statistical evaluations were compared via one-way analysis of variance with Tukey’s post hoc analysis. Significance was defined in all cases as p < .05.
3 | RESULTS
3.1 | Synovial macrophages reduce OA chondrocyte viability
To assess the direct effect of synovial macrophages on chondrocyte and synoviocyte viability, OA cartilage was cocultured with either (a) OA synovium or (b) OA synovium that had macrophages selectively depleted via clodronate (Figure 1). In the OA group, both chondrocyte (Figure 2a,c) and synoviocyte (Figure 2b,c) viability significantly (p < .05) decreased from 85.3 ± 2.6% to 58.2 ± 4.2% and from 72.1 ± 5.5% to 44.6 ± 7.1%, respectively, over 15 days in culture. In comparison, the cell viability in cartilage only and synovium only groups remained unchanged throughout 15 days.
FIGURE 2.
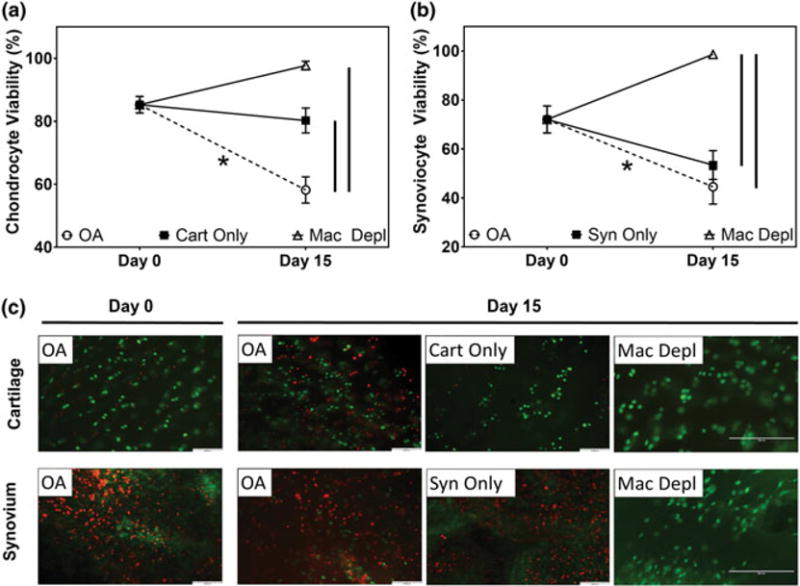
Viability assessments of osteoarthritic cartilage and synovium cocultures. Semiquantitative analysis of (a) articular chondrocyte and (b) synoviocyte viability determined via histological analysis using Live/Dead staining of osteoarthritic joint tissues (n = 5 per study group per time-point) at Day 0 and 15 days of culture. (c) Representative Live/Dead images [scale bar = 100 μm for osteoarthritis (OA), cartilage biopsies only (cart only), synovium biopsies only (syn only) groups, scale bar = 200 μm for macrophage depleted (mac depl) group] of cocultured cartilage and synovium biopsies from samples analysed (n = 5 per study group per time-point; green = viable cells, red = dead cells). * indicates statistical difference comparing between Day 0 and Day 15 within the same study group (p < .05). Vertical lines indicate a statistical difference between connected study groups at Day 15 (p < .05) [Colour figure can be viewed at wileyonlinelibrary.com]
Upon depletion of macrophages from OA synovium, viability of cocultured chondrocytes (97.7 ± 1.4%) and synoviocytes (98.6 ± 0.8%) was maintained through Day 15; these values were significantly (p < .05) greater than the viability of tissue explants in the OA group. Representative Live/Dead images of macrophage depleted synovium (Figure 2c) confirmed a reduction in total cell number within the tissue.
3.2 | Synovial macrophages contribute to progressive cartilage degradation
To further determine if the OA coculture model demonstrates progressive cartilage destruction as observed in vivo and to delineate the direct role of synovial macrophages on promoting cartilage degradation, cartilage was evaluated biochemically and histologically after coculture with native OA synovium or with synovium that had been depleted of macrophages. Mean GAG content of cartilage in the OA group (Figure 3a) demonstrated a significant (p < .05) and progressive 54% decrease from Day 0 to Day 15 (141.9 ± 19.6 μg/mg dry weight and 65.7 ± 12.4 μg/mg dry weight, respectively). However, OA cartilage GAG content in the Cart Only group did not significantly change over time in culture (154.4 ± 24.6 μg/mg dry weight). Similarly, media concentrations of hydroxyproline (HYP; a measure of collagen leaching/degradation) in the OA group were higher (0.015 ± 0.004 μg/mg dry weight) as compared with media collected from Cart Only cultures (0.007 ± 0.002 μg/mg dry weight) indicating progressive collagen leaching (Figure 3b). Histolopathological evaluation and semiquantitative OARSI scoring of OA cartilage in the OA group further corroborated these findings as scores significantly (p < .05) worsened as coculture duration increased (Day 0: 9.8 ± 1.7, Day 15: 15.5 ± 1.8) illustrating reductions in proteoglycan staining initiating at the superficial zone and increased surface fibrillation (Figure 3e). Conversely, OARSI scores did not significantly change over time in cart only cultures (Day 0: 9.8 ± 1.7, Day 15: 7.0 ± 1.5).
FIGURE 3.
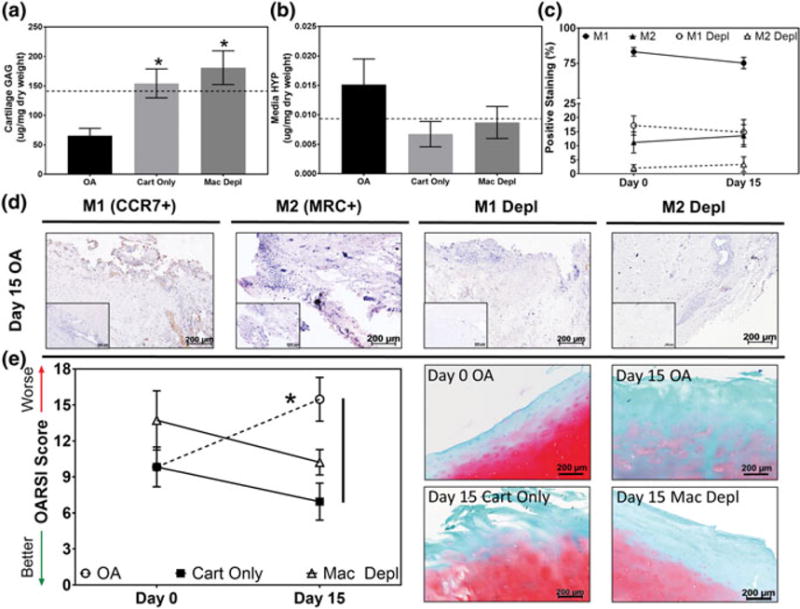
Biochemical and histological assessments of osteoarthritic cartilage and synovium cocultures in the presence or absence of synovial macrophages. Quantitative analysis of (a) articular cartilage glycosaminoglycan (GAG) and (a) media hydroxyproline (HYP) content in osteoarthritis (OA), cartilage only and macrophage depleted (Mac Depl) cocultures following 15 days of coculture (n = 5 per study group per time-point). Dotted line indicates Day 0 GAG or HYP content. *Indicates statistical difference comparing between the OA study group at Day 15 (p < .05). (c) Semiquantitative analysis illustrating the percentage of M1 and M2 polarized macrophages in native (M1: Filled circles, M2: Filled triangles) and Mac Depl (M1: Open circles, M2: Open triangles) OA synovium at Day 0 and following 15 days of coculture in each respective culture conditions (n = 5 per study group per time-point). (d) Representative histological images stained via immunohistochemistry for M1[C-C chemokine receptor Type 7 (CCR7+)] and M2[mannose receptor C (MRC+)] polarized macrophages following 15 days of coculture (n = 5 per study group per time-point). Brown = positive staining; inserts = negative controls; scale bar = 200 μm. (e) Representative histological images (right) and semiquantitative Osteoarthritis Research Society International (OARSI) scoring analysis (left) of safranin-O stained cartilage biopsies (n = 5 per study group per time-point; red = proteoglycan, green = collagen) at Day 0 and following coculture in the respective study groups for 15 days. *indicates statistical difference comparing between Day 0 and Day 15 within the same study group (p < .05). Vertical lines indicate a statistical difference between connected study groups at Day 15 (p < .05) [Colour figure can be viewed at wileyonlinelibrary.com]
In cocultures containing OA cartilage with macrophage depleted synovium, cartilage GAG content and media HYP concentrations were not significantly different compared with Day 0 values (181.0 ± 28.5 μg/mg dry weight and 0.009 ± 0.003 μg/mg dry weight, respectively) (Figure 3a,b). Furthermore, cartilage OARSI scores did not significantly change throughout the study duration in macrophage depleted cocultures (Day 0: 13.7 ± 2.5, Day 15: 10.2 ± 1.1) (Figure 3e).
3.3 | Synovial macrophages and their M1/M2 polarization states persist in vitro
To confirm the relative ratios of M1 and M2 polarized macrophages in human OA synovium prior to (i.e., immediately following explant/harvest) and following coculture with OA cartilage, IHC for CCR7 and MRC was performed, respectively (Figure 3c,d). Prior to culture, 83.2 ± 3.1% of OA synoviocytes stained positively for the M1 marker CCR7, whereas only 11.2 ± 3.8% stained positive for the M2 marker MRC (Figure 3c) yielding an approximate M1:M2 percentage ratio of 7.4:1. These values did not significantly change following coculture for 15 days with OA cartilage (M1: 75.2 ± 4.0%, M2: 13.6 ± 3.9%) resulting in a M1:M2 percentage ratio of 5.5:1. Of note, however, if OA synovium was cultured in the absence of OA cartilage (data not shown), the percentage of M1 synovial macrophages significantly (p < .05) decreased to 56.0 ± 8.0% at Day 15 compared with samples coculture with OA cartilage; however, the percentage of M2 macrophages in these samples remained unchanged (4.8 ± 1.3%).
Following macrophage depletion, the relative percentage of M1 and M2 synovial macrophages expectedly decreased to 17.2 ± 3.4% and 2.1 ± 1.2%, respectively, at Day 0, and thereafter did not change significantly at Day 15 (M1: 14.8 ± 4.5%, M2: 3.4 ± 2.7%; Figure 3c). This resulted in a 4.3:1 M1:M2 percentage ratio in the macrophage depleted OA synovium of at Day 15. Representative histological images depicting M1 and M2 IHC staining prior to macrophage depletion depict positive (brown) punctate staining corresponding with macrophage nuclei throughout the tissue and around blood vessels in native synovium (Figure 3d). In synovium depleted of macrophages, staining for both M1 and M2 markers was relatively absent; some scattered positive staining was observed diffusely throughout the tissues.
3.4 | Synovial macrophages contribute to the maintenance of a proinflammatory and prodegenerative mileiu
To define the amounts of soluble proinflammatory cytokines, chemokines, proteases, and growth factors found within the OA coculture micro-environment, a cytokine array detecting known mediators of human OA was performed (Table 1). Additionally, media samples obtained from explant cocultures depleted of synovial macrophages were analysed and compared with determine the specific contribution of macrophages to cytokine/protease production. In OA explant cocultures, levels of inflammatory mediators including interlukins(IL)-1α, -1β, -6, -8, tumour necrosis factor alpha (TNF-α) and chemokines including macrophage chemotactic protein-1 (MCP-1), C-C motif chemokine ligand 5 [CCL-5/regulated on activation, normal T cell expressed and secreted(RANTES)], granulocyte macrophage colony stimulating factor (GM-CSF), and the aggrecanase ADAMTS-4 were maintained throughout the culture period as no significant differences were observed comparing between Day 0 and 15 values. The chemokines macrophage inflammatory protein (MIP)-1α (CCL3) and MIP-1β (CCL4), and the anti-inflammatory cytokines IL-10 and IL-13 significantly (p < .05) decreased with increasing time in coculture. Conversely, MMP-9 and MMP-13 increased significantly (p < .05) compared with their respective Day 0 values.
TABLE 1.
Cytokine, chemokine, protease, and growth factor profiles of media obtained from human OA cartilage and synovium cocultures in the presence and absence of synovial macrophages
| Category | Mediator | Media concentration (pg/ml)
|
||
|---|---|---|---|---|
| Day 0 OA |
Day 15 OA |
Day 15 Mac depleted |
||
| Proinflammatory cytokines | IL-1α | 11.0 ± 6.1 | 21.9 ± 9.3 | 19.6 ± 2.2 |
| IL-1β | 22.0 ± 10.3 | 4.8 ± 3.2 | ND | |
| TNF-α | 376.6 ± 120.1 | 436.3 ± 158.2 | 319.7 ± 49.0 | |
| IL-6 | 2,000.0 ± 0.0 | 1,768.0 ± 158.1 | 1,548.0 ± 277.0 | |
| IL-8 | 685.7 ± 39.0 | 600.1 ± 38.9 | 537.9 ± 117.9 | |
|
| ||||
| Chemokines | MCP-1 | 277.2 ± 14.4 | 256.4 ± 14.0 | 239.6 ± 25.8 |
| CCL-5/RANTES | 27.0 ± 6.1 | 39.0 ± 14.1 | 23.5 ± 8.1 | |
| GM-CSF | 48.0 ± 18.0 | 19.4 ± 8.7 | 28.9 ± 18.3 | |
| MIP-1α | 52.2 ± 7.1 | 32.5 ± 4.9* | 8.34 ± 2.5** | |
| MIP-1β | 378.4 ± 58.6 | 208.1 ± 42.2* | 7.9 ± 1.2** | |
|
| ||||
| Proteases | MMP-9 | 3,100.0 ± 1,141.0 | 23,204.0 ± 7,533.0* | 4,310.0 ± 2,660.0 |
| MMP-13 | 214.0 ± 103.0 | 1,428.0 ± 328.0* | 24.2 ± 0.3** | |
| ADAMTS-4 | 5.0 ± 1.1 | 4.4 ± 0.9 | N/A | |
|
| ||||
| Anti-inflammatory cytokines | IL-2 | 6.1 ± 3.3 | 4.7 ± 3.1 | 37.3 ± 16.4** |
| IL-4 | N/D | N/D | N/D | |
| IL-10 | 4.8 ± 1.0 | 1.1 ± 0.3* | 1.58 ± 0.5 | |
| IL-13 | 7.9 ± 1.3 | 2.9 ± 0.9* | 1.36 ± 0.9 | |
|
| ||||
| Growth factors | VEGF | 4,000.0 ± 0.0 | 3,020.0 ± 363.4* | 3,817.0 ± 137.3 |
Note. OA = osteoarthritis; IL = interleukin; VEGF = vascular endothelial growth factor; MMP = matrix metalloproteinase; ADAMTS = a disintegrin and metalloproteinase with thrombospondin motifs; MIP = macrophage inflammatory protein; CCL = C–C motif chemokine ligand; RANTES = regulated on activation, normal T cell expressed and secreted; GM-CSF = granulocyte macrophage colony stimulating factor; ND = not detected; N/A = study group not analysed.
Indicates statistical difference compared with Day 0 OA group (p < .05).
Indicates statistical difference compared with Day 15 OA group (p < .05).
Macrophage depletion resulted in significant (p < .05) reductions in IL-1β, MIP-1α, MIP-1β, and MMP-13 as compared with Day 15 cocultures with OA synovium. Additionally, IL-2 was significantly higher in cocultures depleted of synovial macrophages.
3.5 | hAMSCs protect OA chondrocyte viability and prevent cartilage tissue degradation more effectively than hADSCs
To determine and compare the ability of hAMSC and hADSCs to mitigate OA progression using the OA explant coculture model, stem cells were seeded directly on top of OA cartilage explants (i.e., direct contact coculture) following initiation of coculture with synovium (Figure 1). Mean OA chondrocyte viability did not significantly change from Day 0 (85.3 ± 2.6%) to Day 15 (89.8 ± 2.4%) in the hAMSC treated group (Figure 4a,c). Conversely, chondrocyte viability significantly (p < .05) decreased with time in culture in the OA (Day 15: 63.4 ± 3.9%) and hADSC (D15: 58.4 ± 5.0%) groups. OARSI assessments indicated a significant worsening (p < .05) of cartilage ECM integrity and proteoglycan staining intensity with time in culture in the OA group (Day O: 8.0 ± 2.2, Day 15: 13.5 ± 2.4; Figure 4b,c). However, mean OARSI scores for hADSC (Day O: 8.0 ± 2.2, Day 15: 6.8 ± 2.3) and hAMSC (Day O: 8.0 ± 2.2, Day 15: 3.5 ± 1.8) groups remained constant or improved with increasing time in culture, respectively, and thus, by Day 15, a significant (p < .05) difference comparing between OA and hAMSC groups was demonstrated. Safranin-O staining of cartilage illustrated minimal surface fibrillation and the largest areal staining of aggrecan in the hAMSC treated group. Surface fibrillation was found to increase and aggrecan staining intensity decreased in the hADSC group; however, this was not to the same deficit levels as found in the OA group by Day 15 (Figure 4c).
FIGURE 4.
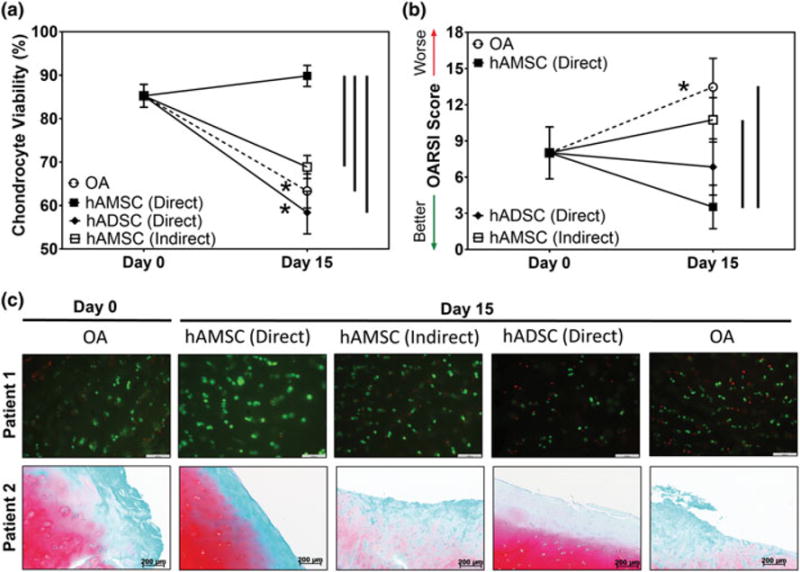
Viability and histological assessments of osteoarthritic cartilage and synovium cocultures. Semiquantitative analysis of (a) articular chondrocyte and (b) synoviocyte viability following coculture with stem cells in direct contact with osteoarthritis (OA) cartilage biopsies or in indirect contact (i.e., seeded on the underside of trans-well inserts) determined via Live/Dead staining of osteoarthritic joint tissues at Day 0 and 15 days of culture (n = 5 per study group per time-point). (c) Representative patient-matched (top row) Live/Dead images (green = viable cells, red = dead cell; scale bar = 100 μm) and (bottom row) safranin-O stained tissue sections (red = proteoglycan, green = collagen; scale bar = 200 μm) from cocultures containing stem cells (n = 5 per study group per time-point). * indicates statistical difference comparing between Day 0 and Day 15 within the same study group (p < .05). Vertical lines indicate a statistical difference between connected study groups at Day 15 (p < .05). hAMSC = human amniotic stem cells; hADSC = human adipose stem cells; OARSI = Osteoarthritis Research Society International [Colour figure can be viewed at wileyonlinelibrary.com]
Histological results were further corroborated by DMMB analysis that indicated that the hAMSC treated group had significantly (p < .05) more GAG (499 ± 101.9 μg/mg dry weight) as compared with all other groups at Day 15 (Figure 5a). No differences in GAG content were noted between the OA (174 ± 54.0 μg/mg dry weight) or hADSC (155 ± 26.3 μg/mg dry weight) groups. Media HYP content (Figure 5b) was not found to be statistically different at Day 15 comparing OA (0.166 ± 0.044 μg/mg dry weight) and hAMSC (0.169 ± 0.081 μg/mg dry weight) groups; however, lower concentrations of media HYP (0.006 ± 0.002 μg/mg dry weight) were found in the hADSC group compared with the OA and hAMSC groups.
FIGURE 5.
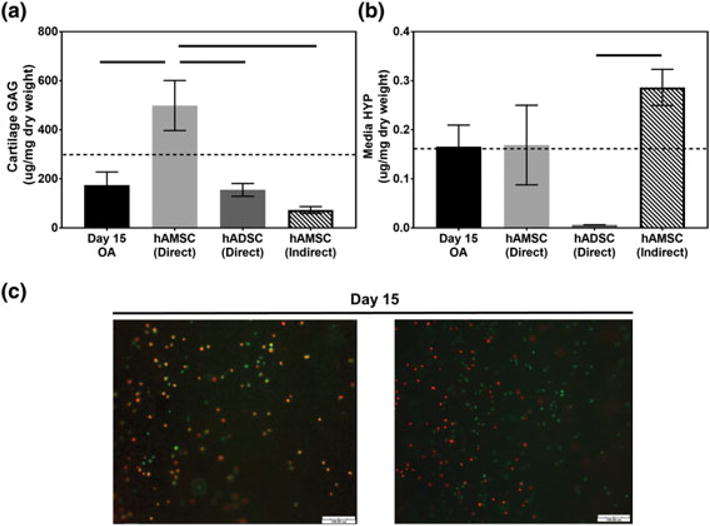
Biochemical and viability assessments of osteoarthritic cartilage and synovium cocultures in the presence of human stem cells. Quantitative analysis of (a) articular cartilage glycosaminoglycan (GAG) and (b) media hydroxyproline (HYP) content in osteoarthritis (OA) cocultures containing human amniotic stem cells (hAMSCs) and human adipose stem cells (hADSCs) following 15 days of coculture (n = 5 per study group per time-point). Dotted line indicates Day 0 GAG or HYP content. Horizontal lines indicate a statistical difference between connected study groups at Day 15 (p < .05). (c) Representative Live/Dead images of the underside of trans-well inserts seeded with hAMSCs (indirect) in OA cocultures for 15 days (n = 5 per time-point; green = viable cells, red = dead cell; scale bar = 100 μm) [Colour figure can be viewed at wileyonlinelibrary.com]
To further define the effect of direct- versus indirect-contact coculture on OA progression, hAMSCs were seeded onto the underside of trans-well inserts as opposed to being placed directly on the OA cartilage (Figure 1). This resulted in significantly (p < .05) lower chondrocyte viability (68.9 ± 2.6%), worse (higher) OARSI scores (10.8 ± 1.8), and lower GAG content (73.0 ± 14.0) at Day 15 in comparison with hAMSCs cultured in direct-contact with OA cartilage. Media HYP content of indirect contact coculture hAMSCs (0.286 ± 0.037 μg/mg dry weight) was not significantly different compared with hAMSCs in direct contact with OA cartilage. Of note, representative Live/Dead images of hAMSCs on the underside of well inserts (Figure 5c) confirmed the presence of viable hAMSCs at Day 15.
3.6 | hAMSCs shift OA synovial macrophages towards an M2 phenotype more effectively than hADSCs
To determine and compare the effect of hAMSCs and hADSCs on OA synovial macrophage phenotype in the OA explant coculture model, the percentage of cells staining positively for M1 (CCR7+) and M2 (MRC+) macrophage markers was quantitatively evaluated using IHC (Figure 6). The percentage of M1 synovial macrophages remained unchanged from Day 0 (84.1 ± 4.3%) to Day 15 (80.6 ± 4.6%) in the OA group (Figure 6a). Conversely, application of both hAMSCs and hADSCs in direct contact with OA cartilage resulted in significantly (p < .05) lower percentages of M1 synovial macrophages at Day 15 (48.6 ± 8.1% and 57.6 ± 8.0%, respectively) compared with their respective Day 0 values (Figure 6a). By Day 15, the percentage of M1 synovial macrophages was significantly (p < .05) lower in the hAMSCs group compared with the OA group. No significant differences in the relative percentage of M2 synovial macrophages was observed in the OA (Day 0: 11.2 ± 3.7%, Day 15: 13.6 ± 3.9%) or direct contact hADSC (Day 15: 11.5 ± 4.2%) groups throughout culture (Figure 6b). However, the hAMSC group demonstrated a significant (p < .05) increase in the percentage of M2 synovial macrophages from Day 0 to Day 15 (37.0 ± 11.2%) that yielded a significantly (p < .05) higher percentage of M2 macrophages compared with the OA and hADSC groups at Day 15 (Figure 6b). The resultant M1:M2 percentage ratios in the OA and direct contact hAMSCs and hADSCs groups were 6:1, 1.3:1, and 5:1, respectively.
FIGURE 6.
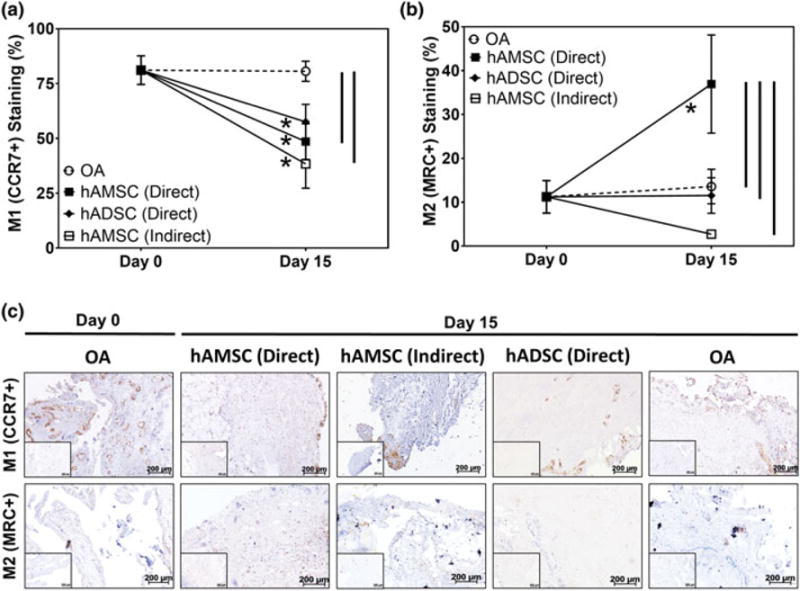
Analysis of synovial macrophage polarization in coculture with and without human stem cells. (a) Semiquantitative analysis illustrating the percentage of M1 and M2 polarized macrophages in cocultures with [human amniotic stem cell (hAMSC) direct: Filled squares, human adipose stem cell (hADSC) direct; filled diamonds, and hAMSC indirect; open squares] and without stem cells [osteoarthritis (OA); open circles] at Day 0 and following 15 days of coculture in respective culture conditions (n = 5 per study group per time-point). * indicates statistical difference comparing between Day 0 and Day 15 within the same study group (p < .05). Vertical lines indicate a statistical difference between connected study groups at Day 15 (p < .05). (d) Representative histological images stained via immunohistochemistry for M1[C-C chemokine receptor Type 7 (CCR7+)] and M2[mannose receptor C (MRC+)] polarized macrophages following 15 days of coculture (n = 5 per study group per time-point). Brown = positive staining, inserts = negative controls, scale bar = 200 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Indirect contact coculture of hAMSCs (i.e., placed on the underside of the trans-well inserts containing OA synovium), the percentages of M1 synovial macrophages (38.4 ± 11.1%) at Day 15 were significantly (p < .05) lower compared with Day 0. Conversely, the percentage of M2 macrophages decreased to 2.7 ± 0.7% by Day 15; however, this was not statistically different from Day 0 values. No significant difference in the percentage of M1 synovial macrophages was observed comparing indirect- and direct-contact hAMSC cocultures; however, direct contact-coculture resulted in significantly (p < .05) higher percentage of M2 synovial macrophages as compared with indirect contact coculture with the same stem cell type.
3.7 | Human stem cell cytokine/protease expression is dependent on stem cell type and tissue contact
To gain further insight into the observed differences in chondroprotection and macrophage polarization observed in the different stem cell treatment groups, media concentrations of cytokines, chemokines, and proteases were evaluated in each treatment group and compared with their respective patient-matched sample controls (i.e., OA coculture without stem cells). Inclusion of hAMSCs in direct contact with OA cartilage resulted in a significant (p < .05) increase in the concentrations of IL-1α, IL-13, MCP-1, MIP-1α, and MMP-9 compared with patient-matched OA controls (Table 2). Direct contact coculture with hAMSCs also resulted in higher concentrations of IL-10 and GM-CSF compared with OA controls; however, these increases did not reach statistical significance (p = .06 and p = .07, respectively). Concentrations of MMP-13 and ADAMTS-4 were lower in the direct-contact hAMSC group compared with OA controls (Table 2). Direct contact coculture of OA cartilage with hADSCs resulted in significantly (p < .05) lower media concentrations of IL-5 compared with patient-matched OA controls (Table 2). Moreover, IL-10, GM-CSF, RANTES/CCL-5, and ADAMTS-4 were higher in cocultures containing hADSCs compared with OA controls. Conversely, MMP-13 and MMP-9 concentrations decreased in cocultures with hADSCs as compared with OA controls.
TABLE 2.
Comparison of select cytokine, chemokine, and protease profiles in media obtained from patient paired human OA cartilage and synovium cocultures in the presence or absence of human stem cells
| Stem cell coculture | Mediator | Day 15 OA |
Day 15 Stem cell treated |
|
|---|---|---|---|---|
| hAMSC (direct) | IL-1α | 6.37 ± 4.64 | 26.32 ± 2.27* | |
| IL-5 | ND | ND | ||
| IL-13 | 0.84 ± 0.84 | 5.03 ± 1.80* | ||
| IL-10 | 0.71 ± 0.33 | 1.48 ± 0.44 | ||
| GM-CSF | 4.96 ± 0.35 | 14.85 ± 4.86 | ||
| RANTES/CCL-5 | 15.58 ± 5.14 | 19.66 ± 5.76 | ||
| MCP-1 | 214.64 ± 65.78 | 266.25 ± 10.09* | ||
| MIP-1α | 24.17 ± 4.73 | 46.26 ± 5.62* | ||
| ADAMTS-4 | 4.28 ± 1.83 | 3.63 ± 0.53 | ||
| MMP-13 | 1,107.50 ± 461.48 | 681.67 ± 288.72 | ||
| MMP-9 | 9,152.48 ± 5,300.98 | 27,760.70 ± 2,239.30* | ||
|
| ||||
| hADSC (direct) | IL-1α | 47.97 ± 25.12 | 6.90 ± 6.90 | |
| IL-5 | 8.10 ± 3.49 | ND | ||
| IL-13 | 6.13 ± 1.21 | 8.92 ± 1.73 | ||
| IL-10 | 1.22 ± 0.46 | 2.40 ± 0.61 | ||
| GM-CSF | 52.20 ± 21.56 | 151.82 ± 102.66 | ||
| RANTES/CCL-5 | 39.82 ± 10.25 | 95.54 ± 47.87 | ||
| MCP-1 | 280.23 ± 26.05 | 241.33 ± 12.26 | ||
| MIP-1α | 43.33 ± 10.95 | 53.29 ± 13.29 | ||
| ADAMTS-4 | 5.03 ± 1.26 | 6.58 ± 1.91 | ||
| MMP-13 | 1,768.00 ± 805.35 | 867.33 ± 260.54 | ||
| MMP-9 | 47,639.43 ± 18,206.35 | 6,503.38 ± 2,535.03 | ||
|
| ||||
| hAMSC (indirect) | IL-1α | 9.01 ± 7.81 | 25.63 ± 3.88 | |
| IL-5 | 2.42 ± 2.10 | ND | ||
| IL-13 | 0.97 ± 0.84 | 1.19 ± 1.03 | ||
| IL-10 | 0.97 ± 0.46 | 0.86 ± 0.40 | ||
| GM-CSF | 2.66 ± 1.16 | 5.30 ± 2.45 | ||
| RANTES/CCL-5 | 87.30 ± 48.30 | 39.86 ± 19.36 | ||
| MCP-1 | 296.95 ± 14.57 | 279.38 ± 20.14 | ||
| MIP-1α | 32.66 ± 11.67 | 48.49 ± 21.76 | ||
| ADAMTS-4 | 3.98 ± 1.64 | 2.38 ± 0.28 | ||
| MMP-13 | 1,407.83 ± 565.69 | 1,450.5 ± 131.75 | ||
| MMP-9 | 21,018.87 ± 8,981.13 | 4,108.67 ± 3,387.54 | ||
Note. IL = interleukin; hAMSC = human amniotic stem cells; hADSC = human adipose stem cells; MMP = matrix metalloproteinase; ADAMTS = a disintegrin and metalloproteinase with thrombospondin motifs; MIP = macrophage inflammatory protein; RANTES = regulated on activation, normal T cell expressed and secreted; CCL = C-C motif chemokine ligand; GM-CSF = granulocyte macrophage colony stimulating factor; MCP = macrophage chemotactic protein; ND = not detected.
Indicates statistical difference compared with Day 15 OA group (p < 0.05).
Finally, indirect contact coculture of hAMSCs with OA cartilage resulted in an increase in IL-1α and GM-CSF and a decrease in RANTES/CCL-5, ADAMTS-4, and MMP-9 compared with OA controls; however, these differences were not statistically significant (Table 2). In general, direct contact coculture with hAMSCs resulted in lower concentrations of MMP-13 and higher concentrations of MMP-9 compared with indirect contact coculture with hAMSCs.
4 | DISCUSSION
Beekhuizen and colleagues previously reported on the maintenance of OA cartilage and synovium viability in a similar explant coculture model to the one described herein (Beekhuizen et al., 2011). Our studies expand significantly upon this work by utilizing a similar in vitro model of OA to investigate several important yet unanswered questions regarding (a) the fidelity by which this model mimics key tissue-level attributes of OA pathogenesis, (b) the specific role that synovial macrophages and their phenotype play in OA, and (c) to evaluate and compare the therapeutic efficacy of different stem cell sources to mitigate OA progression. Key findings from these studies indicate that (a) cocultures of OA cartilage and synovium exhibit hallmarks of OA including impaired chondrocyte viability and progressive cartilage degradation in the presence of a sustained complex milieu of proinflammatory and degradative mediators, (b) the maintenance of macrophage M1:M2 percentage ratio in OA synovium requires the presence of both OA cartilage and synovium that together contribute directly to OA progression in vitro by reducing chondrocyte viability and enhancing cartilage proteoglycan and collagen loss mediated in part by IL-1β and MMP-13, and (c) administration of hAMSCs more effectively mitigated progression of OA compared with hADSCs when placed in direct contact with OA cartilage within the model resulting in enhanced chondroprotection and a shift in the synovial macrophage M1:M2 percentage ratio favouring the maintenance of OA cartilage homeostasis.
Although the pathogenesis of OA is complex and poorly understood, use of in vitro models to study the disease continue to provide valuable insight. The more closely a model reflects the actual disease condition, the more likely it can be used to define the aetiology of the disease and uncover new avenues for therapy. Such models should incorporate the human joint tissues involved and accurately reproduce the hallmarks of OA. One such OA hallmark is chondrocyte apoptosis. The frequency in which apoptotic chondrocytes are detected has been shown to be greater in OA cartilage compared with the normal tissue (Aigner et al., 2001; Aigner & Kim, 2002). Additionally, large-scale studies have illustrated a significant decrease in the expression of OA chondrocyte genes that make them more prone to oxidative stress (Aigner et al., 2006). Furthermore, the presence of proinflammatory mediators and reactive oxygen species, including IL-1β and nitric oxide (NO) have been shown to result in decreased OA chondrocyte viability (Liu-Bryan & Terkeltaub, 2015; Loeser, 2012). Herein, we observed decreases in OA chondrocyte viability with time in coculture that was enhanced in the presence of OA synovium. However, upon depletion of synovial macrophages via clodronate, OA chondrocyte viability was maintained. This was expected considering we have demonstrated herein that M1 macrophages predominate the OA synovium. Thus, elimination of these cells, which are known producers of IL-1β and NO, would account for the improvement in OA chondrocyte viability (G. Wu et al., 2007). It should be noted that reduced chondrocyte viability was not due to cell damage during harvest as all viability analysis was performed in central regions of the cartilage biopsy away from cut edges nor was it due to nutrient deprivation within the coculture system as incorporation of additional cells did not result in increased chondrocyte death.
Progressive deterioration of articular cartilage is one of the most well-known attributes of OA. Degradation has been demonstrated to initiate within superficial zone cartilage resulting in fibrillation and loss of proteoglycan content that gradually proceeds to the deep zone (Goldring & Marcu, 2009). OA cartilage proteolysis has been shown to be driven by the aggrecanases, specifically ADAMTS-4 (Naito et al., 2007), which is up-regulated in OA chondrocytes following stimulation with proinflammatory cytokines (Verma & Dalal, 2011). Loss of proteoglycan subsequently leads to the exposure of Type II collagen that undergoes irreversible proteolytic degradation by MMP-13, MMP-9, and other proteases (Goldring & Marcu, 2009; Reboul, Pelletier, Tardif, Cloutier, & Martel-Pelletier, 2011; Tetlow, Adlam, & Woolley, 2001; W. Wu et al., 2002). The coculture model used herein recapitulates this slowly progressive destruction of cartilage over a period of 2 weeks as indicated via biochemical and histological evaluations. Importantly, inclusion of OA synovium in the coculture system was required to achieve significant proteoglycan loss that appeared to be mediated in part by the persistence of ADAMTS-4. Additionally, collagen degradation increased with time in coculture that can be attributed to increasing concentration of MMP-13 and MMP-9 observed within the model. With respect to macrophage contribution to progressive cartilage destruction, depletion of macrophages reduced cartilage destruction to levels found in OA cartilage only monocultures. This confirms the requirement of paracrine crosstalk between chondrocytes and synovial macrophages for continued cartilage destruction. Furthermore, MMP-9 and MMP-13 levels significantly decreased when macrophages were depleted from OA synovium indicating their direct contribution to the production of these proteases.
Another clinical feature of OA is the presence of a complex milieu of cytokines, chemokines, and proteases found within the synovial fluid. Aspirates of synovial fluid from OA patients demonstrate the presence of proinflammatory cytokines; IL-1α, IL-1β, TNF-α, IL-6, and IL-8, anti-inflammatory cytokines; IL-2, IL-4, IL-10, and IL-13, chemokines; GM-CSF, MCP-1, MIP-1α, and the proteases; MMP-1, -2, -3, -9, and -13 (Partsch et al., 1997; Sohn et al., 2012; Tsuchida et al., 2014; Vangsness, Burke, Narvy, MacPhee, & Fedenko, 2011). These and other mediators are typically found in concentrations that vary from patient to patient and range from one to hundreds of picograms per millilitre. The cytokine and chemokine concentrations within coculture media were found to be comparable with in vivo values. Macrophage depletion from OA synovium resulted in significant reductions in IL-1β, MIP-1α, MIP-1β, and MMP-13 indicating that these cells are responsible for their production. MIPs are effector chemokines secreted by macrophages following stimulation by IL-1β, which functions in a paracrine manner to attract other proinflammatory cells (Maurer & Von Stebut, 2004). Thus, depletion of macrophages concomitant with reduced IL-1β concentrations would expectedly result in significant reductions of these chemokines as was observed in the model. Unexpectedly, TNF-α, IL-6, and IL-8 levels did not significantly decrease after depletion of synovial macrophages. This contrasts with the findings of Bondeson et al. who demonstrated that selective depletion of macrophages in coculture with synovial fibroblasts resulted in significant reductions of TNF-α, IL-6, and IL-8 (Bondeson et al., 2010); however, although macrophages were depleted in our system, the continued presence of OA cartilage likely provided soluble cues maintaining the activated proinflammatory state of the synovial fibroblasts.
Taken together, the model used herein demonstrates the key features of OA. Moreover, selective depletion of synovial macrophages from OA explant cocultures resulted in the attenuation of cartilage tissue destruction demonstrating their critical role in mediating the pathogenesis. In the absence of macrophages, OA chondrocytes and synovial fibroblasts continue to produce proinflammatory mediators; however, the presence of these cytokines was not enough to propagate tissue-level destruction over the time frame investigated.
Considering the critical role that macrophages play in the progression of OA and bearing in mind that these cells can acquire multiple dynamically shifting phenotypes, we wanted to quantitatively define the M1:M2 macrophage ratio in human OA synovium; an evaluation that has yet to be reported in literature. Additionally, we wanted to assess if these ratios were maintained within the in vitro coculture model. Histological analysis of 15 individual OA patient synovial samples immediately following harvest demonstrated an M1: M2 percentage ratio of 7.4:1 indicating the predominance of an M1 phenotype. Total synovial cell number within each sample and the macrophage ratio did not significantly change over 15 days of coculture within each patient sample indicating that the in vitro environment maintained the macrophage phenotype as observed in vivo. Of note, when OA synovium was cultured in the absence of OA cartilage, the number of M1 synovial macrophages dramatically decreased indicating that stimulus from OA cartilage is required to maintain their M1 activated state. Upon depletion of synovial macrophages via clodronate, the M1:M2 percentage ratio decreased to 4.3:1, and the overall number of synovial macrophages decreased by approximately 84% resulting in attenuation of OA cartilage destruction. Although complete elimination of macrophages from the OA micro-environment would not be possible in vivo, results herein suggest that promoting a shift in macrophage polarization towards an M2 phenotype and thus effectively shifting the M1:M2 percentage ratio below that of a 4:1 ratio may be a feasible therapeutic target for mitigating OA. In fact, increasing the relative proportion of M2 to M1 macrophages has demonstrated promise for improving cardiac tissue regeneration in a mouse infarct healing model (Ben-Mordechai et al., 2013). Thus, employing strategies that demonstrate the ability to shift synovial macrophage polarization may be of therapeutic benefit for OA.
Evaluation of stem cells as a potential therapeutic for mitigating OA progression is grounded in their ability to provide chondroprotection and modulate inflammation (Kuroda et al., 2015; Platas et al., 2013). Herein, we illustrate the enhanced chondroprotective effects of hAMSCs as compared with hADSCs when coculture with OA cartilage and synovium. More specifically, direct contact of hAMSCs with OA cartilage resulted in the maintenance of chondrocyte viability and enhanced mitigation of cartilage destruction as indicated by preservation of OA cartilage GAG and HYP content and histopathological scoring. Contrary to the findings of others (Kuroda et al., 2015; Platas et al., 2013), hADSCs did not demonstrate a pronounced chondroprotective effect within the OA coculture system used herein. One plausible explanation for this is that previous studies evaluated hADSCs in simplified culture systems employing supraphysiological levels of either TNF-α or IL-1β alone, respectively (Kuroda et al., 2015; Platas et al., 2013). Because it has been shown that stem cells require priming by inflammatory mediators in order to generate an immunomodulatory and anti-inflammatory effect (Crisostomo et al., 2008), placing hADSCs in an environment with nonphysiological levels of inflammatory mediators could enhance their chondroprotective effect that may not directly correlate with effects observed in our coculture model or in vivo. Thus, our data may suggest that hAMSCs require a lower threshold of inflammatory priming to generate a chondroprotective/anti-inflammatory effect. Further evaluation of this hypothesis is warranted and studies are currently underway in our lab to investigate this hypothesis.
We also demonstrate that hAMSCs more effectively shifted synovial macrophage phenotype as compared with hADSCs in the context of the OA micro-environment that could also result in the observed differences in cartilage destruction between treatment with hAMSCs and hADSCs. This finding was supported by the observed significant increase in the proportion of M2 synovial macrophages in cocultures treated with hAMSCs in direct contact with OA cartilage by Day 15 yielding an M1:M2 macrophage percentage ratio of 1.3:1. Conversely, the M1:M2 macrophage percentage ratio at Day 15 in hADSC treated cocultures was ~5:1. This effective shift in phenotype is further supported by the significantly increased concentration of IL-13, an anti-inflammatory cytokine shown to induce an M2 macrophage shift and that is produced by M2 macrophages themselves (Roszer, 2015), found in the hAMSC direct group. A similar increase in IL-13 was not found when comparing patient-matched OA cocultures with and without hADSCs. ADAMTS-4 and MMP-13 levels in media samples form cocultures treated with hAMSC tended to decrease as well; this was not the case in cocultures with hADSCs. It should be noted that overall synovial cell numbers did not change significantly in any study group over the time-points investigated confirming that the significant decrease in the percentage of M1 macrophages and the concomitant increase in M2 macrophages in the hAMSC cocultures was due to shifting macrophage phenotype and not macrophage death. Similar effects were observed by Abumaree and colleges who found that chorionic membrane derived stem cells were more effective at shifting macrophage polarization towards an M2 phenotype compared with hBMSCs (Abumaree et al., 2013).
Additionally, in regards to the hAMSC direct group, a significant increase in MCP-1 and MIP-1α was also observed in cocultures as compared with patient-matched samples without hAMSCs. However, these values were not statistically different from hADSC treated cocultures or the indirect hAMSC group. Media concentrations of MMP-9 was significantly elevated in cocultures within the direct hAMSC group as compared with patient-matched cocultures without hAMSCs and comparing across the other stem cell treatment groups. This may suggest that the hAMSCs may be attempting to migrate into the OA cartilage as has been previously observed in vivo (Sato et al., 2012); however, further study of this phenomenon is warranted.
Finally, application of hAMSCs in indirect contact was evaluated considering that clinical administration via intra-articular injection likely results in a proportion of those cells depositing on the femoral articular cartilage surface while the remainder home to the synovium (Zachar, Bacenkova, & Rosocha, 2016). Interestingly, the hAMSC indirect group was not as effective at imparting chondroprotection that could be related to cartilage tissue proximity and diffusion limitations of the soluble signals. Additionally, the hAMSC indirect group was not as effective at shifting synovial macrophage polarization towards and M2 phenotype. This was similar to the findings of Abumaree et al. who demonstrated that chorionic stem cells placed in direct contact with human macrophages were the most effective method for shifting macrophage polarization as opposed to indirect contact/stem cell conditioned media (Abumaree et al., 2013). Although it is plausible that the hAMSCs in the indirect group could have extended cell projections through the submicron pores of the trans-well insert, they would likely be in contact with synovial membrane ECM as opposed to the synoviocytes themselves.
As with any studies, limitations were noted herein. First, only two markers were used to discriminate between M1 (CCR7) and M2 (MRC) polarized synovial macrophages. However, these markers have been shown to have the greatest differential expression between M1 and M2 macrophages (Martinez, Gordon, Locati, & Mantovani, 2006). Secondly, it would have been advantageous to represent M1:M2 ratios as raw cell counts; however, patient-to-patient variability in overall synoviocyte number within the biopsies was variable, and thus, the quantitative ratio of M1:M2 macrophages was presented as a percentage of the overall cell number within the representative fields of view. This allows for a quantitative comparative measure across patient samples. Additionally, although we used these ratios to define a proposed therapeutic threshold of M1:M2 macrophage ratio (<4:1), it is possible that overall reductions in macrophage numbers would also be therapeutically beneficial, albeit more difficult to achieve in vivo given the constant blood supply to the synovium. Another perceived limitation was that the direct hAMSC and hADSC groups were not tracked for location or assessed for differences in viability within the coculture condition. However, if viability was in fact different between the study groups, this effect was captured within the studies and would lend further support to the idea that an optimal stem cell source may exist for utilization in the OA micro-environment. Additionally, it would have been beneficial to evaluate the coculture media from the different stem cell treated conditions for additional anti-inflammatory/immunosuppressive paracrine signals include prostaglandin E2, transforming growth factor beta, indoleamine 2,3-dioxygenase, TNF-inducible gene 6 protein, and transforming growth factor beta (Crisostomo et al., 2008; Van Buul et al., 2012), however this can be analysed in future studies. Finally, it should be noted that although this model does recapitulate many of the hallmarks of OA, it lacks some of the dynamic aspects of the disease. This includes continuous mechanical forces commonly borne by the tissues of the knee and involvement of the subchondral bone and local adipose tissue to the progression of OA.
5 | CONCLUSIONS
In conclusion, the in vitro coculture model of patient-matched human OA cartilage and synovium accurately reflects the tissue-level hallmarks and complex cellular signalling milieu commonly observed in the human joint space in vivo and thus serves as valuable tool to evaluate regenerative medicine strategies targeting late-stage OA. We have quantitatively defined the M1:M2 synovial macrophage ratio in human OA synovium and have illustrated the direct impact of synovial macrophages on progressive cartilage destruction. Moreover, we have demonstrated that hAMSCs are more effective at chondroprotection in the OA micro-environment as compared with hADSCs. This was shown to be mediated in part by maintaining enhanced OA chondrocyte viability and more effective shifting of the synovial macrophage M1:M2 percentage ratio below that of a proposed therapeutic threshold value as compared with hADSCs.
Acknowledgments
The authors would like to acknowledge Greenville Health System’s Maternal Fetal Medicine group for assistance with amnion tissue collection. Research reported in this publication was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health (award number 2P20GM103499). The authors of this manuscript have nothing to disclose.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: 2P20GM103499
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
ORCID
Jeremy Mercuri http://orcid.org/0000-0001-8719-6672
References
- Abumaree M, Al Jumah M, Kalionis B, Jawdat D, Al Khaldi A, Abomaray F, Knawy B. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting acrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Reviews. 2013;9(5):620–641. doi: 10.1007/s12015-013-9455-2. [DOI] [PubMed] [Google Scholar]
- Aigner T, Fundel K, Saas J, Gebhard P, Haag J, Weiss T, Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis and Rheumatism. 2006;54(11):3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- Aigner T, Hemmel M, Neureiter D, Gebhard P, Zeiler G, Kirchner T. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritic human articular knee cartilage: A study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis and Rheumatism. 2001;44(6):1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aigner T, Kim H. Apoptosis and cellular vitality issues in osteoarthritic cartilage degeneration. Arthritis and Rheumatism. 2002;46(8):1986–1996. doi: 10.1002/art.10554. [DOI] [PubMed] [Google Scholar]
- Bauer C, Niculescu-Morzsa E, Jeyakumar V, Kern D, Späth S, Nehrer S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. Journal of Inflammation. 2016;13(1):1–9. doi: 10.1186/s12950-016-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekhuizen M, Bastiaansen-Jenniskens Y, Koevoet W, Saris D, Dhert W, Creemers L, Van Osch G. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage. Arthritis and Rheumatism. 2011;63(7):1918–1927. doi: 10.1002/art.30364. [DOI] [PubMed] [Google Scholar]
- Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg M, Abd Elrahman I, Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. Journal of the American College of Cardiology. 2013;62(20):1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Bondeson J, Blom A, Wainwright S, Hughes C, Caterson B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Art. 2010;62(3):647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- Cisternas M, Murphy L, Sacks J, Solomon D, Pasta D, Helmick C. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a US population-based survey. Arthritis Care & Research. 2016;68(5):574–580. doi: 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo P, Wang Y, Markel T, Wang M, Lahm T, Meldrum D, Lahm T. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NFKB—but not JNK—dependent mechanism. Translational Physiology. 2008;46202:675–682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- Goldring M, Marcu K. Review cartilage homeostasis in health and rheumatic diseases. Arthritis Research & Therapy. 2009;11(224):1–16. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley R, Topoluk N, Mercuri J. Tissues reborn: Fetal membrane-derived matrices and stem cells in orthopedic regenerative medicine. Critical Reviews in Biomedical Engineering. 2014;42(3–4):249–270. doi: 10.1615/critrevbiomedeng.2014011591. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kabata T, Hayashi K, Maeda T, Kajino Y, Iwai S, Tsuchiya H. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskeletal Disorders. 2015;16:1–10. doi: 10.1186/s12891-015-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nature Reviews Rheumatology. 2015;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. Aging and osteoarthritis. Current Opinion in Rheumatology. 2012;23(5):492–496. doi: 10.1097/BOR.0b013e3283494005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. The Journal of Immunology. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Maurer M, Von Stebut E. Macrophage inflammatory protein-1. The International Journal of Biochemistry & Cell Biology. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Murray P, Allen J, Biswas S, Fisher E, Gilroy D, Goerdt S, Saeij J. Perspective macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, Okada Y. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathology International. 2007;57(11):703–711. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partsch G, Steiner G, Leeb B, Dunky A, Broll H, Smolen J. Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. The Journal of Rheumatology. 1997;24(3):518–523. [PubMed] [Google Scholar]
- Platas J, Guillén M, Dolores M, Gomar F, Mirabet V, Alcaraz M. Conditioned media from adipose-tissue-derived mesenchymal stem cells downregulate degradative mediators induced by Interleukin-1 beta in osteoarthritic chondrocytes. Mediators of Inflammation. 2013;2013:1–10. doi: 10.1155/2013/357014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, van den Berg WB. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis and Cartilage/OARS, Osteoarthritis Research Society. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Raines A, Shih M, Chua L, Su C, Tseng S, O’Connell J. Efficacy of particulate amniotic membrane and umbilical cord tissues in attenuating cartilage destruction in an osteoarthritis model. Tissue Engineering Part A. 2017;23(1–2):12–19. doi: 10.1089/ten.TEA.2016.0088. [DOI] [PubMed] [Google Scholar]
- Reboul P, Pelletier J, Tardif G, Cloutier J, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. Journal of Clinical Investigation. 2011;26:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators of Inflammation. 2015;2015:1–16. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavedi S, Diaz-Rodriguez P, Erndt-Marino J, Hahn M. A three-dimensional chondrocyte-macrophage oculture system to probe inflammation in experimental osteoarthritis. Tissue Engineering Part A. 2017;23(3–4):101–114. doi: 10.1089/ten.tea.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero A, Watanabe S, Baba H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Research & Therapy. 2012;14(1):R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn D, Sokolove J, Sharpe O, Erhart J, Chandra P, Lahey L, Robinson W. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via toll- like receptor 4. Arthritis Research and Therapy. 2012;14:1–13. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow L, Adlam D, Woolley D. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage associations with degenerative changes. Arthritis and Rheumatism. 2001;44(3):585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Beekhuizen M, Hart M, Radstake T, Dhert W, Saris D, Creemers L. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Research & Therapy. 2014;16(441):1–15. doi: 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buul G, Villafuertes E, Bos P, Waarsing J, Kops N, Narcisi R, Van Osch G. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis and Cartilage. 2012;20(10):1186–1196. doi: 10.1016/j.joca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Vangsness T, Burke W, Narvy S, MacPhee R, Fedenko A. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis. Bulletin of the NYU Hospital for Joint Diseases. 2011;69(2):122–127. [PubMed] [Google Scholar]
- Verma P, Dalal K. ADATMS-4 and ADAMTS-5: Key enzymes in osteoarthritis. Journal of Cellular Biochemistry. 2011;112:3507–3514. doi: 10.1002/jcb.23298. https://doi.org/10.1002/jcb.23298. [DOI] [PubMed] [Google Scholar]
- Wojdasiewicz P, Poniatowski A, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation. 2014;2014:1–19. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Chen T, Chang H, Chiu W, Chang C, Chen R. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. Journal of Cellular Biochemistry. 2007;101(6):1520–1531. doi: 10.1002/jcb.21268. [DOI] [PubMed] [Google Scholar]
- Wu W, Billinghurst R, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole A. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis and Rheumatism. 2002;46(8):2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- Yelin E, Weinstein S, King T. The burden of musculoskeletal diseases in the United States. Seminars in Arthritis and Rheumatism. 2016;46(3):259–260. doi: 10.1016/j.semarthrit.2016.07.013. https://doi.org/10.1016/j.semarthrit.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. Journal of Inflammation Research. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]


