Abstract
Background
Inhibitory control deficits are common in autism spectrum disorder (ASD) and associated with more severe repetitive behaviors. Inhibitory control deficits may reflect slower execution of stopping processes, or a reduced ability to delay the onset of behavioral responses in contexts of uncertainty. Previous studies have documented relatively spared stopping processes in ASD, but whether inhibitory control deficits in ASD reflect failures to delay response onset has not been systematically assessed. Further, while improvements in stopping abilities and response slowing are seen through adolescence/early adulthood in health, their development in ASD is less clear.
Methods
A stop-signal test (SST) was administered to 121 individuals with ASD and 76 age and IQ-matched healthy controls (ages 5–28). This test included “GO trials” in which participants pressed a button when a peripheral target appeared and interleaved “STOP trials” in which they were cued to inhibit button-presses when a stop-signal appeared at variable times following the GO cue. STOP trial accuracy, RT of the stopping process (SSRT), and reaction time (RT) slowing during GO trials were examined.
Results
Relative to controls, individuals with ASD had reduced accuracy on STOP trials. SSRTs were similar across control and ASD participants, but RT slowing was reduced in patients compared to controls. Age-related increases in stopping ability and RT slowing were attenuated in ASD. Reduced stopping accuracy and RT slowing were associated with more severe repetitive behaviors in ASD.
Discussion
Our findings show that inhibitory control deficits in ASD involve failures to strategically delay behavioral response onset. These results suggest that reduced preparatory behavioral control may underpin inhibitory control deficits as well as repetitive behaviors in ASD. Typical age-related improvements in inhibitory control during late childhood/early adolescence are reduced in ASD, highlighting an important developmental window during which treatments may mitigate cognitive alterations contributing to repetitive behaviors.
Keywords: Autism spectrum disorders, inhibition, cognitive development
Introduction
Inhibitory control, or the ability to suppress contextually-inappropriate responses, is critical for adapting behavior to changing and often uncertain environmental demands. Deficits of inhibitory control are common in autism spectrum disorder (ASD; Geurts, van den Bergh, & Ruzzano, 2014) and emerge as early as 24 months old (St John et al., 2016). They also are familial (Mosconi et al., 2010) and associated with restricted, repetitive behaviors (RRBs; Mosconi et al., 2009; South, Ozonoff, & McMahon, 2007). The mechanisms linking inhibitory control deficits and RRBs remain unclear, but these findings suggest that RRBs reflect neurocognitive disturbances affecting patients’ abilities to withhold behaviors that are prepotent but contextually-inappropriate (e.g., talking about a strong interest). Thus, understanding inhibitory control deficits in ASD may provide important insights into the cognitive and pathophysiological processes underlying core clinical features.
Recent studies have begun to specify the cognitive components of inhibitory control deficits in ASD. Guerts and colleagues’ meta-analysis (2014) observed that prepotent response inhibition, or the ability to suppress a previously-reinforced behavioral response, was more severely impaired in ASD (average Effect Size (ES)=0.55) than distractor interference, or the ability to ignore irrelevant information (average ES=0.31). This suggests that patients show greater difficulty inhibiting prepotent behavioral responses, whereas their ability to inhibit attention towards task-irrelevant stimuli is less affected.
Successful inhibition of prepotent responses relies on distinct reactive and proactive control mechanisms (Aron, 2011). Reactive control involves terminating already-initiated behaviors in response to external cues. In contrast, proactive control involves withholding or slowing the initiation of behavioral responses in preparation for stopping during conditions of uncertainty. The stop-signal task (SST) is the most common paradigm used to study reactive and proactive control processes (Logan, 1994). During this task, participants make a behavioral response to a GO cue on the majority of trials, but inhibit their response on a minority of trials when a STOP cue follows the presentation of the GO cue. Task performance has been modeled as a race between independent GO and STOP processes; successful inhibition occurs when the STOP process finishes prior to the GO process (Logan, 1994). Reactive inhibition is measured by estimating the time it takes the STOP process to be completed (i.e., stop-signal reaction time (SSRT)), with shorter SSRTs reflecting faster reactive stopping (Logan, 1994). Proactive inhibition is estimated by the extent to which RT increases when GO and STOP trials are interleaved relative to individuals’ baseline RT when only GO trials are administered. Proactive RT slowing extends the duration of the GO process, thereby increasing the likelihood that the STOP process can interrupt already-initiated behavioral responses in response to a STOP cue (Verbruggen & Logan, 2009). Our group and others have shown that greater RT slowing is associated with greater stopping abilities (Jahfari, Stinear, Claffey, Verbruggen, & Aron, 2010; Schmitt, Ankeny, Sweeney, & Mosconi, 2016; Verbruggen & Logan, 2009).
Studies examining SSTs have found that individuals with ASD make more inhibition errors on STOP trials compared to controls (Ishii-Takahashi et al., 2014; Lemon, Gargaro, Enticott, & Rinehart, 2011), while SSRTs are similar across groups (Adams & Jarrold, 2012; Lemon et al., 2011; Ozonoff & Strayer, 1997). These results suggest that reactive control processes may be relatively intact in patients. Proactive control strategies, in contrast, have not yet been systematically investigated in ASD. Because overlapping, yet distinct, fronto-basal-ganglia circuits support reactive and proactive control (Aron, Robbins, & Poldrack, 2004), systematic comparisons of these component processes may be leveraged to identify the distinct cognitive and neurophysiological mechanisms underpinning inhibitory control impairments and associated RRBs in ASD.
In healthy individuals, prepotent response inhibition and proactive slowing develop throughout childhood and reach adult levels at ~15 years of age (Luna, Garver, Urban, Lazar, & Sweeney, 2004). During adolescence, individuals become less reliant on reactive control and more reliant on proactive control to delay behavioral response onset (Vink et al., 2014). The rate at which inhibitory control improves with age in ASD has been found to be similar (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007), increased (Happe, Booth, Charlton, & Hughes, 2006), and attenuated (Padmanabhan et al., 2015) relative to controls, though inhibitory control deficits in ASD appear to be more profound during adolescence and adulthood relative to childhood (Adams & Jarrold, 2012; Geurts et al., 2014; Ozonoff & Strayer, 1997). Large sample studies across childhood and into adulthood are needed to clarify the timing and maturation of inhibitory control deficits in ASD and thus determine critical time-points during which interventions may be most effective.
In the current study, we examined reactive and proactive processes of inhibitory control in 121 individuals with ASD and 76 healthy controls age 5–28 years. We predicted that individuals with ASD would demonstrate reduced stopping accuracy compared to controls due to reduced proactive RT adjustments, but that groups would show similar SSRTs suggesting reactive control processes are spared. Based on prior findings that inhibitory control deficits become more pronounced in adolescence and early adulthood in ASD, we predicted that age-associated increases in stopping ability and proactive RT slowing would be attenuated in ASD relative to controls. Lastly, we hypothesized that reduced stopping accuracy and proactive RT slowing would be associated with more severe RRBs in ASD.
Methods
Participants
We tested 121 individuals with ASD and 76 age-, nonverbal IQ-, and sex-matched healthy controls using a manual motor SST and a baseline RT task (Table 1). Testing was conducted with identical procedures at the University of Illinois at Chicago (CHI; n=115) and the University of Texas Southwestern (UTSW; n=82). Participants were recruited through community advertisements, and those with ASD were recruited through outpatient clinics.
Table 1.
Demographic and clinical characteristics of ASD and control participants
| ASD (n=121) | Control (n=76) | ||
|---|---|---|---|
| Male n(%) | CHI | 57(80) | 34(76) |
| UTSW | 44(86) | 21(68) | |
| Overall | 101(83) | 55(72) | |
| Age in years | CHI | 12.8(5.0) | 14.9(5.9)~ |
| UTSW | 11.6(3.8) | 11.6(5.1) | |
| Overall | 12.3(4.7) | 13.5(5.8) | |
| Range | 5–26 | 5–28 | |
| Non-verbal IQ | CHI | 101.9(18.7) | 102.7(10.6) |
| UTSW | 101.4(17.6) | 103.9(11.6) | |
| Overall | 101.7(18.1) | 103.2(11.0) | |
| Range | 64–158 | 76–129 | |
| Verbal IQ | CHI | 100.1(18.1) | 108.1(13.4) |
| UTSW | 95.1(18.7) | 111.1(12.9) | |
| Overall | 97.8(18.5)*** | 109.3(13.2) | |
| Range | 58–148 | 82–140 | |
| Full-Scale IQ | CHI | 99.8(17.8) | 106.7(12.0) |
| UTSW | 97.7(17.3) | 108.4(11.2) | |
| Overall | 98.8(17.6)*** | 107.4(11.6) | |
| Range | 69–144 | 76–128 | |
| ADI Social (Range: 5–30) | 20.6(5.5) | – | |
| ADI Communication (Range: 4–26) | 16.2(4.8) | – | |
| ADI RRB (Range: 1–12) | 6.2(2.5) | – | |
| ADOS Social Affectǂ (Range: 0–19) | 9.5(4.0) | – | |
| ADOS RRB (Range: 0–12) | 2.8(2.1) | – | |
| ADOS Comparison Score (Range: 3–10) | 7.1(2.1) | – | |
| RBS-R Total (Range: 0–96) | 29.8(20.3) | – | |
| RBS-R Compulsive (Range: 0–24) | 4.5(5.0) | – | |
| RBS-R Restricted (Range: 0–12) | 3.9(2.8) | – | |
| RBS-R Rituals (Range: 0–18) | 6.1(4.2) | – | |
| RBS-R Sameness (Range: 0–31) | 9.3(7.0) | – | |
| RBS-R Self-Injury (Range: 0–12) | 2.3(2.7) | – | |
| RBS-R Stereotyped (Range: 0–18) | 4.1(3.6) | – | |
Mean (standard deviation), unless otherwise denoted.
p<0.001 for ASD vs Control,
p<.05 for CHI vs UTSW
for Module 4, Communication+Social Interaction Total
ASD participants met DSM-5 criteria for ASD based on the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), and expert clinical opinion. Patients were excluded if they had a known genetic disorder associated with ASD (e.g., Fragile X) or a medical history of non-febrile seizures. Healthy controls were excluded if they scored ≥8 on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), had a history of psychiatric disorder, had a first-degree relative with serious mental illness (e.g., schizophrenia), or had a first- or second-degree relative with ASD. No participants had a history of head injury resulting in loss of consciousness, had consumed caffeine within 24-hours of testing, or used nicotine one-hour prior to testing. The majority of ASD participants were recruited as part of a larger study that excluded individuals who were taking medications known to affect cognitive and sensorimotor abilities (e.g., stimulants; Reilly, Lencer, Bishop, Keedy, & Sweeney, 2008), and thus only a small number of individuals with ASD enrolled in the study were currently taking medications. Two patients were taking a selective-serotonin reuptake inhibitor, but removing these patients from our analyses did not substantively affect results; thus, they were included in final analyses. All participants provided written consent or assent for minors (and written consent from their guardians). All procedures were approved by the local Institutional Review Boards.
Apparatus and Stimuli
Visual stimuli subtending 0.5–1 degree (deg) of visual angle were presented in the horizontal plane at eye-level. Participants were seated approximately 50-cm in front of a 55.9-cm monitor with a 60-Hz refresh rate. All participants used a custom-made button-box that recorded button presses through a USB port with a sampling rate of 125-Hz.
Tasks and Procedures
Baseline Reaction Time (RT) Test
A baseline RT test was administered to establish each individual’s RT during only ‘GO’ trials. During each trial, participants rested their right and left thumbs on buttons corresponding to the locations of the peripheral targets while viewing a white central fixation crosshair for a random interval of 750–1500 ms (Figure 1). ‘GO’ trials consisted of a green circular target (0.75 deg) that appeared 12 deg to the right or left of center. Participants completed 60 GO trials (30 rightward, 30 leftward), and were instructed to press the corresponding button as quickly as possible when the target appeared. If participants did not respond within 650 ms, they received negative feedback (e.g., a red ‘X’ in place of the green circle and the word “faster”).
Figure 1.
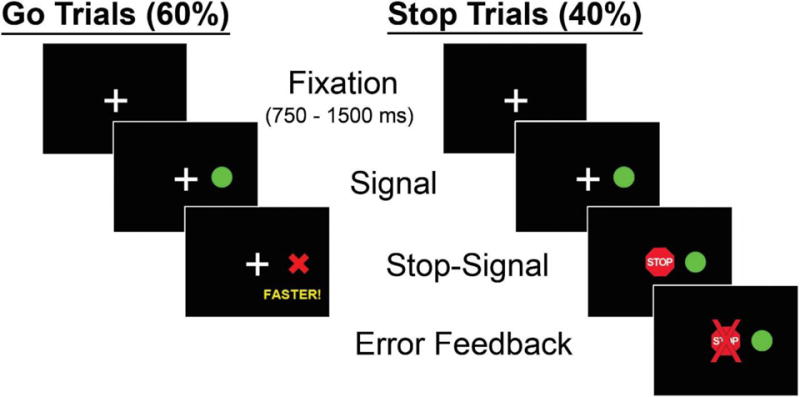
Schematic representation of stop-signal task. During GO trials, participants responded to a peripheral target appearing on the right or left side by pressing the corresponding button. During STOP trials, a centrally-presented red stop-signal appeared at a variable delay following the GO cue. Participants were instructed to inhibit their response when the STOP cue appeared.
Stop-Signal Task (SST)
During ‘STOP’ trials, a centrally-displayed red stop-signal was presented after the green target appeared at varying delays (i.e., stop-signal delay (SSD)), and participants were instructed not to respond. SSDs were sampled continuously in 16.67 ms intervals (matching the monitor refresh rate of 60-Hz) between 50–283 ms. The order of SSD intervals varied randomly, and GO and STOP trials were presented in a pseudorandom order; no more than three consecutive trials of the same type were administered. When participants pressed a button on a STOP trial, a red ‘X’ was displayed in the center to alert them to the error. Four blocks of 63 trials (60% GO and 40% STOP trials interleaved) were administered. Each block of trials was followed by 10 sec of rest.
Clinical Measures
The ADI and ADOS were administered by research-reliable clinicians (LS, SPW, MWM) to confirm ASD diagnoses and quantify clinical symptom severity across social-communication and RRB domains. For the ADI, diagnostic algorithm totals for Social, Communication, and RRB deficits were examined. For the ADOS, algorithm totals for social communication (Modules 2 (n=3) and 3 (n=87): Social Affect; Module 4 (n=31): Communication + Social) and RRB deficits (Modules 2 and 3: RRB; Module 4: Stereotyped Behaviors and Restricted Interests) were examined. Because different items are included in the algorithms for Modules 2 and 3 versus Module 4, we computed alternate Social-Communication and RRB totals for Module 4 based on the corresponding subscale items from Modules 2 and 3. Analyses using identical items across Modules yielded substantively similar results to those using Module-specific algorithm items, so only the latter are reported in final analyses. We also examined Comparison Scores for Modules 2 and 3 (Hus, Gotham, & Lord, 2014), though these were not available for Module 4. The Repetitive Behavior Scale-Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000), a parent-report questionnaire, also was used to quantify RRBs. To estimate general cognitive abilities, participants were administered the Differential Ability Scales, Second Edition (DAS; Elliott, 2007), Wechsler Abbreviated Scales of Intelligence (WASI; Wechsler, 1999)), or Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition (WPPSI; Wechsler, 2012). CHI participants <18 years completed the DAS (n=91) and those >18 years of age completed the WASI (n=24). UTSW participants <6 years completed the WPSSI (n=4) and those >6 years completed the WASI (n=78).
Statistical Analyses
To examine how stopping accuracy varied as a function of SSD, we conducted a 2 (ASD versus controls) × 15 (SSD blocked in 16.67 ms intervals) repeated-measures analysis of variance (ANOVA). Separate one-way ANOVAs were used to compare groups on stopping accuracy, RT slowing, SSRT, and the SSD at which stopping accuracy equaled 50% (p50). We used the quantile method to estimate SSRT (for details, see Band, van der Molen, & Logan, 2003). This method was selected because it best accounts for individual differences in GO RT. To compare baseline and SST GO trials RTs, we conducted a 2 (ASD versus controls) × 2 (baseline versus correct GO trials) repeated-measures ANOVA. We conducted ANCOVAs for each of our outcome measures using VIQ, sex, and site as covariates. Because no substantive differences in results were found with any of the ANCOVA models (Appendix S1.), only results without the covariates are reported.
To determine whether age moderated the relationship between group and SST performance, we used the PROCESS macro developed by Hayes (2013) for SPSS. This analysis is based on Ordinary Least-Squares regression and utilizes bootstrapping procedures (5,000 samples) for investigating moderation effects of one variable on the relationship between two variables known to be related to one another. For models found to be significant, we conducted changepoint analyses using the Johnson-Neyman Technique to determine the youngest age at which performance diverged between groups (Johnson & Fay, 1950; for details see Appendix S1.). All SST outcomes were linearized using a log-transformation prior to moderation analyses.
For individuals with ASD, we examined the relationships between SST performance and clinical issues using Spearman correlations. Although the time elapsed between administration of behavioral and clinical measures was kept to a minimum for the majority of participants (Table S1.), we conducted hierarchical regressions using the enter method with linearized variables to determine the moderating effects of longer intervals between clinical and SST administrations on the strengths of the relationships between SST performance and clinically-rated behaviors. For each hierarchical regression conducted, Model 1 included the SST performance variable, Model 2 included the SST performance variable and the duration between administrations, and Model 3 included the interaction term (SST performance × duration between administrations). For each hierarchical regression, durations between administrations and interaction terms had non-significant effects (p’s > 0.24), thus therefore were not included in final analyses.
Results
Stopping Accuracy
Individuals with ASD demonstrated reduced stopping accuracy compared to controls (Table 2; F(1, 195)=11.94, p=.001, Cohen’s d=.51). Stopping accuracy decreased as SSD increased (Figure 2; F(14,182)=114.25, p<.001, d=1.56), a pattern that was similar for patients and controls (group × SSD interaction: F(14, 182)=1.12, p=.23, d=.15). As we have previously found that few individuals are able to consistently inhibit button presses at SSDs >200 ms (Schmitt et al., 2015), we examined stopping accuracy across a smaller range of SSDs (50–200 ms). For this analysis, stopping accuracy deteriorated more rapidly for individuals with ASD than controls (group X SSD interaction: Fcubic(10, 182)=7.59, p=.01, d=.40). Similarly, the p50 was lower for patients (F(1,195)=11.00, p=.001, d=.49), indicating that patients’ performance fell below chance at shorter SSDs than controls. Individuals with ASD also failed to respond on more GO trials than controls (F(1, 195)=7.68, p=.006, d=.41).
Table 2.
Stop-signal task performance for ASD participants and controls
| ASD | Control | |
|---|---|---|
| SSRT(ms) | 267(48) | 262(48) |
|
|
||
| Baseline RT(ms) | 387(62)** | 358(69) |
|
|
||
| GO RT(ms) | 451(42)* | 464(39) |
|
|
||
| Baseline–GO RT(ms) | 66(44)*** | 106(50) |
|
|
||
| % GO Trial Omissions | 17(13)** | 12(10) |
|
|
||
| % Accuracy on STOP Trials | 55(14)*** | 64(16) |
|
|
||
| p50(ms) | 184(31)*** | 201(37) |
|
|
||
Mean (standard deviation)
RT=reaction time, SSRT=stop-signal reaction time, p50=stop-signal delay at which performance is 50%
p<0.05;
p<0.01;
p<0.001
Figure 2.
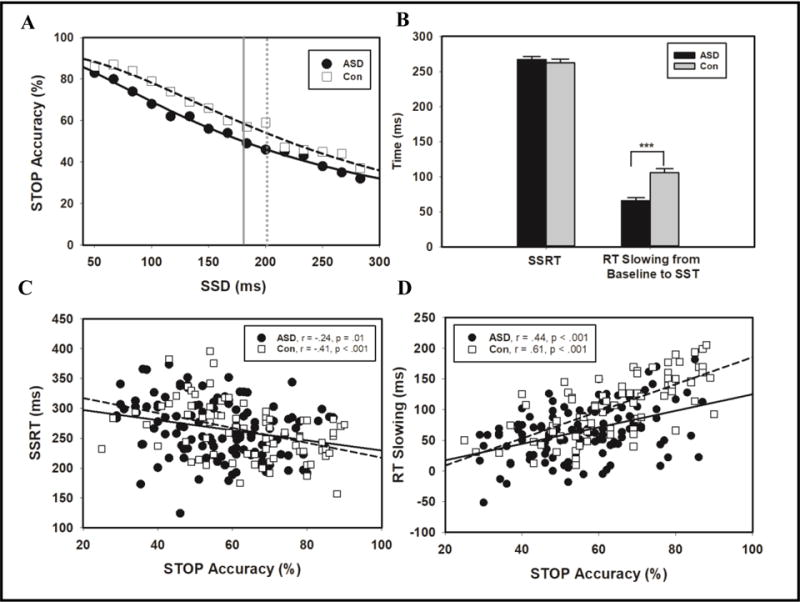
Reactive and proactive inhibitory control in individuals with ASD and controls. STOP trial accuracy across SSDs for individuals with ASD (black circle) and controls (open square; A). The SSD at which performance reached 50% accuracy is indicated by a solid grey line for ASD participants and a dotted grey line for controls. Means for SSRT and RT slowing for individuals with ASD (black) and controls (grey; B). Correlations with linear regression lines for STOP accuracy and SSRT (B) and STOP accuracy and RT slowing (D).
Reaction Times
RT was slower during SST compared to baseline GO trials (F(1, 195)=704.93, p<.001, d=3.89). Both groups showed similar RTs overall (F(1, 195)=.003, p=.96, d=.01), but the task X group interaction was significant (F(1, 195)=27.84, p<.001, d=.78). Compared to controls, individuals with ASD showed slower RTs at baseline (t(190)=3.10, p=.002, d=.44), but faster RTs during the SST (Table 2; t(1, 195)=−2.02, p=.04, d=.32). Thus, individuals with ASD slowed their RTs to a lesser degree than controls (Figure 2; F(1, 195)=33.23, p<.001, d=.85). SSRT did not differ between groups (F(1,195)=.31, p=.54, d=.08).
Relationships between SST Variables
For controls, greater stopping accuracy was associated with shorter SSRTs (r=−.41, p<.001) and greater RT slowing (r=−.61, p<.001). In addition, greater RT slowing was associated with shorter SSRTs (r=−.70, p<.001). For individuals with ASD, greater stopping accuracy was associated with shorter SSRTs (r=−.24, p=.01) and greater RT slowing (r=.44 p<.001). Greater RT slowing was associated with shorter SSRTs for patients (r=−.41, p<.001).
The strength of the relationship between stopping accuracy and RT slowing was stronger for controls than patients (Z=−2.58, p=.01). But, the strengths of the relationships between SSRT and stopping accuracy (Z=1.19, p=.23) and RT slowing (Z=1.82, p=.07) did not differ between groups. The strength of the relationship between stopping accuracy and RT slowing was stronger than that between stopping accuracy and SSRT for controls (Z=2.54 p=.01), but not for individuals with ASD (Z=1.60, p=.11).
Relationships between Age and Inhibitory Control
Quadratic functions provided the best fit for modeling the relationships between age and SST variables across participant groups. For controls, increased age was associated with increased stopping ability (Figure 3A; r2=.36, F(2,73)=20.89, p<.001, Cohen’s f=.56), greater RT slowing (Figure 3B; r2=.56, F(2,73)=46.02, p<.001, f=1.27), and shorter SSRT (r2=.60, F(2,73)=55.57, p<.001, f=1.50). For individuals with ASD, increased age was not significantly related to increased stopping accuracy (Figure 3A; r2=.03, F(2,118)=1.73, p=.18, f=.03), yet age was associated with greater RT slowing (Figure 3B; r2=.25, F(2,118)=18.85, p<.001, f=.33) and shorter SSRT (r2=.33, F(2,118)=29.55, p<.001, f=.49). The relationships between age and stopping ability (Z=−3.53, p<.001), age and RT slowing (Z=−2.78, p=.01), and age and SSRT (Z=−2.53, p=.01) were stronger for controls than patients.
Figure 3.
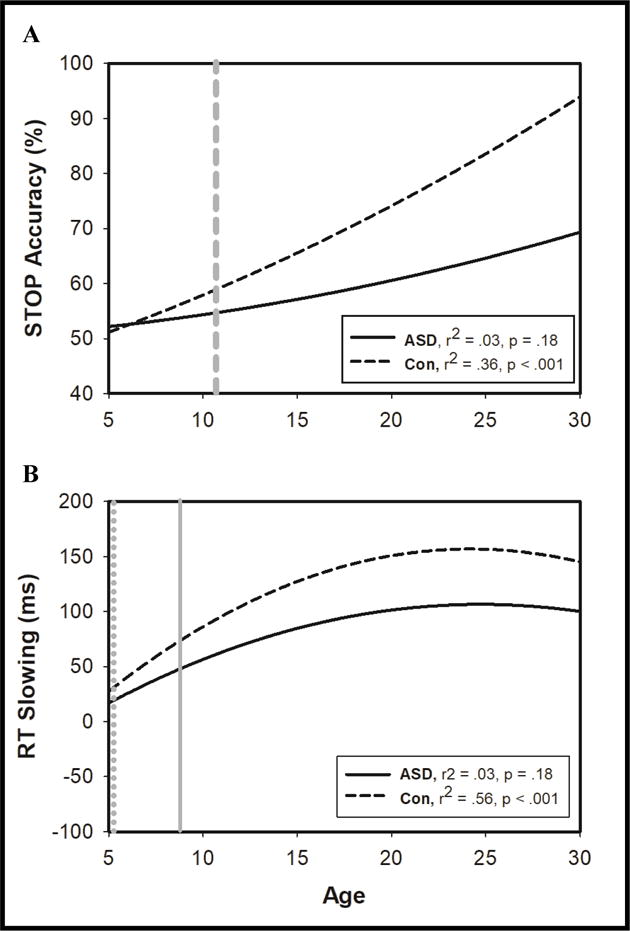
Age-related increases in SST performance. Age-related increases in STOP accuracy for individuals with ASD (solid black line) and controls (dashed black line). Age at which group performance began to diverge is indicated by thick dashed grey line (A). Age-related increases in increased RT slowing both groups, with light grey line indicating when RT slowing began to impact stopping performance for control (dotted grey line) and ASD participants (solid grey line).
Age significantly moderated the relationship between group and stopping accuracy (Figure 3A; r2=.02, F(1,193)=4.53, p=.04, f=.02), such that increased age was associated with greater increases in stopping accuracy for controls relative to patients. Changepoint analyses revealed that groups began to significantly diverge in their stopping ability at age 10.7 years (Table S2.). Age did not significantly moderate the relationships between group and SSRT (r2<.01, F(1,193)=.03, p=.86, f<.01) or group and RT slowing (r2<.01, F(1,193)=.77, p=.38, f<.01).
Age moderated the relationship between RT slowing and stopping accuracy (r2=.10, F(1,193)=26.59, p<.001, f=.10). Changepoint analyses (Table S3.) indicated that the age at which RT slowing began to be significantly associated with increased stopping ability was younger for controls (5.0 years; r2=.07, F(1,76)=9.28, p=.003, f=.08) than patients (8.7 years; r2=.08, F(1,111)=10.36, p<.001, f=.09). Age did not moderate the relationship between SSRT and stopping accuracy (r2<.01, F(1,193)=.11, p=.75, f<.01).
Clinical Correlations
For individuals with ASD, reduced stopping accuracy was associated with more severe clinical ratings of RRBs (RBS-R total: Spearman’s rho(ρ)= −.21, p=.03; RBS-R compulsive: ρ=−.20, p=.03: RBS-R ritualistic: ρ=−.23, p=.02). Reduced RT slowing also was associated with more severe RRBs (RBS-R total: ρ=−.19, p=.04; RBS-R stereotypies: ρ=−.24, p=.01; ADOS RRB: ρ=−.23, p=.02). SSRT was not related to any clinical variables, and no SST variables were associated with social-communication impairments on the ADOS or ADI (p’s<.05).
Discussion
Testing inhibitory control in a large sample of individuals with ASD, we report three key findings. First, we found that inhibitory control deficits in ASD involve a reduced ability to proactively delay the onset of behavioral responses. Yet, the ability to reactively stop behaviors seems to be preserved in patients. Second, age-related improvements in stopping ability were attenuated in ASD, which appears to be, in part, due to reduced rate of improvement in proactive slowing in patients beginning in early childhood. Third, more severe behavioral inhibition and slowing deficits were associated with more severe RRBs. This suggests that disruptions of preparatory processes involved in suppressing behaviors during conditions of uncertainty may manifest clinically in ASD as failures to terminate or delay contextually-inappropriate behaviors.
Impaired Response Inhibition in ASD
Extending previous findings that individuals with ASD have a reduced ability to inhibit prepotent responses (Ishii-Takahashi et al., 2014; Lemon et al., 2011), we found that, compared to healthy controls, patients showed more rapid deterioration of their inhibitory control abilities as SSDs were increased, and they reached chance-levels at a shorter SSD than healthy participants. These findings suggest that individuals with ASD have a reduced capacity to withhold behavioral responses that becomes more severe during longer periods of uncertainty. These results suggest reduced top-down modulation of sensorimotor systems in individuals with ASD, consistent with prior studies indicating increased rates of excitatory relative to inhibitory activity in ASD (Rubenstein & Merzenich, 2003).
Proactive and Reactive Control Processes in ASD
Failures to inhibit behaviors at higher SSDs may reflect deficits of proactive or reactive control processes. Individuals may proactively delay their response onset to increase the opportunity for STOP processes to interrupt context inappropriate behavior (Aron, 2011; Schmitt et al., 2016; Verbruggen & Logan, 2009). Our finding that patients delay their RTs less than controls suggests that patients’ inhibitory control deficits reflect a reduced ability to proactively delay response timing during uncertain conditions. Inhibitory control deficits in ASD also could reflect alterations in reactive control processes as reflected in other patient populations by increased SSRTs (e.g., ADHD; Logan, 1994). Yet, our finding of similar SSRTs in individuals with ASD and controls suggests that reactive control is intact in ASD, consistent with prior studies (Adams & Jarrold, 2012; Lemon et al., 2011; Ozonoff & Strayer, 1997). Furthermore, relative to SSRT, proactive slowing was more strongly associated with stopping ability in patients. Taken together, these results suggest that deficits of preparatory control processes initiated prior to behavioral response onset are the primary mechanisms disrupting inhibitory control in individuals with ASD.
In order to determine whether individuals with ASD could delay response onset within a restricted time-frame as opposed to indefinitely, we required participants to respond within 650 ms. This timing restraint forced participants to make rapid decisions regarding whether to GO or STOP as is often required during the course of daily living (e.g., when deciding whether to cross a street in traffic). Our finding that individuals with ASD had more GO trial omissions than controls suggests that patients attempted to delay response onset, but they did so in an “all-or-nothing” fashion, such that they were less able to effectively modulate RTs to negotiate the balance between speed and accuracy. These results implicate top-down processes involved in strategically delaying response onset and chronometric systems involved in ensuring consistent and accurate timing of behavior. This finding is consistent with prior studies showing reduced precision of movement timing and timing perception in ASD (D’Cruz et al., 2009; Wallace & Happe, 2008). These findings may help explain why prior studies of SST that did not restrict RTs did not identify inhibitory control deficits in ASD (Adams & Jarrold, 2012; Ozonoff & Strayer, 1997). It also is possible that our restriction on the maximum RT led us to include slower RTs from patients as “omission errors”, whereas they may have reflected patients’ tendency to react more slowly during the SST. However, when removing ASD participants with high omission rates (>20%, n=35), group comparisons were not substantively affected, suggesting that inhibitory control deficits more likely reflect reduced slowing and chronometric system alterations. Still, future studies examining the RT of “omission errors” in ASD may help clarify mechanisms contributing to patients’ inhibitory control deficits.
Development of Inhibitory Control in ASD
Extending previous findings (Padmanabhan et al., 2015), we found that for both ASD and control groups, the abilities to inhibit responses and proactively delay response onset mature into early adulthood. Stopping abilities mature rapidly from middle childhood and throughout adulthood in individuals with and without ASD, consistent with previous findings (Luna et al., 2007; Happé et al., 2006). In contrast, proactive control develops more steadily throughout childhood and adolescence and remains relatively stable through early adulthood. Maturational processes of inhibitory and proactive control each are attenuated in ASD, leading to larger gaps in stopping and proactive slowing abilities in patients compared to controls during adolescence/adulthood relative to childhood. Beginning at ~10.7 years, stopping abilities in individuals with ASD and controls begin to diverge and subsequently widen with increasing age. The relatively slower development of inhibitory control in healthy children compared to healthy adolescents may account for prior findings that SST performance is spared in younger samples of individuals with ASD (Adams & Jarrold, 2012; Ozonoff & Strayer, 1997).
We provide novel evidence that proactive control mechanisms emerge early in childhood and support the ability to withhold responses as early as 5.0 years old. Yet, because proactive control is slower to develop in individuals with ASD, it does not contribute significantly to stopping ability until later in childhood (~8.7 years). Delays in proactive control development in ASD thus help account for differences in response inhibition emerging at age ~10.7 years and subsequently widening with increasing age as documented in a study of 30–70 year old individuals with ASD (Powell, Klinger, & Klinger, 2017).
Brain Processes Supporting Inhibitory Control
Numerous studies of healthy individuals have documented that inhibitory control is supported by overlapping, yet distinct fronto-basal-ganglia circuits involving the right inferior frontal cortex (rIFC), pre-supplementary motor area (pre-SMA), internal segment of the globus pallidus (GPi), and subthalamic nucleus (STN; Aron, 2011; Aron et al., 2004). Reactive control relies on rIFC and pre-SMA processes terminating basal ganglia output to the primary motor cortex prior to execution. Proactive control involves STN suppression of basal ganglia output via modulation of the rIFC, which delays the onset of motor responses during conditions of uncertainty (Wessel & Aron, 2017). Thus, our finding of reduced proactive slowing in ASD implicates deficits of STN modulation of rIFC. Padmanabhan and colleagues (2015) recently demonstrated rIFC hypo-activation in adolescents and adults, but not children with ASD during a test of inhibitory control, supporting the hypothesis that inhibitory control impairments in ASD reflect STN-prefrontal cortical dysfunction.
Consistent with this hypothesis, we documented fronto-striatal hypo-activation in ASD during a test in which participants had to flexibly shift away from previously reinforced behaviors under conditions of uncertainty (D’Cruz, Mosconi, Ragozzino, Cook, & Sweeney, 2016). These results indicate that fronto-striatal circuits supporting behavioral flexibility are compromised when trial conditions are uncertain in ASD, and thus may reflect a more generalized problem flexibly adapting behavior during uncertain conditions. Consistent with this hypothesis, frontal and basal ganglia structural abnormalities have been documented in ASD and are associated with more severe RRBs (Langen et al., 2014; Sears et al., 1999). Direct examination of these brain systems during proactive control operations are needed to further investigate the role of fronto-basal-ganglia processes in inhibitory control deficits in ASD.
Clinical Associations
We also found that reduced RT slowing is associated with more severe RRBs in individuals with ASD, extending previous findings that increased response inhibition error rate is associated with more severe RRBs (Mosconi et al., 2009; South et al., 2007). These results suggest that disrupted cognitive processes involved in preparatory control of behavior may manifest clinically as difficulties suppressing prepotent behaviors, or continuing to engage in repetitive behaviors despite cues that they should be inhibited. For example, in novel or unexpected situations, individuals with ASD may display less flexible, but more compulsive/ritualistic behavior because of their reduced ability to proactively withhold and slow execution of contextually-inappropriate behaviors.
It also in possible that RRBs reflect an intolerance to uncertainty and/or anxiety as recently suggested by others (South & Rodgers, 2017). This hypothesis also may explain why RRBs sometimes manifest more frequently during social interaction when uncertainty may be higher. It is likely that RRBs are multi-factorial and reflect multiple cognitive and psychological mechanisms. Further research examining how these mechanisms interact and lead to distinct RRBs is warranted.
While we report multiple new findings, there are several limitations of this study that should be considered. First, while we studied a relatively large number of individuals with ASD, the generalizability of our findings may be limited because we targeted individuals with IQs in the broad range of average and who were not currently taking medications that may have confounded performance. Second, although we document moderate to larger effect sizes for impairments in stopping and slowing abilities, age-related findings showed relatively smaller effect sizes. Longitudinal studies accounting for intra-individual variations in inhibitory control will be important for elucidating these effects. Last, the variable durations between administration of the SST and clinical measures may have limited our estimates of the strength of the relationship between inhibitory control deficits and clinical issues. We expected that longer intervals between SST and clinical administrations could reduce the strengths of these associations, though they were largely unchanged when accounting for the amount of elapsed time. The relatively minimal impact of these intervals likely reflects the fact they were brief for the majority of participants.
Conclusions
Our results implicate deficits in proactive response slowing contribute to inhibitory control impairments in ASD. Additionally, our finding that inhibitory control impairments are related to more severe RRBs suggest shared underlying neurobiological processes involving dysmaturation fronto-basal-ganglia circuitry. Results showing that inhibitory control deficits in ASD become more severe in adolescence and adulthood indicate an expanded window of opportunity as well as vulnerability for these critical clinical symptoms. Furthermore, findings that individuals with obsessive-compulsive disorder (Chamberlain, Blackwell, Fineberg, Robbins, & Sahakian, 2006) and schizophrenia (Ethridge et al., 2014) also demonstrate inhibitory control deficits and fronto-basal-ganglia dysfunction suggests that common neurocognitive endophenotypes may cut across diagnostic boundaries and may be useful for identifying biologically-based targets for informing disease classification and treatment development.
Supplementary Material
Appendix S1. Secondary Analyses Results.
Table S1. Elapsed time (in weeks) between administration of clinical measures and the SST.
Table S2. Changepoint analyses of stopping accuracy comparing individuals with ASD and healthy controls.
Table S3. Changepoint analyses of age moderation of RT slowing effect on stopping accuracy.
Key points.
Deficits of inhibitory control have been repeatedly documented in autism spectrum disorder (ASD), yet the neurocognitive mechanisms contributing to these deficits have not been systematically investigated.
We provide novel evidence that reduced proactive control strategies contribute to inhibitory control deficits in ASD, and these deficits are related to more severe restricted, repetitive behaviours.
We also show that the age-related increases in the ability to withhold and strategically slow behavioural responses are attenuated in individuals with ASD.
Our findings implicate that dysfunctions of the fronto-striatal circuits that support behavioural response inhibition and proactive inhibitory control may underlie core features of ASD, and highlight an important developmental window during which treatments may mitigate cognitive alterations contributing to repetitive behaviours in ASD.
Acknowledgments
NIH Autism Center of Excellence P50HD055751; K23 MH092696, R01 MH112734, University of Kansas Center for Autism Research and Training Research Investment Council Strategic Initiative Grant. JS has consulted to Takeda Pharmaceuticals. MM is a consultant on an investigator-initiated study funded by Novartis.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Conflicts of interest statement: The remaining authors have declared that have no competing or potential conflicts of interest.
References
- Adams NC, Jarrold C. Inhibition in autism: children with autism have difficulty inhibiting irrelevant distractors but not prepotent responses. J Autism Dev Disord. 2012;42(6):1052–1063. doi: 10.1007/s10803-011-1345-3. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112(2):105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. Strategy implementation in obsessive-compulsive disorder and trichotillomania. Psychol Med. 2006;36(1):91–97. doi: 10.1017/S0033291705006124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz AM, Mosconi MW, Ragozzino ME, Cook EH, Sweeney JA. Alterations in the functional neural circuitry supporting flexible choice behavior in autism spectrum disorders. Transl Psychiatry. 2016;6(10):e916. doi: 10.1038/tp.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz AM, Mosconi MW, Steele S, Rubin LH, Luna B, Minshew N, Sweeney JA. Lateralized response timing deficits in autism. Biol Psychiatry. 2009;66(4):393–397. doi: 10.1016/j.biopsych.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales, 2nd edition: Introductory and technical handbook. San Antonio, TX: The Psychological Corporation; 2007. [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Hill SK, Keefe RS, Sweeney JA. Behavioral response inhibition in psychotic disorders: diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr Res. 2014;159(2–3):491–498. doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, van den Bergh SF, Ruzzano L. Prepotent response inhibition and interference control in autism spectrum disorders: two meta-analyses. Autism Res. 2014;7(4):407–420. doi: 10.1002/aur.1369. [DOI] [PubMed] [Google Scholar]
- Happe F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006;61(1):25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis. New York: The Guilford Press; 2013. [Google Scholar]
- Ishii-Takahashi A, Takizawa R, Nishimura Y, Kawakubo Y, Kuwabara H, Matsubayashi J, Kano Y. Prefrontal activation during inhibitory control measured by near-infrared spectroscopy for differentiating between autism spectrum disorders and attention deficit hyperactivity disorder in adults. Neuroimage Clin. 2014;4:53–63. doi: 10.1016/j.nicl.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? J Cogn Neurosci. 2010;22(7):1479–1492. doi: 10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, Fay LC. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15(4):349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Langen M, Bos D, Noordermeer SD, Nederveen H, van Engeland H, Durston S. Changes in the development of striatum are involved in repetitive behavior in autism. Biol Psychiatry. 2014;76(5):405–411. doi: 10.1016/j.biopsych.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Lemon JM, Gargaro B, Enticott PG, Rinehart NJ. Executive functioning in autism spectrum disorders: a gender comparison of response inhibition. J Autism Dev Disord. 2011;41(3):352–356. doi: 10.1007/s10803-010-1039-2. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Guter S, Kapur K, Macmillan C, Sweeney JA. Neurobehavioral abnormalities in first-degree relatives of individuals with autism. Arch Gen Psychiatry. 2010;67(8):830–840. doi: 10.1001/archgenpsychiatry.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, Sweeney JA. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. 2009;39(9):1559–1566. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. J Autism Dev Disord. 1997;27(1):59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Garver K, O’Hearn K, Nawarawong N, Liu R, Minshew N, Luna B. Developmental changes in brain function underlying inhibitory control in autism spectrum disorders. Autism Res. 2015;8(2):123–135. doi: 10.1002/aur.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PS, Klinger LG, Klinger MR. Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. J Autism Dev Disord. 2017 doi: 10.1007/s10803-017-3238-6. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008;68(3):415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview, Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schmitt LM, Ankeny LD, Sweeney JA, Mosconi MW. Inhibitory Control Processes and the Strategies That Support Them during Hand and Eye Movements. Front Psychol. 2016;7:1–14. doi: 10.3389/fpsyg.2016.01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11(5):437–451. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- South M, Rodgers J. Sensory, Emotional and Cognitive Contributions to Anxiety in Autism Spectrum Disorders. Front Hum Neurosci. 2017;11:20. doi: 10.3389/fnhum.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T, Estes AM, Dager SR, Kostopoulos P, Wolff JJ, Pandey J, Piven J. Emerging Executive Functioning and Motor Development in Infants at High and Low Risk for Autism Spectrum Disorder. Front Psychol. 2016;7:1016. doi: 10.3389/fpsyg.2016.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform. 2009;35(3):835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WP, Kahn RS. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum Brain Mapp. 2014;35(9):4415–4427. doi: 10.1002/hbm.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Happe F. Time perception in autism spectrum disorders. Res Autism Spectr Disord. 2008;2:447–455. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Fourth. New York, NY: The Psychological Corporation; 2012. [Google Scholar]
- Wessel JR, Aron AR. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron. 2017;93(2):259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Secondary Analyses Results.
Table S1. Elapsed time (in weeks) between administration of clinical measures and the SST.
Table S2. Changepoint analyses of stopping accuracy comparing individuals with ASD and healthy controls.
Table S3. Changepoint analyses of age moderation of RT slowing effect on stopping accuracy.


