Abstract
Background
Type 2 diabetes (T2D) is a risk factor for hepatocellular carcinoma (HCC). However, it is unknown whether T2D duration or additional metabolic comorbidities further contribute to HCC risk.
Methods
From the Nurses’ Health Study (NHS), 120,826 women were enrolled in 1980, and from the Health Professionals Follow-up Study (HPFS), 50,284 men were enrolled in 1986, and followed through 2012. Physician-diagnosed T2D was ascertained at baseline and updated biennially. Cox proportional hazards regression models were used to calculate age- and multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for incident HCC.
Results
Over 32 years of follow-up (4,488,410 person-years), we documented 112 cases of HCC (69 women, 43 men). T2D was associated with an increased HCC risk (multivariable HR 4.59, 95% CI 2.98–7.07), as was an increasing T2D duration (Ptrend<0.001). Compared to non-diabetics, the multivariable HRs for HCC were 2.96 (95% CI 1.57–5.60) for 0 to <2 years; 6.08 (95% CI 2.96–12.50) for 2 to <10 years; and 7.52 (95% CI 3.88–14.58) for ≥10 years. Increasing number of metabolic comorbidities (T2D, obesity, hypertension, dyslipidemia) was associated with increased HCC risk (Ptrend<0.001); compared to individuals without metabolic comorbidity, those with four metabolic comorbidities had an 8.1-fold increased HCC risk (95% CI 2.48–26.7). In T2D, neither insulin use nor oral hypoglycemic use was significantly associated with HCC risk (HR 2.04 [95% CI 0.69–6.09], and HR 1.45 [95% CI 0.69–3.07] respectively).
Conclusions
T2D is independently associated with increased risk for HCC, in two prospective cohorts of U.S. men and women. This risk is enhanced with prolonged diabetes duration and with comorbid metabolic conditions, suggesting the importance of insulin resistance in the pathogenesis of HCC.
Keywords: metabolic syndrome, hepatocellular carcinoma, insulin resistance, hyperglycemia, prevention, prospective cohort
Introduction
An estimated 400 million individuals have diabetes worldwide, among whom 85–95% have type 2 diabetes 1. In the United States, the incidence of diabetes has tripled since the 1980s 2, and accumulating epidemiologic evidence shows that type 2 diabetes (T2D) is associated with an increased risk for numerous cancers, including colon, kidney, breast, pancreas and liver 3–5. Possible mechanisms of diabetes-related carcinogenesis include inflammatory activation, insulin resistance, hyperinsulinemia, and aberrations in insulin-like growth factor-1 (IGF-1) signaling 6, 7.
With an annual worldwide incidence of 500,000 cases/year, hepatocellular carcinoma (HCC) represents the fifth most common malignancy and the second-leading cause of cancer-related death 8. In the United States, 35% of HCC cases are attributable to nonalcoholic fatty liver disease (NAFLD) or the metabolic syndrome9, and the burden of disease is projected to continue to increase, in parallel with the epidemics of diabetes and obesity 10. Despite an accelerating incidence, the prognosis for HCC remains very poor, with an estimated median survival of 11 months, and an overall ratio of mortality to incidence of 0.95 8. As a result, effective preventive strategies are urgently needed to reduce HCC-related morbidity and mortality.
Recent prospective studies have shown that T2D is an independent risk factor for primary liver cancer incidence and mortality 11–15. However, data are limited regarding potential modifiers of HCC risk among those with diabetes. While some studies suggest that risk of HCC is increased in individuals with the metabolic syndrome 16, 17 or a prolonged duration of diabetes 5, 18–20, others have not found such associations 21, 22. Moreover, available studies lack prospective collection of comprehensive lifestyle data with serial assessments over time, which is important to accurately estimate the risk associated with an exposure over a prolonged time 15.
To address this, we prospectively examined the association between T2D, duration of T2D, the influence of co-occurring metabolic conditions, and HCC risk, in a pooled analysis of the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS).
Methods
Study population
The study designs of the NHS and the HPFS cohorts have previously been described 23, 24. Briefly, the NHS was established in 1976 with 121,700 female registered nurses in the United States, aged 30–55 years at baseline; the HPFS was established in 1986 with 51,529 male health professionals, aged 40–75 years at baseline. In both cohorts, participants initially returned a mailed questionnaire detailing their medical history as well as lifestyle and behavioral risk factors for chronic disease. Follow-up questionnaires were subsequently sent every 2 years, and follow-up rates consistently exceed 90% of available person-years 25.
In this study, we defined baseline as 1980 (NHS) and 1986 (HPFS), when specific behavioral data including alcohol consumption and aspirin medication use were first ascertained. Deaths in either cohort were identified through reports from family members in response to follow-up questionnaires, and from the National Death Index. We excluded individuals with any diagnosis of cancer at baseline (excepting non-melanoma skin cancer), and those with missing information on diabetes status or a missing date of diagnosis for either diabetes or HCC. Both the NHS and HPFS studies were approved by the Institutional Review Board of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health (Boston, MA).
Assessment of T2D
On the baseline and subsequent biennial questionnaires, participants were asked if and on what date they had been diagnosed with diabetes by a physician. Self-reported diabetes resulted in a subsequent mailing requesting additional information regarding diagnosis date, diagnostic testing and symptoms. Beginning in 1986 in both cohorts, questionnaires were updated to query the regular use of insulin or oral hypoglycemic medications. T2D diagnosed before 1988 was defined according to the National Diabetes Data Group criteria 26, and using the American Diabetes Association criteria for cases identified after 1998 27. Duration of diabetes was calculated by subtracting the date of diagnosis from the date of the most recent completed questionnaire. The validity of the supplementary questionnaire for T2D diagnosis has been confirmed by medical record review in prior studies in the NHS and HPFS, with positive predictive value exceeding 97% 28, 29.
Assessment of Hepatocellular Carcinoma
In both NHS and HPFS, self-reported diagnoses of liver cancer were obtained from biennial questionnaires, and all participants reporting liver cancer were asked for permission to acquire and review their medical records, relevant imaging and histopathology. A study physician, blinded to exposure information, reviewed all records to confirm HCC diagnoses, and extracted information from the medical charts regarding anatomic features, the presence of underlying cirrhosis diagnosed by histopathology or by appropriate cross-sectional imaging, and the presence of documented viral hepatitis. Subjects whose diagnoses of HCC could not be confirmed (n=5) were excluded from analyses. A total of 112 confirmed HCC cases were documented between 1980–2012, including 43 cases in men, and 69 cases in women. Of these, 47 cases arose in individuals without underlying cirrhosis. We also documented 31 cases of intrahepatic cholangiocarcinoma (ICC).
Assessment of Covariates
Information on age, height, weight, smoking status, alcohol intake, and personal and family medical history was collected at baseline and on each biennial questionnaire. Body mass index (BMI) was computed from each questionnaire using weight in kilograms divided by height in square meters. Self-reported body weight in both cohorts has previously been highly correlated with technician-measured weights (r=0.96) 30. Leisure time physical activity, expressed as the average number of metabolic equivalents (METs) expended per week, was first assessed in 1986. Information on physician-diagnosed hypercholesterolemia was collected at the cohort baseline and updated on each biennial questionnaire. A validation study from a random subset of NHS and HPFS participants demonstrated that self-reported cholesterol was correlated with serum cholesterol levels (Spearman correlation coefficients of 0.56 in NHS and 0.51 in HPFS)23, 31.
Statistical Analysis
Participants were followed prospectively for the diagnosis of HCC from 1980 (NHS) or 1986 (HPFS) through 2012. Person-time for each participant was calculated from the date of return for the initial questionnaire until date of death, HCC diagnosis, last questionnaire returned, or the end of study follow-up (January 31, 2012 in HPFS; June 1, 2012 in NHS), whichever came first. Status of confirmed T2D was ascertained and updated biennially and modeled as a time-dependent variable, to account for any incident diagnosis of diabetes over study follow-up.
To evaluate the association between T2D and HCC risk, we used Cox proportional hazards modeling to calculate age- and multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI). The following covariates were included: age in years, sex, race, family history of diabetes, smoking status (current, former, never), BMI (kg/m2), alcohol intake (grams/day), physical activity (METs/week), regular aspirin use (≥ 2 aspirin tablets per week), and physician-diagnosed dyslipidemia and hypertension. The proportionality assumption was not violated. We observed no heterogeneity in the association of diabetes with HCC in separate analyses of NHS and HPFS (Pheterogeneity=0.14), therefore individual-level data were pooled and we adjusted for cohort in all analyses.
In stratified analyses, we tested for effect modification by putative HCC risk factors including age, sex, BMI, history of hypertension, history of dyslipidemia, smoking status, alcohol consumption and use of regular aspirin. We tested the significance of interaction using the log likelihood ratio test, comparing the model that included cross-classified categories to a model that included these factors as independent variables.
To determine whether metabolic comorbidities are associated with overall HCC risk, we grouped each participant into one of five categories according to number of comorbid metabolic conditions, which included obesity (BMI ≥ 30kg/m2), hypertension, dyslipidemia and T2D. We tested for linear trend by including the number of comorbidities as a continuous term in the final model. We also conducted an exploratory analysis of antidiabetic medications and HCC risk in those with T2D, using 1986 as baseline.
A series of four sensitivity analyses were conducted. First, we excluded any individual diagnosed with HCC within 4 years of a diagnosis of T2D (n=4). Second, we excluded any individual with HCC found to be associated with either HBV or HCV (n=21). Third, we tested whether the association between T2D and liver cancer risk is specific to HCC, by assessing the relationship between T2D and the second most common histological type of primary liver cancer, intrahepatic cholangiocarcinoma (ICC; n=33). Fourth, to test the relationship between diabetes and risk of non-cirrhotic HCC, we excluded any individuals with confirmed cirrhosis (n=23) or unknown cirrhosis status (n=41) at the time of HCC diagnosis.
All P-values were two-tailed and a P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Among 120,826 women in NHS and 50,284 men in HPFS, we documented 112 incident cases of HCC (69 women, 43 men) over 4,488,410 total person-years of follow-up. Table 1 presents the age-adjusted characteristics of the study population by T2D status, in 1996; the midpoint of follow-up was selected to provide the best representation of characteristics during the follow-up period. Compared to non-diabetics, participants with T2D were more likely to have obesity (39.2% vs. 14.6%), hypertension (57.4% vs. 26.1%), dyslipidemia (51.3% vs. 27.8%), and a family history of diabetes (46.7% vs. 22.8%).
Table 1.
Age-standardized characteristics of study participants from NHS and HPFS cohorts in 1996*
| Characteristics1 | No diabetes (N=143,785) | Type 2 diabetes (N=10,110) |
|---|---|---|
| Women, % | 71.0 | 70.2 |
| Age, years, SD | 62.5 (7.9) | 65.7 (7.5) |
| White race, % | 96.6 | 93.9 |
| Body mass index (BMI), kg/m2, SD | 26.2 (4.7) | 29.6 (5.9) |
| Obesity‡, % | 14.6 | 39.2 |
| History of hypertension, % | 26.1 | 57.4 |
| History of dyslipidemia, % | 27.8 | 51.3 |
| Duration of diabetes, years; median [IQR] | -- | 5.3 [0.8–12.0] |
| Smoking status, % | ||
| • Current | 12.0 | 10.2 |
| • Former | 42.2 | 46.4 |
| • Never | 45.8 | 43.4 |
| Physical activity, MET-hours/week, median [IQR] | 11.9 [6.2–27.1] | 11.5 [2.7–18.4] |
| Alcohol intake, grams/day, median [IQR] | 1.8 [0.0–6.9] | 0.9 [0.0–1.8] |
| Family history of diabetes, % | 22.8 | 46.7 |
| Regular aspirin use 2, % | 29.8 | 36.1 |
| Oral antidiabetic medication use 3, % | -- | 36.4 |
| Insulin use, % | -- | 6.9 |
Abbreviations: BMI, body mass index; MET, metabolic equivalents; IQR, interquartile range; SD, standard deviation.
All data reported as percentage (%) or mean±standard deviation (SD), unless noted otherwise. Except for the data on mean of age, all data shown are age-standardized to the age distribution of study participants.
1996 selected to represent population characteristics at the approximate middle of follow-up
Regular aspirin use was defined as the regular use of at least 2 aspirin pills per week
Oral antidiabetic medication use included any hypoglycemic medications taken by mouth, and did not distinguish by individual type of oral antidiabetic agent
Obesity defined as BMI ≥30kg/m2
Association between T2D and HCC
T2D was associated with a statistically significant increased risk of HCC (Table 2). Among women, the age-adjusted HR of incident HCC in diabetics was 5.80 [95% CI 3.49–9.64], compared to non-diabetics. Further adjustment for race, continuous BMI, smoking status, alcohol intake, physical activity, regular aspirin use and family history of diabetes did not materially change the estimated HR (5.49 [95% CI 3.16–9.51]). Among men, the age-adjusted HR of incident HCC associated with diabetes was 3.42 [95% CI 1.74–6.72], and this association remained significant in the fully-adjusted model (HR 3.34 [95% CI 1.64–6.78]). In the pooled cohort, diabetes remained associated with a statistically significant increased risk of HCC (adjusted HR 4.59 [95% CI 2.98–7.07]). Further accounting for hypertension and dyslipidemia in an additional analysis did not substantially alter the results (HR 4.74 [95% CI 3.04–7.37]). In stratified analyses, the positive associations between T2D and HCC were consistent across all pre-specified strata (all P-interactions > 0.05; Table 4).
Table 2.
Diabetes and risk of HCC in women (1980–2012) and men (1986–2012) in pooled NHS and HPFS cohorts (n=150,652)
| No Diabetes | Diabetes | |
|---|---|---|
| Women | ||
|
| ||
| Cases/Person-years | 44/3,173,654 | 25/212,578 |
| Age-adjusted HR (95%CI) | 1 | 5.80 (3.49–9.64) |
| Model 2‡; (95%CI) | 1 | 5.61 (3.33–9.45) |
| Model 3§; HR (95%CI) | 1 | 5.49 (3.16–9.51) |
|
| ||
| Men | ||
|
| ||
| Cases/Person-years | 31/1,018,938 | 12/83,239 |
| Age-adjusted HR (95%CI) | 1 | 3.42 (1.74–6.72) |
| Model 2‡; (95%CI) | 1 | 3.28 (1.65–6.50) |
| Model 3§; HR (95%CI) | 1 | 3.34 (1.64–6.78) |
|
| ||
| Pooled | ||
|
| ||
| Cases/Person-years | 75/4,192,592 | 37/295,818 |
| Age-adjusted HR (95%CI) | 1 | 4.77 (3.18–7.14) |
| Model 2‡; (95%CI) | 1 | 4.59 (3.04–6.92) |
| Model 3§; HR (95%CI) | 1 | 4.59 (2.98–7.07) |
Abbreviations: HCC, hepatocellular carcinoma; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; PY, person-years; HR, Hazard Ratio; CI, confidence interval
Model 2 = age, race (white vs. non-white) and body mass index (continuous kg/m2), assessed as a time-dependent covariate. The combined analysis was additionally adjusted for sex.
Model 3 = Model 2 + alcohol intake (0 – 4.9 g/day, 5–14.9 g/day, ≥15 g/day), smoking status (current vs. prior vs. never), physical activity (< 3 metabolic equivalent (MET)-hours/week, 3 to 8.9 MET-hours/week, ≥ 9 MET-hours/week), regular aspirin use (non-use vs. use of at least 2 aspirin tablets per week), and family history of diabetes (no vs. yes). The combined analysis was additionally adjusted for sex.
Table 4.
Stratified Analyses: Diabetes and Risk of hepatocellular carcinoma in a pooled cohort of women (1980–2012) and men (1986–2012) in the NHS and HPFS populations
| Variable | No. of HCC Cases1 | Multivariable-adjusted HR (95% CI) | P-interaction | |
|---|---|---|---|---|
|
| ||||
| History of Type 2 Diabetes | ||||
| No | Yes | |||
|
| ||||
| Age, years | 0.15 | |||
| • ≤ 65 | 23 | 1 | 1.21 (0.25–5.73) | |
| • > 65 | 89 | 1 | 5.79 (3.64–9.20) | |
|
| ||||
| Cohort | 0.95 | |||
| • HPFS | 43 | 1 | 3.42 (1.68–6.95) | |
| • NHS | 69 | 1 | 5.48 (3.13–9.60) | |
|
| ||||
| BMI, kg/m2 | 0.38 | |||
| • BMI ≤ 25 | 46 | 1 | 3.92 (1.84–8.38) | |
| • 25 < BMI ≤ 30 | 41 | 1 | 6.75 (3.43–13.29) | |
| • BMI > 30 | 25 | 1 | 3.11 (1.31–7.41) | |
|
| ||||
| Smoking status | 0.18 | |||
| • Never | 37 | 1 | 5.89 (2.86–12.13) | |
| • Ever | 75 | 1 | 3.89 (2.25–6.72) | |
|
| ||||
| Alcohol intake, grams/day | 0.42 | |||
| • < 5 grams | 87 | 1 | 4.72 (2.29–9.71) | |
| • ≥ 5 grams | 25 | 1 | 4.27 (2.19–8.31) | |
|
| ||||
| Regular aspirin use* | 0.38 | |||
| • No | 87 | 1 | 4.16 (2.51–6.90) | |
| • Yes | 25 | 1 | 6.58 (2.68–16.13) | |
|
| ||||
| Family history of diabetes | 0.43 | |||
| • No | 80 | 1 | 4.14 (1.94–8.82) | |
| • Yes | 32 | 1 | 4.98 (2.93–8.47) | |
|
| ||||
| Hypertension | 0.19 | |||
| • No | 61 | 1 | 4.43 (2.50–7.85) | |
| • Yes | 51 | 1 | 4.65 (2.36–9.18) | |
|
| ||||
| Dyslipidemia | 0.126 | |||
| • No | 73 | 1 | 3.22 (1.83–5.67) | |
| • Yes | 39 | 1 | 7.01 (3.80–12.93) | |
Abbreviations: HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; HPFS, Health Professionals’ Follow-up Study; NHS, Nurses’ Health Study; BMI, body mass index
Number of cases of hepatocellular carcinoma (HCC), recorded in each strata
Regular aspirin use defined as regular use of ≥ 2 aspirin tablets per week, vs. non-use
Diabetes duration and HCC risk
The magnitude of HCC risk increased significantly with increasing duration of T2D (Ptrend <0.0001) (Table 3). Compared to non-diabetics, the multivariable-adjusted HR for HCC was 2.96 [95% CI 1.57–5.60] for 0 to <2 years of T2D; HR 6.08 [95% CI 2.96–12.5] for 2 to <10 years of T2D; and HR 7.52 [95% CI 3.88–14.6]) for ≥ 10 years of T2D (Table 3).
Table 3.
Duration of Diabetes and Risk of HCC in women (1980–2012) and men (1986–2012) in pooled NHS and HPFS cohorts (n=150,652)
| Duration of Diabetes, years | Ptrend1 | ||||
|---|---|---|---|---|---|
|
| |||||
| Non-diabetic | 0 to < 2 years | 2 to < 10 years | ≥ 10 years | ||
| Cases/Person-Years | 75/4,194,312 | 13/112,087 | 10/90,905 | 14/91,105 | -- |
| Model 1*, HR (95% CI) | 1 | 3.41 (1.83–6.33) | 5.44 (2.74–10.80) | 6.85 (3.74–12.57) | <0.0001 |
| Model 2‡, HR (95% CI) | 1 | 3.01 (1.61–5.66) | 5.76 (2.84–11.68) | 7.21 (3.82–13.59) | <0.0001 |
| Model 3§, HR (95% CI) | 1 | 2.96 (1.57–5.60) | 6.08 (2.96–12.50) | 7.52 (3.88–14.58) | <0.0001 |
Abbreviations: HCC, hepatocellular carcinoma; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; PY, person-years; HR, Hazard Ratio; CI, confidence interval
Model 1 adjusted for age in months.
Model 2 adjusted for age in months, sex, race (white vs. non-white) and body mass index (continuous kg/m2), as a time-dependent covariate.
Model 3 adjusted Model 2 + alcohol intake (0 – 4.9 g/day, 5–14.9 g/day, ≥15 g/day), smoking status (current vs. prior vs. never), physical activity (< 3 metabolic equivalent (MET)-hours/week, 3 to 8.9 MET-hours/week, ≥ 9 MET-hours/week), regular aspirin use (non-use vs. use of at least 2 aspirin tablets per week), and family history of diabetes (no vs. yes). All covariates are updated over time.
P-trend calculated by including duration of diabetes (months, continuous) in the relevant multivariable-adjusted model
Analysis of antidiabetic medications: 1986–2012
Beginning in 1986 for both cohorts, supplementary questionnaires recorded antidiabetic medications (oral agents vs. insulin ± oral agents vs. none). During the follow-up period 1986–2012, 107 cases of incident HCC were confirmed (3,735,585 total person-years). In multivariable-adjusted Model 2 (Table S1), T2D was associated with a statistically significant increase in HCC risk (HR 5.09 [95% CI 3.28–7.91]); this estimate was not altered after further adjustment for use of either oral hypoglycemics (HR 4.78 [95% CI 3.05–7.49]) or insulin (HR 4.54 [95% CI 2.76–7.45]) (Table S1).
We also tested the hypothesis that among diabetics, antidiabetic medications might impact HCC risk (Table S2). After adjusting for age, gender, race and BMI, we observed a trend toward increased risk with insulin, but this did not reach statistical significance (HR 2.04 [95% CI 0.71–6.19]). Oral antidiabetic medications were not significantly associated with HCC risk (HR 1.45 [95% CI 0.69–3.07]).
Analysis of metabolic comorbidities
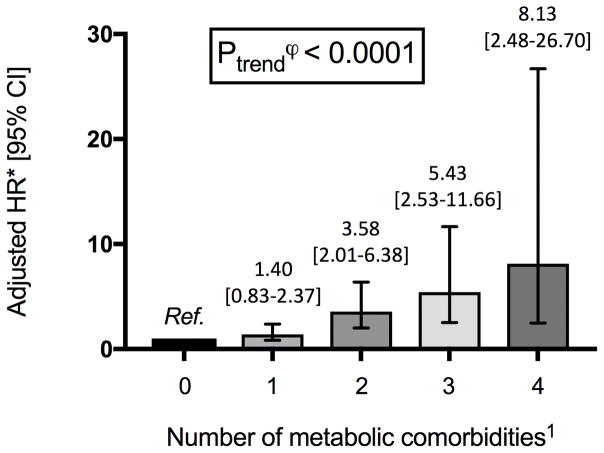
An increasing number of metabolic comorbidities (T2D, obesity [BMI ≥30kg/m2], hypertension and dyslipidemia) was associated with a statistically significant, dose-dependent increase in HCC risk (Ptrend<0.0001) (Figure 1). The greatest HCC risk was observed in individuals with all 4 metabolic comorbidities (HR 8.13 [95% CI 2.48–26.70]), compared to those with no comorbidities (Figure 1).
Figure 1.
Association between increasing number of metabolic comorbid conditions and risk of hepatocellular carcinoma (HCC)
Abbreviations: HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; Ref., reference
1 Metabolic comorbidities include obesity (body mass index, BMI ≥ 30kg/m2), dyslipidemia, hypertension and type 2 diabetes.
ϕ P-trend calculated by including the number of increasing comorbidities as a continuous term, in the fully-adjusted multivariable model.
* Multivariable model adjusted for age (years, continuous), sex, race (white vs. non-white), smoking status (past, current, never), family history of diabetes, alcohol consumption (0 – 4.9 g/day, 5–14.9 g/day, ≥ 15 g/day), physical activity level (metabolic equivalent-hours/week) and regular aspirin use (defined as regular use of ≥ 2 tablets of aspirin/week), with all relevant variables updated over time.
Exploratory and sensitivity analyses
In the first sensitivity analysis, we excluded any case of HCC occurring within 4 years of a T2D diagnosis (n=4 excluded) to minimize reverse causation or detection bias, and our results were similar (adjusted HR 3.99 [95% CI 2.55–6.26]) (Table S3). Second, we excluded any case of HCC associated with underlying HBV or HCV (n=21), and our results were unchanged from the main analysis (HR 4.82 [95% CI 2.97–7.81]) (Table S4). Third, we examined the relationship between T2D and ICC. After accounting for age, sex, race and BMI, T2D was not associated with ICC risk (HR 1.12 [0.38–3.28]) (Table S5).
Finally, we examined the relationship between T2D and risk of non-cirrhotic HCC, of which n=47 cases were documented between 1980–2012. In the final multivariable-adjusted model, T2D was associated with a significantly increased risk of non-cirrhotic HCC (HR 3.05 [95% CI 1.41–6.62]).
Discussion
In two large, prospective cohorts of U.S. men and women, with over 26 year of follow-up, we observed a statistically significant association between the diagnosis of T2D and increased risk for incident HCC. This risk was significantly enhanced in individuals with a prolonged duration of diabetes, and in those with an increasing number of comorbid metabolic conditions. Moreover, the association between diabetes and increased HCC risk remained significant even in those without underlying cirrhosis.
These findings are supported by a growing body of literature demonstrating that T2D is an important HCC risk factor11, 14, including a recent pooled analysis in which T2D was associated with a 2.6-fold increased risk of primary liver cancer 15. Our study extends these data in several important ways. First, we confirmed all cases of HCC, without reliance upon administrative billing codes that may not accurately distinguish HCC from other liver neoplasms, or identify cases of non-cirrhotic HCC. Second, to our knowledge this represents the first large, prospective cohort of men and women to comprehensively account for multiple factors that influence the relationship between T2D and HCC, including diabetes duration, antidiabetic medications, and metabolic comorbidities. Finally, by using prospectively ascertained and serially-updated covariate information, this analysis minimizes exposure misclassification, thus providing a more precise estimation of the magnitude of HCC risk in this population.
Several biologic mechanisms have been proposed to explain the link between T2D and HCC. T2D may promote tumorigenesis via stimulation of IGF-1 signaling, for prolonged insulin resistance and hyperinsulinemia reduce concentrations of IGF binding proteins and increase bioavailable IGF-1 and IGF-II, which stimulate cellular proliferation and inhibit apoptosis 7. IGF overexpression has been shown to promote hepatocarcinogenesis via activation of Wnt signaling through a PI3K/β-catenin mediated pathway 32, and IGF-II/IGF-1 receptor signaling may also enhance self-renewal of hepatic cancer stem cells 33. Additionally, hyperglycemia may further contribute to hepatocarcinogenesis, as accelerated glucose metabolism is a hallmark of many malignancies, including HCC 34, and a recent multinational cohort study found that chronic hyperglycemia related to prolonged type 1 diabetes was similarly associated with increased risk of primary liver cancer35. Finally, it has been shown that in the setting of NAFLD, comorbid diabetes promotes intrahepatic lipid peroxidation and reactive oxygen species formation, which can accelerate DNA damage and culminate in the development of HCC 3, 6, 17.
In this population, we observed a dose-dependent increase in risk for HCC with increasing metabolic comorbidities, supporting a central role for insulin resistance in the pathogenesis of HCC. Each of the comorbidities assessed – obesity, T2D, hyperlipidemia and hypertension – reflect components of the metabolic syndrome, linked through a shared association with overnutrition and insulin resistance 36. Although the association between obesity and HCC risk is well-established 15, less is known about the potential contributions of hypertension or dyslipidemia to the magnitude of that risk, and the limited available data are conflicting 17, 37–39. In one prospective Taiwanese cohort, hypertension was associated with increased HCC-related mortality (HR 2.03 [95% CI 1.53–2.70]) 40, and in two recent analyses of U.S. insurance claims data, positive associations were found between hypertension, dyslipidemia and risk for HCC17, 41. The present study confirms these observations, and further demonstrates in a well-characterized, prospective cohort that increasing metabolic dysfunction is associated with a dose-dependent increase in HCC risk.
In the setting of diabetes or obesity, the pathogenesis of HCC is likely mediated through the progression of NAFLD. Up to 90% of individuals who are diabetic or obese have significant intrahepatic fat accumulation 42, and in longitudinal NAFLD cohorts with paired liver biopsies, the presence of diabetes increases fibrosis progression, while improved glycemic control correlates with fibrosis regression 43. Although HCC is heavily influenced by the presence of underlying cirrhosis, accumulating evidence now shows that nearly 40% of NAFLD-associated HCCs arise in non-cirrhotic livers, particularly in those with evidence of the metabolic syndrome44, 45. To date, however, these data are derived primarily from retrospective studies 44, 46, 47, or from administrative datasets lacking individual patient information or adjudicated outcomes 17, 48, 49. To our knowledge this represents the first prospective analysis utilizing serially-updated exposure information to demonstrate an association between T2D and increased risk for HCC, regardless of underlying cirrhosis status. These data thereby support previous work suggesting that the at-risk population for HCC may be far larger than previously estimated, and highlight the need for future studies to define appropriate strategies for HCC risk stratification and early detection in individuals with and without cirrhosis.
Data are limited regarding the relationship between T2D duration and HCC risk. Most published reports only assess T2D status at one point in time 5, 18–22, an approach that does not capture incident cases arising during study follow-up, and may lead to the attenuation of longitudinal risk estimates. This is particularly relevant when considering the natural history of T2D, which is marked by protracted insulin resistance and compensatory hyperinsulinemia that can precede a formal diagnosis by several years 36. The present study benefitted from prospectively-ascertained and updated data, which provide evidence for an association between an increasing duration of T2D and increased risk of developing HCC.
A body of evidence now suggests that in T2D, antidiabetic medication use may impact HCC risk. In a recent meta-analysis, metformin was associated with a 50% reduction in HCC risk, whereas insulin was associated with a 161% increase in risk 50. This was confirmed in a comparative network meta-analysis of antidiabetic treatments, in which metformin was superior to insulin for HCC risk reduction (RR 0.30, 95% CI 0.18–0.50]) 51. Although we did not find an association between oral antidiabetics and HCC risk reduction, our cohorts lacked information regarding individual oral antidiabetic agents. It is possible that the combination of metformin with oral insulin secretagogues – which likely have opposing effects on HCC risk – resulted in a null association. Given this, we eagerly await future prospective studies with detailed data on individual hypoglycemic agents.
Strengths of this study include a prospective design and a large, well-characterized population, with detailed information on clinical risk factors for HCC, and carefully-adjudicated clinical endpoints28. However, several limitations should be considered. First, participants in these cohorts are predominantly Caucasian healthcare professionals. However, our age-specific incidence of HCC approximates published rates from other U.S. populations 15, and previous studies have demonstrated that the anthropormophic and lifestyle covariates in these cohorts are comparable to the broader population23, 24. Second, this study may be subject to detection bias, if individuals with diabetes are more likely to receive medical attention and come under enhanced surveillance for symptoms suggestive of HCC. However, this seems unlikely as the exclusion of HCC cases arising within 4 years of a T2D diagnosis did not materially impact our results. Third, despite careful adjustment for lifestyle and clinical factors, these cohorts lack information regarding severity of underlying diabetes, thus raising the possibility of confounding by indication. Fourth, we cannot exclude the possibility of residual confounding, particularly as we were unable to ascertain the status of underlying chronic liver disease within the study population. Despite this, the age- and multivariable-adjusted analyses provided similar results, which were not altered after exclusion of individuals with either viral hepatitis-associated HCC or cirrhotic HCC. Finally, we acknowledge that the number of outcomes in certain subgroups is small, however we note that these numbers are similar to other published prospective, epidemiologic studies of HCC.
In conclusion, this study confirms that type 2 diabetes is an independent risk factor for HCC, and demonstrates for the first time in a U.S. population of men and women that the presence of metabolic comorbidities enhance the magnitude of HCC risk, in a dose-dependent fashion. These findings suggest that insulin resistance and the metabolic syndrome play a central role in the pathogenesis of HCC.
Supplementary Material
Acknowledgments
Grant Support:
UM1 CA186107 (Nurses’ Health Study infrastructure grant)
P01 CA87969 (Nurses’ Health Study program grant for cancer research)
UM1 CA167552 (Health Professionals Follow Up Study infrastructure grant)
NIH K24 DK078772 (RTC)
NIH K23DK099422 (KEC)
NIH K23 DK099681 (HK)
NIH T32 CA009001 (TV)
NIH K24 DK098311 (ATC)
NIH K07 CA188126 (XZ)
Career development award, American Gastroenterological Association (HK)
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow Up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data
We also wish to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School.
Footnotes
- Tracey G. Simon: study design, data analysis and interpretation, drafting of the article, critical revision
- Lindsay Y. King: study conception and design, critical revision
- Dawn Q. Chong: data interpretation, critical revision
- Long Nguyen: study design, data interpretation, critical revision
- Yanan Ma: data analysis and interpretation, critical revision
- Trong VoPham: data interpretation, critical revision
- Edward Giovannucci: data interpretation, critical revision
- Charles S. Fuchs: data interpretation, critical revision
- Jeffrey A. Meyerhardt: data interpretation, critical revision
- Kathleen E. Corey: data interpretation, critical revision
- Hamed Khalili: data interpretation, critical revision
- Raymond T. Chung: data interpretation, critical revision
- Xuehong Zhang: data interpretation, critical revision, study oversight
- Andrew T. Chan: data interpretation, critical revision, study oversight
Disclosures and conflicts of interest:
The remaining authors have no disclosures and no conflicts of interest to disclose.
References
- 1.Group IDFDA. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2011. Diabetes Res Clin Pract. 2013;100:277–9. doi: 10.1016/j.diabres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–26. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24:2449–55. doi: 10.1093/annonc/mdt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–65. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 7.Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne) 2014;5:10. doi: 10.3389/fendo.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 9.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–65. doi: 10.1002/cncr.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223–38. doi: 10.1016/j.cld.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130:1639–48. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 12.Lai SW, Chen PC, Liao KF, et al. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 13.Chen HF, Chen P, Li CY. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology. 2010;52:155–63. doi: 10.1002/hep.23641. [DOI] [PubMed] [Google Scholar]
- 14.Koh WP, Wang R, Jin A, et al. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182–8. doi: 10.1038/bjc.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell PT, Newton CC, Freedman ND, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults Cancer Res. 2016;76:6076–6083. doi: 10.1158/0008-5472.CAN-16-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48:172–7. doi: 10.1097/MCG.0b013e3182a030c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–71. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128:635–43. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donadon V, Balbi M, Valent F, et al. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3025–32. doi: 10.3748/wjg.v16.i24.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Vecchia C, Negri E, Decarli A, et al. Diabetes mellitus and the risk of primary liver cancer. Int J Cancer. 1997;73:204–7. doi: 10.1002/(sici)1097-0215(19971009)73:2<204::aid-ijc7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Chlebowski R, Liu S, et al. Diabetes mellitus as a risk factor for gastrointestinal cancers among postmenopausal women. Cancer Causes Control. 2013;24:577–85. doi: 10.1007/s10552-012-9996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan MM, Curley SA, Li D, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–46. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 24.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Stampfer MJ, Colditz GA, et al. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol. 1990;131:1068–71. doi: 10.1093/oxfordjournals.aje.a115598. [DOI] [PubMed] [Google Scholar]
- 26.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 27.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 30.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–2. [PubMed] [Google Scholar]
- 31.Shai I, Rimm EB, Hankinson SE, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110:2824–30. doi: 10.1161/01.CIR.0000146339.57154.9B. [DOI] [PubMed] [Google Scholar]
- 32.Gehmert S, Sadat S, Song YH, et al. The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 (Sfrp2) release. Biochem Biophys Res Commun. 2008;371:752–5. doi: 10.1016/j.bbrc.2008.04.151. [DOI] [PubMed] [Google Scholar]
- 33.Shan J, Shen J, Liu L, et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56:1004–14. doi: 10.1002/hep.25745. [DOI] [PubMed] [Google Scholar]
- 34.Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16:635–49. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carstensen B, Read SH, Friis S, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59:980–8. doi: 10.1007/s00125-016-3884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou JR, Blackburn GL, Walker WA. Symposium introduction: metabolic syndrome and the onset of cancer. Am J Clin Nutr. 2007;86:s817–9. doi: 10.1093/ajcn/86.3.817S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turati F, Talamini R, Pelucchi C, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222–8. doi: 10.1038/bjc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 39.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–61. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang CH, Lee LT, Hung SH, et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59:2207–15. doi: 10.1002/hep.27014. [DOI] [PubMed] [Google Scholar]
- 41.Kasmari AJ, Welch A, Liu G, et al. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med. 2017;130:746.e1–746e7. doi: 10.1016/j.amjmed.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 43.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–55. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–38. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 45.Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–9. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 46.Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601. e1. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee TY, Wu JC, Yu SH, et al. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically non-cirrhotic non-alcoholic fatty liver disease. Int J Cancer. 2017 doi: 10.1002/ijc.30784. [DOI] [PubMed] [Google Scholar]
- 49.Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 50.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–91. doi: 10.1038/ajg.2013.5. quiz 892. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YY, Zhu GQ, Liu T, et al. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci Rep. 2016;6:33743. doi: 10.1038/srep33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.