Abstract
Insulin delivery to the brain has emerged as an important therapeutic target for cognitive disorders associated with abnormal brain energy metabolism. Although insulin is transported across the blood–brain barrier, peripheral routes of administration are problematic due to systemic effects of insulin on blood glucose. Intranasal (IN) administration is being investigated as an alternative route. We conducted a head-to-head comparison of subcutaneous (SC) and IN insulin, assessing plasma and brain pharmacokinetics and blood glucose levels in the mouse. SC insulin (2.4 IU) achieved therapeutically relevant concentrations in the brain (AUCbrain = 2537 h·μIU/mL) but dramatically increased plasma insulin (AUCplasma = 520 351 h·*μIU/mL), resulting in severe hypoglycemia and in some cases death. IN administration of the same dose resulted in similar insulin levels in the brain (AUCbrain = 3442 h·μIU/mL) but substantially lower plasma concentrations (AUCplasma = 354 h·μIU/mL), amounting to a ~ 2000-fold increase in the AUCbrain:plasma ratio relative to SC. IN dosing also had no significant effect on blood glucose. When administered daily for 9 days, IN insulin increased brain glucose and energy metabolite concentrations (e.g., adenosine triphosphate and phosphocreatine) without causing overt toxicity, suggesting that IN insulin may be a safe therapeutic option for cognitively impaired patients.
Keywords: Insulin, intranasal, pharmacokinetics, neurocognitive impairment, glucose, energy metabolism
Graphical Abstract
INTRODUCTION
A growing body of preclinical and clinical evidence suggests that delivery of insulin to the brain may prevent neuronal damage and improve cognition in a broad range of conditions associated with cognitive impairment including diabetes,1,2 substance abuse,3 stroke,4,5 postoperative cognitive dysfunction,6,7 developmental delay,8,9 Parkinson’s disease,10 bipolar disorder,11 Alzheimer’s disease (AD),12,13 and HIV-associated neurocognitive disorders (HAND).14–16 Cognitive deficits in many of these disorders have been linked to oxidative stress in the brain, neuroinflammation, and neurodegeneration.17 When these conditions are modeled in vitro and in vivo, activation of insulin receptors has been shown to be protective.18,19 The neuroprotective effects of insulin appear to involve the induction or preservation of brain glucose metabolism and restoration of the energy storage molecules adenosine triphosphate (ATP) and phosphocreatine,13,20–25 in addition to neurotrophic and anti-inflammatory effects mediated by the direct activation of insulin receptors on neurons.26,27 When delivered to the brain, insulin has been shown to increase glucose uptake13,18–20,25,28 and improve cognitive performance in animals,3,7,15,16,25,29 healthy humans,30–32 and cognitively impaired patients.1,2,8,9,11,33–37 Given the absence of robust neuroprotective therapeutics, insulin delivery to the brain has thus emerged as a promising new therapy for multiple neurological and psychiatric disorders.
A major hurdle to this approach is safely achieving therapeutic levels of insulin in the brain. Although insulin has been shown to actively cross the blood–brain barrier from the periphery,38 subcutaneously administered insulin results in plasma concentrations significantly higher than those observed in central nervous system (CNS) compartments.38,39 For example, subcutaneous (SC) insulin has been shown to achieve brain:plasma ratios of about 1:2000 in mice38,39 and cerebrospinal fluid (CSF):plasma ratios of about 1:200 in humans.40–42 This degree of brain penetration may not allow for sufficient CNS insulin exposure before reaching systemic insulin levels that induce severe hypoglycemia. Moreover, insulin entry into the brain requires active transport, and compromised blood–brain barrier integrity in patients with insulin resistance or cognitive disorders may decrease the ability of peripheral insulin to enter the brain.43
In recent years, intranasal (IN) administration of insulin has been explored as a brain-targeted route of delivery that may avoid systemic hyperinsulinemia and hypoglycemia.44 IN administration has been found to deliver small molecules, biologics, and peptides such as insulin to the brain via absorption through the olfactory epithelium,45,46 bypassing the blood–brain barrier45,47 and diffusing throughout the brain along olfactory and trigeminal perivascular channels as well as through olfactory bulb axonal transport.18,48–50 Pharmacokinetic studies performed in rodents, non-human primates, and healthy humans have validated the ability of IN-administered macromolecules and peptides, including insulin, to penetrate and accumulate in brain regions important for cognition such as the hippocampus and cerebral cortex.51–57 Multiple clinical trials have since been initiated to assess the therapeutic efficacy of IN insulin on cognitive symptoms in various psychiatric and neurological patient populations.58 However, while IN dosing delivers insulin to the brain, absorption into systemic circulation via the nasal respiratory epithelium also occurs to some extent.18
In this study, we performed a head-to-head comparison of insulin exposure in mouse brain and plasma following IN and SC administration. It was recently found that 2.4 IU insulin administered IN in the EcoHIV-infected mouse model of HAND significantly attenuated cognitive impairment.15 We first determined the SC dose of insulin required to achieve similar concentrations in the brain. Using this dose, we then compared plasma and brain insulin pharmacokinetics and blood glucose levels following IN vs SC administration. In order to identify a pharmacodynamic effect of IN insulin in the brain, we also examined its effect on brain energy metabolites.
RESULTS
IN Insulin Achieves Similar Brain but Reduced Plasma Concentrations Relative to SC Insulin
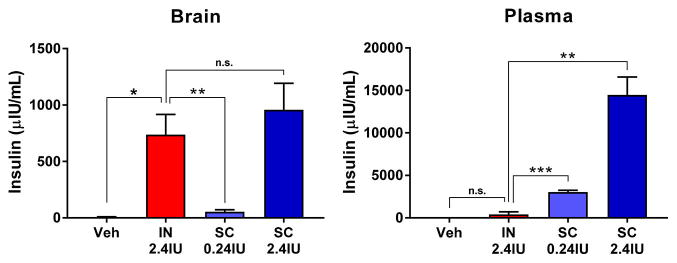
In order to match the brain insulin concentrations achieved by IN administration of 2.4 IU insulin, a dose known to be efficacious in a mouse HAND model,15 we administered two doses (0.24 and 2.4 IU) of SC insulin to fasted mice. At 1 h postadministration, 2.4 IU IN insulin achieved brain insulin concentrations of 737 ± 179 μIU/mL, significantly above basal concentrations represented by vehicle treated mice (Figure 1, left). SC administration of 0.24 and 2.4 IU insulin resulted in dose-dependent increases in brain insulin concentrations; the 2.4 IU SC dose reached similar levels to the same dose administered IN (958 ± 235 μIU/mL). In contrast, 2.4 IU SC insulin administration resulted in dramatically increased plasma insulin concentrations (14 500 ± 2084 μIU/mL) well above those achieved in the brain, whereas 2.4 IU IN insulin administration did not significantly elevate plasma insulin concentrations above basal levels (405 ± 300 μIU/mL; Figure 1, right).
Figure 1.
IN insulin achieves similar brain concentrations with reduced plasma concentrations relative to SC insulin. Brain and plasma were harvested 1 h postadministration of vehicle or two doses of subcutaneous (SC) insulin (0.24 and 2.4 IU) or intranasal (IN) insulin (2.4 IU) in fasted mice. (Left) SC administration resulted in dose-dependent increases in insulin concentrations in the brain that reached similar levels compared with IN insulin at the 2.4 IU dose. (Right) SC insulin at both doses yielded substantially larger increases in plasma insulin concentrations compared with IN dosing (which did increase plasma insulin levels above baseline). Values depict the mean + SEM. Comparison between treatment groups by pairwise t-test; *p < 0.05, **p < 0.01, ***p < 0.001, n.s. not significant; n = 3–6/dose.
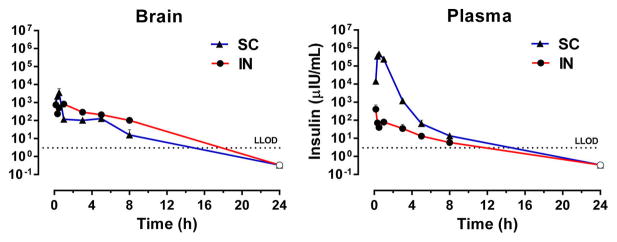
Having achieved similar brain insulin concentrations through IN and SC dosing of 2.4 IU, a full pharmacokinetic evaluation of both routes was conducted in fasted mice. Insulin concentration–time profile comparison in the brain of mice gave similar mean insulin concentrations for both routes of administration at all time points (Figure 2, left). SC insulin achieved a Cmax in the brain slightly higher than the IN route, but similar insulin exposures (Table 1). In contrast, mice receiving SC insulin exhibited substantially higher mean plasma insulin levels at every time point through 8 h postadministration compared to mice administered the same dose of IN insulin (Figure 2, right). Plasma insulin Cmax was 464 108 and 405 μIU/mL after SC and IN administration, respectively (Table 1). Similarly, plasma insulin exposure based on AUC0–t was 520 351 and 354 h·μIU/mL after SC and IN administration, respectively (Table 1). The concentrations of SC insulin peaked at 0.5 h in both plasma and brain, whereas concentrations of IN insulin peaked at 10 min in plasma and 1 h in brain (Table 1). Insulin clearance from both brain and plasma was relatively rapid, with half-lives (t1/2) between 2 and 4 h for both IN and SC routes (Table 1).
Figure 2.
IN insulin achieves similar brain exposure with reduced plasma exposure relative to SC insulin. Plasma and brain were harvested at multiple time points postadministration of insulin (2.4 IU) delivered via the subcutaneous (SC) or intranasal (IN) route. SC administration resulted in (right) substantially higher insulin exposure in plasma but (left) comparable brain exposure relative to IN administration. Dotted line indicates lower limit of detection (LLOD). Values depict the mean + SEM; open points indicate a mean value of zero; n = 3–8/group.
Table 1.
Mean Pharmacokinetic Parameters of IN vs SC Insulin
| Matrix | Route | tmax (h) | Cmax (μIU/mL) | insulin AUC0–t (h·μIU/mL) | t1/2 (h) | AUCbrain:plasma |
|---|---|---|---|---|---|---|
| Plasma | SC | 0.5 | 464184 | 520351 | 2.6 | |
| IN | 0.17 | 405 | 354 | 3.3 | ||
| Brain | SC | 3545 | 2537 | 2.4 | 0.005 | |
| IN | 738 | 3442 | 2.1 | 9.72 |
IN Insulin Substantially Improves the Brain:Plasma Ratio Relative to SC Insulin
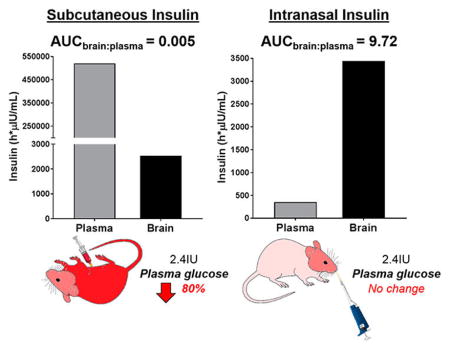
The brain:plasma Cmax ratio following 2.4 IU IN insulin was increased greater than 200-fold relative to the brain:plasma ratio following SC administration of the same insulin dose (Figure 3, left). An analogous comparison of AUC0–t values revealed a ~2000-fold increase in brain:plasma insulin exposure by IN versus SC administration (Figure 3, right).
Figure 3.
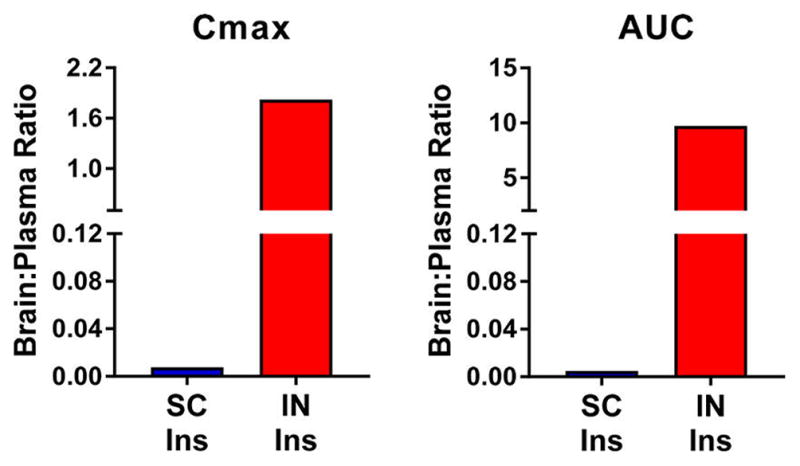
IN insulin substantially improves the brain:plasma ratio relative to SC insulin. Brain to plasma ratios of (left) Cmax and (right) AUC values obtained after subcutaneous (SC) and intranasal (IN) insulin (2.4 IU) administration were calculated. IN administration resulted in a greater than 200- and 2000-fold increases in the brain:plasma insulin Cmax and AUC ratios, respectively. Values depict the mean + SEM or mean; n = 3–8/group.
SC but Not IN Insulin Induces Severe Hypoglycemia
Changes in peripheral glucose represent a potential limiting factor preventing SC insulin from being a viable therapeutic approach for CNS disease. We were thus interested in comparing the effect of SC and IN insulin on blood glucose levels in mice over time. Twenty minutes after SC administration of insulin (2.4 IU), blood glucose levels decreased by 80% and did not return to basal levels for about 3 h. In about 10% of mice, severe hypoglycemia resulted in death. In contrast, mice administered IN insulin exhibited no statistically significant change in blood glucose relative to baseline (main effect of route [F(1,20) = 26.9, p < 0.0001], time [F(4,20) = 9.56, p = 0.0002], and interaction [F(4,20) = 4.14, p = 0.0133]; Figure 4).
Figure 4.
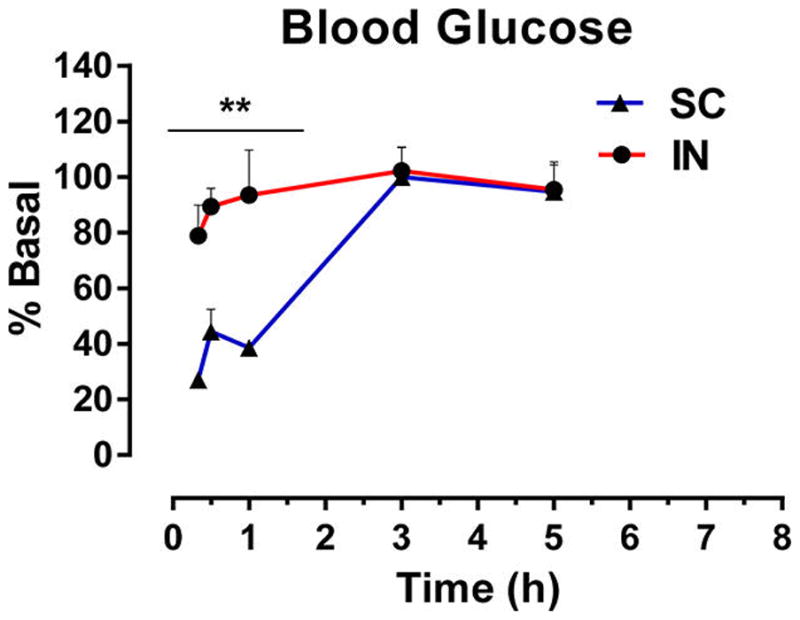
SC insulin induces severe hypoglycemia. Blood glucose was measured at multiple time points postadministration of insulin (2.4 IU) via the subcutaneous (SC) or intranasal (IN) route. SC administration resulted in significant and prolonged reductions in blood glucose and death in about 10% of mice. IN administration of the same dose had no significant effect on blood glucose. Values depict the mean + SEM. Blood glucose changes are compared by two-way ANOVA with Bonferroni posthoc test; **p < 0.01 vs basal for SC; n = 3/group.
Repeated IN Insulin Administration Increases Brain Concentrations of Glucose and Phosphorylated Energy Metabolites
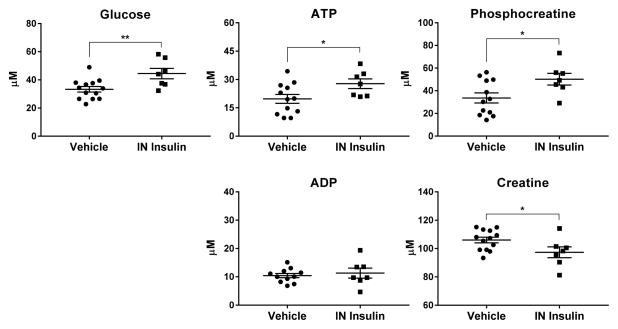
Having demonstrated favorable brain and plasma pharmacokinetics of insulin after IN administration and limited peripheral effects on blood glucose, we next determined if this route of administration could measurably affect brain energy metabolites, a potential pharmacodynamic marker of therapeutic efficacy.29 Glucose and the energy metabolites ATP, ADP, phosphocreatine, and creatine were measured by NMR in homogenized mouse brain tissue following daily doses of 2.4 IU IN insulin for 9 days. Repeated IN insulin administration resulted in increased concentrations of brain glucose [t(18) = 2.95, p = 0.0086], ATP [t(17) = 2.21, p = 0.0412], and phosphocreatine [t(17) = 2.36, p = 0.0304], with a concurrent reduction in creatine [t(18) = 2.22, p = 0.0393] (Figure 5).
Figure 5.
Repeated IN insulin administration increased brain concentrations of glucose and phosphorylated energy metabolites. Tissue was harvested from mice after 9 days of daily intranasal (IN) administration of vehicle or insulin (2.4 IU). Brain energy metabolites were analyzed by NMR. IN insulin administration produced an increase in (top left) glucose and (top middle) ATP as well as in (top right) the energy storage molecule phosphocreatine. There was no change in (bottom left) ADP, but there was a corresponding reduction in (bottom right) creatine. Values are depicted individually as well as the mean ± SEM. Changes due to treatment are compared by t-test; *p < 0.05, **p < 0.01; n = 7–13/group.
DISCUSSION
Insulin delivery to the brain via the IN route is under investigation for the treatment of multiple diseases associated with cognitive impairment linked to oxidative stress-mediated alterations in brain energy metabolism.3,7–9,13,15,18–20,25,28–37 Herein we show that IN administration achieves insulin exposure in the brain similar to SC administration of the same dose (2.4 IU), but it achieves significantly lower peripheral exposure, preventing severe hypoglycemia. Repeated IN dosing of 2.4 IU insulin was also well tolerated and resulted in increased concentrations of glucose and phosphorylated energy substrates in the brain, a potentially neuroprotective and pro-cognitive profile.13,18–20,25,28
Although SC insulin injection has been used for nearly a century to maintain peripheral euglycemia and treat diabetes,59 the behavioral and cognitive effects of insulin in the brain have only recently been recognized and become a major new area of therapeutic interest.60 Insulin is known to be actively transported from circulation across the blood–brain barrier,38 offering the potential for SC dosing to achieve therapeutically relevant insulin brain concentrations. Indeed, we demonstrated significant brain insulin exposure after SC injection in the mouse. However, brain insulin concentrations significantly above basal levels could only be achieved at a SC dose that also increased plasma insulin to dangerous levels, resulting in hypoglycemia and in some cases death.
IN administration of insulin has become a preferred alternative route for brain targeting with more than 50 clinical trials registered in various patient populations that exhibit cognitive impairment.58 Although preliminary clinical responses are promising,1,2,8,9,11,29–33 few pharmacokinetic studies have been performed to determine the optimal IN insulin dose or to establish a pharmacokinetic/pharmacodynamic relationship. This is especially important because IN administration of insulin and other peptides is known to result in systemic exposure via absorption through the nasal respiratory epithelium.51 We thus conducted a direct plasma and brain pharmacokinetic comparison between IN and SC administration of insulin at a dose known to be cognitively enhancing in animal models of cognitive impairment.15 We found that SC administered insulin caused plasma hyperinsulinemia, hypoglycemia, and sometimes death, while IN administered insulin resulted in similar brain exposure but significantly lower plasma insulin concentrations, resulting in no effect on plasma glucose. Although IN dosing in rodents does not perfectly model the use of nasal delivery devices in the clinic,45 these findings support the IN route for selective delivery of insulin to the brain.
Having confirmed that IN insulin administration affords a significant improvement in brain targeting, we subsequently demonstrated that repeated daily administration of IN insulin increased brain concentrations of glucose as well as the cellular energy substrates ATP and phosphocreatine. These effects are consistent with previous investigations into the therapeutic mechanism of action of insulin which has been linked to induction of glucose uptake in the brain28 and restoration of ATP and phosphocreatine.13,18–25,28 Reduced glucose uptake in the brain as well as depletion of ATP and phosphocreatine has been linked to the deleterious effects of oxidative stress, neuroinflammation, and neurodegeneration associated with multiple cognitive disorders.5,17,21,28,61 For example, cerebral hypometabolism has been demonstrated by FDG-PET in cognitively impaired patients with Parkinson’s disease,62,63 AD,13,64–66 and HAND.67–69 In some cases, this deficit appears to occur downstream of insulin resistance in the brain.66,70–72 In AD brain samples and in cultured neurons under oxidative stress, accompanying depletion of ATP and phosphocreatine has also been detected.21–23,73,74 IN insulin has generally been found to reverse these effects, inducing FDG-PET uptake in the brains of rodents19,20,25 and humans13,18,19,28 and promoting the production of neuronal ATP and phosphocreatine.21–23,75 Renewing normal levels of ATP, phosphocreatine, and other energy substrates in the brain may broadly improve cognition in various disease states by meeting the high metabolic demand necessary to preserve neuronal function and cognition.76 It should be noted, however, that conflicting findings have been reported suggesting little or no acute effect of insulin on neuronal glucose uptake at the cellular level.77 This apparent discrepancy could be explained in part by the recent observation that insulin effects on brain metabolism may be mediated by nonspecific vasodilation and increased perfusion rather than by selective effects on glucose transport, but further investigation is required.2
Taken together, the current study offers insight into the potential mechanism of action of IN insulin observed in preclinical models of disease and in cognitively impaired patients. These experiments also support the superiority of IN dosing and the use of this route of administration for brain targeting in the clinic.
METHODS
Mouse Pharmacokinetic Studies
All animal studies were conducted in compliance with NIH guidelines and with the approval of the Johns Hopkins University Institutional Animal Care and Use Committee. For insulin distribution studies, male CD-1 mice were obtained at 20–25 g (Harlan Laboratories, Indianapolis, IN) and maintained on a 12 h light/dark cycle with ad libitum access to food and water, allowing at least 3 days to acclimatize after shipment. The night before the study, mice were fasted prior to insulin administration, but for no longer than 12 h. Human insulin (Humulin-R, Eli Lilly) diluted in saline was then administered via the subcutaneous (SC) or intranasal (IN) route. All mice were briefly anesthetized by isoflurane inhalation prior to dosing. IN insulin was administered to prostrate mice by twice applying 6 μL drops to the surface of each nare for a total volume of 24 μL. At the indicated time point (0.16–24 h), mice were anesthetized by isoflurane inhalation and tissue was harvested. Blood was collected by cardiac puncture. A small drop was used for glucose detection using a commercially available Accu-Check Aviva meter (Roche Diabetes Care, Inc.). The remainder was collected into EDTA-lined tubes and stored on ice prior to isolation of plasma by centrifugation at 10 000 rpm for 8 min at 4 °C. Cortex was extracted and immediately frozen on dry ice.
Mouse Brain Metabolite Studies
For analysis of IN insulin effects on brain energy metabolites, a separate cohort of male C57BL/6 mice at 6 weeks of age was obtained (Harlan Laboratories, Indianapolis, IN) and randomized to receive vehicle or insulin (Humulin, 2.4 IU, IN) once per day for 9 days. For this study, IN dosing was performed in nonanesthetized mice. Fifteen minutes after administration of the final dose, mice were euthanized by CO2 inhalation and immediately exposed to head-focused microwave irradiation (Cober Metabolic Vivostat, model S6F, Cober Electronics, South Norwalk, CT; frequency, 2450 MHz; power, 10.0 kW; 100 ms) to inactivate metabolic enzymes, fix brain tissue, and preserve energy metabolites prior to dissection of striatum. For all studies, tissue samples were stored at −80 °C prior to analyte extraction and analysis by ELISA or NMR for insulin or energy metabolite concentrations, respectively.
Brain Insulin Extraction
Human insulin extraction procedures were adapted from previously established protocols.78 Briefly, on day 1, brain samples were weighed and homogenized in ice-cold acidified ethanol (ethanol: 75, water: 25, 12.1 N HCl: 1.6 v/v) at 50 mg/mL wet weight. The homogenate was further disrupted for 15 min in a bath sonicator. The homogenized samples were left overnight at 4 °C with gentle rocking. On day 2, the homogenates were centrifuged at 16 000–17 200g for 30 min at 4 °C. An aliquot of the supernatants was transferred to fresh Eppendorf tubes, neutralized with 1 M Tris base (pH ~ 10.7, 200 μL Tris/mL sample), and solvent reduced at 45 °C for 1 h in a nitrogen evaporator and for an additional hour in a regular lyophilizer. The lyophilized samples were then reconstituted in “zero standard” (provided with the insulin ELISA kit), vortexed, and incubated overnight at 4 °C. Finally, on day 3, the reconstituted samples were centrifuged at 2470g for 1 min at room temperature and assayed for insulin levels.
Insulin Analysis
Insulin levels in brain and plasma were determined using a commercially available sandwich ELISA kit (Alpco) designed for the quantitative measurement of human insulin and proinsulin in serum, plasma, and cell culture supernatants. Plasma samples diluted with “zero standard” and brain insulin extracts (as obtained above) were assayed per the manufacturer’s instructions. Briefly, insulin in standards, samples, and controls was captured using an anti-human insulin primary antibody coated on 8-well microplate strips (in a 96-well format). A secondary anti-human insulin antibody conjugated to biotin and HRP-conjugated streptavidin was also added along with the samples for the initial incubation. After an hour of incubation at room temperature, with shaking at 700–900 rpm on a microplate shaker, the HRP substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was added to the wells and color was allowed to develop for 30 min with further shaking. Color development was stabilized by the addition of sulfuric acid stop solution, and absorbance intensity was measured at 450 nm. The resulting stabilized color was proportional to the amount of insulin standards, and the curve was fit to a five parameter logistic fit. Insulin levels in the known diabetic control and in the samples were determined using the standard curve.
Brain Metabolite Extraction
To extract metabolites, brain regions were weighed and 30 mg of tissue was immediately transferred to a mortar and pestle frozen with dry ice. Tissue was broken into smaller pieces then transferred to a glass vial to be homogenized by sonication in ice-cold methanol (1 mg = 13 μL). Chloroform was added to the homogenate (1 mg = 13 μL) and vials were vortexed and then incubated on ice for 15 min. Vials were then centrifuged at 1000g at 4 °C. Distilled water was then added (1 mg = 13 μL), followed by vortexing and centrifugation at 1000g. Vials were stored at −20 °C overnight to allow the separation of the aqueous and organic layers. The next day, the sample vials were centrifuged at 3000g at 4 °C for 10 min to further separate the layers. Using a Pasteur pipet, approximately 550 μL of the aqueous layer was carefully transferred to a new glass amber vial and kept on ice. The samples were then dried using a nitrogen dryer. The dried samples were stored at −80 °C and then resuspended in 500 μL of deuterium oxide (D2O) containing 0.05% of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSPd4). Each sample was then transferred to high-quality NMR tube (5 mm outer diameter) for analysis.
NMR
One-dimensional proton (1D 1H) NMR spectra were collected with an Bruker 750 MHz, AVANCE III spectrometer (Bruker BioSpin, Ettingen) using a double-resonance 5 mm Inverse broadband (BBI) probe. The 1D 1H spectra of the tissue extractions were collected using zgpr pulse, where pulse width of the 90° pulse was 7.6 μs and relaxation delay (RD) was 8.0 s; number of repetitions (n) was 128. Water signal was suppressed by weak square pulse on water peak during RD. Free induction decay (FID) was acquired in 65 536 data points with a spectral width of 13 ppm, and FID acquisitions were accumulated 128 times to increase the signal-to-noise ratio (SNR).
Spectra Processing and Metabolite Analysis
The one-dimensional 1H spectra were processed using Topsin 3.0 (Bruker). An exponential line broadening of 0.3 Hz was applied prior to Fourier transformation. Spectra were phase and baseline corrected automatically in Topspin and referenced to the TSP peak at 0.00 ppm. The concentration of individual metabolites was fitted using Chenomx software manually (version 8.3, Alberta, Canada). TSP with a concentration of 0.29 was used as the concentration and chemical shift reference.
Statistical Analysis
For all experiments, mean group values and standard errors were calculated and statistically compared using GraphPad Prism (version 7, GraphPad Software, La Jolla, CA). Single time point insulin concentrations in plasma and brain after IN vs SC administration were compared by pairwise t-test. Time-dependent blood glucose changes were compared by two-way ANOVA with Bonferroni posthoc test. Alterations in brain energy metabolites were each compared by t-test. For all experiments, significance was defined as p < 0.05.
Pharmacokinetic parameters were calculated using WinNonlin (version 5.3, Certara, St. Louis, MO). Area under the curve (AUC) was calculated by the log–linear trapezoidal rule to the end of sample collection by noncompartmental analysis. Insulin t1/2 was estimated in plasma and brain using the first-order equation t1/2 = 0.693/Kel, where Kel (elimination rate constant) is the slope of the linear regression from the natural log percentage of substrate remaining versus incubation time.79
Acknowledgments
Funding
This research was supported by grants including NIMH P30 MH075673 and NIH/NIMH PO1MH105280, and Johns Hopkins PREP R25GM109441.
ABBREVIATIONS
- AD
Alzheimer’s disease
- HAND
HIV-associated neurocognitive disorder
- ATP
adenosine triphosphate
- CNS
central nervous system
- IN
intranasal
- SC
subcutaneous
- CSF
cerebrospinal fluid
Footnotes
Author Contributions
M.T.N. and A.J.G. contributed equally to this work, including the drafting and revision of the manuscript. M.T.N., Y.W., A.A.M., and S.W.Y. performed dosing and collected mouse samples. M.T.N. and A.G.T. performed insulin ELISAs. S.H.K, S.W.Y., and J.X. conducted NMR studies. J.C.M., N.J.H., D.J.V., R.R., and B.S.S. contributed to study design and supervision as well as manuscript conceptualization, drafting, and revision.
Notes
The authors declare no competing financial interest.
References
- 1.Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes, Obes Metab. 2012;14:214–221. doi: 10.1111/j.1463-1326.2011.01490.x. [DOI] [PubMed] [Google Scholar]
- 2.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beirami E, Oryan S, Seyedhoseini Tamijani SM, Ahmadiani A, Dargahi L. Intranasal insulin treatment restores cognitive deficits and insulin signaling impairment induced by repeated methamphetamine exposure. J Cell Biochem. 2018;119:2345. doi: 10.1002/jcb.26398. [DOI] [PubMed] [Google Scholar]
- 4.Lioutas VA, Novak V. Intranasal insulin neuroprotection in ischemic stroke. Neural Regener Res. 2016;11:400–401. doi: 10.4103/1673-5374.179040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37:267–273. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 6.Kawano T, Iwata H, Aoyama B, Nishigaki A, Yamanaka D, Tateiwa H, Eguchi S, Locatelli FM, Yokoyama M. The role of hippocampal insulin signaling on postoperative cognitive dysfunction in an aged rat model of abdominal surgery. Life Sci. 2016;162:87–94. doi: 10.1016/j.lfs.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX. Intranasal Insulin Prevents Anesthesia-Induced Spatial Learning and Memory Deficit in Mice. Sci Rep. 2016;6:21186. doi: 10.1038/srep21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwanenburg RJ, Bocca G, Ruiter SA, Dillingh JH, Flapper BC, van den Heuvel ER, van Ravenswaaij-Arts CM. Is there an effect of intranasal insulin on development and behaviour in Phelan-McDermid syndrome? A randomized, double-blind, placebo-controlled trial. Eur J Hum Genet. 2016;24:1696–1701. doi: 10.1038/ejhg.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt H, Kern W, Giese R, Hallschmid M, Enders A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: an exploratory clinical trial. J Med Genet. 2009;46:217–222. doi: 10.1136/jmg.2008.062141. [DOI] [PubMed] [Google Scholar]
- 10.Poewe W, Seppi K. Insulin signalling: new target for Parkinson’s treatments? Lancet. 2017;390:1628–1630. doi: 10.1016/S0140-6736(17)32101-3. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre RS, Soczynska JK, Woldeyohannes HO, Miranda A, Vaccarino A, Macqueen G, Lewis GF, Kennedy SH. A randomized, double-blind, controlled trial evaluating the effect of intranasal insulin on neurocognitive function in euthymic patients with bipolar disorder. Bipolar Disord. 2012;14:697–706. doi: 10.1111/bdi.12006. [DOI] [PubMed] [Google Scholar]
- 12.Freiherr J, Hallschmid M, Frey WH, 2nd, Brunner YF, Chapman CD, Holscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson LR, Frey WH., 2nd Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2007;2:81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim B-H, Alt J, Rojas C, Hadas E, Kelschenbach J, Borjabad A, Thomas AG, Volsky D, Slusher B. Treatment of EcoHIV-infected Mice with Intranasal Insulin Abrogates Cognitive Impairment and Normalizes Energy Metabolite Alterations Associated with HAND. 13th International Symposium NeuroVirology; June 2–6; San Diego, CA. 2015. p. S36. [Google Scholar]
- 16.Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, Cohen EA, Power C. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J Neurosci. 2016;36:10683–10695. doi: 10.1523/JNEUROSCI.1287-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson PK, Toborek M. Biochemistry & Molecular Biology. Springer; New York: 2014. Neuroinflammation and neurodegeneration. [DOI] [Google Scholar]
- 18.Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30:2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derakhshan F, Toth C. Insulin and the brain. Curr Diabetes Rev. 2013;9:102–116. [PubMed] [Google Scholar]
- 20.Chen Y, Guo Z, Mao YF, Zheng T, Zhang B. Intranasal Insulin Ameliorates Cerebral Hypometabolism, Neuronal Loss, and Astrogliosis in Streptozotocin-Induced Alzheimer’s Rat Model. Neurotoxic Res. 2017:1–9. doi: 10.1007/s12640-017-9809-7. [DOI] [PubMed] [Google Scholar]
- 21.Duarte AI, Proenca T, Oliveira CR, Santos MS, Rego AC. Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes. 2006;55:2863–2870. doi: 10.2337/db06-0030. [DOI] [PubMed] [Google Scholar]
- 22.Duarte AI, Santos MS, Oliveira CR, Rego AC. Insulin neuroprotection against oxidative stress in cortical neurons–involvement of uric acid and glutathione antioxidant defenses. Free Radical Biol Med. 2005;39:876–889. doi: 10.1016/j.freeradbiomed.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta, Mol Cell Res. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Hoyer S, Lannert H. Long-term abnormalities in brain glucose/energy metabolism after inhibition of the neuronal insulin receptor: implication of tau-protein. J Neural Transm Suppl. 2007;72:195–202. doi: 10.1007/978-3-211-73574-9_25. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 2017;173:1–10. doi: 10.1016/j.lfs.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Ishii DN, Glazner GW, Wang C, Fernyhough P. Neurotrophic Effects and Mechanism of Insulin, Insulin-Like Growth Factors, and Nerve Growth Factor in Spinal Cord and Peripheral Neurons. In: Raizada DLK, editor. Molecular and Cellular Biology of Insulin-like Growth Factors and Their Receptors. Springer-Verlag; Boston, MA: 1993. pp. 403–425. [Google Scholar]
- 27.Spielman LJ, Bahniwal M, Little JP, Walker DG, Klegeris A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr Alzheimer Res. 2015;12:684–693. doi: 10.2174/1567205012666150710104428. [DOI] [PubMed] [Google Scholar]
- 28.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 29.Guo Z, Chen Y, Mao YF, Zheng T, Jiang Y, Yan Y, Yin X, Zhang B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci Rep. 2017;7:45971. doi: 10.1038/srep45971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 32.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344. doi: 10.1210/jc.2007-2606. [DOI] [PubMed] [Google Scholar]
- 33.Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 35.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 36.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 37.Reger M, Watson G, Green P, Baker L, Cholerton B, Fishel M, Plymate S, Cherrier M, Schellenberg G, Frey W, Craft S. Intranasal Insulin Administration Dose-Dependently Modulates Verbal Memory and Plasma β-Amyloid in Memory-Impaired Older Adults. J Alzheimer’s Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 40.Baura GD, Foster DM, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cedernaes J, Chapman CD, Brunner Y, Schioth HB, Frieherr J, Benedict C. Intranasal Insulin Targeting to Brain and Cerebrospinal Fluid (CSF): A Review of its Clinical Effects and Mechanisms. Respir Drug Delivery Eur. 2013:59–68. [Google Scholar]
- 42.Wallum BJ, Taborsky GJ, Jr, Porte D, Jr, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D. Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab. 1987;64:190–194. doi: 10.1210/jcem-64-1-190. [DOI] [PubMed] [Google Scholar]
- 43.Neumann KF, Rojo L, Navarrete LP, Farias G, Reyes P, Maccioni RB. Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr Alzheimer Res. 2008;5:438–447. doi: 10.2174/156720508785908919. [DOI] [PubMed] [Google Scholar]
- 44.Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hyperinsulinemia causes activation of the hypothalamus-pituitary-adrenal axis in humans. Int J Obes. 2001;25:S38–40. doi: 10.1038/sj.ijo.0801695. [DOI] [PubMed] [Google Scholar]
- 45.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 46.Nedelcovych M, Dash RP, Tenora L, Zimmermann SC, Gadiano AJ, Garrett C, Alt J, Hollinger KR, Pommier E, Jancarik A, Rojas C, Thomas AG, Wu Y, Wozniak K, Majer P, Slusher BS, Rais R. Enhanced Brain Delivery of (2-(phosphonomethyl)pentanedioic acid) following Intranasal Administration of its gamma-substituted Ester Prodrugs. Mol Pharmaceutics. 2017;14:3248. doi: 10.1021/acs.molpharmaceut.7b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Delivery Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9:S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renner DB, Svitak AL, Gallus NJ, Ericson ME, Frey WH, 2nd, Hanson LR. Intranasal delivery of insulin via the olfactory nerve pathway. J Pharm Pharmacol. 2012;64:1709–1714. doi: 10.1111/j.2042-7158.2012.01555.x. [DOI] [PubMed] [Google Scholar]
- 50.Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35:371–381. doi: 10.1038/jcbfm.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 52.Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH. Delivery of Nerve Growth Factor to the Brain via the Olfactory Pathway. J Alzheimer’s Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- 53.Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Controlled Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 54.Nonaka N, Farr SA, Kageyama H, Shioda S, Banks WA. Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J Pharmacol Exp Ther. 2008;325:513–519. doi: 10.1124/jpet.107.132381. [DOI] [PubMed] [Google Scholar]
- 55.Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH., 2nd Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152:785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Kamei N, Shingaki T, Kanayama Y, Tanaka M, Zochi R, Hasegawa K, Watanabe Y, Takeda-Morishita M. Visualization and Quantitative Assessment of the Brain Distribution of Insulin through Nose-to-Brain Delivery Based on the Cell-Penetrating Peptide Noncovalent Strategy. Mol Pharmaceutics. 2016;13:1004–1011. doi: 10.1021/acs.molpharmaceut.5b00854. [DOI] [PubMed] [Google Scholar]
- 58. [accessed Sept 1, 2017];ClinicalTrialsgov. www.clinicaltrials.gov.
- 59.Quianzon CC, Cheikh I. History of insulin. J Community Hosp Int Med Perspect. 2012;2:18701. doi: 10.3402/jchimp.v2i2.18701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol. 2004;188:43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Ko JH, Katako A, Aljuaid M, Goertzen AL, Borys A, Hobson DE, Kim SM, Lee CS. Distinct brain metabolic patterns separately associated with cognition, motor function, and aging in Parkinson’s disease dementia. Neurobiol Aging. 2017;60:81–91. doi: 10.1016/j.neurobiolaging.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Tang Y, Ge J, Liu F, Wu P, Guo S, Liu Z, Wang Y, Wang Y, Ding Z, Wu J, Zuo C, Wang J. Cerebral Metabolic Differences Associated with Cognitive Impairment in Parkinson’s Disease. PLoS One. 2016;11:e0152716. doi: 10.1371/journal.pone.0152716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 65.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, Jonaitis EM, Sager MA, Asthana S. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurology. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med. 2013;43:349–366. doi: 10.1053/j.semnuclmed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 68.von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57:1601–1607. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- 69.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 70.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 71.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev. 2016;96:1169–1209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 72.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: A new target for disease modification? Prog Neurobiol. 2016;145–146:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 73.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimer’s Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 74.Burklen TS, Schlattner U, Homayouni R, Gough K, Rak M, Szeghalmi A, Wallimann T. The creatine kinase/creatine connection to Alzheimer’s disease: CK-inactivation, APP-CK complexes and focal creatine deposits. J Biomed Biotechnol. 2006;2006:1–11. doi: 10.1155/JBB/2006/35936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henneberg N, Hoyer S. Short-term or long-term intracerebroventricular (i. c.v.) infusion of insulin exhibits a discrete anabolic effect on cerebral energy metabolism in the rat. Neurosci Lett. 1994;175:153–156. doi: 10.1016/0304-3940(94)91102-9. [DOI] [PubMed] [Google Scholar]
- 76.Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’s Disease. Front Endocrinol (Lausanne, Switz) 2014;5:161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes. 2014;63:3992–3997. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rastogi GK, Letarte J, Fraser TR. Immunoreactive insulin content of 203 pancreases from foetuses of healthy mothers. Diabetologia. 1970;6:445–446. doi: 10.1007/BF01212080. [DOI] [PubMed] [Google Scholar]
- 79.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, Garberg P, Postlind H. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453–472. [PubMed] [Google Scholar]