Abstract
Background/Objectives
Behavioral problems in patients with Alzheimer’s disease (AD) impose major management challenges. Current prevention strategies are anchored to cognitive outcomes but behavioral outcomes may provide another, clinically relevant opportunity for pre-emptive therapy. We sought to determine whether personality changes which predispose to behavioral disorders arise during the transition from preclinical AD to mild cognitive impairment (MCI).
Design
Longitudinal observational cohort study.
Setting
Academic medical center.
Participants
277 members of an apolipoprotein E ε4 (APOE ε4) genetically enriched cohort of Maricopa County residents were neuropsychiatrically healthy at entry. Over a mean interval of 7 years 25 developed MCI and had the NEO Personality Inventory-Revised (NEO-PI-R) before and during the MCI transition epoch and were compared with 252 nontransitioners also with serial NEO-PI-R administrations.
Intervention
Longitudinal administration of the NEO-PI-R (and neuropsychological test battery).
Measurements
Change in NEO-PI-R factor scores (Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness) from entry to either the epoch of MCI diagnosis or an equivalent followup duration in nontransitioners.
Results
NEO-PI-R Neuroticism T-scores increased significantly more in MCI transitioners than in nontransitioners (mean: +2.9; 95% CI: [0.9, 4.9]) vs 0 [−0.7, 0.7], P=.02), and Openness decreased more in MCI transitioners than in nontransitioners (−4.8 [−7.3, −2.4] vs −1.0 [−1.6, −0.4], P<.001). Concurrent subclinical but statistically significant changes in behavioral scores worsened in MCI transitioners relative to nontransitioners on measures of depression, somatization, irritability, anxiety, and aggressive attitude.
Conclusion
Personality and subclinical behavioral changes begin during the transition from preclinical AD to incident MCI, and qualitatively resemble the clinically manifest behavioral disorders that subsequently arise in patients with frank dementia.
Keywords: Aging, Preclinical Alzheimer’s disease, mild cognitive impairment (MCI), personality change, behavioral disorder, NEO-Personality Inventory-Revised
INTRODUCTION
Cognitive loss is the most widely recognized consequence of Alzheimer’s disease (AD), and constitutes the defining feature of its symptomatic onset at the stage of mild cognitive impairment (MCI)1 yet behavioral problems may be the most troubling aspect of dementia care2–5. Analogous to the mild cognitive decline that heralds the symptomatic onset of AD and anticipates the inexorable intellectual decline that subsequently undermines the functional capacity of its victims, behavioral changes might also begin early, even perhaps with the transition from the presymptomatic to the MCI stage anticipating the disruptive behaviors that overburden caregivers and prompt medical intervention.
Behavioral problems are often attributed to, or even equated with a change in a patient’s personality, but a distinction should be drawn between them. Personality describes a tendency toward a certain reaction or behavior, not the reaction or behavior itself. While behaviors adapt to varied situations, personality itself remains stable6. For example, a stress prone individual may feel highly stressed driving in traffic yet may feel no stress while watching television whereas an actively depressed person will be depressed regardless of whether they are driving or watching television. Studies seeking to address the way in which AD affects personality have documented an informant’s (typically a spousal caregiver) impressions of how personality changed from the premorbid to the dementia state. These studies are limited, however, by reliance on a proxy’s recollection of what a dementia patient was like before the onset of dementia, and by confusing the consequence that is the impaired behavior of frankly demented patients with earlier true personality changes that predispose to subsequent behavioral disruption7–19.
If personality begins to change as early as the preclinical or MCI stage of AD, then personality assessment might be a way to identify patients at risk of future behavioral problems possibly facilitating earlier (and potentially more benign) intervention, but existing data are limited20,21. To address this need and overcome prior limitations, we have been prospectively administering the NEO Personality Inventory-Revised (NEO-PI-R)22 to cognitively normal members of the Arizona APOE Cohort who are able to complete their own personality questionnaires to determine whether personality changes during the transition from presymptomatic AD to the earliest symptomatic state, incident MCI.
METHODS
Subjects
From January 1, 1994 through December 31, 2016, cognitively normal residents of Maricopa County age 21 years and older were recruited through local media ads, underwent apolipoprotein E (APOE) genotyping and longitudinal neuropsychological assessment every two years. Determination of APOE genotype was performed using Taqman Single Nucleotide Polymorphism assays.
All identified ε4 homozygotes (HMZ) were matched by age, sex, and education to one ε4 heterozygote (HTZ; all with the ε3/4 genotype) and two ε4 non-carriers. Many additional heterozygous persons and non-carriers who were otherwise eligible for enrollment were also recruited so that roughly half of the cohort represents matched quartos and the remaining members were not matched but otherwise fulfilled entry criteria. Each participant had screening tests that included a medical history, neurological examination, the Folstein Mini-Mental State Exam (MMSE), Hamilton Depression (Ham-D) Rating Scale, Functional Activities Questionnaire (FAQ), Instrumental Activities of Daily Living (IADL), and Structured Psychiatric Interview for DSM-III-R. We excluded anyone with potentially confounding medical, neurologic, or psychiatric problems (essentially any condition that might adversely affect cognitive abilities such as end stage organ disease, stroke, or active major depression). None met published criteria for MCI1, AD23, other forms of dementia or major depressive disorder24. Entry criteria included scores of at least 27 on the MMSE (with at least 1 of 3 on the recall subtest), 10 or less on the Ham-D, and perfect scores on the FAQ and IADL. All individuals gave their written, informed consent to participate in the study and have the results of the APOE test withheld from them which was approved by the Mayo Clinic Institutional Review Board.
Data were reviewed at each visit by a neurologist (RJC) and neuropsychologist (DECL) for indications of cognitive impairment. Amnestic MCI was diagnosed in those individuals who endorsed symptoms of memory loss (corroborated by a close informant), exhibited objective decline from previous performance on neuropsychological tests sensitive to memory, and who met published criteria for MCI1. MCI was first suspected based on collected study data, and such individuals were then invited to complete a clinical neurological evaluation (RJC) that included standard laboratory and radiological assessments following which a formal diagnosis was made and provided to the patient.
Members of the Arizona APOE Cohort with two of more NEO-PI-R administrations were considered for study inclusion. Patients diagnosed with incident amnestic MCI who received the NEO-PI-R prior to developing MCI as well as at the time of MCI diagnosis were identified as MCI transitioners. The youngest MCI transitioner was 54 years old at the time of their first NEO-PI-R administration so a lower age cut-off of 50 years at the time of the initial NEO-PI-R administration was chosen for the control group. Those nontransitioners who received the NEO-PI-R serially but did not develop a cognitive disorder were included as controls. The first and last NEO-PI-R administrations, typically the first and second or third, for each member were used to evaluate change in NEO-PI-R factor and facet scores.
Neuropsychological Assessment
A previously described comprehensive neuropsychological battery was administered every two years25, and is summarized in Table 1. Personality was assessed with the NEO-PI-R which defines personality according to five factors: Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness22. It was added to our battery in 2006, and repeated on alternate visits (roughly every four years). Brief operational definitions of these five factors are as follows (adapted from ref. 26): Neuroticism is a tendency to feel anxiety and other negative emotions, Extraversion is a tendency to be outgoing and lead in social contexts, Openness is a tendency to be receptive to new ideas and experiences, Agreeableness is a tendency to be trusting and deferential, and Conscientiousness is a tendency to be organized and rule abiding. Each factor is comprised of six facets. For example, the six facets of the Neuroticism factor all reflect reactivity to stress and include the tendency to experience anxiety, anger, depression, and self-consciousness; the ability to resist temptations and cravings (impulsivity), and a general ability to cope with stress (vulnerability)22..
Table 1.
Neuropsychology Battery
| Test | Scores Used |
|---|---|
| Memory | |
| Auditory Verbal Learning Test [AVLT] | Total Learning [TL], Long Term Memory [LTM] |
| Buschke Free and Cued Selective Reminding Test | Total free [SRT-free] recall |
| Rey-Osterrieth Complex Figure Test [CFT] | Total recall [CFT-recall] |
| Benton Visual Retention Test [VRT] | Total correct |
| Executive | |
| Wisconsin Card Sorting Test [WCST] | Categories completed |
| Paced Auditory Serial Attention Task 3 [PASAT-3] and 2 [PASAT-2] second versions | Total correct for each |
| Wechsler Adult Intelligence Scale-Revised [WAIS-R] Digit Symbol Substitution [DSS] | Age-scaled score |
| Controlled Oral Word Association Test [COWAT] | Total words raw score |
| Language | |
| Boston Naming Test [BNT] 60 item | Total correct |
| Token Test | Total correct |
| WAIS-R Vocabulary | Age-scaled score |
| WAIS-R Similarities | Age-scaled score |
| Visuospatial | |
| Judgment of Line Orientation [JLO] | Total Correct |
| Facial Recognition Test | Corrected long form score |
| Rey-Osterrieth CFT | Copy score |
| WAIS-R Block Design | Age-scaled score |
| Behavior | |
| Personality Assessment Inventory [PAI] | Clinical (CSc), Treatment (TSc), and Interpersonal (ISc) scale T-scores |
| Beck Depression Inventory [BDI] | Total score |
| Hamilton Depression Scale [Ham-D] | Total Score |
| Geriatric Depression Scale [GDS] | Total Score |
Neuropsychological tests administered and the scores used in this study.
The NEO-PI-R is designed to measure personality traits and not psychological abnormality. The scores on the domain and facet scales represent how much of that particular trait an individual holds and does not imply clinical diagnosis or disorder. Therefore, our neuropsychological battery also includes measures of psychopathology (Table 1) including (in addition to the Ham-D which we use as a screening measure) the Beck Depression Inventory, Geriatric Depression Scale (GDS), and Personality Assessment Inventory (PAI). In contrast to the NEO-PI-R, the PAI is designed to measure clinically significant levels of symptomatology related to “clinical diagnosis, treatment planning and screening for psychopathology” (PAI manual page 5). It was not designed to measure the domains of normal personality27.
Statistical Analysis
Baseline demographics, NEO-PI-R scores, and other neuropsychological scores at the time of the first NEO-PI-R administration were summarized within the groups using means and 95% confidence intervals (CI) or relative frequencies and compared between groups using t-tests and chi-squared tests. NEO-PI-R change scores were computed as the score at last NEO-PI-R administration minus the score at the first administration. Personality Assessment Inventory (PAI) change scores were similarly computed and were derived from the same administrations as the first and last NEO-PI-R administrations. NEO-PI-R and PAI change scores were compared between groups using t-tests. To assess within-subject change, the percentage of subjects meeting various thresholds for meaningful change (5-point increase in Neuroticism or 5-point decline in Openness) were compared between groups using chi-squared tests. In a subsequent analysis to adjust for potential differences between groups in time between administrations, scores at the last visit were compared between groups adjusting for score at the first visit and time interval between administrations using analysis of covariance (ANCOVA). To supplement primary univariate and multivariate statistical testing, a novel graphical approach utilizing a multi-sectional ‘fishbone’ plot displays NEO-PI-R domain- and subdomain-specific effect sizes. P values ≤ .05 were considered statistically significant.. Statistical analysis was carried out using SAS software (SAS Version 9, SAS Institute, Cary, NC).
RESULTS
277 members of the Arizona APOE Cohort met inclusion criteria, of which 25 were incident MCI cases and 252 were nontransitioning controls. Subject characteristics at baseline are summarized in Table 2. There was a higher rate of APOE e4 carrier status (80 vs 38.1%, P<.001) among MCI transitioners compared to nontransitioners, but there was no difference in mean age (mean: 62.9; 95% CI: [62.1, 63.8] years), education (16.1 [15.8, 16.3] years), race/ethnicity (83.4% non-Hispanic white), sex (67.9% female), mean number of NEO-PI-R administrations (2.3 [2.3, 2.4]) or interval between first and last NEO-PI-R administration (78.9 [76.2, 81.7] months) (Table 2). Baseline scores did not differ between MCI transitioners and nontransitioners on any personality (NEO-PI-R factor and facet scores) or behavioral (PAI, Ham-D, Beck, GDS) measure. Neuropsychological and behavioral test performances at baseline, summarized in Supplementary Tables S1 and S2, were normal but characterized by lower scores in the MCI transitioner group on memory related measures such as the Auditory Verbal Learning Test Long Term Memory score (7.0 [5.8, 8.2] vs 9.9 [9.5, 10.3], P<.001).
Table 2.
Group Characteristics at First NEO-PI-R Epoch
| MCI Transitioners | Nontransitioners |
P Value |
|
|---|---|---|---|
|
| |||
| n | 25 | 252 | |
|
| |||
| Age in years, mean (95% CI) | 65.5 (63.1, 67.9) | 62.7 (61.8, 63.6) | .06a |
|
| |||
| Female, n (%) | 13 (52.0%) | 175 (69.4%) | .07b |
|
| |||
| APOE e4 carriers, n (%) | 20 (80.0%) | 96 (38.31%) | <.001b |
|
| |||
| Non-Hispanic White, n (%) | 22 (88.0%) | 209 (82.9%) | .64b |
|
| |||
| Education years, mean (95% CI) | 16.4 (15.3, 17.5) | 16.0 (15.8, 16.3) | .46a |
|
| |||
| Inter-NEO Interval in months, mean (95% CI) | 83.8 (73.6, 93.9) | 78.5 (75.6, 81.3) | .28a |
|
| |||
| Number of NEO Administrations, n (%) | .24b | ||
| 2 | 15 (60.0%) | 177 (70.2%) | |
| 3 | 9 (36.0%) | 73 (29.0%) | |
| 4 | 1 (4.0%) | 2 (0.8%) | |
|
| |||
| Neuroticism Factor, mean (95% CI) | 41.9 (38.7, 45.1) | 42.6 (41.5, 43.7) | .68a |
|
| |||
| Extraversion Factor, mean (95% CI) | 50.8 (47.0, 54.6) | 49.0 (47.9, 50.1) | .34a |
|
| |||
| Openness Factor, mean (95% CI) | 53.1 (49.0, 57.1) | 52.3 (51.0, 53.5) | .70a |
|
| |||
| Agreeableness Factor. mean (95% CI) | 53.3 (49.6, 56.9) | 53.6 (52.6, 54.7) | .84a |
|
| |||
| Conscientiousness Factor, mean (95% CI) | 49.0 (45.5, 52.4) | 51.6 (50.5, 52.7) | .15a |
T-test;
Chi-squared test
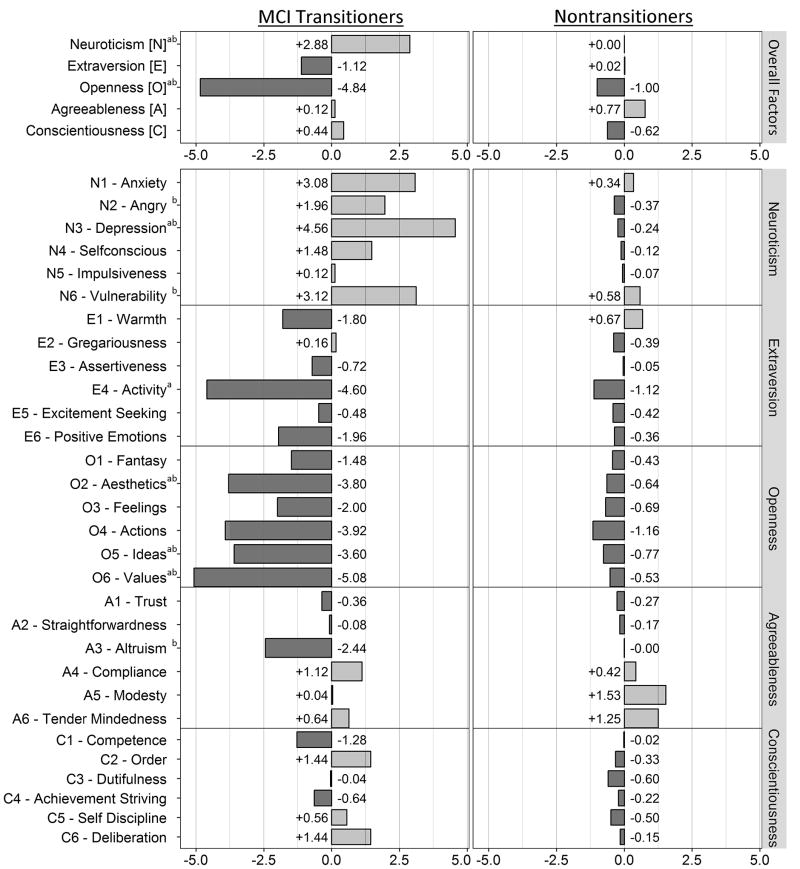
Longitudinally, MCI transitioners exhibited greater decline on multiple neuropsychological measures (as expected) particularly in the domains of memory and executive skills than nontransitioners (Supplementary Table S1). NEO-PI-R changes are summarized in figure 1 and table 3. At the group level Neuroticism T-scores increased more in MCI transitioners than in nontransitioners (+2.9 [0.9, 4.9] vs 0 [−0.7, 0.7], P=.02), and Openness decreased more in MCI transitioners than in nontransitioners (−4.8 [−7.3, −2.4] vs −1.0 [−1.6, −0.4], P<.001). Facet scores that worsened more in MCI transitioners than nontransitioners included the Neuroticism facet score of depression (+4.6 [1.7, 7.4] vs −0.2 [−1.1, 0.6], P<.001), and the Openness facet scores of activity (−4.6 [−7.5, −1.7] vs −1.1 [−2.0, −0.2], P=.02), aesthetics (−3.8 [−7.0, −0.6] vs −0.6 [−1.4, 0.1], P=.01), ideas (−3.6 [−6.2, −1.0] vs −0.8 [−1.5, 0.0], P=.03), and values (−5.1 [−7.3, −2.8] vs −0.5 [−1.4, 0.3], P=.002). Adjusting for the length of time between NEO-PI-R administrations did not change these results. At the individual level, Neuroticism T-scores increased by more than 5 points in 40% of transitioners and 18.3% of nontransitioners (P=.01) while Openness T-score declined by more than 5 points in 48% of transitioners and 21.4% of nontransitioners (P=.003).
Figure 1. Mean Changes in NEO-PI-R Scores by MCI Transition Status.
Fishbone plot of NEO-PI-R changes in MCI transitioners (left) and nontransitioners (right). a Unadjusted T-test P ≤ .05; b ANCOVA adjusted for first NEO-PI-R score and time interval P ≤ .05. Note that all significant differences indicate greater “worsening” in the MCI transitioners relative to the nontransitioners.
Table 3.
NEO-PI-R Change Scores
|
MCI Transitioners [mean (95%CI)] |
Nontransitioners [mean (95%CI)] |
P Valuea | P Valueb | |
|---|---|---|---|---|
| Neuroticism [N] | +2.9 (0.9, 4.9) | 0.0 (−0.7, 0.7) | .02 | .02 |
| Extraversion [E] | −1.1 (−3.4, 1.1) | +0.0 (−0.6, 0.7) | .30 | .49 |
| Openness [O] | −4.8 (−7.3, −2.4) | −1.0 (−1.6, −0.4) | <.001 | <.001 |
| Agreeableness [A] | +0.1 (−2.5, 2.8) | +0.8 (0.0, 1.5) | .60 | .57 |
| Conscientiousness [C] | +0.4 (−2.5, 3.4) | −0.6 (−1.2, 0.0) | .33 | .59 |
| N1-Anxiety | +3.1 (−0.0, 6.2) | +0.3 (−0.6, 1.3) | .08 | .10 |
| N2-Anger | +2.0 (−1.4, 5.3) | −0.4 (−1.2, 0.4) | .09 | .05 |
| N3-Depression | +4.6 (1.7, 7.4) | −0.2 (−1.1, 0.6) | <.001 | <.001 |
| N4-Selfconsciousness | +1.5 (−1.2, 4.2) | −0.1 (−0.9, 0.7) | .24 | .13 |
| N5-Impulsiveness | +0.1 (−2.5, 2.7) | −0.1 (−1.0, 0.9) | .90 | .79 |
| N6-Vulnerability | +3.1 (1.0, 5.3) | +0.6 (−0.3, 1.4) | .07 | .05 |
| E1-Warmth | −1.8 (−5.0, 1.4) | +0.7 (−0.1, 1.4) | .05 | .06 |
| E2-Gregariousness | +0.2 (−2.6, 2.9) | −0.4 (−1.3, 0.5) | .71 | .58 |
| E3-Assertiveness | −0.7 (−3.3, 1.9) | 0.0 (−0.8, 0.7) | .60 | .76 |
| E4-Activity | −4.6 (−7.5, −1.7) | −1.1 (−2.0, −0.2) | .02 | .07 |
| E5-Excitement seeking | −0.5 (−2.7, 1.7) | −0.4 (−1.2, 0.3) | .96 | .95 |
| E6-Positive emotions | −2.0 (−4.5, 0.6) | −0.4 (−1.3, 0.5) | .29 | .66 |
| O1-Fantasy | −1.5 (−4.3, 1.4) | −0.4 (−1.3, 0.5) | .49 | .23 |
| O2-Aesthetics | −3.8 (−7.0, −0.6) | −0.6 (−1.4, 0.1) | .01 | .01 |
| O3-Feelings | −2.0 (−5.5, 1.5) | −0.7 (−1.5, 0.1) | .37 | .35 |
| O4-Actions | −3.9 (−7.6, −0.3) | −1.2 (−2.1, −0.2) | .08 | .24 |
| O5-Ideas | −3.6 (−6.2, −1.0) | −0.8 (−1.5, 0.0) | .03 | .03 |
| O6-Values | −5.1 (−7.3, −2.8) | −0.5 (−1.4, 0.3) | .002 | .003 |
| A1-Trust | −0.4 (−3.0, 2.3) | −0.3 (−1.1, 0.5) | .95 | .90 |
| A2-Straightforwardness | −0.1 (−3.4, 3.2) | −0.2 (−1.1, 0.7) | .96 | .71 |
| A3-Altruism | −2.4 (−6.8, 1.9) | 0.0 (−0.8, 0.8) | .10 | .04 |
| A4-Compliance | +1.1 (−1.8, 4.1) | +0.4 (−0.4, 1.3) | .63 | .61 |
| A5-Modesty | 0.0 (−2.7, 2.8) | +1.5 (0.7, 2.4) | .30 | .35 |
| A6-Tender Mindedness | +0.6 (−3.4, 4.7) | +1.3 (0.2, 2.3) | .73 | .61 |
| C1-Competence | −1.3 (−4.7, 2.1) | 0.0 (−0.9, 0.8) | .4 | .32 |
| C2-Order | +1.4 (−1.7, 4.6) | −0.3 (−1.1, 0.5) | .21 | .47 |
| C3-Dutifulness | 0.0 (−3.5, 3.4) | −0.6 (−1.4, 0.2) | .69 | .80 |
| C4-Achievement striving | −0.6 (−3.8, 2.5) | −0.2 (−1.1, 0.6) | .77 | .65 |
| C5-Self discipline | +0.6 (−3.4, 4.5) | −0.5 (−1.3, 0.3) | .47 | .65 |
| C6-Deliberation | +1.4 (−0.8, 3.7) | −0.2 (−0.9, 0.6) | .22 | .52 |
Unadjusted T-test;
ANCOVA adjusted for first NEO-PI-R score and time interval
Concurrent clinically insignificant but statistically significant changes in behavioral scores revealed worsening in MCI transitioners relative to nontransitioners including measures of depression (PAI-CSc-DEP [+4.2 (1.3, 7.1) vs +0.3 (−0.8, 1.3), P=.04], GDS [+3.8 (1.1, 6.5) vs +0.1 (−0.5, 0.8), P=.003), somatization (PAI-CSc-SOM [+5.4 (0.4, 10.3) vs +1.0 (0.2, 1.8), P=.008]), irritability (PAI-BOR-I [+4.6 (0.4, 8.9) vs −0.2 (−1.1, 0.8), P=.008]), affective anxiety (PAI-ANX-A [+4.4 (1.1, 7.6) vs +0.5 (−0.6, 1.5), P=.05]), and aggressive attitude (PAI-AGG-A [+4.8 (0.3, 9.3) vs −0.7 (−1.7, 0.4), P=.005]) (Supplementary Table S2). No transitioner experienced the new onset of depression, anxiety, or other psychiatric disorder although one transitioner’s husband died during this time prior to MCI transition. This individual had 3 study epochs, and showed transient elevation of depression scores during the second epoch that declined again by the third epoch (HamD 0-9-0, Beck 1-18-14, GDS 11-15-13, and PAI-CSc-DEP T-scores 55-63-57) without commensurate NEO-PI-R T-score changes (Neuroticism 50-49-47, Openness 64-64-61).
DISCUSSION
This is the first study to demonstrate that changes in personality, including increasing Neuroticism and decreasing Openness, coincide with the transition from preclinical AD to incident MCI, with concomitant subclinical changes in behavioral measures of somatization, depression, anxiety, irritability, and aggression. Our findings are based upon longitudinal observations over more than seven years prior to the transition point of incident MCI utilizing the gold standard personality measure, the NEO-PI-R, and are derived directly from patient responses rather than proxy estimates. There were no differences in baseline personality or behavioral scores that might otherwise have suggested a predisposition to these negative changes consistent with the hypothesis that these changes were intrinsic to the disease process itself. These changes were identified at the same time that neuropsychological performance declined to the MCI level yet documented prior to communicating a diagnosis of MCI to the patients so that they do not represent a reaction to diagnosis or a complication of treatment.
All previous studies have relied upon informant recollections of the patient’s premorbid personality, and only about half have utilized the five factor model. With few exceptions, patients have had established dementia which risks biasing retrospective estimates of presymptomatic functioning and possibly overestimating the degree of personality (vs frank behavioral) change. Only two studies have looked at a similar period of transition. Copeland et al employed a semi-structured interview and found no differences between patients and controls in 10 patients transitioning from normal to MCI over the course of 3 years20 while Balsis et al found that individuals with preclinical AD did experience more personality changes than normal nontransitioners based on the Blessed Dementia Scale including increased rigidity, apathy, egocentricity, and impaired emotional control21. Six previous studies utilized the NEO-PI9,11,14,16 or NEO-PI-R19, all in established dementia patients, and all concluded that compared to pre-dementia estimates Neuroticism increased while Extraversion, Openness, and Conscientiousness decreased with dementia. One study11 found Agreeableness declined as well. Another 4 studies7,8,15,18 utilized the Brooks McKinlay Personality Inventory28 in which informants rate 18 dichotomized descriptors, and these also found a high frequency of change from the pre-dementia to the post-dementia state. Studies10,17,21 in which informants utilized the Blessed Dementia Scale29 or CAMDEX30 also found changes related to reduced interest, activity, and cognitive flexibility.
We have previously shown that memory decline is detectable preclinically, roughly ten to fifteen years before expected symptomatic onset25,31, and that that personality impacts longitudinal cognitive trajectories in a normal aging population32. Neuroticism and other personality factors do not change simply as a function of aging, but instead are detectable within the context of symptomatic AD at its earliest clinical stage, and unlike preclinical memory decline, there is no preclinical escalation in depression scores prior to the symptomatic transition to MCI33. Thus our current findings are consistent with a model in which personality changes follow memory decline during the transitional period, and precede clinically symptomatic behavioral disorders that do not (usually) emerge until later in the MCI and early dementia stages.
Behavioral symptoms pose major therapeutic challenges during the course of disease progression, and those that are severe enough to prompt pharmacotherapeutic intervention are not rare. For example, in a large Veterans Administration study, 17.7% of dementia patients in 1999 were taking antipsychotic drugs, and although their use has been declining in the U.S. and elsewhere since a 2006 FDA black box warning34, the prevalence of psychotropic use in Finland in 2011 was 45.0–47.9%35. In France, antidepressant use in dementia patients increased from 26% in 2010 to 31% in 201436. Despite their widespread use, psychotropic agents have been found to be of either inconsistent or no benefit in patients with dementia37, and associated with significant adverse outcomes38. Gilley et al have previously reported that Neuroticism, as estimated from informant ratings of patients with AD, was predictive of depressive symptoms in a cohort or 410 AD patients followed over four years39. If we can correlate early stage personality changes with behavioral outcomes it may be possible to prevent adverse behavioral outcomes. Caregivers and even patients at these early stages may be alerted to impending behavioral risks allowing for earlier life plans (medical proxies, wills, and so forth), and well as potential prevention strategies. Clinical trials will be needed to assess the efficacy of any prevention therapies that may include both pharmacological and nonpharmacological interventions.
The neurobiology of personality is poorly understood. Both structural40 and functional41 MRI studies of MCI patients have shown alterations in frontotemporal cortices that have been associated with behavioral disturbances, and we found concomitant cognitive changes in not only memory but in executive skills as well (which are not seen prior to MCI transition42) during the MCI transition referable to these anatomical regions. Further work is needed to determine whether greater executive decline portends future behavioral impairment, as both share similar anatomical substrates. Chronic stress has itself been associated with reduced functional integration of limbic networks43, reduced gray matter volumes in frontostriatal regions44, and a variety of functional and structural changes in prefrontal and hippocampal regions45 possibly predisposing stress prone individuals to earlier personality changes and subsequent behavioral disturbances. Previous work has shown that personality factors influence cognitive aging trajectories32, and the risk for MCI and AD46 and support the hypothesis that lifestyle choices that are influenced by personality can impact cognitive outcomes, plausibly through modifiable cerebrovascular components of cognitive decline and dementia47. It is less clear if there is an independent effect of personality on AD itself, but whether or not personality impacts neuropathology, it is clear that neuropathology can impact personality. Even at the earliest stage of symptomatic transition illustrated in this study, personality factors start to change in a way that one could expect to foster behavioral problems. Indeed, behavioral symptoms have been shown to be highly prevalent among patients with established MCI and have included, congruent with our findings, depression, apathy, irritability, and anxiety most frequently48.
A limitation of our study is the lack of neuropathological or biomarker evidence of AD neuropathology as the underlying cause of MCI in our patients. To address this we utilized APOE ε4 as a proxy and infer that MCI in APOE ε4 carriers reflects underlying AD as previous research has shown that e4 is a strong predictor of clinical progression to AD in MCI patients49 and the positive predictive value of APOE ε4 for AD in a neuropathological series was 97%50. Another limitation is the relatively small number of MCI transitioners who received the NEO-PI-R serially. Before our findings can be generalized, replication in other cohorts will be needed, although as our study progresses we will be able to add to these numbers as well as identify behavioral outcomes to correlate with NEO-PI-R scores.
In summary, personality changes occur very early in the clinical course of AD, are characterized by increased Neuroticism and decreased Openness, and coincide with subtle, clinically insignificant behavioral changes that qualitatively mirror and anticipate the clinically severe behavioral problems that often complicate dementia care. Further research is needed to determine whether earlier identification of predisposing personality changes might facilitate earlier, safer and more effective treatment or even prevention of behavioral disorders in patients with AD.
Supplementary Material
Supplementary Table 1: Baseline and Change Scores: Neuropsychology
Supplementary Table 2: Baseline and Change Scores: Behavioral Measures
Impact.
We certify that this work is novel. The potential impact of this research on clinical care includes the following: recognizing that even as early as the transition to MCI personality begins to change putting patients at risk for behavioral problems. Further research is needed to correlate specific personality and cognitive profiles during the MCI stage with behavioral outcomes.
Acknowledgments
Richard J. Caselli, MD receives research funding from NIA, Merck, Novartis, and the Arizona Alzheimer’s Consortium, and serves as an advisory medical editor for Clinical Neurology News.
Bryan K. Woodruff, MD receives research funding from Genentech.
Funding Sources: NIA R01AG031581, P30AG19610, and the Arizona Alzheimer’s Research Consortium.
Sponsor’s Role
The funding sources including the National Institute on Aging and the Arizona Alzheimer’s Research Consortium had no role in any aspect of this study including concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
Footnotes
Conflicts of Interest
Blake T. Langlais, BS reports no disclosures.
Amylou C. Dueck, PhD reports no disclosures.
Bruce R. Henslin, BA reports no disclosures.
Travis A. Johnson, BA reports no disclosures.
Charlene Hoffman-Snyder, DNP reports no disclosures.
Dona E. C. Locke, PhD reports no disclosures.
Author Contributions
Richard J. Caselli, MD designed the study, participated in data acquisition, management, and analysis, and drafted the manuscript. Dr. Caselli, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Blake T. Langlais, BS performed the primary statistical analysis under the supervision of Dr. Amylou Dueck, and provided critical revision to the manuscript including drafting the data analysis section.
Amylou C. Dueck, PhD contributed to as well as supervised data analysis that was performed by Blake Langlais, provided the data analytic strategy, and provided critical revision to the manuscript.
Bruce R. Henslin, BA assisted in data acquisition and provided critical revision to the manuscript.
Travis A. Johnson, BA assisted in data acquisition and provided critical revision to the manuscript.
Bryan K. Woodruff, MD assisted in data acquisition and provided critical revision to the manuscript.
Charlene Hoffman-Snyder, DNP assisted in data acquisition and provided critical revision to the manuscript.
Dona E. C. Locke, PhD provided supervision and quality control over all neuropsychological test administration, assisted Dr. Caselli with interpretation of results, and provided critical revisions to the manuscript.
References
- 1.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steel C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry. 1990;147(8):1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- 3.Black BS, Rabins PV, German PS. Predictors of nursing home placement among elderly public housing residents. Gerontologist. 1999;39(5):559–568. doi: 10.1093/geront/39.5.559. [DOI] [PubMed] [Google Scholar]
- 4.Phillips VL, Diwan S. The incremental effect of dementia-related problem behaviors on the time to nursing home placement in poor, frail, demented older people. J Am Geriatr Soc. 2003;51(2):188–193. doi: 10.1046/j.1532-5415.2003.51057.x. [DOI] [PubMed] [Google Scholar]
- 5.de Vugt ME, Stevens F, Aalten P, Lousberg R, Jaspers N, Verhey FR. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. Int Psychogeriatr. 2005;17(4):577–589. doi: 10.1017/S1041610205002292. [DOI] [PubMed] [Google Scholar]
- 6.Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- 7.Petry S, Cummings JL, Hill MA, Shapira J. Personality alterations in dementia of the Alzheimer type. Arch Neurol. 1988;45:1187–1190. doi: 10.1001/archneur.1988.00520350025009. [DOI] [PubMed] [Google Scholar]
- 8.Petry S, Cummings JL, Hill MA, Shapira J. Personality alterations in dementia of the Alzheimer type: a three year follow-up study. J Geriatr Psychiatr Neurol. 1989;2(4):203–207. doi: 10.1177/089198878900200406. [DOI] [PubMed] [Google Scholar]
- 9.Siegler IC, Welsh KA, Dawson DV, et al. Ratings of personality change in patients being evaluated for memory disorders. Alz Dis Assoc Disord. 1991;5(4):240–250. doi: 10.1097/00002093-199100540-00003. [DOI] [PubMed] [Google Scholar]
- 10.Bozzola FG, Gorelick PB, Freels S. Personality changes in Alzheimer’s disease. Arch Neurol. 1992;49:297–300. doi: 10.1001/archneur.1992.00530270117027. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A, Strauss ME, Smyth KA, Whitehouse PJ. Personality changes in Alzheimer’s disease. Arch Neurol. 1992;49:486–491. doi: 10.1001/archneur.1992.00530290070014. [DOI] [PubMed] [Google Scholar]
- 12.Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychol Aging. 1993;8(4):475–480. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- 13.Strauss ME, Pasupathi M. Primary caregivers’ descriptions of Alzheimer patients’ personality traits: temporal stability and sensitivity to change. Alz Dis Assoc Disord. 1994;8(3):166–176. doi: 10.1097/00002093-199408030-00003. [DOI] [PubMed] [Google Scholar]
- 14.Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer’s disease patients: a replication. Psychol Aging. 1994;9(3):464–466. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- 15.Aitken L, Simpson S, Burns A. Personality change in dementia. Int Psychogeriatr. 1999;11(3):263–271. doi: 10.1017/s1041610299005827. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DV, Welsh-Bohmer KA, Siegler IC. Premorbid personality predicts level of rated personality change in patients with Alzheimer disease. Alz Dis Rel Disord. 2000;14(1):11–19. doi: 10.1097/00002093-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Gamble V, Baiyewu O, Perkins AJ, et al. Informant reports of changes in personality predict dementia in a population-based study of elderly African Americans and Yoruba. Am J Geriatr Psychiatry. 2002;10(6):724–732. [PubMed] [Google Scholar]
- 18.Talassi E, Cipriani G, Bianchetti A, Trabucchi M. Personality changes in Alzheimer’s disease. Aging Ment Health. 2007;11(5):526–531. doi: 10.1080/13607860601086603. [DOI] [PubMed] [Google Scholar]
- 19.Pocnet C, Rossier J, Antonietti JP, von Gunten A. Personality traits and behavioral and psychological symptoms in patients at an early stage of Alzheimer’s disease. Int J Geriatr Psychiatry. 2013;28:276–283. doi: 10.1002/gps.3822. [DOI] [PubMed] [Google Scholar]
- 20.Copeland MP, Daly E, Hines V, Mastromauro C, Zaitchik D, Gunther J, Albert M. Psychiatric symptomatology and prodromal Alzheimer’s disease. Alz Dis Assoc Disord. 2003;17(1):1–8. doi: 10.1097/00002093-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. J Gerontol. 2005;60B(2):P98–P101. doi: 10.1093/geronb/60.2.p98. [DOI] [PubMed] [Google Scholar]
- 22.Costa PT, Jr, McCrae RR. NEO-PI-R Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.; 1992. [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 25.Caselli RJ, Locke DE, Dueck AC, et al. The neuropsychology of normal aging and preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(1):84–92. doi: 10.1016/j.jalz.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: how researchers in different psychology and chronic disease epidemiology are collaborating to understand and address health inequities. Psychological Science In the Public Interest. 2010;11(2):53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- 27.Morey L. Personality Assessment Inventory. Psychological Assessment Resources; Lutz, FL: 1991. [Google Scholar]
- 28.Brooks DN, McKinlay W. Personality and behavioural change after severe blunt head trauma: a relative’s view. J Neurol Neurosurg Psychiatry. 1983;46:336–344. doi: 10.1136/jnnp.46.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 30.Roth M, Tym E, Mountjoy CO, et al. CAMDEX: a standardized instrument for the diagnosis of mental disorders in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 31.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caselli RJ, Dueck AC, Locke DE, et al. Impact of Personality on Cognitive Aging: A Prospective Cohort Study. J Int Neuropsychol Soc. 2016;22(7):765–76. doi: 10.1017/S1355617716000527. [DOI] [PubMed] [Google Scholar]
- 33.Locke DE, Dueck AC, Stonnington CM, Knopman DS, Geda YE, Caselli RJ. Depressive symptoms in healthy apolipoprotein E epsilon4 carriers and noncarriers: a longitudinal study. J Clin Psychiatry. 2013;74(12):1256–61. doi: 10.4088/JCP.13m08564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry. 2011;68(2):190–197. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- 35.Tolppanen AM, Voutilainen A, Taipale H, et al. Regional changes in psychotropic use among Finnish persons with newly diagnosed Alzheimer’s disease in 2005–2011. PLoS One. 2017;12(3):e0173450. doi: 10.1371/journal.pone.0173450. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.David R, Manera V, Fabre R, Pradier C, Robert P, Tifratene K. Evolution of the antidepressant prescribing in Alzheimer’s disease and related disorders between 2010 and 2014: results from the French National Database on Alzheimer’s Disease (BNA) J Alz Dis. 2016;53(4):1365–1374. doi: 10.3233/JAD-160238. [DOI] [PubMed] [Google Scholar]
- 37.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 38.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilley DW, Wilson RS, Bienias JL, Bennett DA, Evans DA. Predictors of depressive symptoms in persons with Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2004 Mar;59(2):P75–83. doi: 10.1093/geronb/59.2.p75. [DOI] [PubMed] [Google Scholar]
- 40.Trzepacz PT, Bhamidipati PK, Willis B, et al. Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alz Dement. 2013;9(5 suppl):S95–S104. doi: 10.1016/j.jalz.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamboni G, Drazich E, McCulloch E, et al. Neuroanatomy of impaired self-awareness in Alzheimer’s disease and mild cognitive impairment. Cortex. 2013;49(3):668–678. doi: 10.1016/j.cortex.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Caselli RJ, Dueck AC, Locke DE, Hoffman-Snyder CR, Woodruff BK, Rapcsak SZ, Reiman EM. Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology. 2011;76(16):1383–8. doi: 10.1212/WNL.0b013e3182167147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jovanovic H, Perski A, Berglund H, Savic I. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. NeuroImage. 2011;55:1178–1188. doi: 10.1016/j.neuroimage.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 44.Blix E, Perski A, Berglund H, Savic I. Long-term occupational stress is associated with regional reductions in brain tissue volumes. PloS One. 2013;8:e64065. doi: 10.1371/journal.pone.0064065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Czeh B. Neuropathology of stress. Acta Neuropathologica. 2014;127:109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 47.Bennett DA, Arnold SE, Valenzuel MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathologica. 2014;127:137–150. doi: 10.1007/s00401-013-1226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging. Arch Gen Psychiatry. 2008;65(10):1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen RC, Smith GE, Ivnik RJ, et al. Apilpoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- 50.Welsh-Bohmer KA, Gearing M, Saunders AM, Roses AD, Mirra S. Apolipoprotein E genotypes in a neuropathological series from the Consortium to Establish a Registry for Alzheimer’s Disease. Ann Neurol. 1997;42(3):319–325. doi: 10.1002/ana.410420308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Baseline and Change Scores: Neuropsychology
Supplementary Table 2: Baseline and Change Scores: Behavioral Measures