Abstract
Objectives
To compare the value of clinically measured gait speed with that of the self-reported Medical Outcomes Study 36-item Short-Form Survey Physical Function Index (SF-36 PF) in predicting future preclinical mobility disability (PCMD) in older women.
Design
Prospective cohort study.
Setting
Forty clinical centers in the United States.
Participants
Women aged 65 to 79 enrolled in the Women’s Health Initiative Clinical Trials with gait speed and SF-36 assessed at baseline (1993–1998) and follow-up Years 1, 3, and 6 (N=3,587).
Measurements
Women were categorized as nondecliners or decliners based on changes (from baseline to Year 1) in gait speed and SF-36 PF scores. Logistic regression models were used to estimate incident PCMD (gait speed <1.0 m/s) at Years 3 and 6. Area under the receiver operating characteristic curve (AUC) was used to compare the predictive value of SF-36 PF with that of measured gait speed.
Results
Slower baseline gait speed and lower SF-36 PF scores were associated with higher adjusted odds of PCMD at Years 3 and 6 (all P<.001). For gait speed, decliners were 2.59 times as likely to have developed PCMD as nondecliners by Year 3 and 2.35 times as likely by Year 6. Likewise, for SF-36, decliners were 1.42 times as likely to have developed PCMD by Year 3 and 1.49 times as likely by Year 6. Baseline gait speed (AUC=0.713) was nonsignificantly better than SF-36 (AUC=0.705) at predicting PCMD over 6 years (P=.21); including measures at a second time point significantly improved model discrimination for predicting PCMD (all P<.001).
Conclusion
Gait speed identified PCMD risk in older women better than the SF-36 PF did, although the results may be limited given that gait speed served as a predictor and to define the PCMD outcome. Nonetheless, monitoring trajectories of change in mobility are better predictors of future mobility disability than single measures.
Keywords: physical function, performance, disability, prevention, geriatric assessment
Loss of mobility function has been shown to be the first area in which older adults become disabled,1 leading to greater functional decline and poorer quality of life. Defining early stages of mobility decline, or preclinical mobility disability (PCMD), is critical to preventing onset of functionally limiting disability in older adults. Physical function (PF) assessments are therefore an important component in identifying individuals with PCMD.
Gait speed is a commonly used clinical measure for assessing mobility limitations in older adults and has excellent interrater and test–retest reliability,2 sensitivity to change, and predictive validity.3–5 Slower gait speed (<1.0 m/s) has been associated with greater risk of functional decline and morbidity,2,5,6 and faster gait speed (≥1.0 m/s) has been associated with better prognoses.2,6,7 Self-reported assessments of PCMD can be used when clinical testing is not possible.8,1,8,9 The Medical Outcomes Study 36-item Short-Form Physical Function Index (SF-36 PF)5 is a commonly used, validated questionnaire method with high sensitivity to detect functional status, among other aspects of quality of life.10,11
Clinical PCMD and self-reported assessments of mobility disabilities have been shown to be strong determinants of functional status and early morbidity.10–12 It is unclear how measured gait speed and self-reported PF compare in estimating PCMD risk, particularly in well-functioning older adults with no known precursors.13 Furthermore, small changes in SF-36 PF score and gait speed over 1 year may or may not be clinically meaningful for predicting future mobility disability. In the large Women’s Health Initiative Clinical Trials (WHI-CT) cohort of community-dwelling, ambulatory, postmenopausal women, we determined how well baseline gait speed and SF-36 PF, separately, and 1-year change in each measure, predicted future PCMD at 3- and 6-year follow-up.
METHODS
Study Population
The WHI protocol, study design, eligibility criteria, data collection, and outcomes ascertainment and adjudication have been published.14,15 Briefly, the WHI-CT incorporated a diverse sample of 68,132 postmenopausal women enrolled between 1993 and 1998 from 40 U.S. centers. SF-36 PF scores were obtained using self-administered questionnaires for all enrolled participants. Clinical PF measures, including gait speed, were administered in a 25% random sample of CT women aged 65 and older (n=5,962), of whom 5,739 had complete baseline data for gait speed and SF-36 PF. From this subset, 1,046 women were excluded for having a gait speed less than 1.0 m/s at baseline, suggesting prevalent PCMD. Another 182 were excluded because they had missing gait speed and SF-36 PF data at Year 1, 193 because of missing SF-36 PF (but not gait speed) data at Year 1, and 455 because of missing gait speed (but not SF-36 PF) data at Year 1, yielding a final sample size of 3,587, of whom 3,158 (88%) had gait speed data at Year 3 and 2,871 (80%) at Year 6. All women provided informed consent, and appropriate institutional review boards approved protocols.
Retention and Loss to Follow-Up
Women missing Year 3 gait speed data were slower at baseline than women with Year 3 data available (1.23 vs 1.26 m/s; P=.01); comparing women with and without Year 6 data showed the same result (1.23 vs 1.26 m/s; P=.001). On average, women with gait speed data available at Year 3 were younger, had lower body mass index (BMI), reported better health, and had a lower prevalence of comorbidities (diabetes mellitus, cardiovascular disease (CVD)) than those lost to follow-up. Likewise, women with gait speed data at Year 6 were generally younger, reported more physical activity, consumed more alcohol, were less likely to be current smokers, and reported better health and less CVD than those lost to follow-up (data not shown). Gait speed decliners and nondecliners had similar retention at Year 3 (87.3% vs 88.6%; P=.26), but retention rates differed at Year 6 (78.3% vs 81.3%; P=.03).
Gait speed Assessments
Trained, certified study staff assessed timed gait speed (m/s) at baseline and Years 1, 3, and 6 using standard testing protocols. Reliability, sensitivity to change, and predictive validity have been published elsewhere.3,4,16 Gait speed was assessed as the time it took a participant to complete a 6-m walk performed at usual pace and using ambulatory aids as needed. The test was repeated, and the faster of the two measured times was included in this analysis.
SF-36 PF Measures
The 10-item SF-36 PF subscale of the SF-36 was used to subjectively assess PF17 and comprised questions related to four physical domains: general health perceptions, PF (ability to perform vigorous or moderate-intensity activities that one might do in a typical day), bodily pain, and role limitations (problems with work and other regular daily activities because of physical health). PF scores ranged from 0 to 100, with higher scores indicating better PF.17
Defining Decreases in PF and PCMD
Meaningful decreases in SF-36 PF and gait speed outcomes were defined a priori according to conservative thresholds for change based on a consensus panel definition, literature review, and clinical experience: change in usual-pace gait speed, 0.1 m/s (0.2 mph);18,19 change in SF-36 PF, 10 points.20 Participants were dichotomized based on change from baseline to Year 1: decliners of at least the prespecified magnitude versus nondecliners, defined as those who did not meet meaningful change criteria. PCMD was defined at Year 3 and 6 follow-up as usual-pace gait speed less than 1.0 m/s.7,21,22
Other Covariates
Information on baseline demographic characteristics, lifestyle factors, medical history and self-reported general health, falls history, and depression was collected in standard self-report assessments. Anthropometrics were measured at each clinic visit. BMI was calculated as weight (kg)/height (m)2. Data collection methods and reporting for these variables are described more fully elsewhere.15
Statistical Analysis
Baseline demographic and health characteristics of gait speed–change groups were compared using t-tests for continuous variables and chi-square tests for categorical variables. Crude associations between baseline gait speed and SF-36 PF were measured using Pearson correlation. Associations between baseline measures of gait speed or SF-36 PF scores and PCMD were tested using logistic regression for each follow-up year separately, yielding odds ratios (ORs) and 95% confidence intervals (CIs). Analyses were repeated using change in gait speed and SF-36 PF as predictors of incident PCMD between baseline and Year 3 and again for incident PCMD between baseline and Year 6. We included as covariates any measures or characteristics present at baseline that might confound the relationship between PF and PCMD. Thus, three levels of covariate adjustment were applied: age, clinical trial arm(s), and baseline gait speed; age at menopause, race and ethnicity, BMI, physical activity, alcohol use, and smoking; and self-reported general health and history of hypertension, diabetes mellitus, CVD, and arthritis. Finally, receiver operating characteristic (ROC) curves were constructed and tested to determine the level of discrimination and relative predictive power of gait speed and SF-36 PF at baseline and 1-year declines in gait speed and SF-36 scores for prediction of PCMD at 3- and 6-year follow-up. Differences in area under the ROC curve (AUC) between gait speed and SF-36 PF (or including both measures in the same model) were tested using the logistic linear predictors of each model and the roccomp command in Stata (Stata Corp., College Station, TX).
In sensitivity analyses, potential interactions between baseline gait speed or SF-36 score and decliner status on incident PCMD were tested using likelihood ratio tests. Statistical analyses were conducted using Stata version 14.1 (Stata Corp.); all reported P-values are 2-sided.
RESULTS
One thousand five hundred thirty-one (43%) women were categorized as decliners: having 1-year gait speed that was 0.1 m/s or more slower than at baseline, and the remaining 2,056 (57%) were classified as nondecliners (Table 1). The women had a mean age of 69.6±3.6, with gait speed decliners being slightly older (69.9±3.7) than nondecliners (69.4±3.5) at baseline (P<.001). There were no significant differences in age at onset of menopause, lifestyle and behavioral or clinical factors, or medical history of disease between these two groups at baseline, although non-Hispanic white women were less likely than those in other racial and ethnic groups to be gait speed decliners than nondecliners (P<.05). Additionally, baseline gait speeds were higher in gait speed decliners than nondecliners, whereas gait speed nondecliners reported higher SF-36 scores than decliners at baseline (both P<.05).
Table 1.
Baseline Characteristics According to Change in Gait Speed and Medical Outcomes Study 36-Item Short-Form Physical Function Index (SF-36 PF)
| Characteristic | Total, N=3,587 | Gait speed | SF-36 PF | ||
|---|---|---|---|---|---|
| Nondecliner, n=2,056 | Decliner, n=1,531 | Nondecliner, n=2,802 | Decliner, n=785 | ||
| Gait speed, m/s, mean±SDa†,b‡ | 1.25±0.2 | 1.19±0.2 | 1.34±0.2 | 1.26±0.2 | 1.24±0.2 |
| SF-36 PF score, mean±SD a | 80.8±17.5 | 81.3±17.2 | 80.2±18.0 | 81.1±18.0 | 79.9±15.6 |
| Age, mean±SD a†, b‡ | 69.6±3.6 | 69.4±3.5 | 69.9±3.7 | 69.5±3.5 | 69.9±3.8 |
| Age at menopause, years, mean±SD a† | 48.5±6.5 | 48.6±6.4 | 48.3±6.7 | 48.7±6.4 | 47.8±6.9 |
| Race and ethnicity, n (%)a* | |||||
| Non-Hispanic white | 3198 (89.2) | 1859 (90.3) | 1342 (87.7) | 2503 (89.3) | 695 (88.5) |
| Black | 193 (5.38) | 104 (5.1) | 89 (5.8) | 145 (5.2) | 48 (6.1) |
| Other, unknown | 196 (5.46) | 96 (4.7) | 100 (6.5) | 154 (5.5) | 42 (5.4) |
| Education, n (%)b* | |||||
| ≤High school, vocational | 1285 (36.0) | 736 (36.0) | 549 (36.1) | 974 (35.0) | 311 (39.8) |
| Some college, associate degree | 1042 (29.2) | 580 (28.4) | 462 (30.4) | 835 (30.0) | 207 (26.5) |
| ≥College degree | 1238 (34.7) | 728 (35.6) | 510 (33.5) | 974 (35.0) | 264 (33.8) |
| BMI, kg/m2, mean±SD b† | 27.9±5.3 | 27.9±5.5 | 27.9±5.1 | 27.6±5.2 | 29.0±5.7 |
| Physical activity, metabolic equivalent h/wk, mean±SD b‡ | 12.0±13.2 | 11.8±12.9 | 12.3±13.7 | 12.4±13.5 | 10.7±12.3 |
| Self-reported general health, n (%)b,† | |||||
| Excellent | 601 (16.8) | 355 (17.3) | 246 (16.1) | 505 (18.1) | 96 (12.2) |
| Very good | 1594 (44.5) | 898 (43.7) | 696 (45.6) | 1285 (45.9) | 309 (39.4) |
| Good | 1184 (33.0) | 687 (33.4) | 497 (32.5) | 867 (31.0) | 317 (40.4) |
| Fair, poor | 204 (5.7) | 115 (5.6) | 89 (5.8) | 141 (5.0) | 63 (8.0) |
| Depression (CES-D > 0.06) | 266 (7.6) | 152 (7.5) | 114 (7.57) | 207 (7.5) | 59 (7.7) |
| Alcohol use b‡ | |||||
| Nondrinker | 414 (11.6) | 217 (10.6) | 197 (12.9) | 320 (11.5) | 94 (12.1) |
| Past drinker, n (%) | 648 (18.2) | 373 (18.3) | 275 (18.0) | 474 (17.0) | 174 (22.3) |
| < 1 drink/m | 451 (12.6) | 268 (13.1) | 183 (12.0) | 349 (12.5) | 102 (13.1) |
| < 1 drink/wk | 759 (21.3) | 416 (20.4) | 343 (22.5) | 592 (21.2) | 167 (21.4) |
| 1–7 drinks/wk | 869 (24.4) | 507 (24.8) | 362 (23.7) | 709 (25.4) | 160 (20.5) |
| ≥ 7 drinks/wk | 427 (12.0) | 262 (12.8) | 165 (10.8) | 344 (12.3) | 83 (10.6) |
| Smoking, n (%) | |||||
| Never | 1973 (55.6) | 1130 (55.5) | 843 (55.8) | 1561 (56.3) | 412 (53.3) |
| Former | 1414 (39.9) | 812 (39.9) | 602 (39.8) | 1096 (39.5) | 318 (41.1) |
| Current | 161 (4.5) | 94 (4.6) | 67 (4.4) | 118 (4.3) | 43 (5. 6) |
| Hypertension history, n (%) b‡ | |||||
| Never | 2231 (32.9) | 1282 (63.2) | 949 (62.6) | 1788 (64.6) | 443 (57.2) |
| Untreated | 284 (8.01) | 170 (8.4) | 114 (7.5) | 209 (7.6) | 75 (9.7) |
| Treated | 1030 (29.1) | 577 (28.4) | 453 (29.9) | 773 (27.9) | 257 (33.2) |
| History of diabetes, n (%) b* | 173 (4.8) | 109 (5.3) | 64 (4.2) | 124 (4.43) | 49 (6.3) |
| History of CVD, n (%) b‡ | 635 (18.0) | 368 (18.1) | 267 (17.8) | 470 (17.0) | 165 (21.6) |
| History of arthritis, n (%)b,† | 1862 (52.3) | 1050 (51.4) | 812 (53.5) | 1411 (50.7) | 451 (58.0) |
| History of fracture (aged ≥ 55), n (%) | 797 (24.3) | 467 (25.0) | 330 (23.4) | 632 (24.7) | 165 (23.1) |
| Hormone therapy use, n (%) | |||||
| Never | 1511 (42.2) | 855 (41.7) | 656 (42.9) | 1182 (42.2) | 329 (42.0) |
| Former | 1045 (29.2) | 600 (29.2) | 445 (29.1) | 803 (28.7) | 242 (30.9) |
| Current | 1025 (28.6) | 597 (29.1) | 428 (28.0) | 813 (29.1) | 212 (27.1) |
| Falls in past 12 months, n (%) | |||||
| 0 | 2459 (68.9) | 1428 (69.8) | 1031 (67.6) | 1925 (69.0) | 534 (68.4) |
| 1 | 693 (19.4) | 384 (18.8) | 309 (20.3) | 543 (19.5) | 150 (19.2) |
| 2 | 304 (8.51) | 166 (8.12) | 138 (9.0) | 236 (8.5) | 68 (8.7) |
| ≥ 3 | 115 (3.22) | 67 (3.3) | 48 (3.2) | 86 (3.1) | 29 (3.7) |
P-values indicate overall difference between decliners and nondecliners in gait seed, calculated as gait speed change from baseline to 1-year follow-up.
P-values indicate overall difference between no change or increase and decrease in SF-36 groups, calculated as change from baseline to 1-year follow-up.
P<*.05,
.001;
.01.
Chi-square tests used for categorical variables and t-tests for continuous variables.
Missing data in gait speed model: age at menopause (n=167, 4.7%), body mass index (BMI) (n=23, 0.6%), physical activity (n=6, 0.2%), education (n=22, 0.6%), general health (n=4, 0.1%), alcohol (n=19, 0.5%), smoking (n=39, 1.1%), hypertension (n=42, 1.2%), diabetes (n=2, 0.1%), cardiovascular disease (CVD) (n=56, 1.6%), arthritis (n=27, 0.8%), fracture (n=310, 8.6%), hormone therapy use (n=6, 0.2%), falls (n=16, 0.4%), depression (n=64, 1.8%)
Missing data in SF-36 model: age at menopause (n=239, 5.0%), waist (n=12, 0.3%), BMI (n=31, 0.7%), physical activity (n=13, 0.3%), education (n=31, 0.7%), general health (n=6, 0.1%), alcohol (n=28, 0.6%), smoking (n=56, 1.2%), hypertension (n=54, 1.1%), diabetes (n=4, 0.1%), CVD (n=81, 1.7%), arthritis (n=37, 0.8%), fracture (n=416, 8.8%), hormone therapy use (n=10, 0.2%), falls (n=24, 0.5%), depression (n=106, 2.2%).
SD=standard deviation.
Furthermore, 22% of women in this study sample were categorized as SF-36 PF decliners, having Year 1 SF-36 PF scores 10 points or more lower than at baseline (Table 1). Significant differences in age, age at onset of menopause, education, alcohol use, physical activity levels, and anthropometrics were observed in SF-36 PF decliners but not in nondecliners. SF-36 PF decliners had significantly slower gait speeds and a higher prevalence of some chronic diseases than nondecliners (all P<.05).
Associations Between Baseline Gait Speed or SF-36 and PCMD
A 1-standard deviation (SD) (0.21 m/s) faster baseline gait speed was associated with 47% lower odds of PCMD at Year 3 and 35% lower odds at Year 6 (Table 2). Likewise, a 1-SD (17.5 points) higher baseline SF-36 score was associated with 24% lower odds of new PCMD at Year 3 and 31% lower at Year 6 (all P<.001).
Table 2.
Associations Between Baseline Gait Speed or Medical Outcomes Study 36-Item Short-Form Physical Function Index (SF-36 PF) and Incident Preclinical Mobility Disability (<1 m/s) at Year 3 or 6
| Physical Function Measure | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| OR (95% Confidence Interval) | |||
| Year 3 | |||
| Gait speed, m/s | 0.45 (0.40–0.51) | 0.50 (0.44–0.57) | 0.53 (0.47–0.60) |
| SF-36 PF | 0.62 (0.58–0.68) | 0.70 (0.64–0.76) | 0.76 (0.69–0.85) |
| Year 6 | |||
| Gait speed, m/s | 0.58 (0.53–0.64) | 0.63 (0.57–0.70) | 0.65 (0.59–0.72) |
| SF-36 PF | 0.59 (0.54–0.64) | 0.65 (0.59–0.72) | 0.69 (0.62–0.77) |
Odds ratios (ORs) represent the effect for a 1–standard deviation change in each physical function measure.
All estimates are strongly significant (P<.001).
Adjusted for age and clinical trial arm.
Further adjusted for age at menopause, race and ethnicity, body mass index, physical activity, alcohol use, and smoking.
Further adjusted for self-reported general health and history of hypertension, diabetes mellitus, cardiovascular disease, and arthritis
Predicted New PCMD by Decliners vs Nondecliners
For gait speed decliners, the overall cumulative incidence of PCMD (<1.0 m/s) was 29.5% at Year 3 and 39.3% at Year 6; for SF-36 PF decliners, incidence was 33.3% at Year 3 and 44.9% at Year 6 (Supplementary Table S1). Fully adjusted models revealed that gait speed decliners were 2.59 times as likely to have PCMD at Year 3 (95% CI=2.10–3.17) and 2.35 times as likely at Year 6 (95% CI=1.94–2.86) as nondecliners. A similar positive association with incident PCMD was noted for SF-36 PF decliners, who were 1.42 times as likely to have PCMD at Year 3 (95% CI=1.14–1.76) and 1.49 (95% CI=1.20–1.84) times as likely at Year 6.
Gait Speed Versus SF-36 PF in Predicting Future PCMD
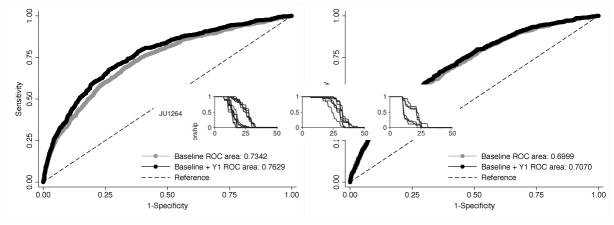
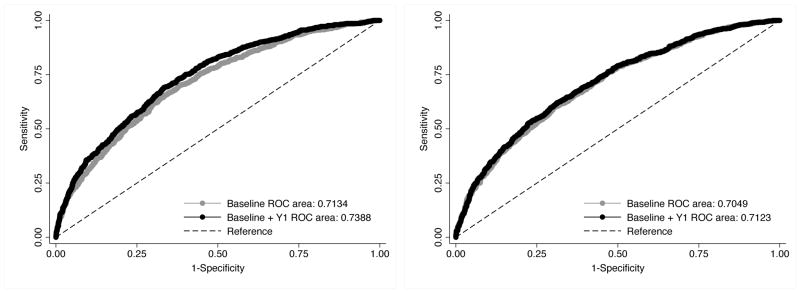
Based on ROC curve analysis, prediction of PCMD at Years 3 and 6 was similar for multivariate models with baseline gait speed (AUC=0.734 and 0.713) and SF-36 (AUC=0.699 and 0.705). These AUCs were significantly different between baseline gait speed and SF-36 at Year 3 (P<.001) but not at Year 6 (P=.21; Supplementary Table S2; Supplementary Figure S1). was by Adding information from the Year 1 SF-36 PF assessment always improved discrimination in predicting PCMD at Year 3 or 6 (all P<.01; Figures 1, 2), and the resulting AUCs significantly differed between gait speed (AUC=0.763 and 0.739) and SF-36 PF (AUC=0.707 and 0.712) at Years 3 and 6 (all P<.001).
Figure 1.
Receiver operating characteristic (ROC) curves for predicting new preclinical mobility disability at Year 3 according to physical function measured at baseline only (gray) or baseline plus 1 year (black). Gait speed, test for difference in ROC curves, P<.001. Medical Outcomes Study 36-Item Short-Form Physical Function Index, test for difference in ROC curves; P=.005.
Figure 2.
Receiver operating characteristic (ROC) curves for predicting new preclinical mobility disability at Year 6 according to physical function measured at baseline only (gray) or baseline plus 1 year (black). Gait speed, test for difference in ROC curves; P<.001. Medical Outcomes Study 36-Item Short-Form Physical Function Index, test for difference in ROC curves; P=.004.
Sensitivity Analyses
No significant interactions were detected between baseline gait speed and baseline SF-36 PF or between gait speed decline and SF-36 PF decline on incident PCMD at Year 3 or 6 (all P>.5).
DISCUSSION
The results of this large, prospective study demonstrate that gait speed is somewhat more strongly associated than SF-36 PF with PCMD risk after 3 and 6 years in ambulatory, community-dwelling women aged 65 and older, supporting its practical application in clinical settings and healthcare research. Nevertheless, the similar predictive ability and discrimination of PCMD using these two metrics suggests that the SF-36 PF is an appropriate, easily obtained surrogate for clinically measured gait speed in screening older women for declining PF and increasing susceptibility to mobility disability over time. Prediction of future PCMD in the present study was significantly stronger when considering measures from two time points than from only one. Thus, our results emphasize the importance of monitoring functional status repeatedly over time to aid clinicians in assessing an individual’s risk of mobility limitations and to guide initiation and intensity of prevention strategies that delay onset of disability in older adults.
Gait speed and other measures of PF tend to decline with advancing age. As expected, 43% of the current study sample experienced declines in gait speed of 0.1m/s or more, and 22% reported SF-36 PF scores of 10 points or more lower over 1 year. Furthermore, women identified as gait speed or SF-36 decliners had a substantial likelihood of having developed PCMD by Years 3 and 6. In fully adjusted models, gait speed decliners had greater PCMD risk at each follow-up (Year 3: OR=2.59; Year 6: OR=2.35) than SF-36 decliners (Year 3: OR=1.42; Year 6: OR=1.49). Similar findings have been reported in other epidemiological studies of older adults.2,7,23 The greater odds of PCMD associated with slowing gait speed than with decline in self-reported PF could be explained, in part, because gait speed was not only an explanatory variable, but was also used to define PCMD.
When evaluating future disability risk in older populations, the approach for selecting the most appropriate functional measure remains unclear.2,9,10,13 In our study, gait speed alone (at baseline or 1-year change) usually performed significantly better in predicting incident PCMD than SF-36 PF, according to the AUC. These findings appear consistent with a previous study2 that recognized gait speed as a “vital sign” for health-related risk of geriatric conditions including “dysmobility” or gait disorders 2,24, but in the present study, single baseline measures of SF-36 PF and gait speed had comparable discrimination between women with and without PCMD (1–3% difference) over 6 years of follow-up, consistent with other investigations,1,8 despite the fact that gait speed and SF-36 PF do not measure the same construct of functional status and disability.9,13 Similar WHI studies have reported that SF-36 performs as well as physical performance measures in defining frailty and predicting risk of falls, hip fracture, and mortality.25
Discrimination of women with future PMCD from those without was nearly 5 percentage points greater for 1-year change in gait speed than SF-36 PF over 6 years. Thus, our findings suggest that gait speed is a more appropriate, more sensitive measure when quantifying risk of incident PCMD, an association that reported declines in SF-36 PF do not modify (Pinteraction>0.2). Hence, routine assessments of gait speed may provide greater value than the SF-36 PF for developing risk profiles underlying various physiological processes (cardiopulmonary or neurological deficits) in nondisabled, healthy older populations and for evaluating an individual’s trajectory from functional independence to disability over time.2,22,26 Regular monitoring of gait speed in older clinical populations may also distinguish presence and severity of subtle functional changes in individuals who do not report functional problems and thus identify persons who may need and use more healthcare services over time.2,27 The SF-36 PF appears to be a suitable alternative measure of gait speed in clinical and epidemiological studies of older women if the primary question is to determine likelihood of future PCMD. Likewise, the SF-36 PF may serve as proxy for clinical gait speed testing if it necessitates additional costs (staff time) or imposes a burden on older adults with other health risks or fatigue.2 Nonetheless, given that difficulties in mobility often precede other age-associated adverse outcomes, including falls, hip fracture, and hospitalization,9,28 the opportune time to intervene in older women may be between baseline and the first year after an initial functional assessment, when the likelihood of promoting recovery and prevent disability onset is high. The utility of baseline self-report and clinical PF and 1-year changes in self-report and clinical PF in predicting future risk of established mobility disability, as well as other health outcomes (all-cause and cause-specific mortality, falls, and hospitalization), will be examined in the WHI.
Strengths and Limitations
This study had several strengths, including the large, prospective study design and availability of clinical and self-reported PF assessments at baseline and 1 year. Furthermore, a 1 m/s cut-point to define new PCMD is based on previous studies that have validated its relevance to functional disability, morbidity, and survival in older adults.6,7,21,22 Some studies have used slower cut-points (≤0.6 or <0.8 m/s)2,7,27,29, which may underestimate risk of future functional disability in individuals with preclinical disability. Our findings suggest that PCMD risk can be identified in older women with faster gait speeds. To our knowledge, a similar criterion for SF-36 PF score that reflects mobility disability has not been established. In our study, we standardized gait speed and SF-36 PF exposures to the standard normal distribution (per 1 SD) to facilitate more direct comparisons of associations with PCMD for each exposure. Alternative measures of subclinical mobility disability (e.g., difficulty walking a one-quarter of a mile, climbing a flight of stairs, performance on a longer walking test) were not available in this cohort. We also acknowledge the possibility of selection bias, because women who were excluded because they were missing Year 1 SF-36 PF or gait speed data (19%) had significantly slower gait speeds than those who were not missing Year 1 measures (data not shown). In addition, self-reported SF-36 PF scores represent personal perspectives of health that mood, expectations, or past experiences that were not included in adjusted analyses may influence differently in different participants.
Conclusion
One-year declines in clinically measured gait speed showed greater sensitivity to change in mobility than SF-36 PF scores alone, although baseline SF-36 PF scores had performance comparable with that of gait speed in discriminating future PMCD cases in older women. The present findings may be limited given that gait speed was used as a primary predictor and to define the PCMD outcome. Nonetheless, clinically measured functional assessments, such as gait speed, may provide additional information regarding the hypothesized mechanism of mobility disability (lower extremity muscle strength) and may thus aid in diagnosis and treatment in clinical settings. Nevertheless, self-reported PF may be a more feasible and practical tool for describing disability risk and prevention in older women when gait speed is not available. The choice to use performance or self-reported measures in clinical and epidemiological studies should be based on the study population, feasibility, and research objectives.9 Additional considerations in healthcare settings should focus on ease and cost of administration in terms of equipment, space, time, patient characteristics, and patient burden (fatigue, physical limitations or disabilities). Regardless of which measure is selected, using two time points, possibly a year or more apart, to assess trajectories of change in mobility provides better clinical value than a single measure in predicting future PMCD.
Supplementary Material
Supplement Figure S1. Receiver operating characteristic (ROC) curves for predicting new pre-clinical mobility disability according to gait speed (gray) or SF-36 (black) at baseline.
Outcome at year 3, test for difference in ROC curves; P<.001
Outcome at year 6, test for difference in ROC curves; P<.21
Supplemental Table S1. Associations between decline (ΔY1-B) in gait speed or SF-36 and pre-clinical mobility disability (<1 m/s) at year 3 or 6
Note: n (%) represents mobility disability prevalence at follow up year;
a Model 1 adjusted for baseline walking speed, age, and clinical trial arm(s)
b Model 2 further adjusted for age at menopause, race/ethnicity, BMI, physical activity, alcohol use, and smoking
c Model 3 further adjusted for self-reported general health and history of hypertension, diabetes, CVD, and arthritis
d Area under ROC curve (AUC) is based on Model 3. Differences in ROC between gait speed decliners and SF-36 decliners and incident mobility disability at year 3 (P<.001), and at year 6 (P=.002)
e All estimates are strongly significant (P<.001) except SF-36 at year 3, model 3 (P=.002)
Supplemental Table S2. Comparing baseline-only to baseline-plus-year-1 modelsa of gait speed and SF-36 scores and pre-clinical mobility disability (<1 m/s) at year 3 or 6, using area under ROC curves
AUC, area under ROC curve; Y1, year 1
a Adjusted for age, clinical trial arms, age at menopause, race/ethnicity, BMI, physical activity, alcohol use, smoking, self-reported general health, and history of hypertension, diabetes, CVD, and arthritis
b P-values represent the difference in AUC of fully adjusted models between baseline alone versus baseline plus year 1 measures
c Differences in AUC between baseline measures of gait speed alone versus SF-36 alone at year 3 (P<.001), and at year 6 (P=.21)
d Differences in ROC between baseline plus year 1 measures of gait speed versus SF-36 at year 3 (P<.001), and at year 6 (P<.001)
Acknowledgments
The authors thank the WHI investigators and staff for their dedication and the study participants for making the program possible. A listing of WHI investigators can be found at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
Footnotes
The research presented in this paper is that of the authors and does not reflect the official policy of the NIH or NHLBI.
Conflict of Interest: The authors declare no conflict of interest and have no financial disclosures to report.
Author Contributions: DRL: study concept and design, data analysis, interpretation of results, writing the manuscript. MLS, BCW, OZ, MJL: consultation on methodology, analysis interpretation. BCW: statistical data analysis, interpretation of results. DOG, NFW, MLJ, BC, HAC, MJL, BC, OZ, JAC, RC, JEM, CAT, MLS: critical revisions to manuscript.
Sponsor’s Role: None.
Financial Disclosure: The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), U.S. Department of Health and Human Services through contracts N268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HSN268201100004C, and HHSN271201100004C.
References
- 1.Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55A:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 2.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: The Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57A:M289–M293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 4.Rosso AL, Lee BK, Stefanick ML, et al. Caregiving frequency and physical function: The Women’s Health Initiative. J Gerontol A Biol Sci Med Sci. 2015;70A:210–215. doi: 10.1093/gerona/glu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 7.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Latham NK, Mehta V, Nguyen AM, et al. Performance-based or self-report measures of physical function: Which should be used in clinical trials of hip fracture patients? Arch Phys Med Rehabil. 2008;89:2146–2155. doi: 10.1016/j.apmr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Syddall HE, Martin HJ, Harwood RH, Cooper C, Aihie Sayer A. The SF-36: A simple, effective measure of mobility-disability for epidemiological studies. J Nutr Health Aging. 2009;13:57–62. doi: 10.1007/s12603-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh JA, Borowsky SJ, Nugent S, et al. Health-related quality of life, functional impairment, and healthcare utilization by veterans: Veterans’ Quality of Life Study. J Am Geriatr Soc. 2005;53:108–113. doi: 10.1111/j.1532-5415.2005.53020.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross-sectional and longitudinal associations between performance and self-report (Zutphen Elderly Study 1990–1993) J Clin Epidemiol. 1996;49:1103–1110. doi: 10.1016/0895-4356(96)00210-7. [DOI] [PubMed] [Google Scholar]
- 14.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 18.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 19.Studenski S. Exercise. In: Landefeld CS, Palmer R, Johnson MA, et al., editors. Current Geriatric Diagnosis and Treatment. New York: McGraw-Hill; 2004. pp. 436–446. [Google Scholar]
- 20.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60A:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 21.Bohannon RW, Williams Andrews A. Normal walking speed: A descriptive meta-analysis. Physiotherapy. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsick EM, Newman AB, Visser M, et al. Mobility limitation in self-described well-functioning older adults: Importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63A:841–847. doi: 10.1093/gerona/63.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouton CP, Espino DV. Health screening in older women. Am Fam Physician. 1999;59:1835–1842. [PubMed] [Google Scholar]
- 25.Zaslavsky O, Zelber-Sagi S, Gray SL, et al. Comparison of frailty phenotypes for prediction of mortality, incident falls, and hip fracture in older women. J Am Geriatr Soc. 2016;64:1858–1862. doi: 10.1111/jgs.14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton A, Fritz SL, Lusardi M. Walking speed: The functional vital sign. J Aging Phys Activity. 2015;23:314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham JE, Fisher SR, Berges IM, Kuo YF, Ostir GV. Walking speed threshold for classifying walking independence in hospitalized older adults. Phys Ther. 2010;90:1591–1597. doi: 10.2522/ptj.20100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Guralnik JM. Disability in older adults: Evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 29.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure S1. Receiver operating characteristic (ROC) curves for predicting new pre-clinical mobility disability according to gait speed (gray) or SF-36 (black) at baseline.
Outcome at year 3, test for difference in ROC curves; P<.001
Outcome at year 6, test for difference in ROC curves; P<.21
Supplemental Table S1. Associations between decline (ΔY1-B) in gait speed or SF-36 and pre-clinical mobility disability (<1 m/s) at year 3 or 6
Note: n (%) represents mobility disability prevalence at follow up year;
a Model 1 adjusted for baseline walking speed, age, and clinical trial arm(s)
b Model 2 further adjusted for age at menopause, race/ethnicity, BMI, physical activity, alcohol use, and smoking
c Model 3 further adjusted for self-reported general health and history of hypertension, diabetes, CVD, and arthritis
d Area under ROC curve (AUC) is based on Model 3. Differences in ROC between gait speed decliners and SF-36 decliners and incident mobility disability at year 3 (P<.001), and at year 6 (P=.002)
e All estimates are strongly significant (P<.001) except SF-36 at year 3, model 3 (P=.002)
Supplemental Table S2. Comparing baseline-only to baseline-plus-year-1 modelsa of gait speed and SF-36 scores and pre-clinical mobility disability (<1 m/s) at year 3 or 6, using area under ROC curves
AUC, area under ROC curve; Y1, year 1
a Adjusted for age, clinical trial arms, age at menopause, race/ethnicity, BMI, physical activity, alcohol use, smoking, self-reported general health, and history of hypertension, diabetes, CVD, and arthritis
b P-values represent the difference in AUC of fully adjusted models between baseline alone versus baseline plus year 1 measures
c Differences in AUC between baseline measures of gait speed alone versus SF-36 alone at year 3 (P<.001), and at year 6 (P=.21)
d Differences in ROC between baseline plus year 1 measures of gait speed versus SF-36 at year 3 (P<.001), and at year 6 (P<.001)