Abstract
As a result of the advancing age of the global population and the progressive increase in lifespan, neurodegenerative disorders continue to increase in incidence throughout the world. New strategies for neurodegenerative disorders involve the novel pathways of the mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) that can modulate pathways of apoptosis and autophagy. The pathways of mTOR and SIRT1 are closely integrated. mTOR forms the complexes mTOR Complex 1 and mTOR Complex 2 and can impact multiple neurodegenerative disorders that include Alzheimer’s disease, Huntington’s disease, and Parkinson’s disease. SIRT1 can control stem cell proliferation, block neuronal injury through limiting programmed cell death, drive vascular cell survival, and control clinical disorders that include dementia and retinopathy. It is important to recognize that oversight of programmed cell death by mTOR and SIRT1 requires a fine degree of precision to prevent the progression of neurodegenerative disorders. Additional investigations and insights into these pathways should offer effective and safe treatments for neurodegenerative disorders.
Neurodegenerative disorders
Neurodegenerative disorders are expected to continue to increase. This is a result of the advancing age of the global population and the progressive increase in lifespan. For example, the incidence of sporadic cases of Alzheimer’s disease (AD) is expected to significantly increase throughout the globe [1–3]. Cognitive disorders such as AD can affect greater than 5 million individuals in the USA alone [1,4]. In addition, ~50 million people suffer from some form of dementia with ~60% of these cases resulting from AD [1,5–7]. The availability of definitive treatments to resolve or prevent the onset of cognitive loss is limited and for the most part such definitive treatments are non-existent [8,9]. Many pathways may lead to cognitive impairment such as cellular injury from β-amyloid (Aβ), tau, excitotoxicity, mitochondrial damage, acetylcholine loss, astrocytic cell injury, oxidative stress, and metabolic dysfunction [10–17]. New strategies for neurodegenerative treatments involve novel pathways of the mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) that can modulate pathways of apoptosis and autophagy.
Programmed cell death
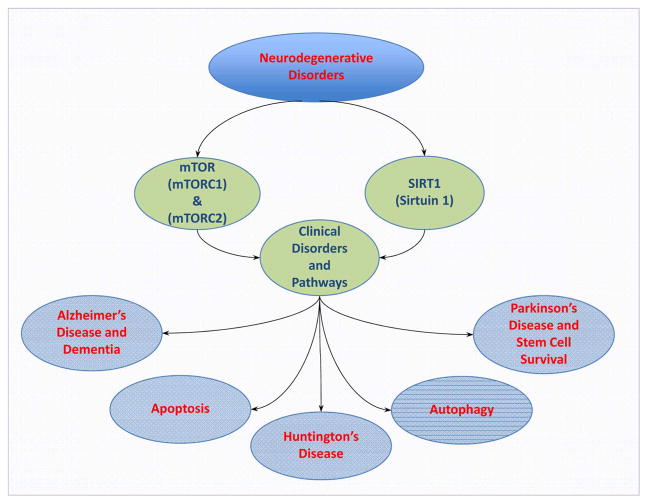
Programmed cell death involves autophagy and apoptosis [18–20] each with different mechanisms [21] (Figure 1). Autophagy recycles components of the cytoplasm in cells for tissue remodeling and eliminates non-functional organelles [18,20,22–24]. Macroautophagy recycles organelles and consists of the sequestration of cytoplasmic proteins and organelles into autophagosomes. Autophagosomes then combine with lysosomes for degradation and recycling [2,25]. Microautophagy involves the invagination of the lysosomal membranes for the sequestration and digestion of cytoplasmic components [26]. Chaperone-mediated autophagy [27] uses cytosolic chaperones to transport cytoplasmic components across lysosomal membranes [28]. Autophagy can be important for clinical aging pathways. Studies with Drosophila demonstrate that neural aggregate accumulation observed with aging is linked to a reduction in the autophagy pathway. These neural aggregates lead to behavior impairments that can be resolved with the maintenance of autophagy pathways in neurons [29]. In addition, autophagy is involved in many degenerative disorders such as cognitive decline [15,16,30], AD [1,31–34], Parkinson’s disease [28,35–37], Huntington’s disease [38–40], diabetes mellitus [15,23,32,41–43], aging processes [44–48], and cardio-renal disease [49]. Autophagy may be particularly important for memory processes in individuals. In some cases, activation of autophagy may decrease neurofibrillary tangles and tau in animal models that suggests a potential treatment for some forms of dementia [31,36,50,51]. Modulation of autophagy also may be important in clinical disorders such as Huntington’s disease to reduce mitochondrial dysfunction and improve motor function [39,40].
Figure 1. Novel treatments for the nervous system.
New treatments for neurodegenerative disorders involve the mTOR and the SIRT1. The pathways of mTOR and SIRT1 are closely aligned to control apoptosis and autophagy. mTOR forms the complexes mTORC1 and mTORC2 and together with SIRT1 can oversee multiple neurodegenerative disorders that include Alzheimer’s disease, dementia, Huntington’s disease, Parkinson’s disease, vascular cell loss, and blockade of stem cell survival.
Apoptosis has an early phase that involves the loss of plasma membrane phosphatidylserine (PS) asymmetry and a later phase that leads to genomic DNA degradation [52–54]. Apoptosis is a process of a series of cascade activation of nucleases and proteases that involve caspases [55,56]. These processes affect both the early phase of apoptosis with the loss of plasma membrane PS asymmetry and a later phase that leads to genomic DNA degradation. Membrane PS asymmetry loss activates inflammatory cells to target, engulf, and remove injured cells [57–60]. Yet, if the engulfment of inflammatory cells can be prevented, functional cells expressing membrane PS residues can be rescued and not be removed from the nervous system [61–64]. Once the destruction of cellular DNA occurs, it is usually not considered to be completely reversible [40]. Apoptosis in the nervous system can be involved in retinal degeneration [62,65], Parkinson’s disease [36,37,66–68], pain sensitivity and neuronal injury [69], Aβ injury [2,70–74], epilepsy [31,75], autism [76], diabetic injury [15,23,42,77–79], and traumatic brain injury [66,80–82].
The mechanistic target of rapamycin
The mTOR is an important pathway during neurodegeneration [83,84] (Figure 1). mTOR is also known as the mammalian target of rapamycin and the FK506-binding protein 12–rapamycin complex-associated protein 1. mTOR governs the transcription of genes, protein formation, proliferation and senescence of cells, cellular metabolism, and cellular longevity [8,17,69,85,86]. mTOR forms the complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [1,31,87,88]. mTORC1 consists of Raptor, the proline-rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR-interacting protein), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8/GβL) [7]. mTORC2 includes Rictor, mLST8, Deptor, the mammalian stress-activated protein kinase-interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) [89–94].
mTOR can modulate diabetes [24,32,66,95–97], neurodegenerative disorders [2,31,36,66,67,98–101], and dementia [11,15,17,102]. mTOR has a significant role in the modulation of autophagy induction [103]. Important in the signaling cascade of mTOR is AMP-activated protein kinase (AMPK). AMPK can prevent mTORC1 activity through the activation of the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex and can lead to the induction of autophagy [34,46,104–106].
mTOR affects neurodegenerative disorders through apoptosis and autophagy [8,18,20]. mTOR activation, in many cases, prevents apoptotic cell death in the nervous system [31,94]. Loss of mTOR activity leads to apoptotic neuronal cell death [107] and aggravation of oxidative stress pathways [108]. mTOR activation can protect against neuronal injury during ischemic preconditioning [109], loss of neurite outgrowth [110], permanent cerebral ischemia [111], cervical spinal cord injury [112], memory loss [113], and Aβ toxicity [11,72–74,114,115].
In contrast with apoptosis in the nervous system, activation of autophagy with the inhibition of mTOR activity can also be neuroprotective [116]. Inhibition of mTOR activity with the induction of autophagy increases cell survival in neonatal models of ischemia [117] and during excitotoxicity [118]. Inhibition of mTOR with autophagy activation results in neural tissue protection and functional improvement in models of spinal cord injury [119]. Autophagy is protective during prion protein disease [120] and as mentioned previously in models of Huntington’s disease [7,121]. In experimental models of AD, disease progression and duration can be associated with dysfunctional autophagic processes as well as inhibition of mTOR activity [122]. Reduction in Aβ production and improved memory function in animal models of AD have been associated with autophagy activation [123].
In some cases, limitations with the induction of autophagy may be required for protection. A reduction in autophagy combined with the activation of mTOR in animal models of traumatic spinal cord injury improves function and increases survival of motor neurons [112]. During ischemic stroke in rodents, blockade of autophagy reduces infarct size and protects cerebral neurons [124]. Autophagy inhibition and activation of mTOR protects dopaminergic neurons during oxidative stress exposure [108]. In tri-cultures of neurons, astrocytes, and microglia that are exposed to inflammatory stressors and Aβ, cell injury rises during autophagy [125]. Autophagy also can impair endothelial progenitor cells, lead to mitochondrial oxidative stress, and block new blood vessel formation during elevated glucose exposure [126]. Cortical interneurons rely upon mTOR activity with reductions in autophagic activity [85]. Trophic factors, such as erythropoietin (EPO), offer protection against hypoxia and oxidative stress in retinal progenitor cells by limiting the induction of autophagy [127]. EPO can prevent neonatal brain damage in the developing rodent during hyperoxia exposure and oxygen toxicity by inhibiting autophagy [128]. Insulin growth factor-1 prevents neuronal injury by preventing the induction of autophagy in Purkinje neurons [129].
Silent mating-type information regulation 2 homolog 1 (Saccharomyces cerevisiae)
mTOR pathways are also dependent on SIRT1 [15,130,131] (Figure 1). SIRT1, a member of the sirtuin family (sirtuin 1), is a histone deacetylase [6,16,40,55,132–135] that can transfer acetyl groups from ε-N-acetyl lysine amino acids onto the histones of DNA to control transcription. Seven identified mammalian homologs of Sir2 include SIRT1 through SIRT7. These histone deacetylases oversee post-translational changes of proteins, cellular proliferation, survival, and senescence. SIRT1 relies upon nicotinamide adenine dinucleotide (NAD+) as a substrate [135–139]. SIRT1 is vital for neurodegenerative disorders [6,140,141] that require the modulation of autophagy and apoptosis [15,27,142,143]. SIRT1 can control stem cell proliferation by modulating autophagic flux [144]. SIRT1 has an inverse relationship with mTOR in embryonic stem cells [46,145] and blocks mTOR to promote autophagy and protect embryonic stem cells during oxidative stress [146]. SIRT1 activation blocks external membrane PS exposure during the early phases of apoptosis in mature cells [58,147–149]. SIRT1 can counteract apoptosis initiated by tumor necrosis factor-α (TNF-α) in endothelial progenitor cells [150]. Loss of SIRT1 expression in endothelial progenitor cells results in apoptotic cell death that can occur in smokers and chronic obstructive disease patients [151]. SIRT1 also drives vascular survival and senescence [77,150,152], cellular metabolism [15,136,145,153–155], atherosclerosis [156–160], lifespan extension [6,161–163], oxidative stress pathways [27,146,155,164–169], neuronal survival and cognition [6,55,170–173], and retinopathy [174].
Future perspectives
Current treatments for neurodegenerative disorders are limited and require novel investigative pathways. Interestingly, mTOR and SIRT1 each offer new directions for the treatment of neurodegenerative disorders. mTOR and SIRT1 also are intimately associated with one another to control the pathways of autophagy and apoptosis. It is important to recognize that oversight of programmed cell death requires a degree of precision that can finely control the level of activation of these pathways such as with autophagy that will block the progression of neurodegenerative disorders rather than worsen these conditions. Additional insights into these pathways should offer effective and safe treatments for neurodegenerative disorders.
Acknowledgments
Funding
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, National Institutes of Health National Institute of Environmental Health Sciences, National Institutes of Health National Institute on Aging, National Institutes of Health National Institute of Neurological Disorders and Stroke, National Institutes of Health American Recovery and Reinvestment Act.
Abbreviations
- AD
Alzheimer’s disease
- AMPK
AMP-activated protein kinase
- Aβ
β-amyloid
- Deptor
DEP domain-containing mTOR-interacting protein
- EPO
erythropoietin
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR Complex 1
- mTORC2
mTOR Complex 2
- PS
phosphatidylserine
Footnotes
Author Contribution
K.M. solely conceived and designed the research, analyzed the results, and completed the writing of the manuscript.
Competing Interests
The Author declares that there are no competing interests associated with the manuscript.
References
- 1.Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46:587–596. doi: 10.3109/07853890.2014.941921. https://doi.org/10.3109/07853890.2014.941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413–1417. doi: 10.4103/1673-5374.139453. https://doi.org/10.4103/1673-5374.139453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schluesener JK, Zhu X, Schluesener HJ, Wang GW, Ao P. Key network approach reveals new insight into Alzheimer’s disease. IET Syst Biol. 2014;8:169–175. doi: 10.1049/iet-syb.2013.0047. https://doi.org/10.1049/iet-syb.2013.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filley CM, Rollins YD, Anderson CA, Arciniegas DB, Howard KL, Murrell JR, et al. The genetics of very early onset Alzheimer disease. Cogn Behav Neurol. 2007;20:149–156. doi: 10.1097/WNN.0b013e318145a8c8. https://doi.org/10.1097/WNN.0b013e318145a8c8. [DOI] [PubMed] [Google Scholar]
- 5.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. https://doi.org/10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Maiese K. SIRT1 and stem cells: in the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7:235–242. doi: 10.4252/wjsc.v7.i2.235. https://doi.org/10.4252/wjsc.v7.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51–60. doi: 10.1016/j.molmed.2012.11.001. https://doi.org/10.1016/j.molmed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res. 2017;14:299–304. doi: 10.2174/1567202614666170718092010. https://doi.org/10.2174/1567202614666170718092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mravec B, Horvathova L, Padova A. Brain under stress and Alzheimer’s disease. Cell Mol Neurobiol. 2018;38:73–84. doi: 10.1007/s10571-017-0521-1. https://doi.org/10.1007/s10571-017-0521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahshin-Majd S, Zamani S, Kiamari T, Kiasalari Z, Baluchnejadmojarad T, Roghani M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: possible involved mechanisms. Peptides. 2016;86:102–111. doi: 10.1016/j.peptides.2016.10.008. https://doi.org/10.1016/j.peptides.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Bellozi PM, Lima IV, Doria JG, Vieira EL, Campos AC, Candelario-Jalil E, et al. Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-β 1-42 induced neurotoxicity and memory impairment. Sci Rep. 2016;6:25226. doi: 10.1038/srep25226. https://doi.org/10.1038/srep25226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement. 2017;13:689–700. doi: 10.1016/j.jalz.2016.10.003. https://doi.org/10.1016/j.jalz.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Rosa M, Malaguarnera L. Chitotriosidase: a new inflammatory marker in diabetic complications. Pathobiology. 2016;83:211–219. doi: 10.1159/000443932. https://doi.org/10.1159/000443932. [DOI] [PubMed] [Google Scholar]
- 14.Hu M, Liu Z, Lv P, Wang H, Zhu Y, Qi Q, et al. Nimodipine activates neuroprotective signaling events and inactivates autophages in the VCID rat hippocampus. Neurol Res. 2017;39:904–909. doi: 10.1080/01616412.2017.1356157. https://doi.org/10.1080/01616412.2017.1356157. [DOI] [PubMed] [Google Scholar]
- 15.Maiese K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen Res. 2016;11:372–385. doi: 10.4103/1673-5374.179032. https://doi.org/10.4103/1673-5374.179032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiese K. Forkhead transcription factors: new considerations for Alzheimer’s disease and dementia. J Transl Sci. 2016;2:241–247. doi: 10.15761/JTS.1000146. https://doi.org/10.15761/JTS.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JA, Lee CH. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. J Vet Sci. 2017;18:11–16. doi: 10.4142/jvs.2017.18.1.11. https://doi.org/10.4142/jvs.2017.18.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. https://doi.org/10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiese K. Autophagy to the rescue. Curr Neurovasc Res. 2017;14:199. doi: 10.2174/1567202614666170724160119. https://doi.org/10.2174/1567202614666170724160119. [DOI] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203–1214. doi: 10.1517/14728222.2012.719499. https://doi.org/10.1517/14728222.2012.719499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–234. doi: 10.1016/j.exger.2010.01.004. https://doi.org/10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Lu Y, Siu HM, Guan J, Zhu L, Zhang S, et al. Identification of novel vacuolin-1 analogues as autophagy inhibitors by virtual drug screening and chemical synthesis. Molecules. 2017;22:E891. doi: 10.3390/molecules22060891. https://doi.org/10.3390/molecules22060891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Rosa M, Distefano G, Gagliano C, Rusciano D, Malaguarnera L. AUTOPHAGY IN DIABETIC RETINOPATHY. Curr Neuropharmacol. 2016;14:810–825. doi: 10.2174/1570159X14666160321122900. https://doi.org/10.2174/1570159X14666160321122900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maiese K. mTOR: driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6:217–224. doi: 10.4239/wjd.v6.i2.217. https://doi.org/10.4239/wjd.v6.i2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White CR, Datta G, Giordano S. High-density lipoprotein regulation of mitochondrial function. Adv Exp Med Biol. 2017;982:407–429. doi: 10.1007/978-3-319-55330-6_22. https://doi.org/10.1007/978-3-319-55330-6_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiese K. Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12:173–188. doi: 10.2174/1567202612666150305110929. https://doi.org/10.2174/1567202612666150305110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiese K. Foxo transcription factors and regenerative pathways in diabetes mellitus. Curr Neurovasc Res. 2015;12:404–413. doi: 10.2174/1567202612666150807112524. https://doi.org/10.2174/1567202612666150807112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener. 2017;12:11. doi: 10.1186/s13024-017-0154-3. https://doi.org/10.1186/s13024-017-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, et al. Aging and autophagic function influences the progressive decline of adult Drosophila behaviors. PLoS ONE. 2015;10:e0132768. doi: 10.1371/journal.pone.0132768. https://doi.org/10.1371/journal.pone.0132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12:379–392. doi: 10.1038/nrneurol.2016.81. https://doi.org/10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 31.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128–148. doi: 10.1016/j.pneurobio.2012.08.001. https://doi.org/10.1016/j.pneurobio.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L. The molecular mechanism of glucagon-like peptide-1 therapy in Alzheimer’s disease, based on a mechanistic target of rapamycin pathway. CNS Drugs. 2017;31:535–549. doi: 10.1007/s40263-017-0431-2. https://doi.org/10.1007/s40263-017-0431-2. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KE, Park JJ. Can co-activation of Nrf2 and neurotrophic signaling pathway slow Alzheimer’s disease? Int J Mol Sci. 2017;18:E1168. doi: 10.3390/ijms18061168. https://doi.org/10.3390/ijms18061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci. 2017;37:2449–2462. doi: 10.1523/JNEUROSCI.3229-16.2017. https://doi.org/10.1523/JNEUROSCI.3229-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang W, Kim HJ, Li H, Jo KD, Lee MK, Yang HO. The neuroprotective effect of erythropoietin on rotenone-induced neurotoxicity in SH-SY5Y cells through the induction of autophagy. Mol Neurobiol. 2016;53:3812–3821. doi: 10.1007/s12035-015-9316-x. https://doi.org/10.1007/s12035-015-9316-x. [DOI] [PubMed] [Google Scholar]
- 36.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82:1245–1266. doi: 10.1111/bcp.12804. https://doi.org/10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, et al. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep. 2016;6:32206. doi: 10.1038/srep32206. https://doi.org/10.1038/srep32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318:33–42. doi: 10.1016/j.yexcr.2011.08.020. https://doi.org/10.1016/j.yexcr.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85:303–315. doi: 10.1016/j.neuron.2014.12.019. https://doi.org/10.1016/j.neuron.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiese K. Foxo proteins in the nervous system. Anal Cell Pathol. 2015;2015:569392. doi: 10.1155/2015/569392. https://doi.org/10.1155/2015/569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurlo T, Rivera JF, Butler AE, Cory M, Hoang J, Costes S, et al. CHOP contributes to, but is not the only mediator of, IAPP induced β-cell apoptosis. Mol Endocrinol. 2016;30:446–454. doi: 10.1210/me.2015-1255. https://doi.org/10.1210/me.2015-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiese K. New insights for oxidative stress and diabetes mellitus. Oxid Med Cell Longev. 2015;2015:875961. doi: 10.1155/2015/875961. https://doi.org/10.1155/2015/875961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi Z, Xia J, Xue X, He Q, Ji L, Ding S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: compared to diet-induced obesity. J Nutr Biochem. 2016;36:31–41. doi: 10.1016/j.jnutbio.2016.07.005. https://doi.org/10.1016/j.jnutbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, et al. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res. 2016;28:303–311. doi: 10.1007/s40520-015-0398-0. https://doi.org/10.1007/s40520-015-0398-0. [DOI] [PubMed] [Google Scholar]
- 45.Maiese K. The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci. 2016;2:185–187. doi: 10.15761/JTS.1000138. https://doi.org/10.15761/JTS.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maiese K. Erythropoietin and mTOR: a “one-two punch” for aging-related disorders accompanied by enhanced life expectancy. Curr Neurovasc Res. 2016;13:329–340. doi: 10.2174/1567202613666160729164900. https://doi.org/10.2174/1567202613666160729164900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milisav I, Poljsak B, Ribaric S. Reduced risk of apoptosis: mechanisms of stress responses. Apoptosis. 2017;22:265–283. doi: 10.1007/s10495-016-1317-3. https://doi.org/10.1007/s10495-016-1317-3. [DOI] [PubMed] [Google Scholar]
- 48.Vaiserman AM, Lushchak OV, Koliada AK. Anti-aging pharmacology: promises and pitfalls. Ageing Res Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. https://doi.org/10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Esterline RL, Vaag A, Oscarsson J, Vora J. MECHANISMS IN ENDOCRINOLOGY: SGLT2 inhibitors; clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. 2018 doi: 10.1530/EJE-17-0832. https://doi.org/10.1530/EJE-17-0832. [DOI] [PubMed]
- 50.Chen X, Kondo K, Motoki K, Homma H, Okazawa H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Sci Rep. 2015;5:12115. doi: 10.1038/srep12115. https://doi.org/10.1038/srep12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frederick C, Ando K, Leroy K, Heraud C, Suain V, Buee L, et al. Rapamycin ester analog CCI-779/Temsirolimus alleviates tau pathology and improves motor deficit in mutant tau transgenic mice. J Alzheimers Dis. 2015;44:1145–1156. doi: 10.3233/JAD-142097. https://doi.org/10.3233/JAD-142097. [DOI] [PubMed] [Google Scholar]
- 52.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-κB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317–1329. doi: 10.1016/j.cellsig.2010.04.009. https://doi.org/10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83:16–26. doi: 10.1016/j.bcp.2011.09.017. https://doi.org/10.1016/j.bcp.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong DZ, Kadir HA, Lee CL, Goh BH. Neuroprotective properties of Loranthus parasiticus aqueous fraction against oxidative stress-induced damage in NG108-15 cells. J Nat Med. 2012;66:544–551. doi: 10.1007/s11418-011-0622-y. https://doi.org/10.1007/s11418-011-0622-y. [DOI] [PubMed] [Google Scholar]
- 55.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–178. doi: 10.1517/14728222.2012.648926. https://doi.org/10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Troy CM, Akpan N, Jean YY. Regulation of caspases in the nervous system implications for functions in health and disease. Prog Mol Biol Transl Sci. 2011;99:265–305. doi: 10.1016/B978-0-12-385504-6.00007-5. https://doi.org/10.1016/B978-0-12-385504-6.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91:601–612. doi: 10.1016/j.exer.2010.07.017. https://doi.org/10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95–112. doi: 10.2174/156720210791184899. https://doi.org/10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shang YC, Chong ZZ, Hou J, Maiese K. Foxo3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009;6:223–238. doi: 10.2174/156720209789630302. https://doi.org/10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/β-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J Mol Neurosci. 2013;49:105–115. doi: 10.1007/s12031-012-9900-8. https://doi.org/10.1007/s12031-012-9900-8. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, et al. Ameliorating the effect of astragaloside IV on learning and memory deficit after chronic cerebral hypoperfusion in rats. Molecules. 2015;20:1904–1921. doi: 10.3390/molecules20021904. https://doi.org/10.3390/molecules20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maiese K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen Res. 2015;10:518–528. doi: 10.4103/1673-5374.155427. https://doi.org/10.4103/1673-5374.155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, et al. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci Rep. 2015;5:7645. doi: 10.1038/srep07645. https://doi.org/10.1038/srep07645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of Schwann cells in vitro. Neurochem Res. 2015;40:698–712. doi: 10.1007/s11064-015-1516-2. https://doi.org/10.1007/s11064-015-1516-2. [DOI] [PubMed] [Google Scholar]
- 65.Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA. Axonal degeneration in retinal ganglion cells is associated with a membrane polarity-sensitive redox process. J Neurosci. 2017;37:3824–3839. doi: 10.1523/JNEUROSCI.3882-16.2017. https://doi.org/10.1523/JNEUROSCI.3882-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2012;13:13830–13866. doi: 10.3390/ijms131113830. https://doi.org/10.3390/ijms131113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G. Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep. 2018;17:131–137. doi: 10.3892/mmr.2017.7897. https://doi.org/10.3892/mmr.2017.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Cen L, Qu S, Wei L, Mo M, Feng J, et al. Enhancing beta-catenin activity via GSK3beta inhibition protects PC12 cells against rotenone toxicity through Nurr1 induction. PLoS ONE. 2016;11:e0152931. doi: 10.1371/journal.pone.0152931. https://doi.org/10.1371/journal.pone.0152931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maiese K. Warming up to new possibilities with the capsaicin receptor TRPV1: mTOR, AMPK, and erythropoietin. Curr Neurovasc Res. 2017;14:184–189. doi: 10.2174/1567202614666170313105337. https://doi.org/10.2174/1567202614666170313105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin N, Xiong LL, Zhang RP, Zheng H, Wang L, Qian ZY, et al. Injection of Abeta1-40 into hippocampus induced cognitive lesion associated with neuronal apoptosis and multiple gene expressions in the tree shrew. Apoptosis. 2016;21:621–640. doi: 10.1007/s10495-016-1227-4. [DOI] [PubMed] [Google Scholar]
- 71.Saleem S, Biswas SC. Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-β-induced neuronal death. J Biol Chem. 2017;292:2571–2585. doi: 10.1074/jbc.M116.744730. https://doi.org/10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 inducible signaling pathway protein 1 (WISP1) targets PRAS40 to govern β-amyloid apoptotic injury of microglia. Curr Neurovasc Res. 2012;9:239–249. doi: 10.2174/156720212803530618. https://doi.org/10.2174/156720212803530618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of β-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging. 2012;4:187–201. doi: 10.18632/aging.100440. https://doi.org/10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10:29–38. doi: 10.2174/156720213804806007. https://doi.org/10.2174/156720213804806007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang BH, Hou Q, Lu YQ, Jia MM, Qiu T, Wang XH, et al. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2018;1678:106–115. doi: 10.1016/j.brainres.2017.10.009. https://doi.org/10.1016/j.brainres.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Liu LM, Ni JF. Rapamycin modulated brain-derived neurotrophic factor and B-cell lymphoma 2 to mitigate autism spectrum disorder in rats. Neuropsychiatr Dis Treat. 2017;13:835–842. doi: 10.2147/NDT.S125088. https://doi.org/10.2147/NDT.S125088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523–535. doi: 10.1111/bph.12496. https://doi.org/10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tulsulkar J, Nada SE, Slotterbeck BD, McInerney MF, Shah ZA. Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity. 2016;24:417–423. doi: 10.1002/oby.21388. https://doi.org/10.1002/oby.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. 2017;61:581–589. doi: 10.1007/s12031-017-0899-8. https://doi.org/10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 80.Maiese K. Charting a course for erythropoietin in traumatic brain injury. J Transl Sci. 2016;2:140–144. doi: 10.15761/jts.1000131. https://doi.org/10.15761/JTS.1000131PMID:27081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye Y, Zhang P, Qian Y, Yin B, Yan M. The effect of pyrroloquinoline quinone on the expression of WISP1 in traumatic brain injury. Stem Cells Int. 2017;2017:4782820. doi: 10.1155/2017/4782820. https://doi.org/10.1155/2017/4782820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S, et al. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017;16:724–736. doi: 10.2174/1871527316666170124164306. https://doi.org/10.2174/1871527316666170124164306. [DOI] [PubMed] [Google Scholar]
- 83.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015;40:107–127. doi: 10.1159/000364974. https://doi.org/10.1159/000364974. [DOI] [PubMed] [Google Scholar]
- 84.Maiese K. Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells. 2015;7:999–1009. doi: 10.4252/wjsc.v7.i7.999. https://doi.org/10.4252/wjsc.v7.i7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ka M, Smith AL, Kim WY. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy. 2017;13:1348–1363. doi: 10.1080/15548627.2017.1327927. https://doi.org/10.1080/15548627.2017.1327927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu X, Li D, Liu J, Diao L, Ling S, Li Y, et al. Dammarane sapogenins ameliorates neurocognitive functional impairment induced by simulated long-duration spaceflight. Front Pharmacol. 2017;8:315. doi: 10.3389/fphar.2017.00315. https://doi.org/10.3389/fphar.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. https://doi.org/10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. https://doi.org/10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–391. doi: 10.4161/oxim.3.6.14787. https://doi.org/10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glidden EJ, Gray LG, Vemuru S, Li D, Harris TE, Mayo MW. Multiple site acetylation of Rictor stimulates mammalian target of rapamycin complex 2 (mTORC2)-dependent phosphorylation of Akt protein. J Biol Chem. 2012;287:581–588. doi: 10.1074/jbc.M111.304337. https://doi.org/10.1074/jbc.M111.304337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10:649–659. doi: 10.1158/1541-7786.MCR-11-0425-T. https://doi.org/10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- 92.Kamarudin MN, Mohd Raflee NA, Syed Hussein SS, Lo JY, Supriady H, Abdul Kadir H. (R)-(+)-α-Lipoic acid protected NG108-15 cells against H2O2-induced cell death through PI3K-Akt/GSK-3β pathway and suppression of NF-κβ-cytokines. Drug Des Devel Ther. 2014;8:1765–1780. doi: 10.2147/DDDT.S67980. https://doi.org/10.2147/DDDT.S67980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res. 2014;11:177–186. doi: 10.2174/1567202611666140408104831. https://doi.org/10.2174/1567202611666140408104831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang Z, Baykal AT, Gao H, Quezada HC, Zhang H, Bereczki E, et al. Mtor is a signaling hub in cell survival: a mass-spectrometry-based proteomics investigation. J Proteome Res. 2014;13:2433–2444. doi: 10.1021/pr500192g. https://doi.org/10.1021/pr500192g. [DOI] [PubMed] [Google Scholar]
- 95.Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. doi: 10.1186/1475-2840-11-45. https://doi.org/10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Burgos-Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors. 2017;43:540–548. doi: 10.1002/biof.1356. https://doi.org/10.1002/biof.1356. [DOI] [PubMed] [Google Scholar]
- 97.Lin Z, Li X, Zhan X, Sun L, Gao J, Cao Y, et al. Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. J Cell Mol Med. 2017;21:3204–3213. doi: 10.1111/jcmm.13224. https://doi.org/10.1111/jcmm.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dijkstra AA, Ingrassia A, de Menezes RX, van Kesteren RE, Rozemuller AJ, Heutink P, et al. Evidence for immune response, axonal dysfunction and reduced endocytosis in the substantia nigra in early stage Parkinson’s disease. PLoS ONE. 2015;10:e0128651. doi: 10.1371/journal.pone.0128651. https://doi.org/10.1371/journal.pone.0128651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo J, Cheng J, North BJ, Wei W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim Biophys Acta. 2017;1868:341–358. doi: 10.1016/j.bbcan.2017.07.001. https://doi.org/10.1016/j.bbcan.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park YS, Park JH, Ko J, Shin IC, Koh HC. mTOR inhibition by rapamycin protects against deltamethrin-induced apoptosis in PC12 cells. Environ Toxicol. 2017;32:109–121. doi: 10.1002/tox.22216. https://doi.org/10.1002/tox.22216. [DOI] [PubMed] [Google Scholar]
- 101.Soltani A, Bahreyni A, Boroumand N, Roshan MK, Khazaei M, Ryzhikov M, et al. Therapeutic potency of mTOR signaling pharmacological inhibitors in the treatment of proinflammatory diseases, current status and perspectives. J Cell Physiol. 2017 doi: 10.1002/jcp.26276. https://doi.org/10.1002/jcp.26276. [DOI] [PubMed]
- 102.Chen L, Zhang Y, Li D, Zhang N, Liu R, Han B, et al. Everolimus (RAD001) ameliorates vascular cognitive impairment by regulating microglial function via the mTORC1 signaling pathway. J Neuroimmunol. 2016;299:164–171. doi: 10.1016/j.jneuroim.2016.09.008. https://doi.org/10.1016/j.jneuroim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Maiese K. Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies. Elsevier and Academic Press; 2016. [Google Scholar]
- 104.Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, et al. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology. 2016;341-343:28–40. doi: 10.1016/j.tox.2016.01.004. https://doi.org/10.1016/j.tox.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Hughes MA, Downs RM, Webb GW, Crocker CL, Kinsey ST, Baumgarner BL. Acute high-caffeine exposure increases autophagic flux and reduces protein synthesis in C2C12 skeletal myotubes. J Muscle Res Cell Motil. 2017;38:201–214. doi: 10.1007/s10974-017-9473-9. https://doi.org/10.1007/s10974-017-9473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang C, Li C, Chen S, Li Z, Ma L, Jia X, et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci Rep. 2017;7:41082. doi: 10.1038/srep41082. https://doi.org/10.1038/srep41082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKα leading to apoptosis of neuronal cells. Lab Invest. 2010;90:762–773. doi: 10.1038/labinvest.2010.36. https://doi.org/10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112:366–376. doi: 10.1111/j.1471-4159.2009.06463.x. https://doi.org/10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- 109.Zare Mehrjerdi F, Aboutaleb N, Habibey R, Ajami M, Soleimani M, Arabian M, et al. Increased phosphorylation of mTOR is involved in remote ischemic preconditioning of hippocampus in mice. Brain Res. 2013;1526:94–101. doi: 10.1016/j.brainres.2013.06.018. https://doi.org/10.1016/j.brainres.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 110.Salto R, Vilchez JD, Giron MD, Cabrera E, Campos N, Manzano M, et al. β-Hydroxy-β-methylbutyrate (HMB) promotes neurite outgrowth in Neuro2a cells. PLoS ONE. 2015;10:e0135614. doi: 10.1371/journal.pone.0135614. https://doi.org/10.1371/journal.pone.0135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404:941–945. doi: 10.1016/j.bbrc.2010.12.085. https://doi.org/10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 112.Walker CL, Walker MJ, Liu NK, Risberg EC, Gao X, Chen J, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS ONE. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. https://doi.org/10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang H, Shi O, Jin Y, Henrich-Noack P, Qiao H, Cai C, et al. Functional protection of learning and memory abilities in rats with vascular dementia. Restor Neurol Neurosci. 2014;32:689–700. doi: 10.3233/RNN-140409. https://doi.org/10.3233/RNN-140409. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Wang YX, Liu T, Law PY, Loh HH, Qiu Y, et al. μ-Opioid receptor attenuates Aβ oligomers-induced neurotoxicity through mTOR signaling. CNS Neurosci Ther. 2015;21:8–14. doi: 10.1111/cns.12316. https://doi.org/10.1111/cns.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao H, Wang ZC, Wang KF, Chen XY. Aβ peptide secretion is reduced by Radix polygalae-induced autophagy via activation of the AMPK/mTOR pathway. Mol Med Report. 2015;12:2771–2776. doi: 10.3892/mmr.2015.3781. https://doi.org/10.3892/mmr.2015.3781. [DOI] [PubMed] [Google Scholar]
- 116.Cai Z, Yan LJ. Rapamycin, autophagy, and Alzheimer’s disease. J Biochem Pharmacol Res. 2013;1:84–90. [PMC free article] [PubMed] [Google Scholar]
- 117.Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury. J Matern Fetal Neonatal Med. 2012;25(suppl 1):30–34. doi: 10.3109/14767058.2012.663176. https://doi.org/10.3109/14767058.2012.663176. [DOI] [PubMed] [Google Scholar]
- 118.Kulbe JR, Mulcahy Levy JM, Coultrap SJ, Thorburn A, Bayer KU. Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res. 2014;1542:12–19. doi: 10.1016/j.brainres.2013.10.032. https://doi.org/10.1016/j.brainres.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sekiguchi A, Kanno H, Ozawa H, Yamaya S, Itoi E. Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma. 2012;29:946–956. doi: 10.1089/neu.2011.1919. https://doi.org/10.1089/neu.2011.1919. [DOI] [PubMed] [Google Scholar]
- 120.Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, et al. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73:99–105. doi: 10.1016/j.neures.2012.03.005. https://doi.org/10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 121.Chan TM, Chen JY, Ho LI, Lin HP, Hsueh KW, Liu DD, et al. ADSC therapy in neurodegenerative disorders. Cell Transplant. 2014;23:549–557. doi: 10.3727/096368914X678445. https://doi.org/10.3727/096368914X678445. [DOI] [PubMed] [Google Scholar]
- 122.Francois A, Rioux-Bilan A, Quellard N, Fernandez B, Janet T, Chassaing D, et al. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J Neuroinflammation. 2014;11:139. doi: 10.1186/s12974-014-0139-x. https://doi.org/10.1186/s12974-014-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, et al. Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci. 2013;33:13138–13149. doi: 10.1523/JNEUROSCI.4790-12.2013. https://doi.org/10.1523/JNEUROSCI.4790-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yin B, Liang H, Chen Y, Chu K, Huang L, Fang L, et al. EGB1212 post-treatment ameliorates hippocampal CA1 neuronal death and memory impairment induced by transient global cerebral ischemia/reperfusion. Am J Chin Med. 2013;41:1329–1341. doi: 10.1142/S0192415X13500894. https://doi.org/10.1142/S0192415X13500894. [DOI] [PubMed] [Google Scholar]
- 125.François A, Terro F, Janet T, Bilan AR, Paccalin M, Page G. Involvement of interleukin-1β in the autophagic process of microglia: relevance to Alzheimer’s disease. J Neuroinflammation. 2013;10:151. doi: 10.1186/1742-2094-10-151. https://doi.org/10.1186/1742-2094-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, et al. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37:1248–1252. doi: 10.1248/bpb.b14-00172. https://doi.org/10.1248/bpb.b14-00172. [DOI] [PubMed] [Google Scholar]
- 127.Sanghera KP, Mathalone N, Baigi R, Panov E, Wang D, Zhao X, et al. The PI3K/Akt/mTOR pathway mediates retinal progenitor cell survival under hypoxic and superoxide stress. Mol Cell Neurosci. 2011;47:145–153. doi: 10.1016/j.mcn.2011.03.010. https://doi.org/10.1016/j.mcn.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 128.Bendix I, Schulze C, Haefen C, Gellhaus A, Endesfelder S, Heumann R, et al. Erythropoietin modulates autophagy signaling in the developing rat brain in an in vivo model of oxygen-toxicity. Int J Mol Sci. 2012;13:12939–12951. doi: 10.3390/ijms131012939. https://doi.org/10.3390/ijms131012939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bains M, Zaegel V, Mize-Berge J, Heidenreich KA. IGF-I stimulates Rab7-RILP interaction during neuronal autophagy. Neurosci Lett. 2011;488:112–117. doi: 10.1016/j.neulet.2010.09.018. https://doi.org/10.1016/j.neulet.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma L, Dong W, Wang R, Li Y, Xu B, Zhang J, et al. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015;116:67–72. doi: 10.1016/j.brainresbull.2015.06.004. https://doi.org/10.1016/j.brainresbull.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 131.Maiese K. MicroRNAs and SIRT1: a strategy for stem cell renewal and clinical development? J Transl Sci. 2015;1:55–57. [PMC free article] [PubMed] [Google Scholar]
- 132.Charles S, Raj V, Arokiaraj J, Mala K. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol Res. 2017;119:1–11. doi: 10.1016/j.phrs.2017.01.022. https://doi.org/10.1016/j.phrs.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 133.Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28–38. doi: 10.1016/j.toxlet.2017.04.018. https://doi.org/10.1016/j.toxlet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 134.Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Sci China Life Sci. 2017;60:1234–1241. doi: 10.1007/s11427-016-9027-6. https://doi.org/10.1007/s11427-016-9027-6. [DOI] [PubMed] [Google Scholar]
- 135.Hwang ES, Song SB. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol Life Sci. 2017;74:3347–3362. doi: 10.1007/s00018-017-2527-8. https://doi.org/10.1007/s00018-017-2527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bruckbauer A, Banerjee J, Cao Q, Cui X, Jing J, Zha L, et al. Leucine-nicotinic acid synergy stimulates AMPK/Sirt1 signaling and regulates lipid metabolism and lifespan in Caenorhabditis elegans, and hyperlipidemia and atherosclerosis in mice. Am J Cardiovasc Dis. 2017;7:33–47. [PMC free article] [PubMed] [Google Scholar]
- 137.Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012;8:89–100. doi: 10.2217/fca.11.76. https://doi.org/10.2217/fca.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li WY, Ren JH, Tao NN, Ran LK, Chen X, Zhou HZ, et al. The SIRT1 inhibitor, nicotinamide, inhibits hepatitis B virus replication in vitro and in vivo. Arch Virol. 2016;161:621–630. doi: 10.1007/s00705-015-2712-8. https://doi.org/10.1007/s00705-015-2712-8. [DOI] [PubMed] [Google Scholar]
- 139.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52:1173–1185. [PMC free article] [PubMed] [Google Scholar]
- 140.Duan W. Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci. 2013;5:36. doi: 10.3389/fnagi.2013.00036. https://doi.org/10.3389/fnagi.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martin A, Tegla CA, Cudrici CD, Kruszewski AM, Azimzadeh P, Boodhoo D, et al. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol Res. 2015;61:187–197. doi: 10.1007/s12026-014-8557-5. https://doi.org/10.1007/s12026-014-8557-5. [DOI] [PubMed] [Google Scholar]
- 142.Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract. 2016;212:310–318. doi: 10.1016/j.prp.2016.02.001. https://doi.org/10.1016/j.prp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 143.Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S, et al. MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget. 2015;6:34446–34457. doi: 10.18632/oncotarget.5920. https://doi.org/10.18632/oncotarget.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mazzoccoli G, Tevy MF, Borghesan M, Delle Vergini MR, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol. 2014;50:137–148. doi: 10.1016/j.exger.2013.10.014. https://doi.org/10.1016/j.exger.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 145.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8:35–48. doi: 10.1517/17460441.2013.736485. https://doi.org/10.1517/17460441.2013.736485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183–1194. doi: 10.1002/stem.1641. https://doi.org/10.1002/stem.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, et al. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/β-catenin-Foxo3a axis. Scientific World Journal. 2014;2014:937051. doi: 10.1155/2014/937051. https://doi.org/10.1155/2014/937051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220–235. doi: 10.2174/156720211796558069. https://doi.org/10.2174/156720211796558069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol Lett. 2013;6:600–604. doi: 10.3892/ol.2013.1400. https://doi.org/10.3892/ol.2013.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, et al. Simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med. 2014;34:177–182. doi: 10.3892/ijmm.2014.1740. https://doi.org/10.3892/ijmm.2014.1740. [DOI] [PubMed] [Google Scholar]
- 151.Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. doi: 10.1002/stem.1488. https://doi.org/10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chiara B, Ilaria C, Antonietta C, Francesca C, Marco M, Lucia A, et al. SIRT1 inhibition affects angiogenic properties of human MSCs. BioMed Res Int. 2014;2014:783459. doi: 10.1155/2014/783459. https://doi.org/10.1155/2014/783459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G. Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J Biol Chem. 2016;291:23318–23329. doi: 10.1074/jbc.M116.737114. https://doi.org/10.1074/jbc.M116.737114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maiese K. Harnessing the power of SIRT1 and non-coding RNAs in vascular disease. Curr Neurovasc Res. 2017;14:82–88. doi: 10.2174/1567202613666161129112822. https://doi.org/10.2174/1567202613666161129112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saboori S, Koohdani F, Nematipour E, Yousefi Rad E, Saboor-Yaraghi AA, Javanbakht MH, et al. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1α and serum antioxidant enzymes in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2016;26:489–494. doi: 10.1016/j.numecd.2015.11.013. https://doi.org/10.1016/j.numecd.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 156.Hung CH, Chan SH, Chu PM, Tsai KL. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol Nutr Food Res. 2015;59:1905–1917. doi: 10.1002/mnfr.201500144. https://doi.org/10.1002/mnfr.201500144. [DOI] [PubMed] [Google Scholar]
- 157.Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58:1941–1951. doi: 10.1002/mnfr.201400161. https://doi.org/10.1002/mnfr.201400161. [DOI] [PubMed] [Google Scholar]
- 158.Kedenko L, Lamina C, Kedenko I, Kollerits B, Kiesslich T, Iglseder B, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112. doi: 10.1186/s12881-014-0112-7. https://doi.org/10.1186/s12881-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luo XY, Qu SL, Tang ZH, Zhang Y, Liu MH, Peng J, et al. SIRT1 in cardiovascular aging. Clin Chim Acta. 2014;437:106–114. doi: 10.1016/j.cca.2014.07.019. https://doi.org/10.1016/j.cca.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 160.Xu S, Bai P, Little PJ, Liu P. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev. 2014;34:644–675. doi: 10.1002/med.21300. https://doi.org/10.1002/med.21300. [DOI] [PubMed] [Google Scholar]
- 161.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. https://doi.org/10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD+-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075–1085. doi: 10.1111/acel.12273. https://doi.org/10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poljsak B, Milisav I. The NAD(+)-depletion theory of ageing: NAD(+) as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity and health span. Rejuvenation Res. 2016 doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 164.Akasaki Y, Alvarez-Garcia O, Saito M, Caramés B, Iwamoto Y, Lotz MK. FOXO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 2014;66:3349–3358. doi: 10.1002/art.38868. https://doi.org/10.1002/art.38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5:159–170. doi: 10.2174/156720208785425666. https://doi.org/10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Colak Y, Yesil A, Mutlu HH, Caklili OT, Ulasoglu C, Senates E, et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointestin Liver Dis. 2014;23:311–319. doi: 10.15403/jgld.2014.1121.233.yck. https://doi.org/10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- 167.Lamoke F, Shaw S, Yuan J, Ananth S, Duncan M, Martin P, et al. Increased oxidative and nitrative stress accelerates aging of the retinal vasculature in the diabetic retina. PLoS ONE. 2015;10:e0139664. doi: 10.1371/journal.pone.0139664. https://doi.org/10.1371/journal.pone.0139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang F, Hu Y, Xu X, Zhai X, Wang G, Ning S, et al. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res. 2015;194:127–138. doi: 10.1016/j.jss.2014.10.004. https://doi.org/10.1016/j.jss.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Zhang XS, Wu Q, Wu LY, Ye ZN, Jiang TW, Li W, et al. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7:e2416. doi: 10.1038/cddis.2016.292. https://doi.org/10.1038/cddis.2016.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bellanti F, Iannelli G, Blonda M, Tamborra R, Villani R, Romano A, et al. Alterations of clock gene RNA expression in brain regions of a triple transgenic model of Alzheimer’s disease. J Alzheimers Dis. 2017;59:615–631. doi: 10.3233/JAD-160942. https://doi.org/10.3233/JAD-160942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo P, Wang D, Wang X, Feng H, Tang Y, Sun R, et al. Effect and mechanism of fuzhisan and donepezil on the sirtuin 1 pathway and amyloid precursor protein metabolism in PC12 cells. Mol Med Rep. 2016;13:3539–3546. doi: 10.3892/mmr.2016.4957. https://doi.org/10.3892/mmr.2016.4957. [DOI] [PubMed] [Google Scholar]
- 172.Maiese K. Forkhead transcription factors: formulating a FOXO target for cognitive loss. Curr Neurovasc Res. 2017;14:415–420. doi: 10.2174/1567202614666171116102911. https://doi.org/10.2174/1567202614666171116102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun Q, Jia N, Wang W, Jin H, Xu J, Hu H. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-β25-35 in rat cortical neurons. Biochem Biophys Res Commun. 2014;448:89–94. doi: 10.1016/j.bbrc.2014.04.066. https://doi.org/10.1016/j.bbrc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 174.Mishra M, Duraisamy AJ, Kowluru RA. Sirt1- a guardian of the development of diabetic retinopathy. Diabetes. 2018 doi: 10.2337/db17-0996. https://doi.org/10.2337/db17-0996. [DOI] [PMC free article] [PubMed]